Abstract
Recent data indicate an important contribution of coagulation factor (F)XII to in vivo thrombus formation. Because fibrin structure plays a key role in clot stability and thrombosis, we hypothesized that FXII(a) interacts with fibrin(ogen) and thereby regulates clot structure and function. In plasma and purified system, we observed a dose-dependent increase in fibrin fiber density and decrease in turbidity, reflecting a denser structure, and a nonlinear increase in clot stiffness with FXIIa. In plasma, this increase was partly independent of thrombin generation, as shown in clots made in prothrombin-deficient plasma initiated with snake venom enzyme and in clots made from plasma deficient in FXII and prothrombin. Purified FXII and α-FXIIa, but not β-FXIIa, bound to purified fibrinogen and fibrin with nanomolar affinity. Immunostaining of human carotid artery thrombi showed that FXII colocalized with areas of dense fibrin deposition, providing evidence for the in vivo modulation of fibrin structure by FXIIa. These data demonstrate that FXIIa modulates fibrin clot structure independently of thrombin generation through direct binding of the N-terminus of FXIIa to fibrin(ogen). Modification of fibrin structure by FXIIa represents a novel physiologic role for the contact pathway that may contribute to the pathophysiology of thrombosis.
Introduction
Blood coagulation culminates in the formation of fibrin, which binds platelets and forms a clot. Fibrin is formed from fibrinogen via cleavage of 2 fibrinopeptides from the Aα- and Bβ-chains N-termini, located in the E-region, by thrombin.1 Fibrinopeptide cleavage exposes binding sites for complementary sites in the D-region, triggering polymerization and the production of protofibrils. Protofibrils aggregate laterally to form fibers, which branch out and form a 3-dimensional network.2 There is increasing evidence that the structure of fibrin regulates thrombosis. Dense fibrin clots with small pores and increased fiber density are more resistant to lysis.3 Structural characteristics affect the mechanical properties of fibrin.4 Both venous and arterial thrombosis has been associated with the formation of an altered fibrin network.5-10
The role of factor (F)XII in hemostasis has long been contested because deficiency in FXII, unlike deficiencies of other coagulation factors, does not lead to bleeding diathesis in humans11 or in mice.12 However, recent in vivo data show that FXII deficiency or inhibition in rodent models reduces thrombus formation while maintaining normal hemostasis.12-15 These findings indicate the existence of FXII-related mechanisms that are preferentially involved in thrombosis but not hemostasis.
Contact activation is triggered by the binding of FXII (80 kDa) to a negatively charged surface and involves the formation of α-FXIIa via autocatalysis. Bound α-FXIIa converts prekallikrein into kallikrein. Kallikrein can further convert α-FXIIa to β-FXIIa by an additional cleavage at R334-N335. α-FXIIa consists of a heavy and light chain that are disulphide linked (80 kDa), whereas β-FXIIa (28 kDa) lacks the heavy chain and loses its capacity to bind to negatively charged surfaces.16 The N-terminal region of FXII (α-FXIIa heavy chain) shows strong homology with tissue-type plasminogen activator (tPA), with the presence of fibronectin type I, epidermal growth factor, and Kringle domains.17,18
In view of the homology between FXII and tPA and in search for a mechanism by which to explain the differential roles of FXII in thrombosis and hemostasis, we hypothesized that FXII(a) interacts with fibrin(ogen) and regulates clot structure and function. We find that FXII(a) binds to fibrin(ogen), largely via its N-terminus, and that this interaction leads to changes in fibrin structure, elasticity, and susceptibility to lysis, in part independently of thrombin generation.
Methods
Reagents
Human plasminogen-free fibrinogen, FXII, α-FXIIa, β-FXIIa, and thrombin were from Enzyme Research Laboratories. Fibrinogen Alexa Fluor–488 was from Invitrogen. Congenital FXII-deficient plasma was from George King Bio-Medical. Immunodepleted FXII–deficient plasma was from American Diagnostica. Corn trypsin inhibitor (CTI), normal citrated plasma immunodepleted of prothrombin, polyclonal sheep anti–human prothrombin, and monoclonal anti–human prothrombin were from Haematologic Technologies. CNBr-activated Sepharose 4B and protein G-Sepharose 4 Fast Flow were from GE Healthcare Bio-Sciences. PPACK (H-D-Phe-Pro-Arg-chloromethylketone, 2HCL) was from Calbiochem. Sulfatides, benzamidine, and BSA were from Sigma-Aldrich. Chromogenic substrates S-2238 and S-2302 were from Chromogenix. Recombinant tPA was from Boehringer Ingelheim. Synthetic phospholipids 1,2-dioleoyl-sn-glycero-3-phosphoserine (DOPS), 1,2-dioeoyl-sn-glycero-3-phosphocholine (DOPC), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were from Avanti Polar Lipids and were prepared by sonication as described earlier (DOPS/DOPC/DOPE, 20 mol/60 mol/20 mol).19 Ecarin was from Pentapharm. Ancrod was from WHO International Laboratory for Biologic Standards, and recombinant hirudin was from Hyphen BioMed. Polyclonal sheep anti–human fibrinogen and polyclonal goat anti–human FXII were from Affinity Biologicals, polyclonal rabbit anti–human fibrinogen (1:200, #A0080) was from Dako, monoclonal mouse anti–human fibrin (1:200, LS-C23559) was from Lifespan Biosciences, and monoclonal mouse anti–human FXII (1:50) was in house.20
Fibrin formation and fibrinolysis by turbidity
Fibrin polymerization of purified proteins or plasma was monitored in low binding polystyrene 96-well plates (Greiner) by the change in turbidity at 405 nm (A405) every 15 seconds, for at least 1.5 hours at 37°C using a ELx808 plate reader (Biotek Instruments).
Thrombin (0-5nM) and CaCl2 (5mM) were incubated at 37°C for 10 minutes in the 96-well plates after which fibrinogen (1 mg/mL), preincubated with α-FXIIa (0-125nM), β-FXIIa (94nM), or FXII (94nM) in HEPES buffer (25mM HEPES, pH 7.4, 150mM NaCl) for 10 minutes at 37°C, was added. All concentrations are final concentrations.
To congenital FXII–deficient plasma (final concentration, 76%) we added FXII (0%-100%; normal FXII concentration, 375nM21 ) and fibrin formation was initiated with sulfatide (4μM), a natural activator of FXII present in mammalian tissue22 and CaCl2 (16mM), in the presence of phospholipid vesicles (4μM). To monitor fibrinolysis, tPA (0.1 μg/mL) was added to the clotting mixture. Clot lysis time (to 50% lysis) was calculated as the time between maximal and half-maximal turbidity.
Inhibition with PPACK
To inhibit the enzymatic activity of α-FXIIa, α-FXIIa (250nM) was incubated with PPACK (1000nM) for 30 minutes at room temperature, in HEPES buffer (25mM HEPES, pH 7.4, 150mM NaCl, 1 mg/mL BSA). After incubation, the free PPACK was extensively dialyzed. Using chromogenic substrates, inhibition of FXIIa (S-2302) and removal of PPACK (determined by the inhibition of thrombin with S-2238) were determined.
Confocal/2-photon microscopy
Fibrinogen (0.5 mg/mL) or diluted plasma (25%) was added to a microchamber (μ-slide VI for Live Cell analysis; ibidi 80601) together with 5% Alexa Fluor–488 fibrinogen. After addition of CaCl2 (5mM) and thrombin (0.625nM), sulfatides (0.4μM), or ancrod (0.1 U/mL), clots were formed for minimally 2 hours in the dark, in a moist atmosphere at room temperature. Experiments in plasma were performed in the presence of phospholipid vesicles (4μM).
Purified fibrinogen was incubated with α-FXIIa (0-30nM), β-FXIIa (0-30nM), or FXII (0-30nM). In congenital FXII–deficient plasma and double-deficient plasma (deficient in FXII and prothrombin), we added purified FXII (0%-100% in undiluted plasma) activated with sulfatides. To plasma immunodepleted of prothrombin, we added hirudin (final concentration, 30nM) to block residual thrombin activity and α-FXIIa (30nM) or CTI (75 μg/mL).
For laser scanning confocal microscopy (Upright Zeiss LSM-510 META Axioplan 2), clots were visualized with a 40×/1.3 NA oil objective, using LSM 510 Version 4.2 software. Alexa Fluor–488 fibrinogen was excited with a 488 nm argon laser. Images were averaged 8 times. For the 2-photon laser scanning microscopy,23 images were recorded with an Eclipse E600FN upright microscope (Nikon) equipped with a Radiance 2100MP optical imaging system (Bio-Rad). Fluorophores were excited by a Spectra-Physics Tsunami Ti:Sapphire laser (Spectra-Physics) mode-locked at 800 nm. Images were obtained with a 40×/1.3 oil NA objective using Lasersharp 2000 Version 6.0 software and fluorescence was detected at 520-560 nm. Further magnification was achieved by optical zoom in the scan head. Images were taken in 5 different areas of the clot. ImageJ Version 1.43 (National Institutes of Health) was used to determine fiber density, by counting the number of fibers crossing lines of 100 μm placed in the image using the plug-in grid.
Scanning EM
Scanning electron microscopy (EM) was carried out as described24 with the following modifications. Clots were formed by mixing fibrinogen (1 mg/mL) with 0.25 U/mL thrombin and 5mM CaCl2 in the absence and presence of α-FXIIa (0, 31.25, or 125nM) in 20mM HEPES, pH 7.4, 150mM NaCl. After 2 hours at room temperature, clots were washed in sodium cacodylate buffer and fixed overnight in 2% glutaraldehyde. Clots were dehydrated with an acetone gradient and sputter coated with platinum palladium. Plasma clots were prepared in a similar manner using immunodepleted FXII–deficient plasma with 0.1 U/mL thrombin, 10mM CaCl2, and 4μM phospholipid vesicles. In some experiments, CTI (75 μg/mL) was added to inhibit FXIIa. Samples were analyzed with a field-emission scanning EM (FEI Quanta 200F) in 10 different areas of the clot and over at least 3 different samples.
Fibrin pore structure
The average pore size of the fibrin clot (expressed as the Darcy constant Ks) was determined in permeation studies, where the flow rate of a buffer through a fibrin clot is measured. FXII-deficient plasma, to which 1%, 10%, or 100% FXII was added, was incubated with sulfatides (1μM), phospholipid vesicles (4μM), and CaCl2 (16mM) for 4 hours in a moist chamber at ambient temperature. Permeation of HEPES buffer (25mM HEPES, pH 7.4, 150mM NaCl) through the clot was quantified as described previously.25 Briefly, using the flow rate and the following equation: Ks = (Q × L × η)/(T × A × P), the Darcy constant Ks in cm2 can be calculated, where Q = volume of liquid (mL), L = clot length (cm), η = viscosity (poise), T = time (s), A = cross-sectional area (cm2), and P = pressure drop (dyne/cm).
Clot viscoelasticity
A magnetic microrheometer, previously described,26,27 was used to examine clot viscoelastic properties. The procedure of Evans et al28 was used to extract the frequency-dependent storage (G′, elastic energy stored during deformation and clot stiffness) and loss (G″, energy dissipated during deformation) modulus. The magnetic microrheometer operates by exerting a force on a 4.5-μm superparamagnetic particle (Dynal) using an external magnetic field generated by 4 electromagnets. The device was used in conjunction with an Olympus IX71 inverted optical microscope incorporating an ultra-long working distance objective (40×/0.55 NA) and CCD camera. Particle tracking, electromagnet control, and image analysis were performed using custom-written Labview Version 7.1 software (National Instruments). Purified fibrinogen (1 mg/mL) was mixed with CaCl2 (5.0mM), TBS buffer (50mM Tris, 150mM NaCl, pH 7.4), magnetic particles suspended in distilled water and FXIIa at 0, 31.25, or 125nM. Thrombin (0.25 U/mL) was added, and the mixture was quickly transferred to a square glass capillary. For plasma clots, FXII-deficient plasma was diluted 6 times in TBS buffer and then mixed with CaCl2 (7.5mM), magnetic particles, and FXIIa.
FXII-FII double-deficient plasma
Congenital FXII–deficient plasma was batch-wise immunodepleted for prothrombin. We coupled polyclonal sheep antihuman prothrombin and monoclonal antihuman prothrombin onto CNBr-activated Sepharose 4B. This Sepharose was added to the plasma and rotated for 0.5 hour. The plasma was removed and the Sepharose washed with high salt buffer (25mM HEPES, pH 7.4, 1M NaCl), followed with low salt buffer (25mM HEPES, pH 7.4, 150mM NaCl). The procedure was repeated until prothrombin concentration did not change. Turbidity measurements showed that the process also removed part of the fibrinogen. Therefore, purified fibrinogen was added to the undiluted plasma to achieve a final concentration of 2 mg/mL.
Prothrombin concentrations were quantified with the chromogenic substrate S2238 in triplicate in a 1:100 final dilution of plasma after complete activation with Ecarin, the venom activator of Echis carinatus (0.5 U/mL).
Thrombin generation
The calibrated automated thrombogram method (thrombinoscope) was used to measure thrombin generation with several modifications. We added purified FXII to congenital FXII–deficient plasma (0%-100%) and to plasma deficient in FXII and prothrombin. Clotting was triggered with 0.4μM sulfatide and 4μM phospholipid vesicles, and determination of thrombin generation was started on addition of fluorogenic Z-Gly-Gly-Arg-AMC substrate with 16mM CaCl2 and was followed continuously in plasma (final concentration, 67%).
Surface plasmon resonance
Binding of FXII, α-FXIIa, and β-FXIIa to fibrinogen and fibrin was analyzed with a Biacore 3000 (BIAcore) as previously described,24,29 with the following modifications. Samples were analyzed in 20mM HEPES, 140mM NaCl, and 2.5mM CaCl2 with 0.05% P-20, pH 7.4, and the experiments were performed at 25°C. Fibrinogen was coated to a carboxy-methyldextra–coated biosensor chip (CM5) by amine coupling, to yield ∼ 1000 response units. Immobilized fibrinogen was converted to fibrin by running 5 U/mL of thrombin at 2 μL/minute for 20 minutes. Thrombin was removed by injecting 1M NaCl.
FXII, α-FXIIa, or β-FXIIa was dialyzed overnight in 20mM HEPES, 140mM NaCl, and 2.5mM CaCl2. Protein concentrations were measured by nanodrop 3.1.0 (Thermo Scientific). Extinction coefficients (1%, 280 nm) of 14.1 and 15.2 were used for calculation of the concentration for FXII, α-FXIIa, and β-FXIIa, respectively.30 FXII, α-FXIIa, or β-FXIIa preparations were injected for 3 minutes at 30 μL/minute, and the dissociation was monitored for 3 minutes. The surface was regenerated with 3M NaCl, pH 7.4, at 30 μL/minute, followed by buffer (60 μL) and re-equilibration with running buffer for 5 minutes. Benzamidine (5mM) was included during dialysis and in the running buffer to prevent FXII activation. Control experiments with gel electrophoresis and amidolytic activity with S2302 showed negligible FXII activity after dialysis (data not shown).
Immunoprecipitation
FXII was immunoprecipitated from normal pooled plasma with a polyclonal antihuman FXII antibody. The immunoprecipitate was isolated from plasma by protein G-Sepharose 4 Fast Flow. The Sepharose was intensively washed, and the extracted protein was subjected to SDS gel electrophoresis. The sample was run on 2 separate 4%-15% acrylamide gradient gels (Bio-Rad) with Tris/glycine buffer. Afterward, the proteins were analyzed by Western blotting and transferred to sheets of Immobilon-P transfer membrane and detected with (1) a polyclonal goat anti–human FXII antibody and (2) a polyclonal sheep anti–human fibrinogen antibody.
Immunostaining
Three thrombi were collected during carotid endarterectomies. The specimens were obtained from the Maastricht Pathology Tissue Collection. Collection, storage, and use of tissue and patient data were performed in agreement with the Code for Proper Secondary Use of Human Tissue in The Netherlands (http://www.fmwv.nl). Paraffin sections (4 μm) were immunohistochemically stained for both fibrin(ogen) and FXII(a) as described.31 Images were captured with a Zeiss Axioskop 40 light microscope equipped with 2.5×/0.06 NA, 10×/0.25 NA, and 40×/0.65 NA objectives, using a Cannon Powershot G9 camera.
Statistical analysis
Data are mean and range or SEM as indicated. Statistical analyses were performed with GraphPad Prism Version 5, using 1-way ANOVA, Bonferroni for posthoc comparison, or t test (GraphPad Software) when appropriate, and P values < .05 were considered statistically significant.
Results
FXIIa and fibrin structure in purified systems
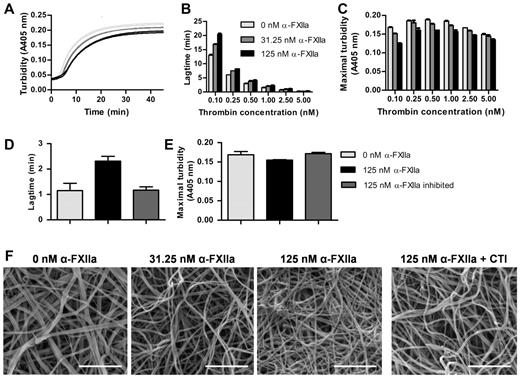
At a fixed thrombin concentration, purified α-FXIIa increased the lag time to fibrin formation and decreased maximal turbidity (Figure 1A). Because thrombin is an important determinant of fibrin formation and clot architecture, we confirmed that these effects of α-FXIIa on clot formation and structure occur at different thrombin concentrations (Figure 1B-C). The effects were strongest at the lower end of the thrombin concentrations tested. Next, fibrin clots were visualized by scanning EM in the presence of α-FXIIa at a fixed thrombin concentration (2.5nM). Figure 1F shows a dose-dependent increase in fibrin structure compactness, with thinner fibers and smaller pores at higher α-FXIIa levels compared with control clots. These findings agree with the lower maximum turbidity observed in the presence of α-FXIIa because lower turbidity is correlated with thinner fibrin fibers.32 The presence of FXII-zymogen or β-FXIIa did not influence fibrin clot structure by turbidimetric assays or microscopy (data not shown). Inhibition of α-FXIIa with PPACK or CTI reversed the effect on fibrin structure, indicating that the proteolytic activity of α-FXIIa is necessary to change fibrin structure (Figure 1D-F).
Effect of α-FXIIa on fibrin polymerization and fibrin structure. (A-E) Human fibrinogen (1 mg/mL) was incubated with α-FXIIa (0-125nM) in HEPES buffer (25mM HEPES, 150mM NaCl, pH 7.4) for 10 minutes at 37°C before clotting was initiated with CaCl2 (5mM) and thrombin (0.1-5nM). Turbidity was monitored every 15 seconds at 405 nm at 37°C. (A) Time course of fibrin clot formation with 3 α-FXIIa concentrations (0, 31.25, and 125nM; each in duplicate) initiated with 0.5nM thrombin. (B) Lag time of fibrin formation and (C) maximal turbidity as a function of thrombin concentration. (D-E) To inhibit α-FXIIa, PPACK (1000nM) was incubated with α-FXIIa and removed via dialysis in HEPES buffer (25mM HEPES, 150mM NaCl, 1 mg/mL BSA, pH 7.4). Clotting was initiated with 1nM thrombin. (D) Lag time of fibrin formation and (E) maximal turbidity. (B-E) Data are mean ± range of 2 separate experiments. (F) Representative scanning EM images of clots (n = 6) prepared by incubating fibrinogen (1 mg/mL) with α-FXIIa (0-125nM), thrombin (2.5nM), and CaCl2 (5mM) in HEPES buffer (20mM HEPES, pH 7.4, 150mM NaCl) for 2 hours at room temperature. To inhibit α-FXIIa, CTI (75 μg/mL) was added to 125nM α-FXIIa. Scale bars represent 1μm.
Effect of α-FXIIa on fibrin polymerization and fibrin structure. (A-E) Human fibrinogen (1 mg/mL) was incubated with α-FXIIa (0-125nM) in HEPES buffer (25mM HEPES, 150mM NaCl, pH 7.4) for 10 minutes at 37°C before clotting was initiated with CaCl2 (5mM) and thrombin (0.1-5nM). Turbidity was monitored every 15 seconds at 405 nm at 37°C. (A) Time course of fibrin clot formation with 3 α-FXIIa concentrations (0, 31.25, and 125nM; each in duplicate) initiated with 0.5nM thrombin. (B) Lag time of fibrin formation and (C) maximal turbidity as a function of thrombin concentration. (D-E) To inhibit α-FXIIa, PPACK (1000nM) was incubated with α-FXIIa and removed via dialysis in HEPES buffer (25mM HEPES, 150mM NaCl, 1 mg/mL BSA, pH 7.4). Clotting was initiated with 1nM thrombin. (D) Lag time of fibrin formation and (E) maximal turbidity. (B-E) Data are mean ± range of 2 separate experiments. (F) Representative scanning EM images of clots (n = 6) prepared by incubating fibrinogen (1 mg/mL) with α-FXIIa (0-125nM), thrombin (2.5nM), and CaCl2 (5mM) in HEPES buffer (20mM HEPES, pH 7.4, 150mM NaCl) for 2 hours at room temperature. To inhibit α-FXIIa, CTI (75 μg/mL) was added to 125nM α-FXIIa. Scale bars represent 1μm.
FXIIa and clot architecture in plasma
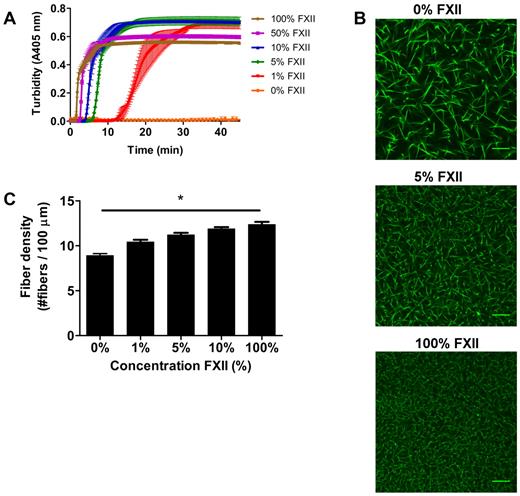
We next investigated the impact of FXIIa on fibrin clot structure in plasma. Turbidity showed a dose-dependent decrease in lag time and maximum turbidity with increasing FXII concentration (Figure 2A). No clotting was observed without the addition of FXII to FXII-deficient plasma within 60 minutes.
Effect of FXII-concentration on fibrin structure in plasma. (A) FXII-deficient plasma was reconstituted with purified FXII, and clotting was initiated via contact activation with sulfatides, in the presence of phospholipid vesicles and CaCl2. Turbidity was monitored every 15 seconds at 405 nm at 37°C. Final concentrations were 76% plasma, variable FXII concentrations (0%-100% of normal plasma concentration), 4μM sulfatides, 4μM phospholipid vesicles, and 16mM CaCl2. Data are mean ± range of 3 measurements. (B) Representative figures of immunofluorescent staining of fibrin clots. FXII-deficient plasma was reconstituted with purified FXII. Alexa Fluor–488 fibrinogen was added, and clotting was initiated with sulfatide, phospholipid vesicles, and CaCl2. Final concentrations were 25% plasma, 5% Alexa Fluor–488 fibrinogen of the total fibrinogen concentration, a range of FXII (0%-100% of normal plasma concentration), 0.4μM sulfatides, 4μM phospholipid vesicles, 5mM CaCl2, 25mM HEPES (pH 7.4), and 150mM NaCl. (C) Fiber density was calculated from the data shown in panel B. Per condition, 2 separate clots were made, images were taken in different areas of the clot, and fiber density was determined in 5 images by counting the number of fibers that cross a line of 100 μm. Bars represent mean ± SEM. *P < .05. Scale bars represent 25 μm.
Effect of FXII-concentration on fibrin structure in plasma. (A) FXII-deficient plasma was reconstituted with purified FXII, and clotting was initiated via contact activation with sulfatides, in the presence of phospholipid vesicles and CaCl2. Turbidity was monitored every 15 seconds at 405 nm at 37°C. Final concentrations were 76% plasma, variable FXII concentrations (0%-100% of normal plasma concentration), 4μM sulfatides, 4μM phospholipid vesicles, and 16mM CaCl2. Data are mean ± range of 3 measurements. (B) Representative figures of immunofluorescent staining of fibrin clots. FXII-deficient plasma was reconstituted with purified FXII. Alexa Fluor–488 fibrinogen was added, and clotting was initiated with sulfatide, phospholipid vesicles, and CaCl2. Final concentrations were 25% plasma, 5% Alexa Fluor–488 fibrinogen of the total fibrinogen concentration, a range of FXII (0%-100% of normal plasma concentration), 0.4μM sulfatides, 4μM phospholipid vesicles, 5mM CaCl2, 25mM HEPES (pH 7.4), and 150mM NaCl. (C) Fiber density was calculated from the data shown in panel B. Per condition, 2 separate clots were made, images were taken in different areas of the clot, and fiber density was determined in 5 images by counting the number of fibers that cross a line of 100 μm. Bars represent mean ± SEM. *P < .05. Scale bars represent 25 μm.
The lag time decreased with increasing levels of FXII, presumably because of increased thrombin generation via FXII activation. To view the corresponding fibrin structure, we performed confocal microscopy experiments. Figure 2B-C show a dose-dependent increase in fiber density with increasing levels of FXII (P < .05). Moreover, scanning EM in plasma at higher FXII concentrations revealed a denser structure with thinner fibers (data not shown) and the permeation constant (Darcy constant Ks), which is a direct measure of the pore size, decreased at higher levels of FXII indicating smaller pore size. The Ks for clots produced with 1μM sulfatides and 1%, 10%, or 100% FXII in FXII-deficient plasma was 33.2, 26.4, and 19.3 × 10−9 cm2, respectively (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Furthermore, turbidimetric lysis assays showed that the clot lysis time in plasma increased and the maximum rate of lysis decreased in a FXII concentration-dependent manner (supplemental Figure 1).
Viscoelastic properties
We used magnetic tweezers equipment to probe the effect of FXIIa on clot viscoelastic properties. We found a nonlinear increase in clot stiffness (G′) in the presence of α-FXIIa. Stiffness of clots made from purified fibrinogen increased nearly 2-fold with 31nM and 1.3-fold with 125nM α-FXIIa (Table 1). The stiffness of plasma clots increased 1.7-fold and 1.2-fold with 31nM and 125nM α-FXIIa, respectively. The nonlinearity of these data suggests that an optimal degree of fiber branching and thickness occurs, which leads to maximal stiffness. At higher α-FXIIa concentration, fibers may become too thin to support maximal stiffness. The loss modulus (G″) and viscous fraction (G″/G′) of the clot did not change significantly with FXIIa.
Effects of FXIIa on viscoelastic properties of fibrin clots produced with purified fibrinogen and plasma
| [α-FXIIa], nM . | Plasma . | Purified fibrinogen . | ||||
|---|---|---|---|---|---|---|
| 0 . | 31 . | 125 . | 0 . | 31 . | 125 . | |
| G′, Pa | 2.89 ± 0.07 | 4.9 ± 0.2 | 3.47 ± 0.05 | 1.95 ± 0.05 | 3.84 ± 0.08 | 2.52 ± 0.04 |
| G″, mPa | 0.8 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.1 | 0.20 ± 0.02 | 0.75 ± 0.02 | 0.32 ± 0.03 |
| G″/G′, 10−3 | 0.28 ± 0.02 | 0.22 ± 0.04 | 0.36 ± 0.02 | 0.10 ± 0.01 | 0.20 ± 0.07 | 0.36 ± 0.03 |
| [α-FXIIa], nM . | Plasma . | Purified fibrinogen . | ||||
|---|---|---|---|---|---|---|
| 0 . | 31 . | 125 . | 0 . | 31 . | 125 . | |
| G′, Pa | 2.89 ± 0.07 | 4.9 ± 0.2 | 3.47 ± 0.05 | 1.95 ± 0.05 | 3.84 ± 0.08 | 2.52 ± 0.04 |
| G″, mPa | 0.8 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.1 | 0.20 ± 0.02 | 0.75 ± 0.02 | 0.32 ± 0.03 |
| G″/G′, 10−3 | 0.28 ± 0.02 | 0.22 ± 0.04 | 0.36 ± 0.02 | 0.10 ± 0.01 | 0.20 ± 0.07 | 0.36 ± 0.03 |
G′ indicates storage modulus or clot stiffness; G″, loss modulus; and G″/G′, viscous fraction of the clot.
Thrombin-independent effects
FXII and prothrombin-deficient plasma.
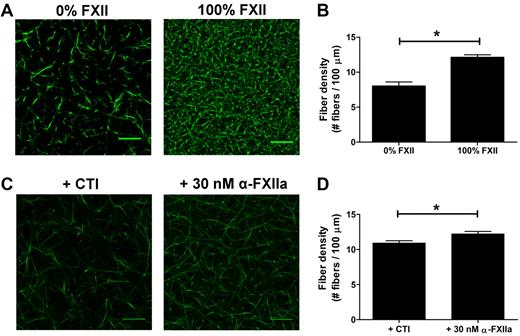
As thrombin influences fibrin clot structure,33 we aimed to investigate whether (at least part of) the effects of FXIIa on fibrin structure in plasma were independent of thrombin generation. First, we compared thrombin generation in FXII-deficient plasma to which purified FXII (up to 100%) was added with that in the absence of FXII. Fully reconstituted FXII-deficient plasma showed a 38-fold increase in thrombin peak height compared with the nonreconstituted plasma (295nM IIa vs 7.7nM IIa; supplemental Figure 2A; supplemental Table 2). To test whether FXII also contributes to a fibrin structure change independent of the effect on thrombin formation, the prothrombin in the FXII-deficient plasma was removed by immunodepletion to yield plasma deficient in both FXII and prothrombin. The prothrombin concentration dropped to 3.2% as measured after activation of the prothrombin with Ecarin and quantification with S-2238. Using this double-deficient plasma, we analyzed the effect of FXII (0%-100%) on fibrin structure. Sulfatides were added to initiate contact activation, and fibrin formation was initiated after 10 minutes by addition of thrombin (0.625nM), phospholipid vesicles, and CaCl2. Confocal microscopy showed a denser fibrin structure at higher FXII levels (Figure 3A-B), despite a marginal increase in thrombin peak height from 13.3nM in the absence of FXII to 16.2nM with 100% FXII (supplemental Figure 2B; supplemental Table 2).
Effect of FXIIa on fibrin fiber density, independent from additional thrombin formation. (A-B) Plasma deficient in FXII and prothrombin was reconstituted with FXII. Sulfatides and Alexa Fluor–488 fibrinogen were added, and clotting was initiated with 0.625nM thrombin, in the presence of phospholipid vesicles and CaCl2. Final concentrations were 25% plasma, 5% Alexa Fluor–488 fibrinogen of the total fibrinogen concentration, FXII (0% or 100% of normal plasma concentration), 0.4μM sulfatides, 4μM phospholipid vesicles, 5mM CaCl2, 25mM HEPES (pH 7.4), and 150mM NaCl. Per condition, 2 separate clots were made, and pictures were taken in different areas of the clot. (C-D) Prothrombin-deficient plasma, in the presence of hirudin, was incubated with the FXIIa inhibitor CTI or with α-FXIIa. Alexa Fluor–488 fibrinogen was added to the plasma, and clotting was initiated by the addition of ancrod and CaCl2. Final concentrations were 25% plasma, 30nM hirudin, 75 μg/mL CTI, 30nM α-FXIIa, 5% Alexa Fluor–488 fibrinogen of the total fibrinogen concentration, 0.1 U/mL ancrod, 5mM CaCl2, 25mM HEPES (pH 7.4), and 150mM NaCl. (B,D) Fiber density was calculated from the data shown in the corresponding panel. Per condition, 2 separate clots were made, pictures were taken in different areas of the clot, and fiber density was determined in 5 pictures by counting the number of fibers that crosses a line of 100 μm. Bars represent mean ± SEM. *P < .05. Scale bars represent 25 μm.
Effect of FXIIa on fibrin fiber density, independent from additional thrombin formation. (A-B) Plasma deficient in FXII and prothrombin was reconstituted with FXII. Sulfatides and Alexa Fluor–488 fibrinogen were added, and clotting was initiated with 0.625nM thrombin, in the presence of phospholipid vesicles and CaCl2. Final concentrations were 25% plasma, 5% Alexa Fluor–488 fibrinogen of the total fibrinogen concentration, FXII (0% or 100% of normal plasma concentration), 0.4μM sulfatides, 4μM phospholipid vesicles, 5mM CaCl2, 25mM HEPES (pH 7.4), and 150mM NaCl. Per condition, 2 separate clots were made, and pictures were taken in different areas of the clot. (C-D) Prothrombin-deficient plasma, in the presence of hirudin, was incubated with the FXIIa inhibitor CTI or with α-FXIIa. Alexa Fluor–488 fibrinogen was added to the plasma, and clotting was initiated by the addition of ancrod and CaCl2. Final concentrations were 25% plasma, 30nM hirudin, 75 μg/mL CTI, 30nM α-FXIIa, 5% Alexa Fluor–488 fibrinogen of the total fibrinogen concentration, 0.1 U/mL ancrod, 5mM CaCl2, 25mM HEPES (pH 7.4), and 150mM NaCl. (B,D) Fiber density was calculated from the data shown in the corresponding panel. Per condition, 2 separate clots were made, pictures were taken in different areas of the clot, and fiber density was determined in 5 pictures by counting the number of fibers that crosses a line of 100 μm. Bars represent mean ± SEM. *P < .05. Scale bars represent 25 μm.
Fibrin formation by ancrod.
To further investigate the direct effect of FXIIa on clot structure in plasma, we clotted prothrombin-depleted plasma (prothrombin < 1%) with the fibrin snake venom activator ancrod in the presence of hirudin, a thrombin inhibitor. We added either α-FXIIa or CTI (a potent inhibitor of FXIIa) to the plasma. Confocal microscopy of these clots showed an increase in the fiber density in the presence of α-FXIIa compared with inhibitor (Figure 3C-D).
FXII(a) binding to fibrin(ogen)
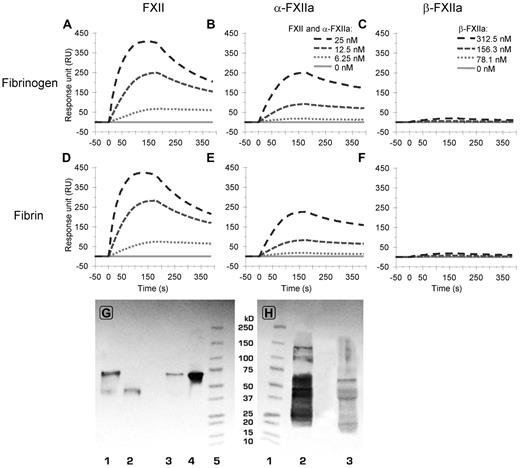
In view of the homology between FXII and tPA, we investigated whether FXII, α-FXIIa, and β-FXIIa bind to fibrinogen and fibrin by surface plasmon resonance.24,29 Figure 4 shows binding of FXII, α-FXIIa, and β-FXIIa to fibrinogen and fibrin at 4 protein concentrations. Supplemental Figures 3 and 4 show all tested concentrations, including separate global kd and ka fittings used for calculation of kinetic constants. FXII and α-FXIIa bound with similar affinity to fibrinogen and fibrin (Figure 4; Table 2). Overall binding response was higher for FXII compared with similar concentrations of α-FXIIa. The affinity of β-FXIIa to fibrinogen and fibrin was 20- to 40-fold lower (Table 2) compared with FXII or α-FXIIa, and a significant binding response was only observed at 300nM β-FXIIa and higher concentrations (Figure 4; supplemental Figure 4). These data indicate the presence of a high-affinity binding site on the heavy chain of FXII and α-FXIIa for fibrinogen and fibrin.
Binding of FXII(a) to fibrin(ogen) analyzed by surface plasmon resonance. (A-B) The binding to fibrinogen (from top to bottom, 25, 12.5, 6.25, and 0nM FXII (A) and α-FXIIa (B), respectively). (C) The (negligible) binding of β-FXIIa to fibrinogen (from top to bottom, 312.5, 156.3, 78.1, and 0nM, respectively). (Bottom panels) Binding of FXII (D), α-FXIIa (E), and β-FXIIa (F) to fibrin at identical concentrations as in panels A to C, respectively. For reasons of clarity, not all tested concentrations are shown in this figure. Supplemental Figures 3 and 4 show the responses for all tested concentrations, including the ka and kd fitting, which were used to determine the Kd values as presented in Table 2. The graphs represent the mean of 3 experiments. (G-H) Western blots after immunoprecipitation of FXII from normal pooled plasma. (G) The blot was stained for FXII with polyclonal antihuman FXII. (H) The blot was stained for fibrinogen with polyclonal antihuman fibrinogen. (G) Lane 1 (0.05 μg nonreduced FXIIa) and lane 2 (0.05 μg reduced FXIIa) are controls, lane 3 is reduced immunoprecipitate, lane 4 is nonreduced immunoprecipitate, and lane 5 is a molecular weight marker. (H) Lane 1 is a molecular weight marker, lane 2 (0.25 μg reduced fibrinogen) is control, and lane 3 is reduced immunoprecipitate. The blots show the presence of zymogen FXII (80 kDa; G) and the Aα-chain (66 kDa), Bβ-chain (52 kDa), and γ-chain (46 kDa) of fibrinogen (H).
Binding of FXII(a) to fibrin(ogen) analyzed by surface plasmon resonance. (A-B) The binding to fibrinogen (from top to bottom, 25, 12.5, 6.25, and 0nM FXII (A) and α-FXIIa (B), respectively). (C) The (negligible) binding of β-FXIIa to fibrinogen (from top to bottom, 312.5, 156.3, 78.1, and 0nM, respectively). (Bottom panels) Binding of FXII (D), α-FXIIa (E), and β-FXIIa (F) to fibrin at identical concentrations as in panels A to C, respectively. For reasons of clarity, not all tested concentrations are shown in this figure. Supplemental Figures 3 and 4 show the responses for all tested concentrations, including the ka and kd fitting, which were used to determine the Kd values as presented in Table 2. The graphs represent the mean of 3 experiments. (G-H) Western blots after immunoprecipitation of FXII from normal pooled plasma. (G) The blot was stained for FXII with polyclonal antihuman FXII. (H) The blot was stained for fibrinogen with polyclonal antihuman fibrinogen. (G) Lane 1 (0.05 μg nonreduced FXIIa) and lane 2 (0.05 μg reduced FXIIa) are controls, lane 3 is reduced immunoprecipitate, lane 4 is nonreduced immunoprecipitate, and lane 5 is a molecular weight marker. (H) Lane 1 is a molecular weight marker, lane 2 (0.25 μg reduced fibrinogen) is control, and lane 3 is reduced immunoprecipitate. The blots show the presence of zymogen FXII (80 kDa; G) and the Aα-chain (66 kDa), Bβ-chain (52 kDa), and γ-chain (46 kDa) of fibrinogen (H).
Surface plasmon resonance analyses of FXII(a) binding to fibrin(ogen)
| . | kd (off-rate, s−1) . | ka (on-rate, M−1s−1) . | Kd (dissociation constant, M) . |
|---|---|---|---|
| FXII to fibrinogen | 2.9 × 10−3 | 9.8 × 105 | 3.0 × 10−9 |
| FXII to fibrin | 2.9 × 10−3 | 11.8 × 105 | 2.5 × 10−9 |
| α-FXIIa to fibrinogen | 1.8 × 10−3 | 4.3 × 105 | 4.1 × 10−9 |
| α-FXIIa to fibrin | 1.8 × 10−3 | 3.9 × 105 | 4.6 × 10−9 |
| β-FXIIa to fibrinogen | 8.2 × 10−4 | 8.7 × 103 | 9.4 × 10−8 |
| β-FXIIa to fibrin | 8.7 × 10−4 | 9.2 × 103 | 9.4 × 10−8 |
| . | kd (off-rate, s−1) . | ka (on-rate, M−1s−1) . | Kd (dissociation constant, M) . |
|---|---|---|---|
| FXII to fibrinogen | 2.9 × 10−3 | 9.8 × 105 | 3.0 × 10−9 |
| FXII to fibrin | 2.9 × 10−3 | 11.8 × 105 | 2.5 × 10−9 |
| α-FXIIa to fibrinogen | 1.8 × 10−3 | 4.3 × 105 | 4.1 × 10−9 |
| α-FXIIa to fibrin | 1.8 × 10−3 | 3.9 × 105 | 4.6 × 10−9 |
| β-FXIIa to fibrinogen | 8.2 × 10−4 | 8.7 × 103 | 9.4 × 10−8 |
| β-FXIIa to fibrin | 8.7 × 10−4 | 9.2 × 103 | 9.4 × 10−8 |
Immunoprecipitation of FXII from normal pooled plasma showed that fibrinogen coprecipitates with FXII. Blotting of the precipitate showed positive staining for FXII and for fibrinogen (Figure 4G-H).
FXII and fibrin(ogen) in carotid thrombi
We next aimed to find evidence for a role of FXII in fibrin structure in vivo. For this, we obtained thrombi from the human carotid artery, removed during carotid endarterectomy, and immunostained these samples for fibrin(ogen) and FXII(a). We observed that carotid artery thrombi stained positive for both FXII(a) and fibrin(ogen) and that the proteins colocalized in the thrombi. The areas that stained positive for FXII(a) coincided with the areas of the thrombus that showed denser fibrin architecture (Figure 5).
Staining of FXII(a) and fibrin(ogen) in human carotid thrombi. Representative images of 3 human carotid thrombi. (A) Overall figure: H&E staining. (B) Immunohistochemical double staining for fibrin(ogen) and CD68 (staining for monocytes/macrophages). (C-D) Immunohistochemical double staining for FXII(a) and CD68 of a human carotid thrombus. (D) The FXII/FXIIa-positive and negative areas are outlined. Original magnifications: A, 2.5; B-C, 10; and D, 40.
Staining of FXII(a) and fibrin(ogen) in human carotid thrombi. Representative images of 3 human carotid thrombi. (A) Overall figure: H&E staining. (B) Immunohistochemical double staining for fibrin(ogen) and CD68 (staining for monocytes/macrophages). (C-D) Immunohistochemical double staining for FXII(a) and CD68 of a human carotid thrombus. (D) The FXII/FXIIa-positive and negative areas are outlined. Original magnifications: A, 2.5; B-C, 10; and D, 40.
Discussion
The structure of a fibrin clot is an emerging determinant of thrombotic events.34-36 Ex vivo clots of patients with premature coronary artery disease are stiffer, form a denser fibrin network, and are more difficult to lyse.7 Our experiments show that FXIIa changes fibrin clot structure in a dose-dependent manner. The effects of FXIIa on fibrin structure are consistent with those observed in patients with thrombosis. Using a combination of deficient plasmas and purified proteins, we show that the effects of FXIIa on fibrin structure are partly independent of the role of FXIIa in thrombin generation. We find that binding of FXII(a) to fibrinogen and fibrin is of high affinity and involves the heavy chain of FXII(a), providing a potential mechanism for the observed direct influence of FXIIa on fibrin structure. Finally, evidence is provided for the colocalization of FXII(a) with areas of dense fibrin(ogen) deposition in human thrombi obtained from patients with carotid artery disease.
Clotting of purified fibrinogen in the presence of α-FXIIa leads to the formation of fibrin with a denser structure. Experiments in congenital FXII-deficient plasma, to which purified FXII was added, also show a denser clot structure. This FXIIa-dependent change in fibrin structure is correlated with decreased fibrinolysis.37 Because the level of thrombin generation in the experiments with FXII-deficient plasma was directly influenced by the FXII concentration, we immunodepleted prothrombin from this plasma. Using this double-deficient plasma, we also observed a denser clot structure at 100% added FXII compared with no addition of FXII. However, this plasma was not entirely prothrombin free, with ∼ 3% residual protein detected. To investigate fibrin formation completely independent of intrinsic pathway-driven thrombin generation, we added hirudin to a commercial prothrombin-depleted plasma to avoid any thrombin activity and induced fibrin formation with ancrod, a snake venom enzyme. We compared the structure of the fibrin clots formed in the presence of FXIIa with those formed in the presence of CTI to inhibit FXIIa formation and/or any FXIIa that might have been formed. We observed significantly denser clot structures in the presence of α-FXIIa, confirming that FXIIa influences clot structure independent of its effects on thrombin.
The contribution of FXII to in vivo thrombin formation has long been debated. Contact activation can be assayed effectively using in vitro coagulation tests, but patients deficient in FXII do not show a bleeding tendency.16 Clinical studies point to a contribution of FXII in arterial thrombosis in humans, but the data are ambiguous.20,38-42 Both low and high levels of FXII, FXIIa, or FXIIa-C1 esterase inhibitor have been associated with an increased risk of thrombosis. Some of the inconsistencies in clinical studies may be related to differences in assay methodologies because different aspects of the contact pathway were determined. In addition, true differences in the effects of FXII(a) related to the populations (young women vs elderly persons) and vascular bed-specific factors (coronary or cerebral thrombosis) may be involved.
Surface plasmon resonance binding experiments show that purified FXII and α-FXIIa are able to bind to purified fibrin and fibrinogen with similar nanomolar affinity. This high binding affinity suggests that FXII and fibrinogen circulate as a complex in plasma, which was confirmed by Western blots and positive staining for fibrinogen after immunoprecipitation of FXII from plasma. Our data indicate that the heavy chain of FXII(a) is involved in this interaction, but the location of the binding site(s) on fibrin(ogen) is unknown. One candidate could be the COOH-terminal two-thirds of the Aα-chains of the fibrinogen molecule, also called αC-region, which is an important determinant of fibrin structure.43 The αC-region is important for lateral aggregation during fibrin polymerization and determines its susceptibility to fibrinolysis.44 Regulators of fibrinolysis, such as tPA and plasminogen, bind to this region and FXII contains several domains that are homologous to tPA.45,46 However, the binding sites for tPA and plasminogen are concealed in fibrinogen and are only exposed on fibrin formation. Therefore, the binding affinity of tPA and plasminogen for fibrin is higher than for fibrinogen.24,29 FXII(a), on the other hand, binds to a site that is exposed in both fibrin and fibrinogen. Turbidity assays with purified proteins showed a delay in lateral aggregation in the presence of α-FXIIa as represented by an increase in lag time. The αC-region of fibrinogen is important for lateral aggregation, and binding of α-FXIIa to this part of the molecule might explain the delay. Furthermore, within the αC region, there is a cluster of negatively charged amino acids (E448-D449 and D452)47 that could serve as a binding site for FXII and α-FXIIa. Further studies will be required to determine the fibrinogen binding site for FXII(a).
FXII and α-FXIIa have the same molecular weight (80 kDa) because activation results from a single cleavage that allows the disulphide bonded heavy and light chains to remain associated. β-FXIIa (28 kDa) is formed after an additional cleavage of α-FXIIa by kallikrein and contains the active site but has lost the heavy chain. β-FXIIa exhibited a 20-40 times lower affinity for fibrin(ogen) than FXII or α-FXIIa. This implies that at least one high-affinity binding site for fibrin(ogen) is located on the heavy chain. The heavy chain of FXII(a) contains several binding sites for negatively charged surfaces, such as in the fibronectin type I domain.48 Despite the similar binding profiles to fibrin(ogen), changes in fibrin clot structure were only observed with α-FXIIa. This implies that both binding and enzymatic activity are involved in α-FXIIa-associated changes in fibrin clot structure. Our findings that CTI and PPACK reversed the effects of FXIIa on fibrin structure appear to be in agreement with the requirement of proteolytic activity. Another possibility is that conformational changes of FXII during activation may lead to the observed effects on fibrin clot structure. CTI and PPACK are known to inhibit the proteolytic activity of FXIIa; however, this does not exclude that they can affect the conformation of FXIIa. Future studies will need to be performed to investigate this further.
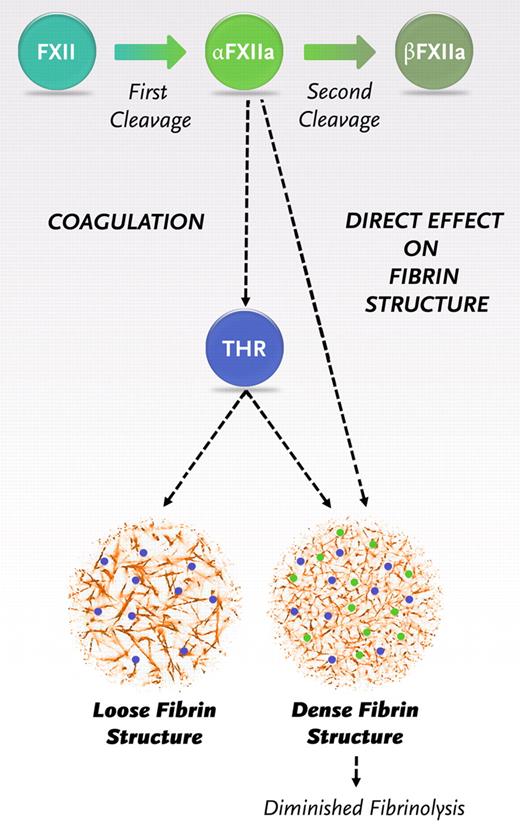
Once triggered, the coagulation pathway interacts to generate a burst of thrombin that will convert fibrinogen to fibrin. Our findings indicate the need for a revision of the coagulation pathway with a new role for α-FXIIa in fibrin formation, additional to its role in thrombin generation (Figure 6). After the first cleavage, FXII is converted to α-FXIIa. α-FXIIa promotes thrombin formation via the intrinsic pathway of coagulation but also directly increases the fiber density within the clot and makes it resistant to fibrinolysis. After a second cleavage, β-FXIIa is formed, which does not influence the fibrin structure. Hence, the ratio of α- over β-FXIIa can be expected to be an important determinant of the structure and function of the fibrin clot.
Schematic representation of the new insights in the role of FXIIa in fibrin clot formation. Activaton of FXII leads to the formation of 2 forms of activated FXII: α-FXIIa (2-chain molecule composed of a heavy chain and a light chain held together by a disulfide bond, the same molecular weight as FXII) and further proteolytic cleavage results in β-FXIIa (loss of the heavy chain). α-FXIIa can initiate thrombin formation via the intrinsic pathway of coagulation by activating FXI. FXII and α-FXIIa can both bind with the same affinity to fibrinogen and fibrin, and binding of α-FXIIa leads to a direct effect on fibrin structure.
Schematic representation of the new insights in the role of FXIIa in fibrin clot formation. Activaton of FXII leads to the formation of 2 forms of activated FXII: α-FXIIa (2-chain molecule composed of a heavy chain and a light chain held together by a disulfide bond, the same molecular weight as FXII) and further proteolytic cleavage results in β-FXIIa (loss of the heavy chain). α-FXIIa can initiate thrombin formation via the intrinsic pathway of coagulation by activating FXI. FXII and α-FXIIa can both bind with the same affinity to fibrinogen and fibrin, and binding of α-FXIIa leads to a direct effect on fibrin structure.
We found in vivo evidence for the role of FXIIa in fibrin structure and function because in human carotid thrombi, FXII(a) colocalized with fibrin(ogen), and denser fibrin(ogen) depositions were observed in FXII(a)–positive areas. Therefore, higher levels of FXII(a) may contribute to the denser fibrin(ogen) structures that stabilize the thrombus. Interestingly, previous studies using animal models found that FXIIa was associated with increased thrombus formation through an interaction with platelets, which provide a surface for FXII activation.12,49 Our findings suggest that the role of FXIIa is not limited to platelet-driven thrombus formation, but that effects on fibrin clot structure and function play an additional role in thrombus stabilization.
FXII was discovered as a coagulation protein, but many functions of FXIIa have been discovered (eg, complement activation,50 fibrinolysis,11 angiogenesis,51,52 and bradykinin formation11 ). It has been reported that FXIIa shows profibrinolytic activity via direct activation of plasminogen. However, FXIIa is a very poor enzyme in activating plasminogen compared with tPA and will be overruled when tPA is present.11 When we add tPA to the system, we observed that the clot lysis time increased dose-dependently with the α-FXII concentration. Therefore, in these experiments, the additional thrombin formation and direct effects of FXIIa on clot structure appear to be more important than any profibrinolytic effect that FXIIa may have through plasminogen to plasmin conversion.
Our study provides evidence for a distinct contribution of FXII in a concentration-dependent manner to pathologic thrombus formation via the formation of compact fibrin structures with increased fiber density and reduced pore size. In vivo, many components participate in the formation of a thrombus. Disruption of the vessel wall or atherosclerotic plaque exposes the subendothelium, which leads to platelet activation and thrombin formation. The platelets form aggregates, which are stabilized by fibrin fibers. FXII and α-FXIIa bind to fibrinogen and fibrin, through the N-terminal heavy chain of FXII. The direct effects of FXIIa on fibrin structure and function are synergistic with its indirect effects on fibrin formation through enhanced thrombin generation, contributing a novel mechanism that consolidates the fibrin clot. Collagen, within the subendothelium, amyloid deposits, and polyphosphates, released from platelets, are able to activate FXII.49,53,54 During the process of thrombus formation, FXII bound to fibrinogen can be activated and modulate fibrin structure. These findings indicate direct regulation of the fibrin fraction of the thrombus by FXIIa and suggest that interference with FXIIa-mediated effects on fibrin may prove useful for the treatment of thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Beekers for excellent technical assistance. Confocal imaging was performed in the faculty of Biological Sciences Bio-imaging facility in Leeds and 2-photon imaging was performed at the Department of Biomedical Engineering at The Cardiovascular Research Institute Maastricht.
This work was supported by The Netherlands Heart Foundation (grant 2008B120), the British Heart Foundation, and the Medical Research Council.
Authorship
Contribution: J.K. assisted in study design, performed research and statistical analysis, analyzed and interpreted data, and wrote the manuscript; J.W.P.G.-R. designed study and research, performed research, analyzed and interpreted data, and wrote the manuscript; H.P., N.J.M., J.I.B., and P.A. performed research and interpreted data; S.M. performed research; G.T. analyzed and discussed results; H.t.C. provided general advice during the study and critically reviewed the manuscript; and R.A.S.A. designed study and research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affilation for N.J.M. is School of Medicine and Dentistry, Institute of Medical Sciences, University of Aberdeen, Aberdeen, United Kingdom.
Correspondence: José W. P. Govers-Riemslag, Laboratory for Clinical Thrombosis and Haemostasis, Department of Internal Medicine, Cardiovascular Research Institute Maastricht, Maastricht University Medical Centre, Universiteitsingel 50, PO Box 616, Box 8, 6200 MD Maastricht, The Netherlands; e-mail: j.govers@maastrichtuniversity.nl.
References
Author notes
J.K. and J.W.P.G.-R. contributed equally to this study.