Abstract
Studies have correlated elevated plasma factor VIII (FVIII) with thrombosis; however, it is unclear whether elevated FVIII is a proinflammatory biomarker, causative agent, or both. We raised FVIII levels in mice and measured the time to vessel occlusion (TTO) after ferric chloride–induced injury. Compared with control (saline-infused) mice, elevated FVIII had no effect after longer (3-minute) carotid artery injury, but it shortened the TTO after shorter (2-minute) injury (P < .008). After injury, circulating thrombin-antithrombin (TAT) complexes were lower after short versus long injury (P < .04), suggesting short treatment produced less coagulation activation. TAT levels in FVIII-infused mice were higher than in controls after short, but not longer, injury. Accordingly, elevated FVIII had no effect on in vitro thrombin generation or platelet aggregation triggered by high tissue factor, but it increased thrombin generation rate and peak (2.4- and 1.5-fold, respectively), and it accelerated platelet aggregation (up to 1.6-fold) when initiated by low tissue factor. Compared with control mice, elevated FVIII stabilized thrombi (fewer emboli) after short injury, but it had no effect after longer injury. TTO and emboli correlated with TATs. These results demonstrate dependence of FVIII activity on extent of vascular injury. We propose elevated plasma FVIII is an etiologic, prothrombotic agent after moderate but not extensive vascular damage.
Introduction
Elevated factor VIII (FVIII) levels have been consistently and positively associated with primary and recurrent venous thromboembolism (VTE; odds ratio [OR], 2.0-10.8].1-8 For example, the Leiden Thrombophilia Study2 showed FVIII concentrations > 1.5 U/mL led to an OR > 5, and Kyrle et al4 showed relative risk of VTE recurrence was 6.6 in patients with FVIII levels greater than 2.34 U/mL. FVIII concentrations > 2 U/mL have been associated with an OR of VTE recurrence as high as 10.8.3
In contrast to VTE, the role of FVIII in arterial thrombosis is controversial. Using broad definitions of coronary heart disease (CHD) encompassing atherosclerosis, angina, transient ischemic attack, acute and nonacute myocardial infarction, and death, several studies have associated elevated FVIII with CHD, stroke, or both, with ORs ranging from 1.2-2.65.9-12 However, associations between FVIII activity and CHD13 or ischemic heart disease12 were lost after multivariate adjustment for diabetes and von Willebrand factor (VWF) levels, respectively. Importantly, because FVIII is increased in diseases that induce an acute phase response, including myocardial infarction,14 surgery,15 and sepsis,16 it is unclear whether FVIII's association with either venous or arterial thrombosis simply reflects an ongoing prothrombotic inflammatory process, or whether it is a direct, causative mechanism in the thrombosis etiology and therefore a therapeutic target, or both.
Studies examining the role of elevated FVIII in murine thrombosis models also have shown discord. Mice infused with recombinant human FVIII to 250% of normal demonstrate FVIII accumulation in thrombi and significantly enhanced thrombus size after photochemical (Rose Bengal)–induced carotid injury.17 In addition, inhibiting FVIII activity with a monoclonal antibody blocks thrombus formation in a murine inferior vena cava (IVC) stenosis model and in baboons implanted with arteriovenous shunts.18,19 In contrast, elevated FVIII (20% murine FVIII plus 280% infused human FVIII) does not promote microvascular or vena cava occlusion in VWF-deficient mice.20,21 Together, these studies suggest a role for FVIII in arterial and venous thrombosis but additional modifiers, such as VWF, modulate its relative impact.
In the current study, we used murine models of thrombosis in the vein and artery to assess the role of elevated FVIII in thrombus formation and stability, and we used ex vivo and in vitro methods to identify the operant biochemical mechanisms. Elevated FVIII augmented thrombus formation and stability after mild, but not severe ferric chloride (FeCl3) injury. FVIII's effects were manifested via its ability to increase thrombin generation and accelerate platelet aggregation initiated by low, but not high, TF activity. These results demonstrate dependence of plasma FVIII activity on the extent of vascular injury. We propose elevated plasma FVIII is an etiologic, prothrombotic agent in situations where there is moderate, but not extensive, vascular damage.
Methods
Proteins and materials
Dulbecco modified Eagle medium with high glucose/2mM l-glutamine, 0.05% trypsin/ethylenediamine tetraacetic acid, and PBS (10mM phosphate, pH 7.1, 150mM NaCl) were from Invitrogen. Human monoclonal purified FVIII (Hemofil M) was from Baxter. FVIII-deficient (hemophilia A, < 1% FVIII activity) platelet-poor plasma (PPP) was from HRF (Raleigh, NC). Kontact (aPTT reagent) was from Thermo Fisher Scientific. Thrombin fluorogenic substrate (Z-glycine-glycine-arginine-AMC) and calibrator (α2-macroglobulin/thrombin) were from Diagnostica Stago. Factor Xa chromogenic substrate (Pefachrome FXa) and Pefabloc FG (glycine-proline-arginine-proline) was from Pentapharm. Thrombin receptor activation peptide (serine-phenylalanine-leucine-leucine-arginine-asparagine, TRAP) was from Bachem California. Collagen was from Chrono-Log. Innovin was from Siemens. Mouse anti–human tissue factor (TF) antibody (HTF-1) was a kind gift from Dr Ronald Bach (University of Minnesota). Nonimmune mouse IgG antibody (MOPC-1), adenosine 5′-diphosphate (ADP), FeCl3, and BSA were from Sigma-Aldrich. Goat anti–mouse and anti–rabbit peroxidase-conjugated antibodies were from Calbiochem. Monoclonal anti–fibrin antibody (59D8) was the generous gift of Drs Marschall Runge (Department of Medicine, University of North Carolina [UNC]) and Charles Esmon (Oklahoma College of Medicine). Biotinylated secondary antibodies were from Vector Laboratories. Target Retrieval Solution was from Dako North America. Tenecteplase was the generous gift of Genentech. Corn trypsin inhibitor and factor X were from Haematologic Technologies. Contact-inhibited normal pooled plasma (NPP) was prepared from whole blood from 40 healthy subjects (50% female, 68% nonwhite) in a protocol approved by the UNC Institutional Review Board, as described previously.22
Murine thrombosis and thrombolysis models
Procedures were approved by the UNC Institutional Animal Care and Use Committee. Mice (6-8-week-old male C57BL/6, Charles River Laboratories) were anesthetized with 1.5% to 2% isoflurane in 2% oxygen, and the left saphenous vein was exposed under an SZX12 dissecting microscope (Olympus) and catheterized as described previously.22,23 FVIII or vehicle (saline or saline/BSA) was administered through the catheter on a per weight basis (blood volume [milliliters] is 7% of body weight [grams]) < 5 minutes before injury. Published studies demonstrate human FVIII binds murine VWF,17 has comparable cofactor activity as murine FVIII,17 and promotes coagulation after tail clipping and vessel injury in hemophilic mice.17,24-27 In addition, previous studies28,29 and our findings (FVIII activity assay, see “FVIII activity assay”) show FVIII has sufficient half-life in murine circulation for these experiments. The endogenous FVIII concentration in mice (1 U/mL, 100%)30 was raised by infusing human FVIII to 285% (total murine plus human FVIII) of normal, consistent with levels associated with thrombosis in humans.1-12
The saphenous vein and carotid artery thrombosis models were performed as described previously.22 In brief, the right saphenous vein was exposed, treated with 5% FeCl3 (0.31M FeCl3 on 0.5 × 2-mm filter paper) for 3 minutes, and washed with warm saline. Saphenous vein blood flow was monitored auditorily by Doppler ultrasonic flow probe. On separate mice, the right common carotid artery was exposed after midline cervical incision, dried, treated with 10% FeCl3 (0.62M on 0.5 × 0.5-mm filter paper) for 3 or 2 minutes, and washed with warm saline. After injury, carotid blood flow was monitored via Doppler transonic flow probe. In both models, the time to occlusion (TTO) was defined as the time between FeCl3 administration to the indicated vessel and lack of flow for 60 consecutive seconds. Experiments were stopped at 45 minutes if no occlusion occurred. Embolization was defined as a rapid increase in blood flow of at least 0.2 mL/min in the 5 minutes after stable occlusion occurred. Either 10 minutes after stable occlusion or after 45 minutes without stable occlusion, blood was drawn from the IVC into 3.2% sodium citrate and processed to PPP by centrifugation at 5000g for 10 minutes.
Thrombolysis was assessed in mice subject to 3-minute FeCl3 carotid artery thrombosis as described previously.22 In brief, after 5 consecutive minutes of blood flow below 0.1 mL/min, mice were infused with tenecteplase (5 mg/kg) through the saphenous vein catheter while continuously monitoring blood flow.
FVIII activity assay
FVIII activity was measured in PPP from FVIII-infused mice not subject to thrombosis. Five and 30 minutes after FVIII infusion, blood was drawn from the IVC into 3.2% sodium citrate and processed to PPP. Murine PPP was mixed with human FVIII-deficient PPP (5% murine PPP and 95% FVIII-deficient PPP), and clotting was initiated with Kontact aPTT reagent (40% total volume) and calcium chloride (10mM, final). The clot formation rate was measured in a SpectraMax Plus 340 plate reader (Molecular Devices)31 and compared with a standard curve created by mixing mouse PPP spiked with FVIII (to 150%-300%) with FVIII-deficient plasma. Five and 30 minutes after FVIII infusion, FVIII activity was 285% and 220%, respectively (endogenous murine FVIII plus infused human FVIII).
Measurement of circulating TAT complexes
Citrated blood samples were drawn from the IVC of mice subject to the FeCl3 carotid artery thrombosis model. Thrombin-antithrombin (TAT) levels were measured by ELISA (Enzygnost TAT micro ELISA; Siemens). Samples showing hemolysis were excluded.
Cell culture
Primary human aortic smooth muscle cells (SMCs; Lonza Walkersville) were cultured as directed in 5% CO2 at 37°C and used between passages 3 and 6 to reduce phenotypic drift.
Phospholipid vesicles
Phosphatidylcholine (egg), phosphatidylethanolamine (soy), and phosphatidylserine (porcine brain) were from Avanti Polar Lipids. Large unilamellar vesicles (41% phosphatidylcholine, 44% phosphatidylethanolamine, 15% phosphatidylserine) were made by extrusion, as described previously.32
TF activity
Thrombin generation
Thrombin generation was measured by calibrated automated thrombography using a Fluoroskan Ascent fluorometer (ThermoLabsystem), as described previously.33 In briefly, recalcified (16mM, final), lipidated (100μM, final) NPP was spiked with FVIII to 2, 3, or 4 U/mL (200%, 300%, and 400% of normal, respectively), final, and immediately added to washed SMC monolayers (67.7% plasma, final). Thrombin generation parameters (lag time and peak) were calculated using Thrombinoscope Version 3.0.0.29 software (Thrombinoscope BV). The thrombin generation rate was calculated by dividing peak height by the difference from time to peak and lag time.
H&E staining and immunohistochemistry
Fixed tissues were dehydrated and paraffin embedded, and consecutive, 5-μm sections were cut and mounted at the UNC Lineberger Comprehensive Cancer Center Animal Histopathology Core. H&E staining was used to visualize the thrombus. Immunohistochemistry (IHC) for fibrin(ogen) was performed with monoclonal antibody 59D8, as described previously.22 Negative controls were stained simultaneously in the absence of primary antibody.
Platelet aggregation
Platelet aggregation was performed as described on an optical aggregometer 470 (Chrono-Log).22 Aggregation was triggered by TRAP (50 μg/mL, final), collagen (2 μg/mL, final), ADP (2.5μM, final), or TF (1:30 000 [high TF] or 1:200,000 [low TF]). For experiments triggered with TF, Pefabloc FG (5 mg/mL, final; Centerchem) was included to inhibit fibrin polymerization.
Statistical methods
For TTO, conditions were compared pairwise by a log-rank test because of censored observations at 45 minutes (no stable occlusion). For TATs and embolization analysis, conditions were compared by Mann-Whitney U tests to account for both unequal variance and nonnormality of outcomes. For in vitro thrombin generation assays, significant differences between groups were identified by a 1-way ANOVA with homogeneity tests and residual plots to check statistical assumptions. To analyze the effect of FVIII concentration on thrombin generation parameters (lag time, rate, and peak height), a 1-sided Dunnett posthoc test using 1 U/mL FVIII (100% FVIII) as the index group (on SMCs of varying confluence, as indicated) was used, with a controlled type-I error rate no larger than .05 in the multiple testing between experimental and index groups. For platelet aggregation data, normalized lag times where ratios were calculated between 200%, 300%, and 400% to 100% FVIII were tested by a 1-sided t test with a null hypothesis no smaller than 1. Bivariate correlation analyses between TTO, TAT, and number of emboli were presented in Pearson coefficient (denoted as R) that indicates the degree of linear dependence. Statistical analyses were performed using SPSS 16.0 (SPSS). P values < .05 were considered significant.
Results
Elevated FVIII shortens the TTO in a saphenous vein thrombosis model
FeCl3 application to the saphenous vein of control (100% FVIII, saline-infused) mice for 3 minutes produced occlusive thrombi in 20.3 (6.0) minutes (median, [range]). Consistent with epidemiologic studies showing a significant (up to 10.8-fold) increased risk of VTE in patients with elevated FVIII,1-8 285% FVIII (final) significantly shortened the TTO to 12.7 (2.8) minutes (median, [range], P < .001) in the saphenous vein. Infusing nonspecific protein (BSA, equivalent w/w) did not shorten the TTO versus control mice. In additional, the shortened TTO was not because of VWF in the FVIII preparation; Hemofil M contains only trace human VWF (0.083 μg of VWF/U FVIII).34 These findings indicate FVIII's effect was specific, and they confirm a direct, pathogenic contribution of elevated FVIII in a murine model of thrombosis in a vein.
Elevated FVIII shortens the TTO after short (2-minute), but not longer (3-minute), FeCl3 treatment to the carotid artery
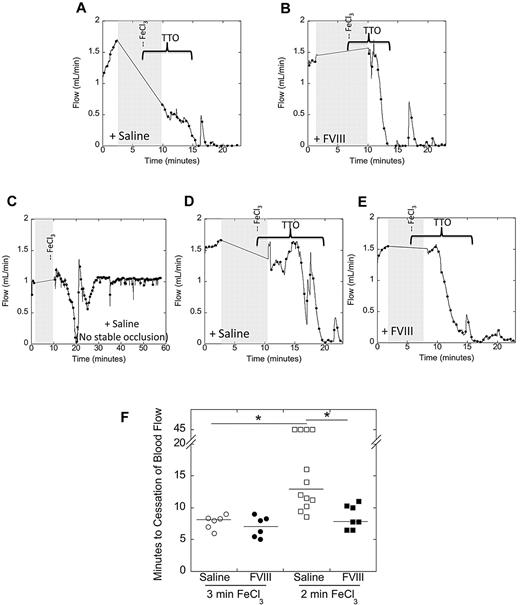
In contrast to VTE, the role of FVIII in arterial thrombosis is unclear. We therefore tested the ability of FVIII to accelerate arterial clotting after 3-minute FeCl3 application to the carotid artery. Representative flow tracings are shown in Figure 1A and B. Control mice developed occlusive thrombi in 8.1 minutes (median; Figure 1F). Surprisingly, although mice with hypercoagulability because of elevated fibrinogen demonstrate significantly shortened TTO under these conditions,22 mice with elevated FVIII did not (7.1 minutes, median; P = .6; Figure 1F).
Elevated FVIII shortens the TTO after 2-minute, but not 3-minute, FeCl3 injury to the carotid artery. C57Bl/6 mice were infused with saline or FVIII to 285% of normal. Thrombosis was induced by 10% FeCl3 application to the carotid artery for the indicated time (3 or 2 minutes), and the TTO was determined by flow probe. In vessels that did not occlude, the TTO was recorded as 45 minutes. (A-E) Representative flow tracings following 3- (A-B) or 2-minute (C-E) FeCl3 application to control (saline-infused, A,C,D) and FVIII-infused (B,E) mice. Gray shaded area represents time of vessel drying, FeCl3 administration, and vessel washing, during which flow could not be monitored. Brackets indicate the time from FeCl3 placement to the time of vessel occlusion. (F) Quantification of TTO data. Each point represents a separate mouse. Lines show median values to accommodate censored data at 45 minutes. *P < .05.
Elevated FVIII shortens the TTO after 2-minute, but not 3-minute, FeCl3 injury to the carotid artery. C57Bl/6 mice were infused with saline or FVIII to 285% of normal. Thrombosis was induced by 10% FeCl3 application to the carotid artery for the indicated time (3 or 2 minutes), and the TTO was determined by flow probe. In vessels that did not occlude, the TTO was recorded as 45 minutes. (A-E) Representative flow tracings following 3- (A-B) or 2-minute (C-E) FeCl3 application to control (saline-infused, A,C,D) and FVIII-infused (B,E) mice. Gray shaded area represents time of vessel drying, FeCl3 administration, and vessel washing, during which flow could not be monitored. Brackets indicate the time from FeCl3 placement to the time of vessel occlusion. (F) Quantification of TTO data. Each point represents a separate mouse. Lines show median values to accommodate censored data at 45 minutes. *P < .05.
Given studies demonstrating differential sensitivity to intrinsic pathway components with extent of FeCl3 injury,35,36 we then tested a second set of conditions in which FeCl3 was applied to the carotid artery for only 2 minutes. Representative flow tracings are shown in Figure 1C through E. Compared with 3-minute FeCl3 injury, control mice took significantly (P < .005) longer to form stable thrombi after 2-minute FeCl3 injury (13 minutes, median; Figure 1F). Interestingly, unlike the 3-minute injury model, mice with elevated FVIII exhibited a significantly (P < .005) shorter TTO than control mice in this 2-minute injury model (7.8 minutes, median; Figure 1F). Infusing BSA did not shorten the TTO compared with control mice. These data indicate elevated FVIII promotes arterial thrombosis after short, but not longer, application of FeCl3.
Elevated FVIII increases thrombin generation in vivo after short, but not longer, FeCl3 treatment
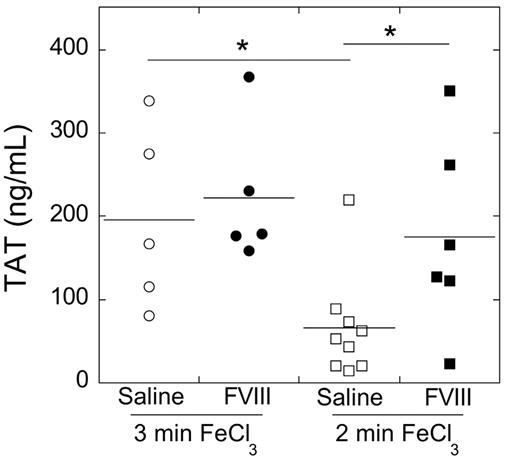
Our TTO data indicated duration of FeCl3 application influenced the impact of FVIII on thrombogenesis. To characterize the effect of FeCl3 injury and FVIII on in vivo procoagulant activity (thrombin generation), we measured circulating TATs. Uninjured mice had a TAT level of 17.4 ± 2.26 ng/mL (mean ± SD). Control mice subject to either 3- or 2-minute FeCl3 treatment had TAT levels of 195 ± 108 or 66.2 ± 63.1 ng/mL, respectively (Figure 2). TAT levels in control mice were significantly (P < .05) higher after 3-minute injury than 2-minute injury, indicating 3-minute FeCl3 application causes higher procoagulant activity than 2-minute FeCl3 application. Compared with controls, elevated FVIII did not increase TAT levels in the 3-minute injury model (222 ± 85.2 ng/mL; P = .5), but it caused significantly higher TAT levels than controls after 2-minute (175 ± 115 ng/mL; P < .05) FeCl3 application (Figure 2). Across all mice, TAT levels correlated with TTO values (R = 0.388, P = .055), suggesting differences in TTO resulted from differential procoagulant activity at the site of injury.
Both time of FeCl3 injury and elevated FVIII influence TAT complex formation. Citrated blood samples were collected via IVC puncture from mice subject to the FeCl3 carotid artery thrombosis model. TAT levels were measured by ELISA. Samples showing hemolysis were excluded. Each point represents a separate mouse. Lines show mean values. *P < .05.
Both time of FeCl3 injury and elevated FVIII influence TAT complex formation. Citrated blood samples were collected via IVC puncture from mice subject to the FeCl3 carotid artery thrombosis model. TAT levels were measured by ELISA. Samples showing hemolysis were excluded. Each point represents a separate mouse. Lines show mean values. *P < .05.
Elevated FVIII increases thrombin generation in an in vitro model of mild, but not severe, vascular injury
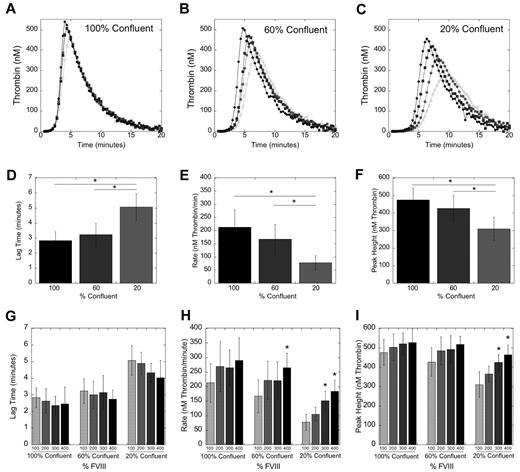
Given findings associating TTO with circulating TAT levels, we hypothesized that the duration of FeCl3 application determined the extent of vascular injury, and consequently, the relative impact of extrinsic (TF-dependent) and intrinsic (FVIII-dependent) contributions to thrombin generation. To specifically interrogate the relationship between vascular procoagulant activity and plasma FVIII levels on thrombin generation kinetics, we modeled vascular injury in vitro by culturing primary SMCs at 100%, 60%, and 20% confluence to model high, medium, and low levels of vascular damage, respectively. Wang et al previously showed SMC TF has a central role in FeCl3-induced vascular injury.37 SMC cultures at 100%, 60%, and 20% confluence expressed 5.5 ± 0.74, 3.7 ± 0.66, and 0.82 ± 0.32pM TF, respectively. We first incubated recalcified NPP (1 U/mL FVIII [100%]) with SMCs and measured thrombin generation (Figure 3D-F). Lipids (100μM, final) were included to compensate for different lipid concentrations in cultures with different SMC plating densities. Similar to thrombin generation triggered by 5 and 1pM soluble TF,38 decreasing cell density (decreasing TF activity) significantly (P < .05) prolonged the lag time and decreased the thrombin generation rate and peak height (Figure 3D-F).
Elevated FVIII increases thrombin generation in an in vitro model of mild, but not severe, TF (SMC) exposure. Recalcified (16mM, final) human NPP spiked with FVIII or HBS (20mM HEPES pH 7.4, 150mM NaCl) was added to SMC monolayers of varying densities. Thrombin generation was measured by calibrated automated thrombography, as described previously.22,33 (A-C) Thrombin generation curves representative of 5 independent experiments with SMC at 100% (A), 60% (B), and 20% (C) confluence. Symbols are 100% (open circles), 200% (closed circles), 300% (closed squares), and 400% (closed diamonds) FVIII, final. (D-F) Mean values (± SD) for lag (D), rate (E), and peak height (F) of thrombin generation for all 5 experiments with human NPP (1 U/mL FVIII) at different SMC confluence levels. *P < .05 versus indicated group. (G-I) Mean values (± SD) for the lag (G), rate (H), and peak height (I) of thrombin generation for all 5 experiments, at varying FVIII levels. *P < .05 versus 1 U/mL within each confluence level.
Elevated FVIII increases thrombin generation in an in vitro model of mild, but not severe, TF (SMC) exposure. Recalcified (16mM, final) human NPP spiked with FVIII or HBS (20mM HEPES pH 7.4, 150mM NaCl) was added to SMC monolayers of varying densities. Thrombin generation was measured by calibrated automated thrombography, as described previously.22,33 (A-C) Thrombin generation curves representative of 5 independent experiments with SMC at 100% (A), 60% (B), and 20% (C) confluence. Symbols are 100% (open circles), 200% (closed circles), 300% (closed squares), and 400% (closed diamonds) FVIII, final. (D-F) Mean values (± SD) for lag (D), rate (E), and peak height (F) of thrombin generation for all 5 experiments with human NPP (1 U/mL FVIII) at different SMC confluence levels. *P < .05 versus indicated group. (G-I) Mean values (± SD) for the lag (G), rate (H), and peak height (I) of thrombin generation for all 5 experiments, at varying FVIII levels. *P < .05 versus 1 U/mL within each confluence level.
We then determined the effect of FVIII on thrombin generation at each confluence level by incubating SMCs with recalcified, lipidated NPP spiked with FVIII (to 2 U/mL [200%], 3 U/mL [300%], and 4 U/mL [400%], final; Figure 3). Elevated FVIII nonsignificantly shortened the lag time in reactions with SMCs at all confluences (Figure 3G); this effect was more pronounced at lower SMC confluences. At 100% confluence (high TF), FVIII had no effect on the rate or peak height (Figure 3H-I). At 60% confluence (medium TF), 400% FVIII increased the rate (P < .05), but not the peak height. At 20% confluence (low TF), both 300% and 400% FVIII significantly (P < .005) increased the rate and peak height (Figure 3H-I). The significantly faster rate of thrombin generation in 20% SMC (Figure 3H-I) was consistent with the shorter TTO in the 2-minute FeCl3 injury model in vivo.
Together, these in vivo and in vitro data show FeCl3-induced vascular damage induces procoagulant activity. Elevated FVIII augments thrombin generation and consequently, thrombus formation, after mild, but not severe vascular injury.
Elevated FVIII does not alter thrombus morphology or increase thrombus fibrin(ogen) content
We showed previously hyperfibrinogenemic mice exhibit increased thrombus fibrin(ogen) deposition.22 We therefore tested whether elevated FVIII and its subsequent increase in thrombin generation in the 2-minute injury model similarly increased thrombus fibrin(ogen) content. H&E staining showed similarly extensive, occlusive thrombi with similar vessel diameters, containing distinct regions of anucleate, proteinaceous material, and erythrocytes in thrombi from control and FVIII-infused mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). IHC of thrombi from both control and FVIII-infused mice demonstrated weak fibrin staining concentrated primarily at the luminal edge of proteinaceous regions. There were no obvious differences in fibrin staining intensity between control and FVIII-infused mice (supplemental Figure 1), suggesting thrombus fibrin content was unchanged by elevated FVIII. Together, these findings indicate FVIII did not shorten the TTO by increasing thrombus fibrin content.
Elevated FVIII accelerates platelet aggregation when triggered with low, but not high, TF
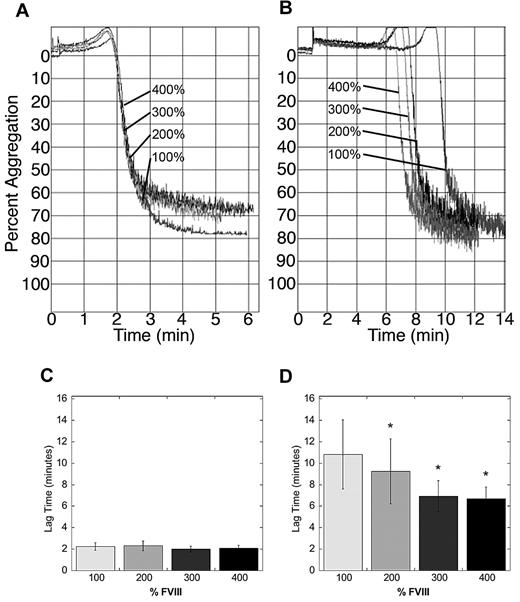
To determine whether FVIII-dependent, increased thrombin generation accelerated platelet incorporation into the thrombus, we raised FVIII levels in human PRP with additional human FVIII, and triggered platelet aggregation with TRAP, collagen, ADP, or TF. Elevated FVIII had no effect on platelet aggregation initiated by TRAP, collagen, or ADP, showing that FVIII does not impact platelet function in the absence of thrombin generation (data not shown). In reactions triggered by TF, FVIII had no effect on either the rate or maximum amplitude of platelet aggregation (Figure 4A). Interestingly, however, whereas elevated FVIII had no effect on the lag time in reactions triggered with high TF, elevated FVIII dose-dependently shortened the lag time of platelet aggregation in reactions triggered by low TF (P < .05, Figure 4A-B). These data are consistent with the FVIII-dependent shortening in TTO after 2-, but not 3-minute injury, and they suggest elevated FVIII shortened the TTO by accelerating platelet activation at the injury site.
Elevated FVIII accelerates the onset of platelet aggregation when initiated with low, but not high, TF. Platelet aggregation in human PRP spiked with human FVIII (final concentrations indicated) was triggered by recalcification and addition of TF and monitored by turbidity as described in “Methods” and Machlus et al.22 Graphs shown are from one experiment, representative of 4 to 6 experiments at each TF concentration. (A) High TF (1:30 000 Innovin, final). (B) Low TF (1:200,000 Innovin, final). Note difference in x-axis (time) scaling in panels A and B. (C-D) Quantification of lag time data initiated with high (C) and low (D) TF, respectively. Graphs show mean ± SEM. *P < .05 versus 100% FVIII.
Elevated FVIII accelerates the onset of platelet aggregation when initiated with low, but not high, TF. Platelet aggregation in human PRP spiked with human FVIII (final concentrations indicated) was triggered by recalcification and addition of TF and monitored by turbidity as described in “Methods” and Machlus et al.22 Graphs shown are from one experiment, representative of 4 to 6 experiments at each TF concentration. (A) High TF (1:30 000 Innovin, final). (B) Low TF (1:200,000 Innovin, final). Note difference in x-axis (time) scaling in panels A and B. (C-D) Quantification of lag time data initiated with high (C) and low (D) TF, respectively. Graphs show mean ± SEM. *P < .05 versus 100% FVIII.
Elevated FVIII increases thrombus stability after mild, but not severe, vascular injury
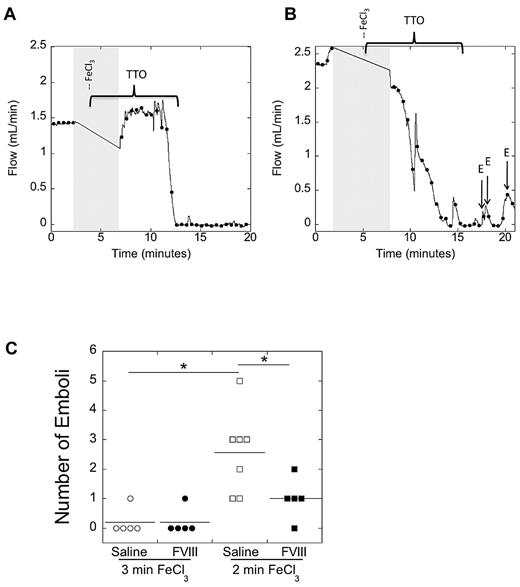
Finally, because both shorter TTO and higher procoagulant activity have previously been associated with increased thrombus stability,22,33,39 we examined thrombus stability in the carotid artery of mice as functions of both duration of FeCl3 treatment and FVIII level. Representative flow tracings from experiments with control (saline-infused) mice after 3- and 2-minute injury are shown in Figure 5A and B, respectively. All mice treated with FeCl3 for 3 minutes demonstrated stable occlusion (< 0.1 mL/min blood flow for 5 consecutive minutes), whereas some mice treated with FeCl3 for 2 minutes did not. We quantified clot stability by counting the number of embolic events (rapid increase in blood flow of at least 0.2 mL/min) in the 5 minutes after the TTO. Mice produced significantly (P < .05) more emboli after 2-minute FeCl3 injury than 3-minute injury (Figure 5C). In the 2-minute injury model, FVIII-infused mice produced significantly (P < .05) fewer emboli than control mice; however, there was no difference in number of emboli between control and FVIII-infused mice after 3-minute injury (Figure 5C). Across all mice, the number of emboli was significantly, positively associated with TTO values (R = 0.495, P = .019) and significantly, negatively associated with TAT values (R = 0.614, P = .005).
Both lengthier FeCl3 treatment and elevated FVIII increase thrombus stability. C57Bl/6 mice were infused with saline or FVIII to 285% of normal. Thrombosis was induced by 10% FeCl3 application to the carotid artery for the indicated time (3 or 2 minutes). After stable occlusion, flow was monitored for 5 minutes to count embolic events (defined as a rapid increase in blood flow of at least 0.2 mL/min). (A-B) Representative flow tracings after 3- (A) and 2-minute (B) FeCl3 application to control (saline-infused) mice, respectively. Gray shaded area represents time of vessel drying, FeCl3 administration and vessel washing, during which flow could not be monitored. Brackets indicate the time from FeCl3 placement to the time of vessel occlusion. Arrows show emboli (marked with E) in the 5 minutes after stable occlusion. (C) Quantification of embolization data. Each point represents a separate mouse. Mice that did not achieve stable occlusion were excluded. Lines show mean values. *P < .05.
Both lengthier FeCl3 treatment and elevated FVIII increase thrombus stability. C57Bl/6 mice were infused with saline or FVIII to 285% of normal. Thrombosis was induced by 10% FeCl3 application to the carotid artery for the indicated time (3 or 2 minutes). After stable occlusion, flow was monitored for 5 minutes to count embolic events (defined as a rapid increase in blood flow of at least 0.2 mL/min). (A-B) Representative flow tracings after 3- (A) and 2-minute (B) FeCl3 application to control (saline-infused) mice, respectively. Gray shaded area represents time of vessel drying, FeCl3 administration and vessel washing, during which flow could not be monitored. Brackets indicate the time from FeCl3 placement to the time of vessel occlusion. Arrows show emboli (marked with E) in the 5 minutes after stable occlusion. (C) Quantification of embolization data. Each point represents a separate mouse. Mice that did not achieve stable occlusion were excluded. Lines show mean values. *P < .05.
Because control and FVIII-infused mice thrombosed with FeCl3 for 3 minutes demonstrated stable occlusion, we further examined thrombus stability by infusing these mice with the tissue plasminogen activator derivative tenecteplase as described previously.22 Consistent with their similar TTO, TATs, and number of emboli, there was no difference in tenecteplase-induced thrombolysis; all mice showed complete return of blood flow (data not shown). In total, these data suggest longer FeCl3 treatment (increased vascular injury) produces highly stable thrombi irrespective of intrinsic pathway components, whereas shorter treatment (less injury) produces unstable thrombi. Elevated FVIII stabilizes thrombi after mild, but not severe, vascular injury.
Discussion
Epidemiologic studies have repeatedly correlated elevated plasma FVIII levels with VTE.1-8 However, the role of FVIII in arterial thrombosis is controversial. In addition, for both venous and arterial thrombosis, it is unclear whether elevated FVIII is merely a proinflammatory biomarker,14-16 a causative mechanism in the disease etiology, or both. We acutely elevated FVIII in mice and examined their susceptibility to thrombosis, and we used ex vivo and in vitro methods to elucidate biochemical mechanisms for the observed effects. Our findings confirm an etiologic, prothrombotic role for elevated FVIII in veins. Moreover, our findings show elevated FVIII (1) shortened the TTO after short (2-minute), but not longer (3-minute) FeCl3 treatment to the carotid artery; (2) increased thrombin generation in mice after short but not longer FeCl3 treatment; (3) augmented the thrombin generation rate and peak in vitro with low, but not high, TF (SMC) exposure; (4) accelerated the onset of platelet aggregation initiated by low TF; and (5) promoted the formation of more stable thrombi (fewer emboli) after short, but not longer injury in mice. Together, these findings demonstrate an etiologic role for FVIII in thrombosis, but suggest dependence of plasma FVIII activity on the extent of vascular injury.
Previously, we and others have shown dose-dependent effects of TF activity on thrombin generation in vitro.33,38,40 Our data quantifying circulating TAT complexes in mice subject to 2- and 3-minute FeCl3 injuries extend the in vitro findings with soluble and cellular TF to the in vivo models and provide a rationale for the differences in TTO and thrombus stability. Compared with shorter FeCl3 treatment, longer FeCl3 application produced higher circulating TAT levels, suggesting prolonged FeCl3 treatment produced a more severe injury with higher exposure of cellular procoagulant activity. Our findings do not rule out the possibility that longer FeCl3 treatment also increased platelet deposition by exposing more subendothelial collagen than the shorter injury. However, we were able to recapitulate effects of longer and shorter FeCl3 treatment on platelet aggregation and thrombin generation by varying the soluble TF concentration (Machlus et al38 ; Figure 4), or SMC confluence in vitro (Figure 3), suggesting differential exposure of procoagulant (TF) activity differentiates these treatments. Any effect on platelets of collagen exposure between the 2- and 3-minute injury models would occur distinctly from, but potentially in addition to, effects of procoagulant activity. Importantly, our observation that TAT measurements correlate with thrombus formation (TTO) and stability measurements provides a means of quantifying differences in extent of vascular injury in different in vivo models. This information may enable reconciliation of differences in outcome measures between studies using different thrombosis models.
Patients with elevated FVIII demonstrate elevated circulating prothrombin fragment 1.2 and TAT complexes,5 and we and others have shown elevated FVIII increases thrombin generation in vitro38,41-43 and in vivo.43 Interestingly, the in vitro studies show a stronger effect of elevated FVIII in reactions triggered with low TF than high TF. It is therefore of considerable interest that elevated FVIII shortened the TTO and improved thrombus stability in the carotid artery after shorter, but not longer, FeCl3 application. Previous studies of intrinsic pathway components have demonstrated dose-dependent effects of FeCl3 treatment on thrombosis; mice lacking intrinsic pathway factors XI or IX develop occlusive thrombi in the carotid artery after high (10%), but not lower (3.5% or 5%), FeCl3 treatment.35,36 Although previous studies hypothesized increased FeCl3 injury reduces sensitivity to intrinsic factors by increasing TF/VIIa activity,35,36 our study is the first to show a mechanism for differences in mild and severe FeCl3 injury: differential procoagulant activity, measured by TAT complex formation. Indeed, the sensitivity of intrinsic pathway components is perhaps best demonstrated by differences in the prothrombin time and partial thromboplastin time tests. Whereas the prothrombin time detects clotting deficiencies because of extrinsic and common pathway components, its high TF concentrations mask effects of factors VIII or IX deficiency. Lower TF concentrations in a partial thromboplastin time reveal FVIII or factor IX deficiencies. To our knowledge, our study is the first to demonstrate an interaction between extent of vascular injury and effect of plasma hypercoagulability in vivo. This interaction suggests more severe injury (longer application of FeCl3) exposes a sufficiently high amount of TF activity to negate effects of elevated FVIII. In contrast, less severe injury (shorter application of FeCl3) exposes less TF activity, enabling a role for FVIII in procoagulant activity.
Our data demonstrating an etiologic role for FVIII in thrombosis are consistent with previous findings that elevated FVIII promotes Rose Bengal–induced thrombosis,17 stabilizes thrombi (decreases microvascular thrombus embolization),20 and normalizes the blood clotting time in VWF-deficient mice20 and that FVIII inhibition blocks thrombus formation in murine and baboon thrombosis models.18,19 By comparing severe and mild vascular injuries, our study illuminates specific contributions of vascular procoagulant activity not apparent in the prior reports. Our findings differ somewhat from observations that elevated FVIII (to 250%) increases thrombus size.17 In our study, thrombi in control and FVIII-infused mice seemed morphologically similar, with no obvious differences in thrombus size (vessel diameter) or final fibrin content. Differences between these findings may reflect the injury model (FeCl3 vs Rose Bengal), the method of quantification (IHC of fully formed occlusive thrombi vs transillumination during thrombus formation that reflects the rate of thrombus growth), or both.17 Although we were unable to measure the rate of thrombus growth in vivo, our in vitro data indicate faster platelet activation (Figure 4) and slightly faster fibrin polymerization (data not shown), consistent with their findings. Brill et al previously showed that elevated FVIII (300% of normal, 20% murine FVIII, and 280% human FVIII) does not promote venous occlusion in VWF-deficient mice, suggesting VWF is necessary for at least part of FVIII's procoagulant effects.20,21 Our study extends these findings by demonstrating a critical role for the extent of vascular injury in FVIII-dependent thrombin generation and thrombogenesis.
Besides elevated FVIII, epidemiologic studies also have correlated elevated fibrinogen levels (hyperfibrinogenemia) with thrombotic risk.2,8,44 We showed previously that like elevated FVIII, elevated fibrinogen shortens the TTO in both saphenous vein and carotid artery and increases thrombus stability.22 However, in contrast to elevated factor VIII, hyperfibrinogenemia shortens the TTO in the 3-minute FeCl3 carotid injury model. Moreover, elevated fibrinogen increases thrombus fibrin content,22 whereas elevated FVIII did not. Rather, elevated FVIII accelerated platelet aggregation in a low TF/thrombin generation-dependent mechanism. Because we and others have shown that high thrombin concentrations produce dense fibrin networks that are resistant to lysis,33,39,45 elevated FVIII also may alter the structure of fibrin present in the thrombi of mice treated with FeCl3 for 2 minutes. Unfortunately, although IHC provides quantitative information about fibrin content, which was not altered by elevated FVIII (supplemental Figure 1), it does not reveal information about fibrin network structure (individual fiber thickness or density). Regardless, the differences between these findings demonstrate fundamental differences in the hypercoagulable mechanisms of elevated fibrinogen and elevated FVIII, and they suggest antithrombotic strategies could be tailored for a specific hypercoagulability (blocking thrombin generation/activity vs blocking fibrin formation).46
Our findings on the relationship between vascular injury and effect of FVIII on thrombosis inform epidemiologic studies showing either moderate or no relationship between FVIII and arterial thrombosis.9,10,12,13 Using the presence of carotid plaque as a marker of mild or moderate disease, Pan et al showed a significant association between heart disease and FVIII level (OR = 2.65).9 In contrast, using a broad definition of CHD that encompassed both mild and severe disease (definite, probable, or silent myocardial infarction or definite CHD death), Folsom et al found no correlation between CHD and FVIII activity (OR = 1.0).13 Interestingly, plaque rupture is associated with exposure of high levels of TF (reviewed in Brill47 ). In light of our data, differences in the conclusions of these studies may reflect the severity of the study inclusion event. FVIII activity probably contributes to thrombosis only when there is mild TF exposure, and it is unlikely to contribute after a severe event that exposes high TF, such as plaque rupture.
Given a causative role for FVIII in thrombosis, it is tempting to speculate about the potential efficacy of antithrombotic strategies that target FVIII activity in venous and arterial thrombosis. Elevated FVIII is strongly (2- to 10.8-fold) associated with VTE, and partial inhibition of FVIII activity with a monoclonal antibody blocks thrombus formation in a murine IVC stenosis model and in baboons implanted with arteriovenous shunts.18,19 In addition, patients with hemophilia A are protected from venous thrombosis.48 These data and our own findings in the saphenous vein thrombosis model suggest FVIII inhibition may protect against VTE. However, hemophilia patients are only partially protected against ischemic heart disease49 and epidemiologic studies inconsistently associate elevated FVIII with arterial thrombosis. Together with our data, these observations suggest FVIII inhibition would have only moderate efficacy in preventing arterial events triggered by high TF exposure.
This study has potential limitations. First, we used human FVIII to increase circulating levels in the mouse. However, our data and published studies demonstrate human FVIII binds murine VWF,17 has comparable cofactor activity as murine FVIII,17 is stable in murine circulation,28,29 is incorporated into murine clots,17 is inactivated by murine-activated protein C (Steve Pipe, University of Michigan, personal communication, June 21, 2011), and supports clot formation in mice.17,24-27 Second, the FeCl3/saphenous vein injury model is not commonly used as a model of venous thrombosis; however, our findings are consistent with published data from other venous models and effectively demonstrate the pathologic activity of elevated human FVIII in thrombi formed in murine veins.17-19 Finally, the FeCl3 thrombosis model does not fully recapitulate the inflammatory process associated with atherosclerosis or plaque rupture in humans or mice, but rather, models acute thrombosis after vascular injury. However, FeCl3 exposes SMC TF and produces platelet-rich thrombi similar to those seen in arterial clots.37,47,50
In conclusion, our results show that both vascular damage and plasma hypercoagulability modulate thrombogenesis. After limited arterial injury, FVIII directly promotes thrombosis via increased thrombin generation resulting in accelerated platelet activation and increased thrombus stability. Elevated FVIII does not promote thrombosis after severe vascular injury. These findings provide a rationale for the controversy surrounding the role of FVIII in arterial thrombosis; in mice, FVIII's prothrombotic effects depend on the extent of injury that exposes procoagulant cells and triggers thrombin generation. Although few diagnostic algorithms simultaneously consider markers of tissue damage and plasma hypercoagulability when assessing thrombosis risk, integrating these pieces of information may refine approaches to understand pathophysiologic mechanisms promoting thrombosis in humans.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jessica Cardenas, Zach Rotfus, Dr Jian-Guo Wang, and Laura Gray for excellent technical assistance; and Dr Robert Campbell for thoughtful discussions.
This study was supported by funding from the National Institutes of Health (R01HL094740, A.S.W.), the National Hemophilia Foundation (A.S.W.), the American Heart Association (10PRE3720011, K.R.M.), and the National Center for Research Resources (TraCS award UL1RR025747, A.S.W.).
National Institutes of Health
Authorship
Contribution: K.R.M. performed experiments, analyzed data, and wrote the manuscript; F.-C.L performed statistical analysis and reviewed the manuscript; and A.S.W. designed and supervised the study, analyzed data, and wrote the manuscript.
Conflict of interest: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 815 Brinkhous-Bullitt Bldg, CB no. 7525, Chapel Hill, NC 27599-7525; e-mail: alisa_wolberg@med.unc.edu.