Abstract
Familial hemophagocytic lymphohistiocytosis (FHL) is a rare, genetically heterogeneous autosomal recessive immune disorder that results when the critical regulatory pathways that mediate immune defense mechanisms and the natural termination of immune/inflammatory responses are disrupted or overwhelmed. To advance the understanding of FHL, we performed gene expression profiling of peripheral blood mononuclear cells from 11 children with untreated FHL. Total RNA was isolated and gene expression levels were determined using microarray analysis. Comparisons between patients with FHL and normal pediatric controls (n = 30) identified 915 down-regulated and 550 up-regulated genes with more than or equal to 2.5-fold difference in expression (P ≤ .05). The expression of genes associated with natural killer cell functions, innate and adaptive immune responses, proapoptotic proteins, and B- and T-cell differentiation were down-regulated in patients with FHL. Genes associated with the canonical pathways of interleukin-6 (IL-6), IL-10 IL-1, IL-8, TREM1, LXR/RXR activation, and PPAR signaling and genes encoding of antiapoptotic proteins were overexpressed in patients with FHL. This first study of genome-wide expression profiling in children with FHL demonstrates the complexity of gene expression patterns, which underlie the immunobiology of FHL.
Introduction
Familial hemophagocytic lymphohistiocytosis (FHL; MIM 267700) is a rare, genetically heterogeneous, often fatal, immune disorder of autosomal recessive inheritance. Disease-causing mutations have been found in the PRF1,1 UNC13D,2 STX11,3 RAB27A,3 and STXBP2 (Munc18-2),4 which encode proteins involved in the granule dependent cytotoxic pathway. An acquired form of hemophagocytic lymphohistiocytosis also exists in association with systemic infections (infection-associated hemophagocytic syndrome), malignant diseases (lymphoma-associated hemophagocytic syndrome), or autoimmune disorders.5 Most familial cases present in infancy, although it can occur in older children or young adults.6
Defective NK-cell function is frequently observed in patients with FHL with the occurrence of uncontrolled proliferation and activation of polyclonal T lymphocytes, NK cells, and macrophages, which infiltrate multiple organs, including the liver, spleen, and central nervous system that eventually leads to death of patients with FHL.1-3,7,8 Previous studies have reported elevated blood levels of proinflammatory cytokines, including interleukin-6 (IL-6), IL-8, IL-18, macrophage inflammatory protein-1A, macrophage-colony stimulating factor, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α).6,9,10 High plasma levels of soluble IL-2 receptor (CD25), sCD95-ligand, and sCD163 have also been reported; other studies showed elevated plasma levels of IL-12 and IL-10.7,10 Characteristic laboratory findings also include elevated levels of ferritin, triglycerides, transaminases, bilirubin, and lactate dehydrogenase and decreased fibrinogen levels.5-8 Hemophagocytosis is a hallmark of cytokine-driven macrophages and histiocytes.5 Some of these clinical and laboratory characteristics of FHL are also observed in severe sepsis and related syndromes, including systemic inflammatory response syndrome (SIRS), multiorgan dysfunction syndrome, and macrophage activation syndrome.11
In this study, the gene expression profiles of peripheral blood mononuclear cells (PBMCs) from patients with FHL were compared with those of PBMCs from pediatric controls to gain insights into the genome-level expression profiles associated with the disease.
Methods
Subjects, sample collection, and genetic analysis
Parents of patients provided written informed consent for enrollment in this study approved by the Institutional Review Boards of Cincinnati Children's Hospital Medical Center, in accordance with the Declaration of Helsinki. Whole blood was collected from 11 patients with active FLH before therapy was started. The control group included 30 healthy children (11 males and 19 females) with a median age of 4.5 years. The technical details of the mutational analysis of PRF1, UNC13D, RAB27A, SH2D1A, and STX11 using polymerase chain reaction (PCR) and direct sequencing have been described previously.12-14
Total RNA isolation, Affymetrix GeneChip hybridization, image acquisition, and data analysis
PBMCs were separated by Ficoll gradient centrifugation, placed in Trizol (Invitrogen), and stored at −80°C. Total RNA was isolated and purified using the RNeasy Micro kit (QIAGEN) and examined using the Bioanalyzer 2100 system (Agilent Technologies). Labeled cDNA was synthesized from the total RNA using the OvationBiotin RNA Amplification and Labeling System (NuGEN) and hybridized to Affymetrix U133 plus 2.0 GeneChips (Affymetrix). Data quality was assessed using the standard metrics of the CCHMC Affymetrix Core, which included an assessment of the positive and negative control features of the arrays. To reduce chip-to-chip variation, expression values were derived using the RMA preprocessing method implemented in the GeneSpring GX 7.3 analysis platform (Agilent Technologies). Differential expression values were identified using analysis of variance and/or Student t test with a significance value of P < .05 and a fold-change cut-off of 2-fold. The complete microarray dataset has been deposited in the Gene Expression Omnibus at the National Center for Biotechnology Information and is accessible through GEO Series accession number GSE 26050.
Gene lists were also analyzed using Ingenuity Pathway Analysis (IPA) Version 8.8 software (Ingenuity Systems; http://www.ingenuity.com) to identify any over-represented biologic pathways. IPA assigns biologic functions to genes based on literature data and information in the Kyoto Encyclopedia of Genes and Genomes to form networks and create canonical pathways of specific biologic processes.15
Flow cytometric and NK-cell cytotoxicity analyses
Relative and absolute numbers of T cells, NK cells, and B cells were determined using previously described procedures.13,14,16 Each cell population is presented here as a percentage of the total PBMC count. NK-cell activity was assessed after coincubation of PBMC preparations (effector cells) with 51Cr-labeled K562 target cells at various effector/target cell ratios.17-19
Real-time RT-PCR
Real-time reverse-transcribed polymerase chain reactions (RT-PCRs) were performed using the RT2 Profiler PCR Array Human Innate and Adaptive Immune Response Platform (PAHS-052), Inflammatory Cytokines & Receptors (PAHS-011), and IFN and Receptor Array (PAHS-064) from SA Biosciences according to the manufacturer's instructions. Quantitative PCR was carried out on an ABI 7500 instrument (Applied Biosystems). For each set of duplicates, the mean value of each gene was determined and used to calculate changes in each level (ie, FHL vs control).
Results
Patient characteristics
Patients in this study fulfilled the diagnostic criteria for FHL according to the Histiocyte Society's diagnostic guidelines20 (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Three patients (P35, P59, and P101) were born to consanguineous parents. Nucleotide sequence analysis identified biallelic disease-causing mutations in PRF1 in patients P33, P59, and P35 (Table 1). Patients P66, P76, P94, P96, P98, P101, and P1002 carried wild-type PRF1, UNC13D, and STX11 and were considered to carry disease-causing mutations of as-yet-to-be identified gene(s). Patient P92 had a 1993(−2) a > c splice site mutation in intron 21 of one allele of UNC13D. UNC18-2 was not tested for the presence of mutations, as this study was carried out before the discovery of that genetic defect in FHL patients.
Genetic and clinical characteristics of patients with FHL
| Patient . | Sex . | Age . | PRF1 . | UNC13D . | STX11 . | NK function in lytic units* . | Perforin MCF NK cells† . |
|---|---|---|---|---|---|---|---|
| P33 | Female | 2 months | 50delT + Q481P | ND | ND | 0.0 | 0.0 |
| P35 | Male | 7 years | hV50M | ND | ND | 9.2 | 57 |
| P59 | Male | 3 months | h50delT | ND | ND | 0.0 | 0.0 |
| P1002 | Female | 9 years | wt | wt | wt | 57.4 | 501 |
| P101 | Male | 6 years | wt | wt | wt | 15.3 | 440 |
| P66 | Female | 15 years | wt | wt | wt | 0.1 | 100 |
| P76 | Male | 5 years | wt | wt | wt | 13.3 | 634 |
| P92 | Female | 1 months | wt | 1993(−2) a > c + wt | wt | 1.0 | 552 |
| P98 | Male | 1 months | wt | wt | wt | 0.0 | 781 |
| P94 | Male | 5 years | wt | wt | wt | 0.0 | 181 |
| P96 | Male | 16 months | wt | wt | wt | 7.4 | 404 |
| Patient . | Sex . | Age . | PRF1 . | UNC13D . | STX11 . | NK function in lytic units* . | Perforin MCF NK cells† . |
|---|---|---|---|---|---|---|---|
| P33 | Female | 2 months | 50delT + Q481P | ND | ND | 0.0 | 0.0 |
| P35 | Male | 7 years | hV50M | ND | ND | 9.2 | 57 |
| P59 | Male | 3 months | h50delT | ND | ND | 0.0 | 0.0 |
| P1002 | Female | 9 years | wt | wt | wt | 57.4 | 501 |
| P101 | Male | 6 years | wt | wt | wt | 15.3 | 440 |
| P66 | Female | 15 years | wt | wt | wt | 0.1 | 100 |
| P76 | Male | 5 years | wt | wt | wt | 13.3 | 634 |
| P92 | Female | 1 months | wt | 1993(−2) a > c + wt | wt | 1.0 | 552 |
| P98 | Male | 1 months | wt | wt | wt | 0.0 | 781 |
| P94 | Male | 5 years | wt | wt | wt | 0.0 | 181 |
| P96 | Male | 16 months | wt | wt | wt | 7.4 | 404 |
Normal controls: 5-35 lytic units (mean, 11.3 lytic units).
Normal controls: 244-502 MCF.
PCR assays failed to detect the presence of Epstein-Barr virus, cytomegalovirus, or other herpes viruses in the serum of all patients in this study.
Subpopulations of PBMC, NK-cell cytolytic activity, and perforin expression
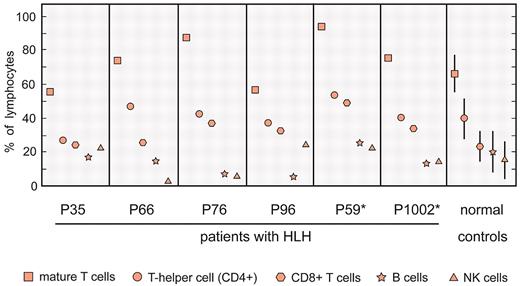
When the overall number of isolated PBMCs was sufficient, the cellular composition of each sample was determined. As shown in Figure 1, the relative cellular composition of PBMC samples was similar in patients with FHL and controls, with the exception of the T-cell composition in patients P66 and P76 and NK-cell composition in patients P66 and P76. The percentages and absolute number of B cells in patients P76 and P96 were lower than those in controls. As shown in Table 1, an absence or decrease of NK-cell cytolytic activity was noted in 6 of the 11 patients.
Flow cytometric analyses of PBMCs isolated from patients with FHL before initiation of treatment. The staining of samples and flow cytometric analyses were performed as described previously.16,17 These results are shown as a percentage of the total PBMC fraction. For normal controls, the range of the percentage of cell subtypes is shown. In the case of P59 and P1002, samples for microarray and flow cytometric analyses were taken at different times.
Flow cytometric analyses of PBMCs isolated from patients with FHL before initiation of treatment. The staining of samples and flow cytometric analyses were performed as described previously.16,17 These results are shown as a percentage of the total PBMC fraction. For normal controls, the range of the percentage of cell subtypes is shown. In the case of P59 and P1002, samples for microarray and flow cytometric analyses were taken at different times.
Differences in genome-wide expression between patients with FHL and controls
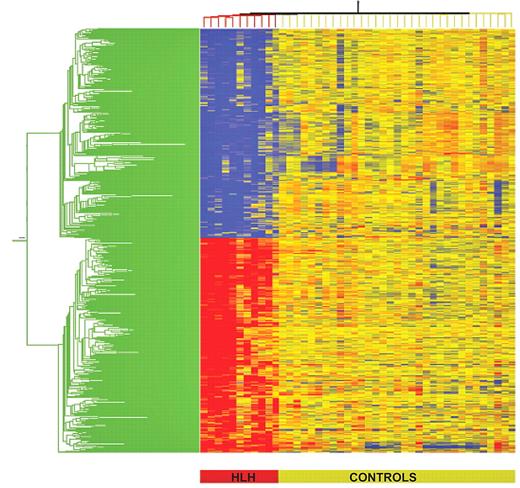
To test the hypothesis that FHL is characterized by distinctive alterations in gene expression, gene expression profiling was performed. Two-group analysis of variance comparisons were carried out (5% Benjamini-Hochberg false discovery rate) on both controls and FHL patients, followed by the use of an expression filter that selected only genes with at least 2.5-fold expression difference. These statistical and expression filters identified 2054 (1284 down- and 870 up-regulated) probe-sets representing 1465 unique and predicted genes that are differentially expressed in patients with FHL compared with that in controls. These 2054 probe-sets were then subjected to two-dimensional cluster analysis, as shown in Figure 2. All patients with FHL are clustered together on the left half of the map in a homogeneous manner, demonstrating the relative unity of gene regulation in PBMCs. Inspection of the data for these 1465 genes showed that 915 had decreased expression and 550 were up-regulated in patients with FHL compared with controls (a list of up-regulated and down-regulated genes is shown in supplemental Tables 2 and 3). The number of down-regulated genes was 2 times more than the number of up-regulated genes. Differences in fold changes for differentially expressed genes on the microarray ranged from a decrease of 26.3-fold in the expression of CX3CR1 (fractalkine receptor gene) to an increase of 192.3-fold in the expression of CXCL3 (encoding a chemokine ligand with c-x-c motif; supplemental Tables 2 and 3).
Hierarchical clustering of differentially expressed genes in FHL versus controls. Differentially regulated genes were identified using analysis of variance (false discovery rate 5%) followed by Tukey post-hoc testing. Individual patients are oriented in columns and expression level for each gene oriented in rows. Red, yellow, and blue colors represent expression levels that are greater than, equal to, or less than the mean expression levels, respectively, in all samples. The color-coded bar at the bottom of the heat-map represents controls in yellow and patients (P59, P33, P59 P1002, P101, P76, P92, P94, P98, P66, and P96) in red.
Hierarchical clustering of differentially expressed genes in FHL versus controls. Differentially regulated genes were identified using analysis of variance (false discovery rate 5%) followed by Tukey post-hoc testing. Individual patients are oriented in columns and expression level for each gene oriented in rows. Red, yellow, and blue colors represent expression levels that are greater than, equal to, or less than the mean expression levels, respectively, in all samples. The color-coded bar at the bottom of the heat-map represents controls in yellow and patients (P59, P33, P59 P1002, P101, P76, P92, P94, P98, P66, and P96) in red.
Signaling pathway and functional ontology analyses of genes differentially expressed in patients with FHL
To determine the biologic meaning of the gene lists, the down- and up-regulated genes, which had at least a 2-fold expression difference, were uploaded to the IPA application, and the enrichment of canonical pathways and molecular networks was inspected. A pathway was considered over-represented if the P value was ≤ .05, as determined by Fisher exact test. The P value was proportional to the number of genes in a list that corresponds to a given canonical pathway. It should be noted that interpreting the pathways and functions of a set of differentially expressed genes could assess only genes of known functions.
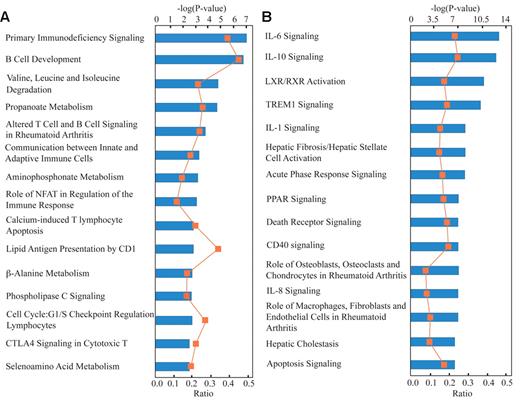
IPA-dependent analyses yielded 39 biologically relevant, over-represented, canonical pathways of the down-regulated gene list related to FHL. Fifteen of these (based on P values) are shown in Figure 3A. These pathways are primarily related to the adaptive immune system, apoptosis, immunodeficiency signaling, NFκB, lipid antigen presentation by CD1, B-cell development, CTLA4 signaling, as well as calcium-mediated apoptosis and other processes related to amino acid metabolism. Three canonical pathways associated with amino acid metabolism were also over-represented in the down-regulated gene list (Figure 3A).
Ingenuity Pathway Analysis of differentially expressed genes in patients with FHL. The top 15 over-represented canonical pathways of the down-regulated (A) and up-regulated (B) genes found in FHL patients compared with controls. Canonical pathways for probe sets indicate down-regulated genes (A) and up-regulated genes (B) that were identified using analysis of variance (P < .05) and fold change (2-fold) cut-offs. Category names are presented on the y-axis. The x-axis indicates the −log (P value) of the over-representation analysis. Orange squares represent ratios calculated from the number of genes in the dataset of a given pathway divided by the total number of molecules in the canonical pathway.
Ingenuity Pathway Analysis of differentially expressed genes in patients with FHL. The top 15 over-represented canonical pathways of the down-regulated (A) and up-regulated (B) genes found in FHL patients compared with controls. Canonical pathways for probe sets indicate down-regulated genes (A) and up-regulated genes (B) that were identified using analysis of variance (P < .05) and fold change (2-fold) cut-offs. Category names are presented on the y-axis. The x-axis indicates the −log (P value) of the over-representation analysis. Orange squares represent ratios calculated from the number of genes in the dataset of a given pathway divided by the total number of molecules in the canonical pathway.
Identical analyses were also conducted on up-regulated genes, and 48 over-represented pathways were identified. Fifteen of these are shown in Figure 3B and include IL-10, IL-6, IL-8, IL-17, TREM, and PPAR pathway signaling; acute phase response signaling; and LXR/RXR activation pathway. The 15 canonical pathways shown in Figure 3 are represented as 92 down-regulated and 85 up-regulated genes (supplemental Tables 4-5).
Comparative analyses of networks assembled by down-regulated genes in FHL and control patients
IPA analysis of the down-regulated genes identified several gene networks with IPA scores more than 7, indicating a less than 10−7 chance that the genes in the network were associated together solely because of random events. The top 5 networks with down-regulated genes are shown in supplemental Table 6. Network 1 received a score of 34 and was assembled from 30 genes that correlate with cell-to-cell signaling and hematologic system development and functionality, as well as immune and lymphatic system development and functionality. This network had NFκB in the center and was overlaid with canonical pathways of primary immunodeficiency signaling, B-cell development, and communication between innate and adaptive immune cells. Network 2 was associated with metabolic diseases, protein trafficking, and cell cycles and was overlaid with canonical pathways, including propanoate metabolism; valine, leucine, and isoleucine degradation; and aminophosphonate metabolism. Network 3 had TP53 in the center and was found to be correlated with genes associated with DNA replication, recombination, and repair; nucleic acid metabolism; and small molecule biochemistry. Network 4, with 27 focus molecules and a computed IPA score of 29, correlated with functions, such as cellular assembly and organization, molecular transport, and protein trafficking. Network 5, with an IPA score of 28 and assembled from 27 genes, correlated with programmed cell death and muscular and genetic disorders.
Comparative analyses of networks assembled by up-regulated genes in FHL and control patients
Network 1 of the up-regulated gene list had an IPA score of 43 and was assembled from 31 genes (supplemental Table 7). This network correlated with cellular functions, such as cellular growth and proliferation, gene expression, RNA damage, cell signaling, genetic disorders, and metabolic diseases. In network 2, TNF and TNF-induced genes (TNFAIP3 and TNFAIP6), TNFAIP1 interacting protein 1 (TNIP1), and tumor necrosis factor superfamily member 9 (TNFSF9) were found in the center connecting to other up-regulated genes in this network. Network 3 had NFκB in the center, which plays an important role in intracellular regulation of immune response, inflammation, and cell cycle regulation. Network 4 correlated with cancer and dermatologic diseases and conditions and was assembled from ERK in the center and various up-regulated genes coding for transcriptional regulators (ZNF165, HEY1, RUNX1, KLF5, and BRG2) and growth factors (JAG1, EREG, HBEGF, and INHBA). Network 5 correlated with cell death, cell cycle, and cellular development and was assembled from CDKN1A and E2F1 in the center and various components regulating cell cycle and cell proliferation.
In a complex disease, such as FHL, multiple interactions of diverse components in the immune system and the complexity of the various intracellular pathways must be considered in the interpretation of genome-wide gene expression experiments. Although it is presently difficult to understand the biologic outcomes of these interaction patterns in the FHL pathomechanism, some of the genes identified by microarray analysis that are of potential interest in relation to this disease will be highlighted here. To ensure that the gene expression profiles accurately reflected the down-regulation of immune function-related genes, representative genes that were differentially expressed in patients with FHL were assessed using real-time RT-PCR analysis. These included some genes key to the canonical pathways and gene networks. Real-time PCR was performed on samples from patients P33, P35, and P1002, in addition to 2 patients (EH-05 and MW) who were not included in the microarray analysis (Figures 4, 5). The real-time RT-PCR results were in close agreement with microarray data.
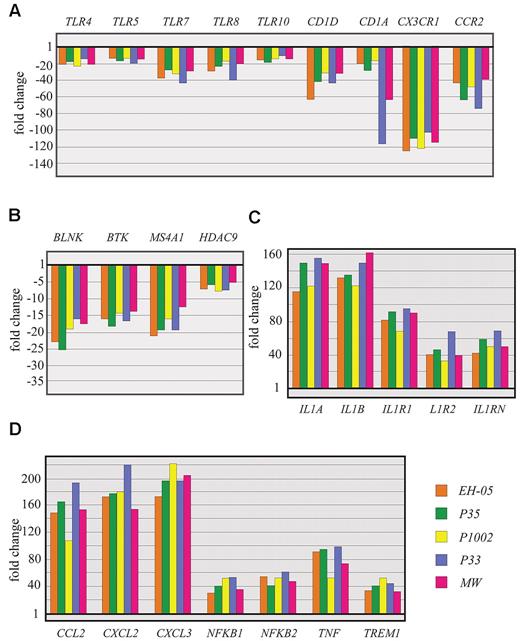
Quantitative RT-PCR analysis of differentially expressed genes in patients with FHL: verification of selected microarray results. Quantitative RT-PCR study of selected genes coding for Toll-like receptors (A), proteins involved in B-cell growth (B), and differentiation and proinflammatory proteins (C-D). Three (P33, P35, and P1002) of the 5 patients selected for quantitative RT-PCR analysis of the genes were also included in microarray analysis. Patients EH-05 and MW were not included in the microarray analysis. The expression level of each gene in each sample was first normalized to the expression of 18S RNA in that sample and then normalized to the expression of that gene in normal PBMCs obtained from children diagnosed as healthy.
Quantitative RT-PCR analysis of differentially expressed genes in patients with FHL: verification of selected microarray results. Quantitative RT-PCR study of selected genes coding for Toll-like receptors (A), proteins involved in B-cell growth (B), and differentiation and proinflammatory proteins (C-D). Three (P33, P35, and P1002) of the 5 patients selected for quantitative RT-PCR analysis of the genes were also included in microarray analysis. Patients EH-05 and MW were not included in the microarray analysis. The expression level of each gene in each sample was first normalized to the expression of 18S RNA in that sample and then normalized to the expression of that gene in normal PBMCs obtained from children diagnosed as healthy.
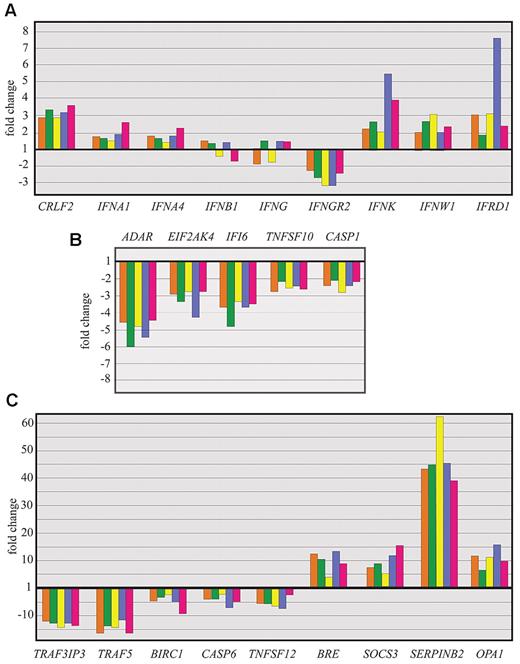
Quantitative RT-PCR analysis of differentially expressed genes in patients with FHL: verification of selected microarray results. Quantitative RT-PCR analysis of selected genes coding for IFNs (A), IFN-inducible genes (B), and proteins involved in apoptosis regulation (C).
Quantitative RT-PCR analysis of differentially expressed genes in patients with FHL: verification of selected microarray results. Quantitative RT-PCR analysis of selected genes coding for IFNs (A), IFN-inducible genes (B), and proteins involved in apoptosis regulation (C).
Genes associated with a wide range of innate immune defense mechanisms are down-regulated in FHL
Microarray analysis identified several immune function-related genes not previously known to be affected in patients with FHL. The expression of Toll-like receptor genes,21 including TLR4, TLR5, TLR7, TLR8, and TLR10, was reduced by more than 3-fold regardless of the genetic cause of the disease (Figure 4A; supplemental Table 2). Genes, such as TIRAP, SARM1, and TRAM, which are activated in signaling cascades by individual TLRs, were also down-regulated. The expression of CD1 and its isoforms22 (CD1A, CD1C, and CD1D) was also reduced in all 11 patients with FHL. The expression of CD1A and CD1D was also analyzed by quantitative RT-PCR in 6 patients and compared well with the microarray observed gene expression changes (Figure 4A; supplemental Table 2). CD1 and its isoforms mediate the presentation of nonpeptide, lipid, and glycolipid antigens to T cells, an important role in the detection and effective clearance of various pathogens.22 The down-regulation of CD1D has attracted particular attention because the encoded protein restricts NK T cells and has potent immunomodulatory properties, including tumor surveillance, maintenance of self-tolerance, and anti-infectious defenses.22
Genes that enhance NK and CTL function and responsiveness are down-regulated in patients with FHL
Four of the 15 canonical pathways associated with down-regulated genes were related to NK and CTL functions and cytotoxicity. KLRF1 showed decreased expression (supplemental Table 2). KLRF1 encodes a lectin-like receptor, which is involved in NK-mediated cytolysis and is expressed on essentially all human NK cells and a subset of effector memory CD8+ T cells with an inflammatory NK-like phenotype.23 All 11 patients demonstrated down-regulated expression of KLRG1 (supplemental Table 2). KLRG1 encodes a member of the superfamily of inhibitory receptors, which binds to major histocompatibility complex class I ligands on target cells and inhibits NK cells from attacking cells expressing class I antigens at normal levels, such as healthy tissues as opposed to virally infected or transformed cells.24
CX3CR1, one of the most down-regulated genes, codes for 7 transmembrane-spanning G protein-coupled receptors that exhibit reduced expression in patients with FHL (Figure 4A; supplemental Table 2). It is expressed in NK cells, monocytes, activated macrophages, and some lymphocyte subpopulations.25 The protein encoded by this gene is a receptor for fractalkine (CX3CL1), a transmembrane protein and chemokine involved in the adhesion and migration of leukocytes.26 CX3CR1/CX3CL1, a crucial component of optimal host defense against microbial infection, activates the killing functions of phagocytes and augments iNOS-mediated NO generation and proinflammatory cytokine production through the NFκB signaling pathway.27 CX3CR1/CX3CL1 signaling is also required for DC-mediated NK-cell activation.
The relative expression of 2B4 (CD244), PLCγ1, and LAT, which encode for proteins known to be involved in the NK-cell signaling pathway and NK cell-mediated cytotoxicity,28 was also suppressed (supplemental Table 2).
B-cell functions are also impaired in patients with all forms of FHL
A number of genes with well-characterized roles in B-cell differentiation and function (BTK, BLNK, CD19, CD79A, CCR2, FCGR2B, CD22, CR2/CD21, and CD72) were found to be suppressed in the 11 patients with FHL (Figure 4B; supplemental Table 2). Proteins encoded by FCGR2B, CD22, and CD72 contain immunoreceptor tyrosine-based motifs in their cytoplasmic tails; these are known as B-cell immune response regulators.29 BLNK (adaptor protein B-cell linker) is required for B-cell proliferation.30 B-lymphocytes lacking BLNK do not proliferate in response to B-cell antigen receptor engagement.30 A recent review of B-cell numbers and functions in newly diagnosed patients with FHL indicated that a significant proportion of patients demonstrate low B-cell counts and hypogammaglobulinemia (A.H.F., unpublished data, July 1, 2010).
Overexpression of genes coding for pro-inflammatory cytokines
Defects in cytolytic function in FHL patients lead to the expansion of CD8+ T cells, which secrete large quantities of cytokines and shed off their receptors. Among the signaling pathways studied, data revealed a unique landscape where the induction of certain pathways can limit the inflammatory response (eg, the induction of IL-10 signaling and the apparent inhibition of the inflammatory response was coupled with the activation of certain pathways, which promote the inflammatory response [eg, IL-6 and acute phase response signaling]). Genes coding for CSF2, IL-10, IL-1A, IL-1B, IL-1RN, and IL-6 showed more than a 10-fold increase in expression levels (Figure 4C; supplemental Table 3). Genes coding for chemokines (CCL2, CCL20, CCL3, CXCL1, and IL-8) and chemokine receptors (CXCR1, IL-1R1, and IL-1RN) showed high levels of expression in FHL patients. Inflammatory response genes, such as NFKB1 and PTGS2, showed more than a 4-fold increase in expression levels (Figure 4C-D; supplemental Table 3). These increased expression levels of chemokines, cytokines, and their receptors contribute to the proinflammatory responses observed in activated macrophages.6
Three members of the IL-1 family31 (IL-1A, IL-1B, and IL-18) and 2 genes coding for receptors of IL-1 (IL-R1A and IL-R2) were overexpressed by more than 10-fold in patients with FHL. Genes known to be induced by IL-1B (pentraxin) or potentially involved in IL-1B secretion (KCNJI5) were also up-regulated. In FHL patients, IL-1B expression may elicit the rapid activation of a cellular network of genes particularly implicated in inflammatory responses that may create a cellular environment favorable for the inhibition of apoptosis.
The pentraxin-related gene (PTX3) and TNFAIP3/A20, which code for TNF-α-induced protein 3/A20 and TNFAIP6, were also among the overexpressed genes (Figure 5C; supplemental Table 3). The protein encoded by TNFAIP3/A20 is a zinc finger protein and has been shown to inhibit NFκB activation as well as TNF-mediated apoptosis.32 A previous knockout study on Tnfaip3, a similar gene in mice, suggested that its genetic product is critical for controlling inflammation by terminating TNF-induced NFκB responses.32
TREM1 (a triggering receptor expressed on myeloid cells-1) and genes in the TREM1 signaling pathway33 (CD83, ITGB1, ICAM1, and TNF) were found to be up-regulated (Figure 4C; supplemental Table 3). TREM1 amplifies acute inflammatory responses by enhancing degranulation and secretion of proinflammatory mediators. Genes associated with TREM1 signaling (CCL2, CCL7, FCGR2B, and NKkB2) were also up-regulated and FCGR2B was down-regulated (supplemental Tables 2, 3).
Genes in 2 signaling pathways related to lipid metabolism34 (LXR/RXR activation and PPARα/RXRα signaling), which paralleled stimulation of ABCA1, the ATP-binding cassette transporter, were up-regulated in FHL patients (supplemental Table 3). The genes of the PPAR canonical signaling pathway,35 including PPARD, PPARG, and other genes associated with the peroxisome proliferator-activated receptor-δ pathway, were among the up-regulated genes (supplemental Table 3). Liver dysfunction and damage to liver cells were indicated by the overexpression of EDN1, ICAM1, and MMP9 involved in the hepatic fibrosis/hepatic stellate canonical pathway.36
IFN-γ and IFN-γ-responsive genes were not among the genes differentially expressed in PBMCs of patients with FHL and controls
Comparison of transcript levels of patients with FHL and controls revealed that IFNs and IFN-inducible genes were not among the differentially expressed genes. Based on the microarray analysis, to further investigate the significance of this observation, we examined the expression of IFNs and IFN-inducible genes. IFN-responsive genes with IFN-responsive elements, such as ADAR, EIF2AK2, IFI16, and TNSF10, were down-regulated (Figure 5B; supplemental Table 3). Others, such as MX1, MyD88, NMI, STAT1, and TRAF3, were not among the differentially expressed genes. IFN-γ and IFN-γ-responsive genes may have been actively suppressed. IFN-γ receptor 2 was also found to be down-regulated (Figure 5A; supplemental Table 3). CCL5, which is induced by IFN-γ and TNF-α, was also down-regulated (Figure 5A; supplemental Table 5). Moreover, the function of one of the up-regulated genes, TNFAIP3/A20 (Figure 5B) is to down-regulate the activity of IRF-3, a key transcription factor for the induction of IFN-γ.37 Another highly up-regulated gene in FHL, SOCS3 (Figure 5B), which operates through the JAK/STAT signaling pathways, lessens the responsiveness of monocyte/macrophages to IFN-γ.38
Decreased expression of proapoptotic genes and increased expression of antiapoptotic genes in FHL patients
Apoptosis is a highly regulated process, which is essential for the maintenance of immune homeostasis by rapid disposal of cells that are abnormal, misplaced, nonfunctional, or potentially dangerous to the organism. FHL is characterized by the nonmalignant accumulation of immune cells. Apoptosis-related pathways were found to be one of the most significant biologic processes involved in FHL. The expression of genes (TRAF5, TRAF3IP3, and CASP6)39 related to the positive regulation of apoptosis and the expression of perforin and granzymes A, K, and H found in CD8+ T cells and NK cells were markedly down-regulated (Figure 5C; supplemental Table 2). The perforin/granzyme system is impaired in these patients either by the inactivating of mutations in PRF1 (observed in patients P33, P59, and P35) or by down-regulation of the perforin/granzyme system, secondary to an unknown genetic cause of the disease.
The genes TNFSF12 (TWEAK) and TNFSF13, which belong to the TNF ligand family and are expressed in hematopoietic tissues, were found to be down-regulated in FHL patients (Figure 5C; supplemental Table 2). These cytokines can induce apoptosis via multiple pathways of cell death in a cell type-specific manner and promote the transition to Th1-based adaptive immunity.39
One of the overexpressed antiapoptotic genes was SERPINB2 (Figure 5C; supplemental Table 3), which encodes plasminogen activator inhibitor type 2, a cytoprotective retinoblastoma-binding protein that protects retinoblastoma from calpain cleavage, consequently increasing retinoblastoma levels and enhancing cell survival.40 Another overexpressed gene was BRE (Figure 5C; supplemental Table 3). The BRE protein binds to the cytoplasmic domains of TNFR-1 and Fas and can attenuate death receptor-initiated apoptosis.41 Transgenic BRE mice are significantly more resistant to Fas-mediated lethal apoptosis than wild-type mice.41
Discussion
To the best of our knowledge, the present study is the first report of gene expression signatures in patients with active FHL and offers new insights into the molecular pathogenesis of this disease. In FHL, autosomal recessive genetic defects disrupt the mechanisms responsible for target cell and activated T-cell apoptosis, and undermine the elimination of antigen-presenting cells and contraction of immune response. As a consequence, CD8+ T cells, NK cells, and macrophages remain activated and mutually stimulate each other. Therefore, the use of PBMCs is a reasonable starting point for the analysis of the global gene expression of this disease. Comparisons of patients with FHL and controls revealed that approximately 8% of the gene probes of the entire array was differentially expressed in PBMCs of patients with FHL (both up- and down-regulated) compared with that in PBMCs of controls, reflecting broad alterations in the gene expression of these cells. Some of the genes (IL-6, IL-8, IL-10, TNF, and M-CSF) identified in this study as overexpressed in patients with FHL confirm previous findings,6,8-10,12 accentuate basic principles on FHL, and support the analytic methods used and the new findings presented here.
It was hypothesized that the PBMC, the most accessible tissue for analysis of gene expression, would reflect pathologic events occurring in FHL patients. However, gene expression patterns might be influenced by quantitative variations within PBMC subsets. No significant changes were observed in the subcell counts of the patients analyzed, demonstrating that even without separation of peripheral blood cells into subsets, subtle and distinguishable differences in the gene expression profiles of patients with FHL and healthy subjects can be readily identified.
The expression levels of more genes were down-regulated than up-regulated in FHL patients. The down-regulated genes code for proteins involved in signaling and regulation of transcription, as well as immune responses and defense mechanism pathways, suggesting that extensive genetic suppression of immunity in FHL patients is central to the disease process and presents at an early stage of FHL. Consequently, susceptibility to secondary infections in FHL patients may be intrinsic to the disease and not directly related to the immunosuppressive drugs used to target hyper-activated T cells and histiocytes, as has been assumed for several years.6
The “cytokine storm” has been considered as a hallmark of the immunologic phenotype of FHL. Gene expression data suggest that the underlying mechanism of FHL includes an imbalance of cytokine homeostasis, massive up-regulation (> 50-fold) of genes encoding proinflammatory proteins (IL-8, IL-6, CCL3, and CCL4), and a moderate (0.58- to 10-fold) increase in the expression of genes coding for anti-inflammatory proteins (TGF-β, IL-1RA, TNFAIP3, and IL-10). This pattern has also been observed in graft-versus-host disease, acute respiratory distress syndrome, and SIRS.42 There were no significant increases in the up-regulation of genes that include IFN (α, β, or γ). The presence of increased levels of IFN-γ in the plasma of patients with FHL has been indicated in several independent case studies of FHL.7-10,12 Abnormal and excessive production of IFN-γ has also been observed in a murine model of FHL type 2, although the genetic background of this model is uniquely prone to viral induction of FHL.43 Osugi et al reported no increase in IFN-γ levels in patients with FHL, regardless of the level of IL-10 in serum.44 In another study by Nagasawa et al, 2 of 4 patients with FHL showed no elevated levels of plasma IFN-γ.45 Serum level of IFN-γ was not elevated in 2 of 5 patients with FHL.46 In a recent review of 33 patients with FHL, at the Cincinnati Children's Hospital Medical Center, eligible for hemopoietic cell transplantation, 18 showed low serum level (< 5 U/mL) of IFN-γ in the active stage of the disease before the prehemopoietic cell transplantation therapy started (A.H.F., unpublished data, July 1, 2010). In summary, these observations suggest that PBMCs from patients with FHL display different gene expression program for IFN genes, which may depend on the diversity of triggering agents of the disease.
Patients with severe acute respiratory syndrome showed a pattern similar to FHL, with several up-regulated genes in the innate immune system, but not IFN-γ or IFN-γ-induced genes.47 After measles virus infection, IFN-γ and IFN-γ-regulated genes are not up-regulated.48 Similarly, gene expression profiling of PBMCs in patients with systemic juvenile idiopathic arthritis showed a low level of IFN gene expression and a relative paucity of genes whose expression is induced by IFN-γ.17
Recent gene expression studies have shown that sJIA is associated with the up-regulation of innate immune pathways, including IL-6, TLR/IL-1R, and PPAR signaling pathways, and the down-regulation of gene networks involving NK-cell, T-cell, and major histocompatibility complex-related biologic processes, including antigen presentation.49 An unexpected finding in sJIA patients was the highly up-regulated erythropoiesis signature of 67 genes, which normally expressed only in immature red blood cells.17 The presence of this signature is thought to be a reflection of increased turnover of red blood cells, perhaps secondary to hemophagocytosis. Of these 67 genes, 12 (ANXA3, CA1, CD34, ELL2, HBB, HBG1, HBM, PINK1, PLEK2, SESN3, a member of the solute carrier family [SLC], and SNCA) were also highly up-regulated in FHL patients. These 12 genes include erythropoiesis-related genes that code for fetal and embryonic hemoglobins. Members of the SLC family of transporter genes are also among the genes up-regulated in both FHL and sJIA patients. According to the literature, to date, the overexpression of genes unique to erythropoiesis have only been observed in patients with FHL or sJIA and do not overlap with the list of erythropoiesis genes up-regulated in patients with sickle cell anemia or with severe hemolysis and consequent prolonged erythropoiesis.49
Several up-regulated signaling pathways (TREM1, LXL/RXR, PPAR, and acute phase response signaling) in FHL are also over-represented in SIRS and are associated with poor outcomes after pediatric septic shock.42 Of the 26 down-regulated genes, 7 (ABHD, DNAJC3, DPEP, HRB2, RGS2, SLC8A, and SRPK2) expressed in nonsurvivors of pediatric septic shock42 were among the down-regulated genes observed in FHL patients, whereas 18 (CCRL2, CEACAM1, CLEC, DDIT4, EMP1, ENPP2, G0S2, GPR171, IL8, MAFF, PDE4D, RGS1, ROCK, SLC39A8, SOCS1, THBS1, TNFAIP3, and TRIB1) of the 34 up-regulated genes42 in nonsurvivors of pediatric septic shock were among the up-regulated genes observed in FHL patients, underscoring the similarities between these 2 conditions.
The data of this study reveal some gene expression patterns common between FHL, SIRS, and pediatric septic shock. Signaling pathways represented by down- and up-regulated genes in patients with FHL related to innate immunity, immune response, defense response, and inflammation were also represented by differentially expressed genes in patients with SIRS and pediatric septic shock. In addition, 24 genes differentially expressed in FHL, compared with controls, were also a part of the 60 differentially expressed genes found in both survivors as well as nonsurvivors after pediatric sepsis.42,50 CX3CR1, which was found to be down-regulated in both nonsurvivors and survivors, was one of the most suppressed genes in patients with FHL. Cells presenting a proinflammatory, Th1, and/or cytotoxic phenotype preferentially express CX3CR1. Therefore, the down-regulation of CX3CR1 expression in patients with FHL or septic shock might be related to decreased Th1 immune responses that are present in both groups. These data corroborate, at the genomic level, the long-standing concept that FHL, SIRS, sepsis, and septic shock share common features. Gene expression in FHL reflects the delicate balance between extremely complex proinflammatory and anti-inflammatory events reminiscent of SIRS and septic shock.
FHL is a challenge for the immune system. The autosomal recessive form of the disease results from a defect in the granule-dependent cytotoxic pathway, followed by an intrinsic imbalance in negative and positive regulatory networks involved in pathogen detection and innate and adaptive immunity. In summary, the gene expression pattern observed in patients with active FHL suggests that the primary disease-causing mutation generates a self-fueling process of dysregulated immune responses with extensive down-regulation of genes related to innate and adaptive immune responses, as well as proapoptotic signals, along with the up-regulation of genes coding for proinflammatory cytokines and antiapoptotic factors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Hector Wong and Michael Jordan for helpful discussions regarding this study.
This work was supported by the National Institute of Allergy and Infectious Diseases (grants R21AI079759-02 and R21 AI076746-01) and the Histiocytosis Association of America.
National Institutes of Health
Authorship
Contribution: J.S. designed the research, analyzed the data, and wrote the manuscript; M.G.B. performed statistical analyses of microarray data and reviewed the manuscript; S.V.N. performed the research and quantitative RT-PCR experiments; S.M.-L. and J.V. performed the research; K.Z. performed the genetic analyses; K.A.R. contributed vital reagents and reviewed the manuscript; A.A.G. contributed vital reagents and reviewed the manuscript; and A.H.F. selected and evaluated the patients and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandra H. Filipovich, Division of Bone Marrow Transplantation and Immunodeficiency, Cincinnati Children's Hospital Medical Center, 3333 Burnett Ave, Cincinnati, OH 45229; e-mail: Lisa.Filipovich@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal