Abstract
Donor-recipient human leukocyte antigen mismatch level affects the outcome of unrelated cord blood (CB) transplantation. To identify possible “permissive” mismatches, we examined the relationship between direction of human leukocyte antigen mismatch (“vector”) and transplantation outcomes in 1202 recipients of single CB units from the New York Blood Center National Cord Blood Program treated in United States Centers from 1993-2006. Altogether, 98 donor/patient pairs had only unidirectional mismatches: 58 in the graft-versus-host (GVH) direction only (GVH-O) and 40 in the host-versus-graft or rejection direction only (R-O). Engraftment was faster in patients with GVH-O mismatches compared with those with 1 bidirectional mismatch (hazard ratio [HR] = 1.6, P = .003). In addition, patients with hematologic malignancies given GVH-O grafts had lower transplantation-related mortality (HR = 0.5, P = .062), overall mortality (HR = 0.5, P = .019), and treatment failure (HR = 0.5, P = .016), resulting in outcomes similar to those of matched CB grafts. In contrast, R-O mismatches had slower engraftment, higher graft failure, and higher relapse rates (HR = 2.4, P = .010). Based on our findings, CB search algorithms should be modified to identify unidirectional mismatches. We recommend that transplant centers give priority to GVH-O-mismatched units over other mismatches and avoid selecting R-O mismatches, if possible.
Introduction
Cord blood (CB) from unrelated donors has become an important source of hematopoietic stem cells (HSCs), expanding significantly the opportunities to find donors for patients requiring bone marrow replacement.1-6 A major advantage of CB has been the ability to transplant grafts that are partially human leukocyte antigen (HLA) mismatched with the patient because of a relatively low incidence and severity of GVHD for the level of mismatch, a probable consequence of immunologic tolerance of this neonatal HSC source.7-9 Indeed, the vast majority of CB transplantations to date (now estimated to be > 30 000 patients worldwide) were performed with grafts having 1 or 2 HLA-A, -B, and -DRB1 mismatches.10
Possible mechanisms for tolerance in CB transplantation are just now being elucidated.11,12 For example, we recently reported improved clinical outcomes, including a possible antileukemia effect, in patients with hematologic malignancies when a noninherited maternal antigen (NIMA) of the CB donor matches the patient's mismatched antigen.13 The effect, if any, of donor/recipient mismatch vector or direction, with its implied immunologic mechanisms, is another obvious area to explore. In the vast majority of mismatched CB grafts transplanted to date, a mismatched HLA antigen is present in both recipient and donor; thus, the mismatch is bidirectional (ie, in both graft-versus-host [GVH] and host-versus-graft or rejection directions). However, when the donor is homozygous at an HLA locus but the patient has 2 antigens identified (one matching the donor) at that locus, only donor cells have an HLA target and the mismatch is in the GVH direction with no rejection mismatch. If all mismatched loci have this kind of mismatch, these are GVH-only (GVH-O) mismatches. Conversely, if the patient is homozygous at a locus but the donor has 2 antigens identified (1 matching the patient) at that locus, only host cells have an HLA target and the mismatch is in the rejection direction. When all mismatched loci have this type of mismatch, these are rejection-only (R-O) mismatches. When there are 2 or more HLA mismatches, various combinations of mismatch direction are possible.
For this study, we hypothesized that the effects of mismatch vector would be most apparent in CB grafts with unidirectional mismatches. GVH-O grafts, for example, should exhibit better engraftment (decreased graft rejection) compared with bidirectional mismatches but retain a risk for GVHD or perhaps have an increased risk. R-O grafts, on the other hand, should have increased graft rejection and decreased GVHD rates compared with bidirectional mismatches. In view of associations between GVHD and graft versus leukemia (GVL) effects, R-O grafts might also carry an increased risk of relapse.14,15 Furthermore, these relationships may be reflected in transplantation-related mortality (TRM), overall mortality, and treatment failure (relapse or death).
Methods
CB unit collection, processing, and testing
Methods of CB collection, testing, processing, freezing, and storage have been described in detail elsewhere.2,4,6,16,17 Mothers gave New York Blood Center (NYBC) Institutional Review Board–approved written informed consent to donate their infant's CB to any patient who might need it in accordance with the Declaration of Helsinki. Typing for the class I HLA-A and -B antigens was performed at a low-to-intermediate resolution level (including splits of broad antigens) with serologic testing confirmed by DNA methods. Typing for the class II DRB1 locus was at the high resolution (allele) level.
CB unit matching and selection for transplantation
Transplant centers selected CB units for transplantation based on the unit's pre-freeze total nucleated cell (TNC) dose per kilogram of patient body weight and the match between the patient's HLA type and that of the CB unit. Patient HLA Laboratory Reports were submitted by transplant centers, most of which also provided patient samples to NYBC for confirmatory typing. Before release for transplantation, we retested the CB unit using blood from a segment of tubing integral to the cryopreservation bag to confirm both the HLA type and the identity of the unit. Confirmatory typing was performed using molecular techniques. CB grafts were classified as HLA-A, -B, and -DRB1 matched or 1 or 2 antigen/allele mismatched. Unit selection did not include matching for other HLA loci (C, DP, or DQ). Mismatch vector was generally not considered in unit selection.
Study inclusion criteria and patient follow-up
Through the end of 2006, the NYBC National Cord Blood Program had provided 2462 CB units from unrelated CB donors to 2258 patients in transplant centers in the United States and 32 other countries. Patients gave written informed consent for CB transplantation and reporting of outcomes according to Food and Drug Administration Investigational New Drug guidelines. Consecutive United States patients transplanted with single CB units for treatment of hematologic malignancies or for genetic or acquired nonmalignant diseases were potentially eligible for the current study (n = 1390). Patients transplanted for solid tumors (n = 11, all with neuroblastoma) and those who received a CB graft as a “rescue” transplant after failure of an autologous marrow transplant (n = 3) were excluded. Patients who received units with 3 (n = 81) or 4 (n = 5) mismatches also were excluded as were those in which the splits of a broad class I antigen match were not identified (n = 12) and those without high resolution DR typing (n = 3). These exclusions left 1275 eligible patients, of whom 1202 had outcome data reported (94% of those eligible) and are the subject of this analysis.
Transplant centers were asked to report information on patient characteristics, including diagnosis and stage of disease at the time of transplant, conditioning regimen, GVHD prophylaxis, and posttransplantation outcome data at 3, 6, and 12 months and annually thereafter. Patients with acute lymphoblastic leukemia, acute myelocytic leukemia, or chronic myelocytic leukemia were classified as low, intermediate, or high risk according to disease stage using International Bone Marrow Transplant Registry criteria.18 Centers reported on the dates of neutrophil and platelet engraftment, on the dates of onset, severity, and organ involvement of acute and chronic GVHD, on date of relapse, and on date and causes of death. Reports were reviewed for completeness and consistency by one of the authors (A.S.) and had ambiguities resolved, as needed, with the transplant center staff. The analytic dataset was checked for accuracy against the original reports by 2 of the coauthors (C.E.S. and D.S.). Follow-up through the first year after transplant or until patient's death was available in 1064 of the 1202 (89%) patients. Among survivors, 73% have had follow-up data reported through at least 1 year after transplantation (median, 26 months).
Transplant outcome end points
Outcome end points followed standard definitions. Time to myeloid engraftment was defined as the first of 3 consecutive days of absolute neutrophil cell count ≥ 500/μL (ANC 500). When chimerism studies were available (n = 627), patients with no donor cells detected (n = 65, 10%) were considered to have graft failure and were censored at the time they achieved host-derived ANC 500. The time of platelet engraftment was defined as the time to first platelet count ≥ 50 000/μL (platelet 50 000) that persisted for at least 7 consecutive days without transfusion. Acute GVHD was reported, but no date of onset was provided in 44 patients; for these cases, the onset was assigned as the average time of those with known onset (28 days). Similarly, 26 patients had chronic GVHD reported but no date of onset. We assigned the onset for these cases as day 100.
Among patients with hematologic malignancies, analysis of relapse was based on either clinical or cytogenetic relapse. TRM in these patients was defined as death while the patient was in remission, and treatment failure was defined as relapse or death with time to treatment failure based on the first event. Among patients without hematologic malignancies, all deaths were considered transplant-related. Surviving patients were censored at the time of last follow-up.
Data analysis and statistical methods
For analytic purposes, CB unit/patient pairs were classified by the number of HLA mismatches and by mismatch direction as having: only bidirectional mismatches (all mismatches in both the GVH and rejection directions), only unidirectional mismatches (as described in “Introduction”), or combinations of mismatch direction (examples are presented in Table 1).
Examples of HLA-A, -B, and -DRB1 mismatches and mismatch direction
| HLA matched | |||||
| Patient | A2 | A68 | B8 | B65 | DRB1*0102 DRB1*0301 |
| CBU | A2 | A68 | B8 | B65 | DRB1*0102 DRB1*0301 |
| 1 HLA mismatch, bidirectional | |||||
| Patient | A1 | A2 | B8 | B50 | DRB1*0301 DRB1*0701 |
| CBU | A1 | A2 | B8 | B45 | DRB1*0301 DRB1*0701 |
| 2 HLA mismatches, both bidirectional | |||||
| Patient | A2 | A68 | B61 | B57 | DRB1*0411 DRB1*1103 |
| CBU | A2 | A68 | B61 | B70 | DRB1*0411 DRB1*0101 |
| 1 HLA mismatch, GVH-O | |||||
| Patient | A2 | A30 | B7 | B58 | DRB1*1102 DRB1*1503 |
| CBU | A2 | A– | B7 | B58 | DRB1*1102 DRB1*1503 |
| 2 HLA mismatches, GVH-O | |||||
| Patient | A3 | A68 | B49 | B53 | DRB1*0804 DRB1*1303 |
| CBU | A– | A68 | B49 | B53 | DRB1*0804 DRB1*—- |
| 1 HLA mismatch, R-O | |||||
| Patient | A2 | A– | B7 | B44 | DRB1*0401 DRB1*1501 |
| CBU | A2 | A3 | B7 | B44 | DRB1*0401 DRB1*1501 |
| 2 HLA mismatches, R-O | |||||
| Patient | A24 | A29 | B44 | B– | DRB1*—- DRB1*1302 |
| CBU | A24 | A29 | B44 | B57 | DRB1*0701 DRB1*1302 |
| 2 HLA mismatches, 1 bidirectional & 1 GVH | |||||
| Patient | A2 | A11 | B7 | B35 | DRB1*0407 DRB1*1501 |
| CBU | A2 | A– | B7 | B35 | DRB1*1201 DRB1*1501 |
| 2 HLA mismatches, 1 bidirectional & 1 rejection | |||||
| Patient | A– | A33 | B45 | B63 | DRB1*0102 DRB1*0301 |
| CBU | A24 | A33 | B45 | B58 | DRB1*0102 DRB1*0301 |
| 2 HLA mismatches, 1 GVH & 1 rejection | |||||
| Patient | A– | A2 | B7 | B37 | DRB1*0301 DRB1*1501 |
| CBU | A1 | A2 | B7 | B37 | DRB1*—- DRB1*1501 |
| HLA matched | |||||
| Patient | A2 | A68 | B8 | B65 | DRB1*0102 DRB1*0301 |
| CBU | A2 | A68 | B8 | B65 | DRB1*0102 DRB1*0301 |
| 1 HLA mismatch, bidirectional | |||||
| Patient | A1 | A2 | B8 | B50 | DRB1*0301 DRB1*0701 |
| CBU | A1 | A2 | B8 | B45 | DRB1*0301 DRB1*0701 |
| 2 HLA mismatches, both bidirectional | |||||
| Patient | A2 | A68 | B61 | B57 | DRB1*0411 DRB1*1103 |
| CBU | A2 | A68 | B61 | B70 | DRB1*0411 DRB1*0101 |
| 1 HLA mismatch, GVH-O | |||||
| Patient | A2 | A30 | B7 | B58 | DRB1*1102 DRB1*1503 |
| CBU | A2 | A– | B7 | B58 | DRB1*1102 DRB1*1503 |
| 2 HLA mismatches, GVH-O | |||||
| Patient | A3 | A68 | B49 | B53 | DRB1*0804 DRB1*1303 |
| CBU | A– | A68 | B49 | B53 | DRB1*0804 DRB1*—- |
| 1 HLA mismatch, R-O | |||||
| Patient | A2 | A– | B7 | B44 | DRB1*0401 DRB1*1501 |
| CBU | A2 | A3 | B7 | B44 | DRB1*0401 DRB1*1501 |
| 2 HLA mismatches, R-O | |||||
| Patient | A24 | A29 | B44 | B– | DRB1*—- DRB1*1302 |
| CBU | A24 | A29 | B44 | B57 | DRB1*0701 DRB1*1302 |
| 2 HLA mismatches, 1 bidirectional & 1 GVH | |||||
| Patient | A2 | A11 | B7 | B35 | DRB1*0407 DRB1*1501 |
| CBU | A2 | A– | B7 | B35 | DRB1*1201 DRB1*1501 |
| 2 HLA mismatches, 1 bidirectional & 1 rejection | |||||
| Patient | A– | A33 | B45 | B63 | DRB1*0102 DRB1*0301 |
| CBU | A24 | A33 | B45 | B58 | DRB1*0102 DRB1*0301 |
| 2 HLA mismatches, 1 GVH & 1 rejection | |||||
| Patient | A– | A2 | B7 | B37 | DRB1*0301 DRB1*1501 |
| CBU | A1 | A2 | B7 | B37 | DRB1*—- DRB1*1501 |
Matched antigens are shown in regular type and mismatched antigens are in bold. Bidirectional means that mismatch was in both the GVH and rejection directions.
CBU indicate cord blood unit; GVH, graft-versus-host; GVH-O, graft-versus-host only; R-O, rejection only; and HLA, human leukocyte antigen.
For time to myeloid engraftment, analyses were limited to the first 77 days after transplantation (3 patients who engrafted after day 77 were counted as not engrafting during the period of analysis). Analysis of acute GVHD was limited to patients who engrafted and, to accommodate a few cases with late onset, included the first 150 days after transplantation. Analysis of chronic GVHD was limited to patients who engrafted and survived to at least 100 days after transplantation.
The significance of differences between categorical variables was estimated by χ2 or Fisher exact test (2-tailed) and between means of continuous variables by Student t test. The probability of transplantation outcome end points was calculated by Kaplan-Meier and, when competing outcome events needed to be taken into account, by cumulative incidence methods.19-21 In estimating cumulative probabilities of engraftment, GVHD, and relapse, the competing event was death. Relapse was the competing event for TRM in patients with hematologic malignancies. Cox regression was used to estimate hazard ratios (HRs) and significance of differences in univariate and multivariate analyses.22
As the focus of this study, HLA match and mismatch direction was retained in all multivariate analyses. Other possible covariates examined for inclusion in multivariate analyses were those reported in previous studies of CB and bone marrow transplantation: TNC dose, patient age and ethnicity, patient and donor sex, ABO and Rh match, pretransplantation cytomegalovirus antibody status, diagnosis, stage of disease for hematologic malignancies, prior transplantation, conditioning regimen (myeloablative vs nonablative), and GVHD prophylaxis. We also included the year of transplantation and transplant center experience as variables possibly contributing to outcome. As a measure of center experience, we grouped together centers that had transplanted 50 or more patients with CB units from NYBC for comparison with patients from centers with fewer transplants. Given our recent findings with the NIMA-matched transplants,13 mismatched patient/CB graft pairs were also classified by the presence of a match in the patient to the donor's NIMA. NIMA match was retained as a variable in all multivariate analyses. Variables having a P value > .05 were dropped stepwise from multivariate analyses in order of highest value until all remaining values were ≤ .05. Statistically significant variables remaining in the models are listed in table footnotes.
Results
Patient and CB graft characteristics
Study patients ranged up to 67 years in age (40% were 10 years of age or older; median age, 7 years), 44% were nonwhite, and nearly two-thirds were transplanted for leukemia (28% of whom had advanced acute lymphoblastic leukemia, acute myelocytic leukemia, or chronic myelocytic leukemia; Table 2; patient characteristics). Some important patient and graft characteristics changed over time. For example, the average TNC dose of CB grafts used in this study population increased over time, especially in transplantations performed in the most recent 4 years (89% of 2003-2006 patients got a unit with a TNC dose ≥ 2.5 × 107/kg compared with 76% for earlier patients, P < .001). HLA match also improved in the more recent transplantations (50% of 2003-2006 patients had 0 or 1 mismatch compared with 41% for earlier transplants, P = .006).
Characteristics of 1202 study participants
| Patient characteristics . | Number (%) . |
|---|---|
| Sex | |
| Male | 715 (59%) |
| Female | 487 (41%) |
| Age, y | |
| 0-1 | 267 (22%) |
| 2-5 | 266 (22%) |
| 6-9 | 193 (16%) |
| 10-15 | 181 (15%) |
| ≥ 16 | 295 (25%) |
| (mean = 12.3 and median = 7.2, range = 1 mo-67 y) | |
| Ethnic background (unknown = 18) | |
| Asian | 49 (4%) |
| African | 228 (19%) |
| Hispanic | 211 (18%) |
| Native American | 10 (1%) |
| Middle Eastern | 21 (2%) |
| White | 660 (56%) |
| Other | 5 (0.4%) |
| Diagnosis | |
| Leukemia | 771 (64%) |
| ALL | 353 (29%) |
| AML | 301 (25%) |
| CML | 81 (7%) |
| Other | 36 (3%) |
| Myelodysplasia | 58 (5%) |
| Lymphoma | 38 (3%) |
| Genetic | 294 (24%) |
| Metabolic | 92 (8%) |
| Immune deficiency | 86 (7%) |
| Bone marrow failure | 69 (6%) |
| Histiocytosis | 30 (2%) |
| Red blood cell disease | 16 (1%) |
| Platelet disease | 1 (0.1%) |
| Severe aplastic anemia | 32 (3%) |
| Other | 9 (1%) |
| Stage of ALL, AML, CML (unknown = 31, including 10 known not to be advanced by IBMTR criteria) | |
| Early | 163 (23%) |
| Intermediate | 327 (46%) |
| Advanced | 214 (30%) |
| Prior transplant (unknown = 70) | |
| None | 1031 (91%) |
| Allogeneic | 52 (5%) |
| Autologous | 46 (4%) |
| Unknown type | 3 (0.3%) |
| Preparatory regimen (unknown = 131) | |
| Myeloablative | 987 (92%) |
| Nonmyeloablative | 84 (8%) |
| GVHD prophylaxis (unknown = 219) | |
| Cyclosporine and Steroids | 610 (62%) |
| Any methotrexate | 167 (17%) |
| Tacrolimus (without methotrexate) | 112 (11%) |
| Other (without methotrexate) | 94 (10%) |
| Center experience | |
| < 50 NYBC CBUs | 665 (55%) |
| ≥ 50 NYBC CBUs | 537 (45%) |
| Year of transplant | |
| 1993-1996 | 224 (19%) |
| 1997-1999 | 459 (38%) |
| 2000-2002 | 238 (20%) |
| 2003-2006 | 281 (23%) |
| HLA mismatch (HLA-A, -B, -DRB1) | |
| 0 mismatches | 72 (6%) |
| 1 bidirectional mismatch | 364 (30%) |
| 2 bidirectional mismatches | 526 (44%) |
| GVHD only | 58 (5%) |
| Rejection only | 40 (3%) |
| 2 MM, bidirectional + GVHD | 83 (7%) |
| 2 MM, bidirectional + rejection | 54 (4%) |
| 2 MM, GVHD + rejection | 5 (0.4%) |
| NIMA match | |
| 0 mismatches | 72 (6%) |
| NIMA match | 82 (7%) |
| No NIMA match | 1005 (84%) |
| Unknown | 43 (4%) |
| Total nucleated cell dose (x 107/kg) | |
| 0.7-2.4 | 255 (21%) |
| 2.5-4.9 | 407 (34%) |
| 5.0-9.9 | 306 (25%) |
| ≥ 10.0 | 234 (19%) |
| Geometric mean = 4.9 × 107/kg, median = 4.5 × 107/kg, range = 0.7-70.9 | |
| Patient characteristics . | Number (%) . |
|---|---|
| Sex | |
| Male | 715 (59%) |
| Female | 487 (41%) |
| Age, y | |
| 0-1 | 267 (22%) |
| 2-5 | 266 (22%) |
| 6-9 | 193 (16%) |
| 10-15 | 181 (15%) |
| ≥ 16 | 295 (25%) |
| (mean = 12.3 and median = 7.2, range = 1 mo-67 y) | |
| Ethnic background (unknown = 18) | |
| Asian | 49 (4%) |
| African | 228 (19%) |
| Hispanic | 211 (18%) |
| Native American | 10 (1%) |
| Middle Eastern | 21 (2%) |
| White | 660 (56%) |
| Other | 5 (0.4%) |
| Diagnosis | |
| Leukemia | 771 (64%) |
| ALL | 353 (29%) |
| AML | 301 (25%) |
| CML | 81 (7%) |
| Other | 36 (3%) |
| Myelodysplasia | 58 (5%) |
| Lymphoma | 38 (3%) |
| Genetic | 294 (24%) |
| Metabolic | 92 (8%) |
| Immune deficiency | 86 (7%) |
| Bone marrow failure | 69 (6%) |
| Histiocytosis | 30 (2%) |
| Red blood cell disease | 16 (1%) |
| Platelet disease | 1 (0.1%) |
| Severe aplastic anemia | 32 (3%) |
| Other | 9 (1%) |
| Stage of ALL, AML, CML (unknown = 31, including 10 known not to be advanced by IBMTR criteria) | |
| Early | 163 (23%) |
| Intermediate | 327 (46%) |
| Advanced | 214 (30%) |
| Prior transplant (unknown = 70) | |
| None | 1031 (91%) |
| Allogeneic | 52 (5%) |
| Autologous | 46 (4%) |
| Unknown type | 3 (0.3%) |
| Preparatory regimen (unknown = 131) | |
| Myeloablative | 987 (92%) |
| Nonmyeloablative | 84 (8%) |
| GVHD prophylaxis (unknown = 219) | |
| Cyclosporine and Steroids | 610 (62%) |
| Any methotrexate | 167 (17%) |
| Tacrolimus (without methotrexate) | 112 (11%) |
| Other (without methotrexate) | 94 (10%) |
| Center experience | |
| < 50 NYBC CBUs | 665 (55%) |
| ≥ 50 NYBC CBUs | 537 (45%) |
| Year of transplant | |
| 1993-1996 | 224 (19%) |
| 1997-1999 | 459 (38%) |
| 2000-2002 | 238 (20%) |
| 2003-2006 | 281 (23%) |
| HLA mismatch (HLA-A, -B, -DRB1) | |
| 0 mismatches | 72 (6%) |
| 1 bidirectional mismatch | 364 (30%) |
| 2 bidirectional mismatches | 526 (44%) |
| GVHD only | 58 (5%) |
| Rejection only | 40 (3%) |
| 2 MM, bidirectional + GVHD | 83 (7%) |
| 2 MM, bidirectional + rejection | 54 (4%) |
| 2 MM, GVHD + rejection | 5 (0.4%) |
| NIMA match | |
| 0 mismatches | 72 (6%) |
| NIMA match | 82 (7%) |
| No NIMA match | 1005 (84%) |
| Unknown | 43 (4%) |
| Total nucleated cell dose (x 107/kg) | |
| 0.7-2.4 | 255 (21%) |
| 2.5-4.9 | 407 (34%) |
| 5.0-9.9 | 306 (25%) |
| ≥ 10.0 | 234 (19%) |
| Geometric mean = 4.9 × 107/kg, median = 4.5 × 107/kg, range = 0.7-70.9 | |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; GVH, graft-versus-host; GVHD, graft-versus-host disease; IBMTR, International Blood and Marrow Transplant Registry; MM, mismatch; NIMA, noninherited maternal antigen; and NYBC, New York Blood Center.
Among the CB unit/patient pairs in the study, 890 had only bidirectional mismatches, 58 had GVH-O mismatches (51 with a single mismatch and 7 with 2), 40 had R-O mismatches (30 with a single mismatch and 10 with 2 mismatches), and 69 had fully matched grafts. The remaining 145 pairs had combinations of mismatch direction (Table 2). Within the HLA mismatch category, patients with unidirectional mismatches generally did not differ from those with bidirectional mismatches. The only exception was GVH-O graft recipients who were more likely to have had a prior allogeneic transplantation (11% vs 5%, P = .029) and to have received nonmyeloablative conditioning (21% vs 6%, P < .001). Both of these variables were associated with a lower engraftment rate. Prior allogeneic transplantation was also associated with increased mortality. These variables were included as covariates in multivariate analyses of study end points where appropriate to help compensate for the differences between patient groups (Tables 3 and 4 footnotes). There was some overlap between mismatch direction and the presence of a NIMA match, a factor associated with better outcomes.13 Among patients with GHV-O mismatched grafts, 4 (7%) also had a NIMA match, no different from the rate among those with only bidirectional mismatches (8%), whereas patients with R-O mismatches had no grafts that matched a donor's NIMA. NIMA match was included in all multivariate analyses regardless of significance.
Multivariate analyses of engraftment and GVHD
| End point: variable . | Number . | HR (95% CI) . | P . |
|---|---|---|---|
| ANC ≥ 500/mm3 during first 77 days after transplantation* (time unknown on 50 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 69 | 1.5 (1.1-2.0) | .006 |
| 1-2 MM/GVHD only | 57 | 1.6 (1.2-2.2) | .003 |
| 1-2 MM/Rejection only | 37 | 0.7 (0.4-1.1) | .095 |
| 1 Bidirectional MM | 342 | 1.0 (Reference) | |
| 2 Bidirectional MM | 509 | 0.9 (0.8-1.1) | .484 |
| 2 MM: Bidirectional + GVHD | 80 | 0.9 (0.7-1.2) | .588 |
| 2 MM: Bidirectional + rejection | 53 | 0.8 (0.6-1.2) | .304 |
| 2 MM: GVHD + rejection | 5 | 0.9 (0.4-2.2) | .806 |
| Platelet count ≥ 50 000/mm3 during first 9 mo after transplantation† (time unknown on 95 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 67 | 2.0(1.4-2.7) | < 0.001 |
| 1-2 MM/GVHD only | 54 | 1.6 (1.1-2.4) | .009 |
| 1-2 MM/Rejection only | 33 | 1.0 (0.6-1.7) | .993 |
| 1 bidirectional MM | 331 | 1.0 (Reference) | |
| 2 bidirectional MM | 492 | 1.1 (0.9-1.3) | .548 |
| 2 MM: bidirectional + GVHD | 75 | 1.4 (0.9-2.1) | .096 |
| 2 MM: bidirectional + rejection | 50 | 1.1 (0.7-1.7) | .666 |
| 2 MM: GVHD + rejection | 5 | 1.4 (0.4-4.4) | .566 |
| Acute grade 3-4 GVHD during first 150 d after transplantation among patients who engrafted‡ (data not reported on 61 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 58 | 0.3 (0.1-0.9) | .022 |
| 1-2 MM/GVHD only | 45 | 0.6 (0.3-1.4) | .241 |
| 1-2 MM/rejection only | 22 | 0.6 (0.2-1.8) | .354 |
| 1 bidirectional MM | 262 | 1.0 (Reference) | |
| 2 bidirectional MM | 368 | 1.3 (0.97-1.9) | .074 |
| 2 MM: bidirectional + GVHD | 59 | 1.0 (0.5-1.7) | .889 |
| 2 MM: bidirectional + rejection | 38 | 0.7 (0.3-1.8) | .505 |
| 2 MM: GVHD + rejection | 5 | 0.0 (0.0- > >) | .942 |
| Chronic GVHD during first 3 y after transplantation among patients who engrafted and survived to day 100§ (data not reported on 135 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 42 | 1.0 (0.6-1.9) | .939 |
| 1-2 MM/GVHD only | 35 | 1.2 (0.6-2.3) | .591 |
| 1-2 MM/rejection only | 13 | 0.9 (0.3-2.8) | .811 |
| 1 bidirectional MM | 162 | 1.0 (Reference) | |
| 2 bidirectional MM | 208 | 1.3 (0.8-1.9) | .256 |
| 2 MM: bidirectional + GVHD | 36 | 1.6 (0.9-3.0) | .103 |
| 2 MM: bidirectional + rejection | 25 | 0.9 (0.4-2.2) | .843 |
| 2 MM: GVHD + rejection | 3 | 3.1 (0.7-12.9) | .122 |
| End point: variable . | Number . | HR (95% CI) . | P . |
|---|---|---|---|
| ANC ≥ 500/mm3 during first 77 days after transplantation* (time unknown on 50 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 69 | 1.5 (1.1-2.0) | .006 |
| 1-2 MM/GVHD only | 57 | 1.6 (1.2-2.2) | .003 |
| 1-2 MM/Rejection only | 37 | 0.7 (0.4-1.1) | .095 |
| 1 Bidirectional MM | 342 | 1.0 (Reference) | |
| 2 Bidirectional MM | 509 | 0.9 (0.8-1.1) | .484 |
| 2 MM: Bidirectional + GVHD | 80 | 0.9 (0.7-1.2) | .588 |
| 2 MM: Bidirectional + rejection | 53 | 0.8 (0.6-1.2) | .304 |
| 2 MM: GVHD + rejection | 5 | 0.9 (0.4-2.2) | .806 |
| Platelet count ≥ 50 000/mm3 during first 9 mo after transplantation† (time unknown on 95 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 67 | 2.0(1.4-2.7) | < 0.001 |
| 1-2 MM/GVHD only | 54 | 1.6 (1.1-2.4) | .009 |
| 1-2 MM/Rejection only | 33 | 1.0 (0.6-1.7) | .993 |
| 1 bidirectional MM | 331 | 1.0 (Reference) | |
| 2 bidirectional MM | 492 | 1.1 (0.9-1.3) | .548 |
| 2 MM: bidirectional + GVHD | 75 | 1.4 (0.9-2.1) | .096 |
| 2 MM: bidirectional + rejection | 50 | 1.1 (0.7-1.7) | .666 |
| 2 MM: GVHD + rejection | 5 | 1.4 (0.4-4.4) | .566 |
| Acute grade 3-4 GVHD during first 150 d after transplantation among patients who engrafted‡ (data not reported on 61 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 58 | 0.3 (0.1-0.9) | .022 |
| 1-2 MM/GVHD only | 45 | 0.6 (0.3-1.4) | .241 |
| 1-2 MM/rejection only | 22 | 0.6 (0.2-1.8) | .354 |
| 1 bidirectional MM | 262 | 1.0 (Reference) | |
| 2 bidirectional MM | 368 | 1.3 (0.97-1.9) | .074 |
| 2 MM: bidirectional + GVHD | 59 | 1.0 (0.5-1.7) | .889 |
| 2 MM: bidirectional + rejection | 38 | 0.7 (0.3-1.8) | .505 |
| 2 MM: GVHD + rejection | 5 | 0.0 (0.0- > >) | .942 |
| Chronic GVHD during first 3 y after transplantation among patients who engrafted and survived to day 100§ (data not reported on 135 patients) | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 42 | 1.0 (0.6-1.9) | .939 |
| 1-2 MM/GVHD only | 35 | 1.2 (0.6-2.3) | .591 |
| 1-2 MM/rejection only | 13 | 0.9 (0.3-2.8) | .811 |
| 1 bidirectional MM | 162 | 1.0 (Reference) | |
| 2 bidirectional MM | 208 | 1.3 (0.8-1.9) | .256 |
| 2 MM: bidirectional + GVHD | 36 | 1.6 (0.9-3.0) | .103 |
| 2 MM: bidirectional + rejection | 25 | 0.9 (0.4-2.2) | .843 |
| 2 MM: GVHD + rejection | 3 | 3.1 (0.7-12.9) | .122 |
CBU indicates cord blood unit; CI, confidence interval; GVHD, graft-versus-host disease; MM, mismatch; NIMA, noninherited maternal antigen; NYBC, New York Blood Center; HR, hazard ratio; and TNC, total nucleated cell.
Other significant predictors included in the multivariate model for time to ANC 500 were TNC dose (continuous log transformed), prior transplant (none vs allogeneic vs autologous vs unknown), preparatory regimen (myeloblative vs nonmyeloablative vs unknown), GVHD prophylaxis (cyclosporin and steroids vs any methotrexate vs any tacrolimus without methotrexate vs other without methotrexate vs unknown) and year of transplant (1993-2002 vs 2003-2006). Match between recipient and donor for noninherited maternal antigens (NIMAs) was forced into the model even when not significant.
Other significant predictors included in the model for time to platelet 50 000 were TNC dose (continuous log transformed), patient age (< 10 y vs ≥ 10 y), patient ethnicity (White vs other vs unknown), prior transplant (none vs allogeneic vs autologous vs unknown), preparatory regimen (myeloblative vs nonmyeloablative vs unknown), GVHD prophylaxis (cyclosporin and steroids vs any methotrexate vs any tacrolimus without methotrexate vs other without methotrexate vs unknown) and year of transplant (1993-2002 vs 2003-2006). NIMA match also was forced into the model even when not significant.
Other significant predictors included in the model for acute GVHD were patient age (< 10 y vs ≥ 10 y), GVHD prophylaxis (cyclosporin and steroids vs any methotrexate vs any tacrolimus without methotrexate vs other without methotrexate vs unknown) and transplant center experience with NYBC CBUs (< 50 vs ≥ 50 patients transplanted). NIMA match also was forced into the model even when not significant.
Other significant predictors included in the model for chronic GVHD were patient age (< 10 y vs ≥ 10 y), patient ethnicity (White vs other vs unknown) and transplant center experience with NYBC CBUs (< 50 vs ≥ 50 patients transplanted). NIMA match also was forced into the model even when not significant.
Multivariate analyses of relapse and survival end points during the first 3 years after transplantation
| End point: variable . | Number . | HR (95% CI) . | P . |
|---|---|---|---|
| Patients with hematologic malignancies (total number = 870) | |||
| Relapse* | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.7 (0.3-2.0) | 0.547 |
| 1-2 MM/GVHD only | 35 | 0.6 (0.3-1.3) | 0.181 |
| 1-2 MM/rejection only | 23 | 2.4 (1.2-4.8) | 0.010 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 0.8 (0.5-1.1) | 0.109 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.6-1.8) | 0.965 |
| 2 MM: bidirectional + rejection | 42 | 1.0 (0.5-1.9) | 0.988 |
| 2 MM: GVHD + rejection | 3 | 0.0 (0.0- > >) | 0.958 |
| Transplantation-related mortality† | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.4 (0.2-0.8) | 0.010 |
| 1-2 MM/GVHD only | 35 | 0.5 (0.2-1.04) | 0.062 |
| 1-2 MM/rejection only | 23 | 1.0 (0.5-2.1) | 0.933 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 1.3 (1.00-1.7) | 0.047 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.6-1.6) | 0.982 |
| 2 MM: bidirectional + rejection | 42 | 1.4 (0.9-2.3) | 0.152 |
| 2 MM: GVHD + rejection | 3 | 2.2 (0.5-9.0) | 0.279 |
| Treatment failure (relapse or death)† | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.5 (0.3-0.8) | 0.004 |
| 1-2 MM/GVHD only | 35 | 0.5 (0.3-0.9) | 0.016 |
| 1-2 MM/rejection only | 23 | 1.4 (0.9-2.4) | 0.147 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 1.1 (0.9-1.4) | 0.218 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.7-1.4) | 0.972 |
| 2 MM: bidirectional + rejection | 42 | 1.4 (0.96-2.1) | 0.080 |
| 2 MM: GVHD + rejection | 3 | 1.3 (0.3-5.1) | 0.744 |
| Overall mortality† | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.5 (0.3-0.8) | 0.007 |
| 1-2 MM/GVHD only | 35 | 0.5 (0.3-0.9) | 0.019 |
| 1-2 MM/rejection only | 23 | 1.4 (0.8-2.4) | 0.193 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 1.1 (0.9-1.4) | 0.217 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.7-1.5) | 0.880 |
| 2 MM: bidirectional + rejection | 42 | 1.3 (0.9-2.0) | 0.154 |
| 2 MM: GVHD + rejection | 3 | 1.4 (0.4-5.8) | 0.614 |
| Patients with other diseases (total number = 332) | |||
| Transplantation-related mortality‡ | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 24 | 0.8 (0.4-1.7) | 0.532 |
| 1-2 MM/GVHD only | 23 | 0.9 (0.4-1.9) | 0.782 |
| 1-2 MM/rejection only | 17 | 0.9 (0.3-2.2) | 0.766 |
| 1 bidirectional MM | 115 | 1.0 (Reference) | |
| 2 bidirectional MM | 112 | 1.4 (0.9-2.0) | 0.140 |
| 2 MM: bidirectional + GVHD | 27 | 1.8 (0.95-3.2) | 0.072 |
| 2 MM: bidirectional + rejection | 12 | 1.8 (0.8-4.1) | 0.146 |
| 2 MM: GVHD + rejection | 2 | 2.1 (0.3-15.3) | 0.473 |
| End point: variable . | Number . | HR (95% CI) . | P . |
|---|---|---|---|
| Patients with hematologic malignancies (total number = 870) | |||
| Relapse* | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.7 (0.3-2.0) | 0.547 |
| 1-2 MM/GVHD only | 35 | 0.6 (0.3-1.3) | 0.181 |
| 1-2 MM/rejection only | 23 | 2.4 (1.2-4.8) | 0.010 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 0.8 (0.5-1.1) | 0.109 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.6-1.8) | 0.965 |
| 2 MM: bidirectional + rejection | 42 | 1.0 (0.5-1.9) | 0.988 |
| 2 MM: GVHD + rejection | 3 | 0.0 (0.0- > >) | 0.958 |
| Transplantation-related mortality† | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.4 (0.2-0.8) | 0.010 |
| 1-2 MM/GVHD only | 35 | 0.5 (0.2-1.04) | 0.062 |
| 1-2 MM/rejection only | 23 | 1.0 (0.5-2.1) | 0.933 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 1.3 (1.00-1.7) | 0.047 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.6-1.6) | 0.982 |
| 2 MM: bidirectional + rejection | 42 | 1.4 (0.9-2.3) | 0.152 |
| 2 MM: GVHD + rejection | 3 | 2.2 (0.5-9.0) | 0.279 |
| Treatment failure (relapse or death)† | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.5 (0.3-0.8) | 0.004 |
| 1-2 MM/GVHD only | 35 | 0.5 (0.3-0.9) | 0.016 |
| 1-2 MM/rejection only | 23 | 1.4 (0.9-2.4) | 0.147 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 1.1 (0.9-1.4) | 0.218 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.7-1.4) | 0.972 |
| 2 MM: bidirectional + rejection | 42 | 1.4 (0.96-2.1) | 0.080 |
| 2 MM: GVHD + rejection | 3 | 1.3 (0.3-5.1) | 0.744 |
| Overall mortality† | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 48 | 0.5 (0.3-0.8) | 0.007 |
| 1-2 MM/GVHD only | 35 | 0.5 (0.3-0.9) | 0.019 |
| 1-2 MM/rejection only | 23 | 1.4 (0.8-2.4) | 0.193 |
| 1 bidirectional MM | 249 | 1.0 (Reference) | |
| 2 bidirectional MM | 414 | 1.1 (0.9-1.4) | 0.217 |
| 2 MM: bidirectional + GVHD | 56 | 1.0 (0.7-1.5) | 0.880 |
| 2 MM: bidirectional + rejection | 42 | 1.3 (0.9-2.0) | 0.154 |
| 2 MM: GVHD + rejection | 3 | 1.4 (0.4-5.8) | 0.614 |
| Patients with other diseases (total number = 332) | |||
| Transplantation-related mortality‡ | |||
| HLA mismatch and mismatch direction | |||
| 0 MM | 24 | 0.8 (0.4-1.7) | 0.532 |
| 1-2 MM/GVHD only | 23 | 0.9 (0.4-1.9) | 0.782 |
| 1-2 MM/rejection only | 17 | 0.9 (0.3-2.2) | 0.766 |
| 1 bidirectional MM | 115 | 1.0 (Reference) | |
| 2 bidirectional MM | 112 | 1.4 (0.9-2.0) | 0.140 |
| 2 MM: bidirectional + GVHD | 27 | 1.8 (0.95-3.2) | 0.072 |
| 2 MM: bidirectional + rejection | 12 | 1.8 (0.8-4.1) | 0.146 |
| 2 MM: GVHD + rejection | 2 | 2.1 (0.3-15.3) | 0.473 |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; CML, chronic myeloid leukemia; GVHD, graft-versus-host disease; IBMTR, International Blood and Marrow Transplant Registry; MM, mismatch; NIMA, noninherited maternal antigen; NYBC, New York Blood Center; HR, hazard ratio; TNC, total nucleated cell; and CMV, cytomegalovirus.
The only other significant predictor in the multivariate model for relapse risk was IBMTR risk category (early stage ALL, AML or CML vs intermediate stage ALL, AML or CML vs late stage ALL, AML or CML vs unknown stage ALL, AML or CML vs other leukemia vs myelodysplasia vs other hematological malignancy) and GVHD prophylaxis (cyclosporin and steroids vs any methotrexate vs any tacrolimus without methotrexate vs other without methotrexate vs unknown). NIMA match was forced into the model even though not significant.
Among patients with hematological malignancies, the other significant predictors in the multivariate models for TRM, overall mortality and treatment failure were patient age (< 10 years vs ≥ 10 years), prior transplant (none vs allogeneic vs autologous vs unknown), pretransplant CMV antibody status (negative vs positive vs unknown) IBMTR risk category (see footnote *), transplant center experience with NYBC CB units (< 50 vs ≥ 50 patients transplanted) and year of transplant (1993-2002 vs 2003-2006). TNC dose (continuous log transformed) was a significant predictor for TRM and overall mortality but not for treatment failure when age was included in the analysis. Patient ethnicity (White vs other vs unknown) was a significant predictor for TRM but not for overall mortality or treatment failure. NIMA match was forced into the models even when not significant.
Among patients with other diseases, TNC dose (continuous log transformed) and prior transplant (none vs allogeneic vsautologous vs unknown) were significant predictors for TRM. NIMA match was forced into the model even though not significant.
Outcome end points
Data on time to ANC 500 were available for 1151 patients, 76% of whom achieved this end point and on platelet 50 000 for 1108 patients, 51% of whom achieved this end point (cumulative incidences). Among patients who engrafted, 21% had grade 2 and 24% had grade 3-4 acute GVHD. Among those who engrafted and survived to day 100 after transplantation, 35% had chronic GVHD with 86% of cases occurring within the first year after transplantation. Relapse was reported in 196 patients transplanted for a hematologic malignancy (23% cumulative incidence), 83% of cases within the first year. Altogether, 704 patients are known to have died, giving overall survival rates of 46% at 1 year, 38% at 3 years, and 36% at 5 years. Among patients with hematologic malignancies, relapse accounted for 26% of deaths.
Faster engraftment with matched and GVH-O grafts
Myeloid engraftment was significantly faster with grafts having GVH-O mismatches compared with the reference group (patients given grafts with 1 bidirectional mismatch) and was similar to that of fully matched grafts (Table 3). This association with time to ANC 500 was most apparent after day 20 after transplantation (Figure 1A). Recipients of R-O mismatched grafts, on the other hand, tended to have a lower rate of myeloid engraftment than patients given grafts with 1 bidirectional mismatch (P = .095, Table 3), a relationship that was significant for patients who survived past day 20 but had not yet engrafted (HR = 0.4, P = .010). This pattern of a later effect on engraftment with HLA mismatch and mismatch direction differed from that of TNC dose, which was strongest during the first 20 days (Figure 1B). Associations between mismatch direction and myeloid engraftment were seen both in patients with hematologic malignancy and in those with other diseases when analyzed separately, although neither subset reached statistical significance (data not shown). GVH-O grafts, like HLA-matched grafts, were also associated with improved platelet engraftment (Table 3).
Cumulative probability of achieving ANC 500 during the first 77 days after transplantation. (A) Relationship to HLA mismatch (MM) number and direction. The number of patients in each mismatch category known to be alive without ANC 500 at time after transplantation are as follows: 0 MM: day 0, 59; day 21, 33; day 42, 3; 1 bidirectional MM: day 0, 342; day 21, 315; day 42, 33; 2 bidirectional MMs: day 0, 509; day 21, 309; day 42, 52; GVH-O MM: day 0, 57; day 21, 30; day 42, 2; rejection only MM: day 0, 37; day 21, 21; day 42, 8. (B) Relationship to TNC dose × 107/kg.
Cumulative probability of achieving ANC 500 during the first 77 days after transplantation. (A) Relationship to HLA mismatch (MM) number and direction. The number of patients in each mismatch category known to be alive without ANC 500 at time after transplantation are as follows: 0 MM: day 0, 59; day 21, 33; day 42, 3; 1 bidirectional MM: day 0, 342; day 21, 315; day 42, 33; 2 bidirectional MMs: day 0, 509; day 21, 309; day 42, 52; GVH-O MM: day 0, 57; day 21, 30; day 42, 2; rejection only MM: day 0, 37; day 21, 21; day 42, 8. (B) Relationship to TNC dose × 107/kg.
No association between GVH-O or R-O grafts and GVHD
Increased risk of relapse with R-O grafts in patients with hematologic malignancies
Among patients with hematologic malignancies, those given R-O grafts had increased relapse risk (HR = 2.4, P = .010, Table 4), with similar trends in both myelogenous (HR = 2.1, P = .113) and nonmyelogenous malignancies (HR = 2.0, P = .151). Because R-O grafts were associated with an increased incidence of graft failure, we also examined the relapse risk for R-O graft recipients who engrafted (n = 12). This subset also had an increased risk of post-transplantation relapse of a similar order of magnitude (HR = 2.0, P = .117).
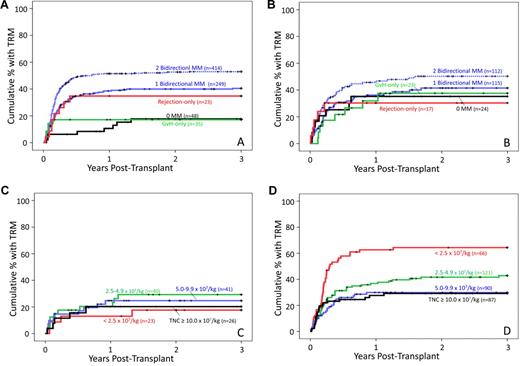
Decreased TRM, overall mortality, and treatment failure with matched and GVH-O grafts in patients with hematologic malignancies
TRM (Figure 2A), overall mortality, and treatment failure rates were reduced for GVH-O graft recipients compared with the 1-bidirectional-mismatch reference group (Table 4) and were similar to those given HLA-matched grafts. R-O graft recipients, on the other hand, had no significant increase in TRM or, despite their higher relapse rate, in overall mortality and treatment failure (Table 4). Mismatch direction was not associated with TRM among the patients with other diseases (Figure 2B; Table 4).
Cumulative probability of TRM during the first 3 years after transplantation. (A) Patients with hematologic malignancies: Relationship to HLA mismatch (MM) number and direction. The number of patients in each mismatch category known to be alive without relapse at time after transplantation are as follows: 0 MM: day 0, 48; year 1, 28; year 2, 20; year 3, 16; 1 bidirectional MM: day 0, 249; year 1, 81; year 2, 56; year 3, 49; 2 bidirectional MMs: day 0, 414; year 1, 123; year 2, 90; year 3, 76; GVH-O MM: day 0, 35; year 1, 20; year 2, 16; year 3, 3; rejection only MM: day 0, 23; year 1, 4; year 2, 3; year 3, 3. (B) Patients with other diseases: relationship to HLA mismatch (MM) number and direction. The number of patients in each mismatch category known to be alive without relapse at time after transplantation are as follows: 0 MM: day 0, 24; year 1, 22; year 2, 7; year 3, 5; 1 bidirectional MM: day 0, 115; year 1, 54; year 2, 37; year 3, 26; 2 bidirectional MMs: day 0, 112; year 1, 49; year 2, 37; year 3, 28; GVH-O MM: day 0, 23; year 1, 12; year 2, 8; year 3, 6; rejection only MM: day 0, 17; year 1, 6; year 2, 5; year 3, 3. (C) All patients (n = 130) given HLA-matched or GVH-only mismatched CB grafts: relationship to TNC dose × 107/kg. (D) All patients (n = 364) given CB grafts with 1 bidirectional mismatch: relationship to TNC dose × 107/kg.
Cumulative probability of TRM during the first 3 years after transplantation. (A) Patients with hematologic malignancies: Relationship to HLA mismatch (MM) number and direction. The number of patients in each mismatch category known to be alive without relapse at time after transplantation are as follows: 0 MM: day 0, 48; year 1, 28; year 2, 20; year 3, 16; 1 bidirectional MM: day 0, 249; year 1, 81; year 2, 56; year 3, 49; 2 bidirectional MMs: day 0, 414; year 1, 123; year 2, 90; year 3, 76; GVH-O MM: day 0, 35; year 1, 20; year 2, 16; year 3, 3; rejection only MM: day 0, 23; year 1, 4; year 2, 3; year 3, 3. (B) Patients with other diseases: relationship to HLA mismatch (MM) number and direction. The number of patients in each mismatch category known to be alive without relapse at time after transplantation are as follows: 0 MM: day 0, 24; year 1, 22; year 2, 7; year 3, 5; 1 bidirectional MM: day 0, 115; year 1, 54; year 2, 37; year 3, 26; 2 bidirectional MMs: day 0, 112; year 1, 49; year 2, 37; year 3, 28; GVH-O MM: day 0, 23; year 1, 12; year 2, 8; year 3, 6; rejection only MM: day 0, 17; year 1, 6; year 2, 5; year 3, 3. (C) All patients (n = 130) given HLA-matched or GVH-only mismatched CB grafts: relationship to TNC dose × 107/kg. (D) All patients (n = 364) given CB grafts with 1 bidirectional mismatch: relationship to TNC dose × 107/kg.
Among patients given either HLA-matched or GVH-O-mismatched grafts (n = 130), TNC dose bore no discernable relationship to TRM (Figure 2C), contrasting with the pattern in patients given grafts with 1 bidirectional mismatch (Figure 2D). Indeed, the 33 older children and adults (≥ 10 years of age) had a TRM risk that was actually somewhat lower than that of younger patients (univariate HR = 0.4, P = .098), even though the older patients had received a substantially lower average TNC dose (geometric mean = 2.4 vs 6.8 × 107/kg, respectively, P < .001).
Combinations of mismatch direction had no association with transplantation outcome
CB grafts having 2 HLA mismatches with various combinations of mismatch direction showed no consistent or significant association with transplant outcome end points compared with patients given grafts having 1 bidirectional mismatch (Tables 3 and 4) or with those having 2 bidirectional mismatches (data not shown).
Discussion
As in prior reports, this study confirms the superior outcomes after transplantation of HLA-matched (“6/6”) CB units, taking into account cell dose effects.1-7,13,23 Unique to this study, we have shown the importance of mismatch direction on engraftment, relapse, and mortality when all mismatched antigens/alleles were unidirectional. GVH-O mismatches had outcomes that were as good as those that were fully matched for HLA-A, -B, and -DRB1, irrespective of TNC dose, with results that were as good for older children and adults as for young children. R-O grafts, on the other hand, had a lower engraftment rate and carried a higher risk of relapse. Our finding that mismatch direction had an impact on outcome is remarkable given that we did not take into account mismatches at high resolution for class I antigens (HLA-A and HLA-B) or mismatches for HLA-C or -DQ. Such mismatches, however, have not been shown as yet to be relevant in CB transplantation as they have been for bone marrow from unrelated donors.24,25 Of practical relevance, moreover, the HLA match level used in this study is the same as that currently used in national and international search algorithms for CB grafts.
Two previous studies of CB transplantation from unrelated donors reported an association between myeloid engraftment and the number of mismatches in the GVH direction.26,27 Another study in adult recipients of related, mismatched bone marrow reported increased graft failure with increasing number of class I rejection mismatches and increased risk of acute GVHD with increasing number of class II GVH mismatches.28 Data from these studies, however, are somewhat ambiguous. Grafts with no mismatch, for example, were counted as having both 0 GVH and 0 rejection mismatches. In addition, most patients in these studies would have received grafts with combinations of mismatch direction or only bidirectional mismatches (Table 1). Most importantly, none identified the relevance of unidirectional mismatches. Thus, their findings are difficult to compare with ours or to apply in donor selection.
The importance of unidirectional mismatches found in our study permits a simple, practical application of our findings in CB unit selection (ie, identifying unidirectional mismatches in search reports and giving priority to GVH-O mismatches). CB may, indeed, present a special opportunity to take advantage of GVH-O grafts because severe GVHD is less frequent and generally easier to control than with bone marrow transplants from unrelated donors.7-9,29 Indeed, GVH-O grafts may be an inappropriate choice in bone marrow and peripheral blood transplants from unrelated donors. Of note in this regard, GVH-O–equivalent mismatches are generally avoided in solid organ transplantation in which accompanying donor lymphocytes can cause fatal GVHD, except for renal transplants where transfer of donor lymphocytes with the graft is minimal.30-32
Failure to detect an increase in severe acute or chronic GVHD among recipients of GVH-O CB grafts in our study compared with those with bidirectional mismatches is reassuring and should dispel any hesitation to use such grafts. Potential associations could have been confounded and obscured, however, by unidentified mismatches at high resolution or at other HLA or minor histocompatibility loci or by tolerance, in some cases, acquired from exposure to maternal noninherited antigens.11-13,33,34 Studies that include evaluation of such factors, probably requiring a larger number of cases having unidirectional mismatches, would be needed to address their simultaneous impact.
Our observations should have special relevance for older children and adults for whom a single CB unit that provides a sufficient TNC dose is rarely found. Although CB has become more accepted for older patients in recent years, many are now given double-unit transplants in an effort to overcome cell dose limitations.35 Our data suggest a possible alternative: the apparent absence of a cell dose effect on TRM with GVH-O-mismatched and HLA-matched CB grafts may justify their use for older children and adults as single-unit transplants, a strategy that could also reduce cost and mitigate a barrier to transplantation for some patients. Supporting this approach, it was recently shown that CB grafts that are HLA-A, -B, and -DRB1 matched (at the level of resolution used for our current study) had better survival than bone marrow grafts that were allele level-matched at 4 loci (HLA-A, -B, -C, and -DRB1), despite having nearly a log lower TNC dose.6 Thus, our new findings also suggest that GVH-O CB grafts might also be preferred over matched bone marrow from an unrelated donor. Such a possibility could be evaluated by direct comparison between CB and bone marrow transplants from data in national and international registries.29
Our findings fulfilled most of our predictions and further support the premise that HLA-related immunologic responses have an impact on CB transplant outcome. These clinical data, however, cannot discern whether the effects of mismatch direction reflect active immune responses from host or CB cells or the absence of such responses. Improved engraftment seen with GVH-O grafts could, in theory, be the result of either the absence of immune-based host rejection of the graft or an active GVH response by CB cells that may help clear host cells from the marrow stem cell niche.36 Indeed, immunologic responsiveness of CB cells affecting engraftment has been reported in double-unit CB transplants, and experimental studies in mice have documented CB donor T-cell reactivity in the early post-transplantation period.37-40 However, the finding that HLA-matched CB grafts also have improved engraftment might favor absence of rejection as the mechanism. Poor engraftment with R-O grafts, on the other hand, suggests an active host immune response, possibly of greater impact when not balanced by concomitant GVH effects as might be expected with bidirectional mismatches. Immunologic rejection of R-O grafts could account for their increased risk of relapse, although our finding that relapse risk seems to persist after engraftment might also suggest weak GVL mechanisms. Studies to elucidate possible mechanisms are warranted and may further refine our ability to identify optimal HSC sources.
Based on our observations, we would now define an optimal CB graft as either matched for HLA-A, -B (low-intermediate resolution), and DRB1 (high resolution) or GVH-O mismatched. Although our data did not detect significant differences in outcomes between these 2 types of grafts, the trend toward a higher incidence of acute GVHD with GVH-O than with HLA-matched grafts might favor selection of an HLA-matched CB unit when both are available.
The practical implication of our study is that including HLA mismatch direction in search procedures permits easy identification of grafts with unidirectional mismatches, helping transplant centers give priority to GVH-O as well as avoid R-O grafts. The benefit of giving priority to GVH-O mismatches will be realized through CB units that are homozygous at 1 or more loci. Among units donated to our program (now > 55 000), 28% are homozygous at 1 or more HLA-A, -B, or -DR locus (at the level of resolution used in the present study), 3.4% at 2 loci, and 0.7% at all 3. Inclusion of GVH-O mismatches in the search algorithm should more than double the chance of finding an optimal CB graft.41 Homozygosity occurs most often, of course, with the most frequent antigens. Consequently, patients with a common antigen at a given locus along with an uncommon one should be more likely to find suitable GVH-O grafts. Ethnic minority patients who have some white ancestry also should have a better chance of finding GVH-O mismatched CB units in inventories, such as those in the United States and Europe, which have a large proportion of white donors. The fact that ethnic groups differ in their frequent antigens, however, reinforces the need for CB banks to include donors from diverse ethnic backgrounds to maximize the chance that all patients, regardless of ethnicity, have access to an HSC source that optimizes their chance of survival.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the obstetricians in collaborating hospitals who supported the program, the mothers who generously donated their infants' CB to the common good, Pablo Rubinstein for the conceptual inspiration for this study, Jon J. van Rood for his scientific input and review of the manuscript, the NYBC National Cord Blood Program staff who performed all of the tasks needed to ensure the quality of CB units, and the transplant centers that reported on outcome data to the NYBC.
The NYBC National Cord Blood Program was initially supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant HL48031 for 1992-1995) and since then by the Starr Foundation and the NYBC and Food and Drug Administration–approved cost reimbursement.
National Institutes of Health
Authorship
Contribution: C.E.S. designed the study, carried out the analysis, interpreted the data, and wrote the paper; A.S. collected and validated the follow-up data, interpreted data, and cowrote the paper; C. Carrier and C. Carpenter performed the HLA typing and confirmed HLA mismatch assignments; and D.S. was responsible for collecting and verifying the transplant outcome data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andromachi Scaradavou, New York Blood Center, National Cord Blood Program, 45-01 Vernon Blvd, New York, NY 11101; e-mail: ascaradavou@nybloodcenter.org.