Abstract
Many different biochemical signaling pathways regulate integrin activation through the integrin cytoplasmic tail. Here, we describe a new role for α-actinin in inside-out integrin activation. In resting human platelets, α-actinin was associated with αIIbβ3, whereas inside-out signaling (αIIbβ3 activation signals) from protease-activated receptors (PARs) dephosphorylated and dissociated α-actinin from αIIbβ3. We evaluated the time-dependent changes of the αIIbβ3 activation state by measuring PAC-1 binding velocity. The initial velocity analysis clearly showed that PAR1-activating peptide stimulation induced only transient αIIbβ3 activation, whereas PAR4-activating peptide induced long-lasting αIIbβ3 activation. When αIIbβ3 activation signaling dwindled, α-actinin became rephosphorylated and reassociated with αIIbβ3. Compared with control platelets, the dissociation of α-actinin from αIIbβ3 was only transient in PAR4-stimulated P2Y12-deficient platelets in which the sustained αIIbβ3 activation was markedly impaired. Overexpression of wild-type α-actinin, but not the mutant Y12F α-actinin, increased its binding to αIIbβ3 and inhibited PAR1-induced initial αIIbβ3 activation in the human megakaryoblastic cell line, CMK. In contrast, knockdown of α-actinin augmented PAR-induced αIIbβ3 activation in CMK. These observations suggest that α-actinin might play a potential role in setting integrins to a default low-affinity ligand-binding state in resting platelets and regulating αIIbβ3 activation by inside-out signaling.
Introduction
Integrins and their ligands play key roles in development, immune responses, leukocyte traffic, hemostasis, and cancer and are at the core of numerous human diseases.1 Many integrins are expressed with their extracellular domains in a default low-affinity ligand-binding state. The main platelet integrin, αIIbβ3, also known as GPIIb/IIIa, is present at a high density on circulating platelets. It is inactive on circulating platelets; if it were not, platelets would bind their main ligand, fibrinogen, from the plasma and aggregate, leading to thrombosis. This inactivation is important for the biologic function of integrins, as is most evident from assessments of their status on circulating blood cells. However, the molecular mechanisms of their being set to an inactive, low-affinity state remain unknown.
High-affinity ligand binding requires activation of integrins through conformational changes regulated by inside-out signaling.2 Integrin cytoplasmic domains play a pivotal role in integrin signaling because the cytoplasmic tails of the integrin α and β subunits are directly accessible to the intracellular signaling apparatus, namely the integrin activation complex (IAC).3 Moreover, ligand binding to the integrin induces outside-in signaling that leads to integrin clustering and subsequent recruitment of actin filaments to the integrin cytoplasmic domain. From the perspective that this recruitment occurs by a complex of interacting cytoskeletal proteins, many studies have focused on the components of the IAC, including talin, kindlin, filamin, and α-actinin. The binding of talin to the integrin β subunit cytoplasmic tail is a common final step in the activation process.4-8 Kindlins bind to the more C-terminal of the NPxY motifs in β-integrin tails and modulate integrin activation.9-11 Filamin binding to β integrin cytoplasmic tails is competitive with that of talin.12 Although these studies suggest a model in which multiple proteins jockey for position on the β integrin tail, how cells orchestrate the process remains less well understood.
α-Actinin plays multiple important roles in the cell.13,14 It links the cytoskeleton to different transmembrane proteins in a variety of junctions, regulates activity of several receptors, and serves as a scaffold connecting the cytoskeleton to diverse signaling pathways. α-Actinin binds to the β integrin cytoplasmic tail,15,16 and recent studies have shown that the interaction between α-actinin and the integrin β2 tail modulates integrin affinity.17,18 For regulating α-actinin function, 4 main mechanisms have been identified to date: processing by proteases, binding to phosphatidylinositol intermediaries, phosphorylation by tyrosine kinases, and binding to calcium. For tyrosine phosphorylation regulation, a second wave of protein tyrosine phosphorylation that is strictly dependent on both ligand binding to αIIbβ3 and cytoskeleton organization was observed in platelets stimulated by thrombin, phorbol myristate acetate, or immobilized fibrinogen.19 Platelet adhesion and spreading on fibrinogen, mediated by the integrin αIIbβ3, trigger a robust and sustained phosphorylation of focal adhesion kinase and α-actinin by outside-in signaling.20,21 Focal adhesion kinase phosphorylates α-actinin, which lowers its affinity for actin.22 Dephosphorylation of α-actinin is regulated by the protein tyrosine phosphatase (PTP) SHP-1 (also named PTP1C, SHPTP-1, SHP, HCP, and PTPN6),23 a main PTP expressed in platelets. Although the regulation of α-actinin by outside-in signaling has been well characterized, its role in inside-out signaling remains to be determined.
Here, we show that α-actinin is associated with resting αIIbβ3 in platelets. Inside-out signaling from thrombin receptors, protease-activated receptor 1 (PAR1) and PAR4, dephosphorylated and dissociated α-actinin from αIIbβ3. Protease-activated receptor 1–activating peptide (PAR1-AP) and PAR4-AP induce transient and sustained αIIbβ3 activation, respectively. When the αIIbβ3 activation signaling dwindled, α-actinin reassociated with αIIbβ3 on rephosphorylation. Our observations suggest an emergent picture of α-actinin as having a role in keeping integrins in a default low-affinity ligand-binding state and regulating integrin activation.
Methods
Preparation of human platelets
Platelets were taken from healthy donors as approved by the institutional review board of Osaka University and were prepared as described previously24 with some modifications. In brief, venous blood was obtained from volunteers with acid citrate dextrose solution (National Institute of Health formula A) as an anticoagulant, used at a 1:6 vol/vol ratio. Platelet-rich plasma was obtained by centrifugation at 250g for 10 minutes. After incubation with 0.5μM prostaglandin E1 for 15 minutes, platelets were isolated by centrifugation of the platelet-rich plasma at 750g for 10 minutes. The pellet was washed twice with PIPES (Piperazine-1,4-bis(2-ethanesulfonic acid)) saline buffer (0.15M NaCl, 20mM PIPES, pH 6.5). The washed platelets were resuspended in Walsh buffer (137mM NaCl, 2.7mM KCl, 1mM MgCl2, 3.3mM NaH2PO4, 3.8mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1% glucose, 0.1% bovine serum albumin [BSA], pH 7.4) to a density of 4 × 108 platelets/mL and allowed to sit for 30 minutes before use.
Antibodies
The monoclonal anti–α-actinin antibody (BM-75.2) and anti–talin antibody (8d4) were purchased from Sigma-Aldrich. The monoclonal anti–α-actinin antibody (H-2) and horseradish peroxidase (HRP)–conjugated anti–mouse immunoglobulin M (IgM) were purchased from Santa Cruz Biotechnology. Anti–vasodilator-stimulated phosphoprotein (VASP) monoclonal antibody (IE273) was purchased from ImmunoGlobe. The antiphosphotyrosine (4G10) antibody and anti–phospho-VASP-Ser239 (16C2) were purchased from Upstate Cell Signaling Solutions. PAC-1, the monoclonal, ligand-mimetic, αIIbβ3-specific antibody that binds specifically to activated αIIbβ3, the allophycocyanin-conjugated monoclonal anti-CD25 antibody, allophycocyanin-conjugated anti-CD42b antibody, and phycoerythrin-conjugated anti-CD42b antibody were purchased from BD Biosciences. peridinin chlorophyll protein complex–cyanine 5.5 (PerCP-Cy5.5)–conjugated anti-CD25 antibody was purchased from eBioscience. The polyclonal anti-αIIbβ3 antibody was a gift from Dr Thomas J. Kunicki (The Scripps Research Institute), and the monoclonal anti-αIIbβ3 antibody that activates αIIbβ3, PT25-2,25 was a gift from Drs. Makoto Handa and Yasuo Ikeda (Keio University). HRP-conjugated secondary antibodies, anti–mouse IgG (H+L), and anti–rabbit IgG (H+L) were purchased from Cell Signaling Technology. Fluorescein isothiocyanate (FITC)– or phycoerythrin-conjugated anti–mouse IgM (μ) was purchased from Caltag Laboratories.
Chemicals
PAR1-AP (SFLLRN), thrombin, and prostaglandin E1 were purchased from Sigma-Aldrich. PAR4-AP (AYPGKF) was purchased from GenixTalk. AR-C69931MX, a P2Y12-specific antagonist, was a gift from Astra-Zeneca. FK633, an αIIbβ3-specific antagonist,26 was a gift from Astellas Pharma Inc. Protein phosphatase inhibitor-1 (PTPI-1) was purchased from Calbiochem.
Immunoprecipitation
Aliquots of washed platelets (4 × 108/mL) were incubated with PAR1-AP (25μM), PAR4-AP (150μM), or thrombin (0.2 U/mL) at room temperature. Reactions were stopped by lysis of platelets with an equal volume of 2 × neutral detergent lysis buffer (15mM HEPES, 150mM NaCl, 2% [vol/vol] Triton X-100, 10mM EGTA [ethylene glycol tetraacetic acid], and 1mM Na3VO4, pH 7.4, plus complete protease inhibitors purchased from Roche Applied Science). Insoluble debris was cleared from the lysate by centrifugation at 13 000g for 4 minutes at 4°C. Supernatants were precleared with protein G-sepharose (GE Healthcare) for 1 hour. Precleared lysates were added to the newly prepared protein G with 1 μg of antibody and incubated at 4°C with constant rotation. Immunoprecipitates were washed 3 times, and proteins were eluted from the beads by incubation of the immunoprecipitates with 20 μL of 3 × sodium dodecylsulfate (SDS) sample buffer (62.5mM Tris [tris(hydroxymethyl)aminomethane], pH 6.8, 25% [vol/vol] glycerol, 2% [vol/vol] SDS, 5mM 2-mercaptoethanol, and 0.01% bromophenol blue) at 96°C for 5 minutes.
Electrophoresis of proteins and immunoblotting
Proteins were separated by continuous SDS–polyacrylamide gel electrophoresis on 4%-20% gels and electrophoretically transferred to Immobilon-P phenylmethlsulfonyl fluoride membranes (Millipore). Membranes were blocked by incubation with 2% (wt/vol) BSA in TBST (150mM NaCl, 50mM Tris, and 0.1% [vol/vol] Tween 20, pH 7.4). Primary antibodies were diluted in 2% (wt/vol) BSA in TBST. After incubation with primary antibodies, membranes were washed with TBST and incubated with HRP-conjugated secondary antibodies for 1 hour at room temperature. After further washing of the membrane, signals were detected by enhanced chemiluminescence. When necessary, membranes were immersed in Restore Western blot stripping buffer (Pierce Chemical) and incubated at room temperature for 30 minutes before extensive washing and reprobing with the appropriate antibody.
Cell culture, plasmids, and transfections
Mammalian expression plasmids, including pcDNA/α-actinin, were a gift from Dr Beatrice Haimovich (University of Medicine and Dentistry of New Jersey). The mutant α-actinin carrying a phenylalanine at position 12 (Y12F) was generated as described.22 CMK cells were maintained in culture as described previously.27 Ribavirin was not used in this study. The plasmid encoding the extracellular and transmembrane domains of the Tac subunit of the human interleukin-2 receptor was generated as described.28 Nucleofection was performed with Nucleofector II (Amaxa Biosystems) according to the manufacturer's instructions. CMK cells were nucleofected with 10 μg/cuvette Tac subunit of the human interleukin-2 receptor–encoding plasmid and 20 μg/cuvette α-actinin–encoding plasmid. Cells were analyzed 20 hours after nucleofection. The short hairpin RNAs (shRNAs) lentiviral particules were generated as described.4 5′-GGAAGCCAGGCATGTGGTTCTGATCATTGGAAGCTTGCGATGATTAGGACTACATGCCTGTCTTCCTTTTTT-3′ and 5′-GGCCAGCTTCTCGTAGTCTTCCATAAGCTGAAGCTTGAGCTTATGGAGGATTATGAGAAGCTGGCTTTTTTT-3′ oligonucleotide sequences were used to construct control and α-actinin shRNA, respectively. The α-actinin shRNA sequence chosen is specific for human α-actinin-1 and is 82% conserved in the human α-actinin-4 nucleotide sequence. shRNA viral vectors were produced by cloning the siRNA cassette into the FG12 lentiviral transgene vector in which DsRed2 was substituted for enhanced green fluorescent protein. In plasmids encoding wild-type or mutant α-actinin, 3 silence mutations were generated to prevent annealing with the α-actinin shRNA.
Flow cytometry and platelet aggregometry
Aliquots of washed platelets and FITC–PAC-1 were incubated with PAR1-AP (25μM), PAR4-AP (150μM), or thrombin (0.2 U/mL) at room temperature for various times. Binding of PAC-1 to platelets or CMK cells was assessed by flow cytometry with the use of FACSCalibur (Becton Dickinson).29 The initial velocity of bound PAC-1 was analyzed as described.30 In brief, washed platelets were mixed with PAR-AP at time “zero.” At different time points from 2 minutes to 20 minutes, 20 μL of FITC–PAC-1 was added, and 30 seconds after the addition of PAC-1, 50 μL of platelet suspension was diluted into Walsh buffer, and bound PAC-1 was measured by flow cytometry. PAR1-AP–induced PAC-1 binding to CMK cells was analyzed on a gated subset of live (propidium iodide negative) differentiated (strongly CD42b+) transfected (strongly CD25+ or DsRed2+) cells. Specific binding was assessed as total binding minus binding in the presence of 10μM FK633. Intracellular α-actinin expression was assessed by flow cytometry as previously described.4 In brief, CMK cells were fixed with 0.5% paraformaldehyde, permeabilized with 0.05% saponin, and incubated for 30 minutes at room temperature with anti–α-actinin monoclonal antibody. After washing, the cells were incubated another 30 minutes with FITC-conjugated goat anti–mouse IgM. Cells were washed and resuspended in 500 μL of phosphate-buffered saline then analyzed by flow cytometry.
Presentation of data
Data are presented as mean ± SEM of ≥ 3 individual experiments from different blood donors. Analysis of statistical significance was performed with Student paired t tests, and differences were considered significant when P < .05. Immunoblots shown are representatives of 3 different experiments and were analyzed by scanning densitometry and quantified with ImageJ Version 1.40g (National Institutes of Health).
Results
Dynamic changes in the interaction between αIIbβ3 and α-actinin in platelets
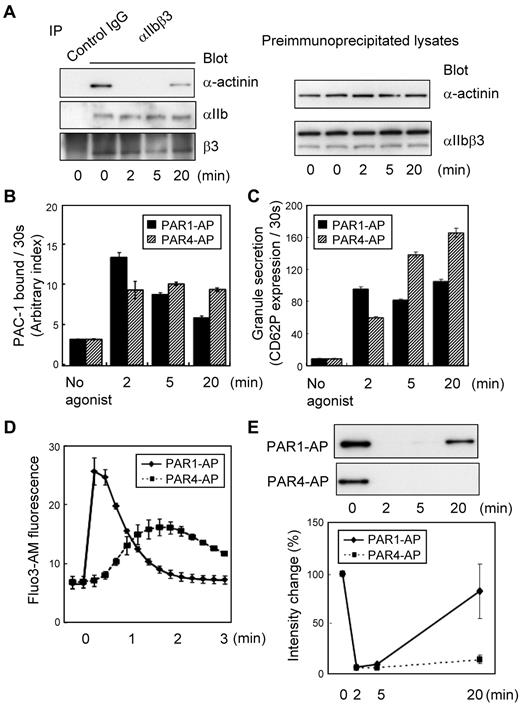
Human washed platelets were stimulated with 25μM PAR1-AP under nonstirring conditions for ≤ 20 minutes to explore the role of α-actinin in inside-out signaling. Immunoprecipitation with polyclonal anti-αIIbβ3 followed by immunoblotting with anti–α-actinin showed that, in resting platelets, α-actinin was already associated with resting αIIbβ3 (Figure 1A). When platelets were stimulated with PAR1-AP, α-actinin was dissociated from αIIbβ3. Some actin-binding proteins moved to the Triton X-100–insoluble fraction from the Triton X-100–soluble fraction in response to platelet activation; however, the amounts of α-actinin, talin, and αIIbβ3 in the Triton X-100–soluble fraction of PAR1-AP–activated platelets were similar to those of resting platelets under our experimental conditions. This finding suggests that the dissociation was not the result of the translocation of α-actinin into the Triton X-100–insoluble fraction. This dissociation remained unaffected even in the presence of 10μM of the αIIbβ3-specific peptidomimetic antagonist FK633, suggesting that the dissociation is independent of αIIbβ3-mediated outside-in signaling (data not shown). Interestingly, α-actinin rebound to αIIbβ3 at 20 minutes after PAR1-AP stimulation.
Dynamic changes in the interaction between αIIbβ3 and α-actinin in platelets. Washed human platelets were stimulated with PAR1-AP (25μM) or PAR4-AP (150μM) under nonstirring conditions for the time indicated. (A) αIIbβ3 was immunoprecipitated from lysates prepared from human platelets stimulated with PAR1-AP. Immunoprecipitates were then subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotted with anti–α-actinin antibody. Immuno-blots were stripped and reprobed with anti-αIIbβ3 antibody. Preimmunoprecipitated lysates were also subjected to SDS-PAGE and immunoblotted with the same series of antibodies. (B) FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. PAC-1 binding/30 seconds was normalized for integrin expression levels. (C) Phycoerythrin-conjugated anti-CD62P was added to the activated platelets and incubated for only 30 seconds to evaluate granule secretion. (D) Intracellular calcium mobilization was assessed by monitoring Fluo3-AM fluorescence by flow cytometry. (E) αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody. Immuno-blots shown are representative of 3 different experiments and analyzed by scanning densitometry and quantified with ImageJ (National Institutes of Health).
Dynamic changes in the interaction between αIIbβ3 and α-actinin in platelets. Washed human platelets were stimulated with PAR1-AP (25μM) or PAR4-AP (150μM) under nonstirring conditions for the time indicated. (A) αIIbβ3 was immunoprecipitated from lysates prepared from human platelets stimulated with PAR1-AP. Immunoprecipitates were then subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotted with anti–α-actinin antibody. Immuno-blots were stripped and reprobed with anti-αIIbβ3 antibody. Preimmunoprecipitated lysates were also subjected to SDS-PAGE and immunoblotted with the same series of antibodies. (B) FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. PAC-1 binding/30 seconds was normalized for integrin expression levels. (C) Phycoerythrin-conjugated anti-CD62P was added to the activated platelets and incubated for only 30 seconds to evaluate granule secretion. (D) Intracellular calcium mobilization was assessed by monitoring Fluo3-AM fluorescence by flow cytometry. (E) αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody. Immuno-blots shown are representative of 3 different experiments and analyzed by scanning densitometry and quantified with ImageJ (National Institutes of Health).
To clarify the physiologic relevance of the α-actinin dissociation to αIIbβ3 activation, we measured the amounts of PAC-1 binding after PAR1-AP or PAR4-AP stimulation. FITC–PAC-1 was incubated with activated platelets for 2 minutes, 5 minutes, and 20 minutes. Under PAR4-AP stimulation, the levels of PAC-1 binding increased as the incubation time extended. However, with PAR1-AP stimulation, the level of PAC-1 binding for a 20-minute incubation was similar to (or even lower than) that for a 5-minute incubation, suggesting that the number of activated αIIbβ3 molecules decreased at 20 minutes after PAR1-AP stimulation (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
To evaluate more precisely the dynamic changes in the αIIbβ3 activation state, we performed initial velocity analysis for PAC-1 binding that has recently been developed.30 In brief, FITC–PAC-1 was added to the activated platelets at the indicated time points after stimulation and incubated for only 30 seconds to obtain the PAC-1 binding velocity at the time points in question. The velocity of PAC-1 binding reflects the relative numbers of activated αIIbβ3 at those time points. PAC-1 binding was normalized for integrin expression levels. This initial velocity analysis clearly showed that PAR1 stimulation induced only transient αIIbβ3 activation, whereas PAR4 induced long-lasting αIIbβ3 activation (Figure 1B). Moreover, we assessed granule secretion and calcium mobilization under these conditions. These agonists induced different kinetics in CD62P expression and intracellular calcium mobilization, as detected by Fluo3-AM fluorescence (Figure 1C-D). These characteristics are consistent with the observed transient and sustained αIIbβ3 activation induced by PAR1-AP and PAR4-AP, respectively (Figure 1E). These data suggest that the dissociation of α-actinin may be related to αIIbβ3 activation.
Tyrosine phosphorylation of α-actinin regulates the interaction between α-actinin and αIIbβ3 in platelets
Because the α-actinin function appears to be regulated, in part, by tyrosine phosphorylation, we then examined the tyrosine phosphorylation state of α-actinin. The tyrosine-phosphorylated α-actinin was detectable as a faint band by immunoprecipitation with α-actinin antibody (BM-75.2 or H-2) followed by immunoblotting with 4G10 (supplemental Figure 1B). Accordingly, we modified our methods, and the platelet lysate was first subjected to immunoprecipitation with monoclonal antibody 4G10, followed by immunoblotting with anti–α-actinin antibody. In resting platelets, the anti–α-actinin antibody recognized the single 105-kDa protein (Figure 2A; supplemental Figure 1C). When stimulated with PAR1-AP, α-actinin was rapidly dephosphorylated. Although we have not excluded that this band was coprecipitated α-actinin with phosphoproteins, the phosphorylation profiles of immunoprecipitated α-actinin were almost the same as the faint band at 105 kDa in supplemental Figure 1B, suggesting that α-actinin itself was phosphorylated. α-Actinin was rephosphorylated at 20 minutes after PAR1-AP stimulation, whereas PAR4-AP induced sustained dephosphorylation of α-actinin. In addition to these agonists, we also examined adenosine diphosphate (ADP) and U46619-induced αIIbβ3 activation (supplemental Figure 2). ADP and U46619 induced transient and sustained αIIbβ3 activation, respectively. Again, α-actinin dissociation and de-phosphorylation induced by ADP and U46619 were transient and sustained, respectively. Thus, the kinetics of α-actinin phosphorylation seemed to synchronize with its interaction with αIIbβ3 (Figures 1E,2A).
Kinetics of tyrosine phosphorylation of α-actinin during platelet activation and inhibition of SHP-1 by PTPI-1. Washed human platelets were stimulated with PAR1-AP (25μM) or PAR4-AP (150μM) for the time indicated. (A) Tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. Immunoblots were analyzed by scanning densitometry and were quantified with ImageJ. (B) Washed human platelets were incubated at room temperature for 2 minutes in the presence of PTPI-1 (50μM). The platelets were then stimulated with PAR1-AP (25μM) for the time indicated. αIIbβ3 or tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. (C) FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. Error bars represent SEMs of 3 experiments.
Kinetics of tyrosine phosphorylation of α-actinin during platelet activation and inhibition of SHP-1 by PTPI-1. Washed human platelets were stimulated with PAR1-AP (25μM) or PAR4-AP (150μM) for the time indicated. (A) Tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. Immunoblots were analyzed by scanning densitometry and were quantified with ImageJ. (B) Washed human platelets were incubated at room temperature for 2 minutes in the presence of PTPI-1 (50μM). The platelets were then stimulated with PAR1-AP (25μM) for the time indicated. αIIbβ3 or tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. (C) FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. Error bars represent SEMs of 3 experiments.
It has been shown that the PTP SHP-1 regulates the dephosphorylation of α-actinin.23 To examine whether dephosphorylation of α-actinin regulates the dissociation of α-actinin from αIIbβ3 and integrin activation, we examined the effects of PTPI-1, a specific inhibitor of SHP-131 and PTP1B. Figure 2B shows that PTPI-1 inhibited the dephosphorylation of α-actinin. In addition, PTPI-1 markedly inhibited the activation of αIIbβ3 as well as the dissociation of α-actinin (Figure 2C). These results suggest that tyrosine phosphorylation of α-actinin regulates the interaction between α-actinin and αIIbβ3.
Interaction between α-actinin and integrin in platelets from a patient with Glanzmann thrombasthenia or P2Y12 deficiency
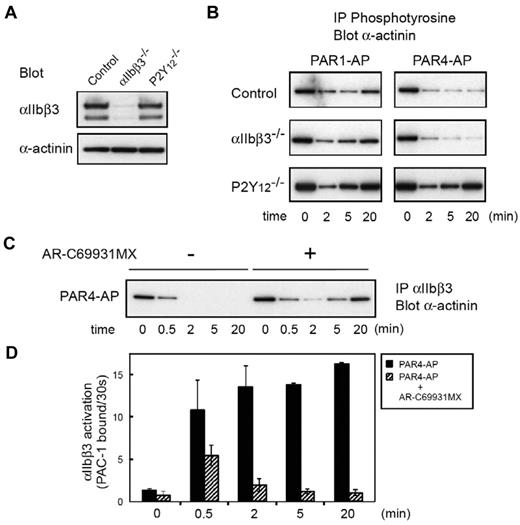
To examine further the role of α-actinin in inside-out signaling, we analyzed platelets from a patient with Glanzmann thrombasthenia or P2Y12-ADP receptor deficiency.24 Figure 3A shows the expression profiles of αIIbβ3 and α-actinin in both patients. In Glanzmann thrombasthenia platelets, the phosphotyrosine profile of α-actinin was almost the same as that of control platelets under both PAR1-AP and PAR4-AP stimulation, confirming that αIIbβ3 outside-in signaling does not mediate these changes. In sharp contrast, PAR4-AP stimulation failed to induce the sustained dephosphorylation of α-actinin in P2Y12-deficient platelets. Similarly, compared with control platelets, dephosphorylation of α-actinin induced by PAR1-AP was also disrupted at earlier time points in P2Y12-deficient platelets (Figure 3B). This early disruption of the dephosphorylation of α-actinin is consistent with our previous finding that P2Y12-mediated signaling is essential for sustained αIIbβ3 activation.29 Indeed, the blockade of P2Y12 with AR-C69931MX impaired the PAR4-AP–induced sustained αIIbβ3 activation, leading to the reassociation of α-actinin with αIIbβ3 (Figure 3C-D).
Changes in α-actinin phosphorylation and its interaction with αIIbβ3 in platelets from patients with Glanzmann thrombasthenia or P2Y12 deficiency. (A) Platelet lysates from patients with Glanzmann thrombasthenia or patients with P2Y12 deficiency were subjected to SDS–polyacrylamide gel electrophoresis and immunoblotted with anti-αIIbβ3 antibody or anti–α-actinin antibody. (B) Washed platelets were stimulated with PAR1-AP (25μM) or PAR4-AP (150μM) for the time indicated. Tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. (C) Washed normal platelets were stimulated with PAR4-AP (150μM) for the time indicated after incubation with AR-C69931MX (1μM) for 2 minutes. αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody. (D) Washed normal platelets were stimulated with PAR4-AP (150μM) for the time indicated after incubation with AR-C69931MX (1μM) for 2 minutes. FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. Error bars represent SEMs of 3 experiments.
Changes in α-actinin phosphorylation and its interaction with αIIbβ3 in platelets from patients with Glanzmann thrombasthenia or P2Y12 deficiency. (A) Platelet lysates from patients with Glanzmann thrombasthenia or patients with P2Y12 deficiency were subjected to SDS–polyacrylamide gel electrophoresis and immunoblotted with anti-αIIbβ3 antibody or anti–α-actinin antibody. (B) Washed platelets were stimulated with PAR1-AP (25μM) or PAR4-AP (150μM) for the time indicated. Tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. (C) Washed normal platelets were stimulated with PAR4-AP (150μM) for the time indicated after incubation with AR-C69931MX (1μM) for 2 minutes. αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody. (D) Washed normal platelets were stimulated with PAR4-AP (150μM) for the time indicated after incubation with AR-C69931MX (1μM) for 2 minutes. FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. Error bars represent SEMs of 3 experiments.
We have previously reported that the sustained αIIbβ3 activation induced by thrombin could be disrupted by inhibiting P2Y12-mediated signaling even after thrombin stimulation.29 Washed platelets were stimulated with thrombin at 0.2 U/mL. Two minutes later, we added AR-C69931MX (1μM) to the thrombin-stimulated platelets. We confirmed that AR-C69931MX still disrupted the sustained αIIbβ3 activation even under these conditions (Figure 4A). Interestingly, the sustained dephosphorylation and the dissociation of α-actinin from αIIbβ3 were disrupted by adding AR-C69931MX (Figure 4B-C). The blockade of P2Y12 was confirmed by the VASP phosphorylation state.32 These results suggest that the interaction between α-actinin and αIIbβ3 is, at least in part, regulated by P2Y12-mediated signaling but not by αIIbβ3 outside-in signaling.
Effects of the blockade of P2Y12 on α-actinin and αIIbβ3. Washed human platelets were incubated with thrombin (0.2 U/mL). The P2Y12 antagonist, AR-C69931MX (1μM), was added after 2 minutes of thrombin stimulation. (A) FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. (B) αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody. (C) Tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. Preimmunoprecipitated lysates were also subjected to SDS–polyacrylamide gel electrophoresis and immunoblotted with anti–phospho-VASP (Ser239) antibody or anti-VASP antibody. Error bars represent SEMs of 3 experiments.
Effects of the blockade of P2Y12 on α-actinin and αIIbβ3. Washed human platelets were incubated with thrombin (0.2 U/mL). The P2Y12 antagonist, AR-C69931MX (1μM), was added after 2 minutes of thrombin stimulation. (A) FITC–PAC-1 was added to the activated platelets after stimulation and incubated for 30 seconds to obtain the PAC-1 binding velocity at the time indicated. (B) αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody. (C) Tyrosine-phosphorylated proteins were immunoprecipitated then immunoblotted with anti–α-actinin antibody. Preimmunoprecipitated lysates were also subjected to SDS–polyacrylamide gel electrophoresis and immunoblotted with anti–phospho-VASP (Ser239) antibody or anti-VASP antibody. Error bars represent SEMs of 3 experiments.
α-Actinin modulates agonist-induced αIIbβ3 activation in CMK cells
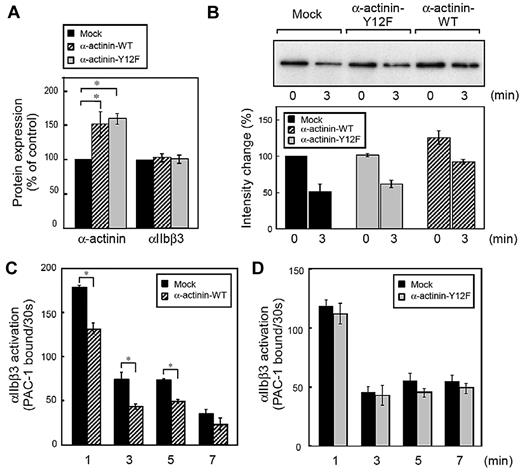
Finally, we used direct genetic manipulation to examine the effect of α-actinin on αIIbβ3 activation in human megakaryoblastic cell line CMK cells. Because the tyrosine residue at position 12 was reported as the main site of tyrosine phosphorylation in α-actinin,23 we overexpressed wild-type α-actinin and Y12F α-actinin in CMK cells. As shown in Figure 5A, the expression of α-actinin was increased to 130%-170% of endogenous levels without affecting αIIbβ3 expression. Next, we examined the interaction between α-actinin and αIIbβ3 in CMK cells. Like platelets and primary megakaryocytes,4 CMK cells can activate αIIbβ3 by the stimulation of physiologic agonists such as PAR1-AP.27,30 Similar to platelets, α-actinin was associated with αIIbβ3 in unstimulated CMK cells. When stimulated with PAR1-AP, α-actinin dissociated from αIIbβ3. Overexpression of wild-type α-actinin, but not the mutant Y12F α-actinin, increased the basal association between α-actinin and αIIbβ3 (Figure 5B).
Effects of α-actinin on αIIbβ3 inside-out signaling in CMK cells. Human megakaryoblastic CMK cells were transiently transfected with plasmids encoding for α-actinin and Tac subunit of the human interleukin-2 receptor. Protein expression and integrin activation were assessed 20 hours after transfection. (A) Intracellular α-actinin and surface αIIbβ3 were determined by flow cytometry. Bar charts represent specific antibody binding to highly transfected cells (CD25-allophycocyanin fluorescence > 50) normalized to mock plasmid (pcDNA3.1)–transfected cells. Transfected CMK cells were incubated with PAR1-AP (50μM) for the time indicated. αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody(B). Bar charts represent PAC-1 binding to wild-type α-actinin (C) or mutant α-actinin (D) transfected cells. Error bars represent SEMs of 3 experiments. *P < .05.
Effects of α-actinin on αIIbβ3 inside-out signaling in CMK cells. Human megakaryoblastic CMK cells were transiently transfected with plasmids encoding for α-actinin and Tac subunit of the human interleukin-2 receptor. Protein expression and integrin activation were assessed 20 hours after transfection. (A) Intracellular α-actinin and surface αIIbβ3 were determined by flow cytometry. Bar charts represent specific antibody binding to highly transfected cells (CD25-allophycocyanin fluorescence > 50) normalized to mock plasmid (pcDNA3.1)–transfected cells. Transfected CMK cells were incubated with PAR1-AP (50μM) for the time indicated. αIIbβ3 was immunoprecipitated then immunoblotted with anti–α-actinin antibody(B). Bar charts represent PAC-1 binding to wild-type α-actinin (C) or mutant α-actinin (D) transfected cells. Error bars represent SEMs of 3 experiments. *P < .05.
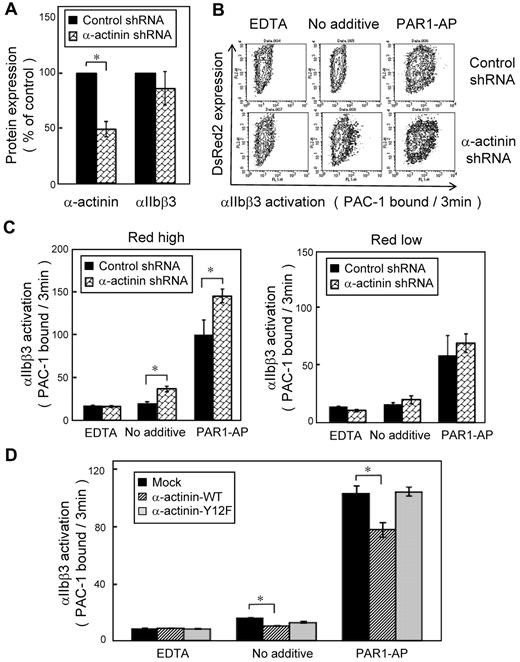
We then performed PAC-1 binding velocity analysis on the gated subset of live, transfected, and CD42b+ CMK cells stimulated with PAR1-AP, as described in the experimental procedures. CMK cells bound little PAC-1 in the absence of agonists. When stimulated with PAR1-AP, most mock-transfected cells bound PAC-1 (supplemental Figure 3A). Overexpression of wild-type α-actinin decreased PAC-1 binding velocity (Figure 5C) as well as the amounts of PAC-1 binding in the conventional PAC-1 binding assay (supplemental Figure 3C). The α-actinin expression has a concentration-dependent effect. High expression of α-actinin suppressed PAC-1 binding induced by PAR1-AP, but low expression of α-actinin did not (supplemental Figure 3B). In contrast, overexpression of the mutant Y12F α-actinin did not decrease PAC-1 binding velocity (Figure 5D). To examine further the effects of α-actinin, α-actinin expression was knocked down by shRNA in CMK cells. Knockdown was maximal at 10 days after infection, and shRNA induced 40%-60% reduction in the α-actinin expression (Figure 6A). Although adequate α-actinin reduction may not be obtained in CMK cells, decreased level of α-actinin augmented αIIbβ3 activation induced by PAR1-AP. Again, transduction levels (DsRed2 expression) have a concentration-dependent effect. High expression of DsRed2 augmented PAC-1 binding induced by PAR1-AP, but low expression of DsRed2 did not (Figure 6B-C). In contrast to PAR1-AP, PAR4-AP induced little PAC-1 binding velocity in CMK cells when stimulated with such high concentration as 1mM (supplemental Figure 4A). We assessed whether knockdown of α-actinin affects this PAR4-AP condition in CMK. PAC-1 binding induced by PAR4-AP was significantly augmented in the knockdown cells as well as PAR1-AP (supplemental Figure 4B). Finally wild-type α-actinin and Y12F α-actinin were overexpressed in α-actinin shRNA-transduced CMK cells. Wild-type α-actinin, but not the mutant Y12F α-actinin, normalized the augmented αIIbβ3 activation induced by PAR1-AP in CMK cells (Figure 6D). These results suggest that the binding of α-actinin might be involved in αIIbβ3 activation.
Knockdown of α-actinin augmented PAR1-AP–induced PAC-1 binding in CMK. Lentiviral particles encoding a shRNA for α-actinin or a control shRNA were transduced to CMK cells. (A) Intracellular α-actinin and surface αIIbβ3 were determined by flow cytometry. (B) CMK cells were stimulated with PAR1-AP (50μM) under nonstirring conditions with FITC–PAC-1 for 3 minutes. In contour plots, PAC-1 binding is shown on the x-axis, and transduction of the lentiviral particles is estimated by DsRed2 expression on the y-axis. (C) Bar charts represent PAC-1 binding to highly transduced cells (DsRed2 fluorescence > 250) and to less transduced cells (DsRed2 fluorescence < 50). (D) α-Actinin shRNA–transduced CMK cells were transiently transfected with plasmid encoding for wild-type or mutant α-actinin. Cells were stimulated with PAR1-AP (50μM) under nonstirring conditions with FITC–PAC-1 for 3 minutes. Error bars represent SEMs of 3 experiments in PAR1-AP and EDTA and of 8 experiments in no additive condition. *P < .05.
Knockdown of α-actinin augmented PAR1-AP–induced PAC-1 binding in CMK. Lentiviral particles encoding a shRNA for α-actinin or a control shRNA were transduced to CMK cells. (A) Intracellular α-actinin and surface αIIbβ3 were determined by flow cytometry. (B) CMK cells were stimulated with PAR1-AP (50μM) under nonstirring conditions with FITC–PAC-1 for 3 minutes. In contour plots, PAC-1 binding is shown on the x-axis, and transduction of the lentiviral particles is estimated by DsRed2 expression on the y-axis. (C) Bar charts represent PAC-1 binding to highly transduced cells (DsRed2 fluorescence > 250) and to less transduced cells (DsRed2 fluorescence < 50). (D) α-Actinin shRNA–transduced CMK cells were transiently transfected with plasmid encoding for wild-type or mutant α-actinin. Cells were stimulated with PAR1-AP (50μM) under nonstirring conditions with FITC–PAC-1 for 3 minutes. Error bars represent SEMs of 3 experiments in PAR1-AP and EDTA and of 8 experiments in no additive condition. *P < .05.
Discussion
Here, we focused on α-actinin as a member of the IAC and assessed its potential role in integrin-reversible activation. Resting αIIbβ3 was already associated with α-actinin, and inside-out signaling by PARs induced a dissociation of α-actinin from αIIbβ3. This dissociation was regulated by dephosphorylation of α-actinin and associated with reversible αIIbβ3 activation, as evidenced by the stimulation of PAR1-AP and PAR4-AP. Overexpression of wild-type α-actinin, but not a mutant with a tyrosine-phosphorylation defect (Y12F), inhibited αIIbβ3 activation in a megakaryocytic cell line, CMK, in which αIIbβ3 is activated by PAR1-AP. Knockdown of α-actinin augmented PAR-AP–induced PAC-1 binding in CMK. Thus, our observations suggest that α-actinin may play a role in keeping αIIbβ3 in a low-affinity state.
Thrombin activates human platelets through proteolytic activation of 2 protease-activated receptors, PAR1 and PAR4.33 PAR1 is a high-affinity receptor for platelet activation at low concentrations of thrombin, whereas PAR4 is a low-affinity receptor that mediates thrombin signaling at high concentrations. Consistent with previous reports,34,35 we showed distinct kinetics of signaling from these PARs by evaluation of intracellular calcium mobilization and P-selectin translocation. PAR1 triggered a rapid and transient increase in intracellular calcium, whereas PAR4 triggered a slower but more prolonged response. Accordingly, we used PAR1-AP and PAR4-AP to examine the role of α-actinin in integrin activation. To assess the activation state of αIIbβ3 more precisely, we performed initial velocity analysis at a specific time point. The kinetic approach with the use of flow cytometry has been proposed for assessing the dynamics of αIIbβ3 activation,36 and we have modified it as the initial velocity analysis for assessing reversible αIIbβ3 activation. On-rate of PAC-1 binding reflects the number of activated receptors, and the initial velocity analysis showed that PAR1 stimulation induced only transient activation. In addition, only 25% of αIIbβ3 was kept activated at 20 minutes after stimulation compared with the amount of activated αIIbβ3 at 2 minutes after stimulation.
In contrast, PAR4 induced long-lasting αIIbβ3 activation, and the amount of activated αIIbβ3 was almost the same at 2 minutes and 20 minutes after stimulation. The association/dissociation behavior of α-actinin with αIIbβ3 is apparently related to the distinct αIIbβ3 activation kinetics induced by PAR1-AP, PAR4-AP, ADP, and U46619.
We have shown that continuous interaction between released endogenous ADP and P2Y12 is critical for sustained αIIbβ3 activation induced by thrombin.29 In this context, the sustained αIIbβ3 activation induced by PAR4-AP was markedly impaired in P2Y12-deficient platelets. In addition, the dissociation and dephosphorylation of α-actinin induced by PAR4-AP as well as by PAR1-AP was markedly impaired. Moreover, after αIIbβ3 activation was completed by thrombin stimulation, the addition of AR-C69931MX disrupted the dissociation and dephosphorylation of α-actinin as well as the sustained αIIbβ3 activation. PTPI-1, an inhibitor of SHP-1, inhibited the dissociation as well as the dephosphorylation of α-actinin induced by PAR1-AP. These data suggest a close association between the dissociation and dephosphorylation of α-actinin and regulation of these changes, at least in part, by P2Y12-mediated signaling.
Cellular control of integrin activation requires transmission of a signal from the cytoplasmic tails to the extracellular domains. One may argue that the activation state of αIIbβ3 is an intrinsic state of the integrin itself and not a property of platelets per se.37 However, this concept is based on the property of exogenous αIIbβ3 expressed on Chinese hamster ovary cells which is not activated by several platelet agonists. In this study, we have demonstrated that endogenous αIIbβ3 expressed on CMK cells can be activated by PAR-APs. Recent studies have identified some intracellular adapters, enzymes, and substrates necessary for αIIbβ3 activation and the tails function as regulatory scaffolds.38 Among cytoplasmic proteins associated with β3 tail, talin4,8 and kindlins9 are now well established as being essential for integrin activation. Recently, it has been shown that talin binding is sufficient to activate integrin αIIbβ339 and that a talin membrane contact would be required for integrin activation.39,40 In this context, α-actinin may modulate the accessibility of talin to the β3 tail or to plasma membrane because putative α-actinin binding sites have been reported within the membrane proximal region of the β3 cytoplasmic tail.15,41 In platelets α-actinin was no longer associated with αIIbβ3 after PAR-AP stimulation. This may imply that α-actinin knockdown would not further enhance the extent of integrin activation. However, α-actinin knockdown significantly augmented PAC-1 binding induced by 1mM PAR4-AP as well as 50μM PAR1-AP in CMK. Unlike platelets, CMK needed a higher concentration of agonists to get enough levels of activated αIIbβ3. Even under those conditions, the levels of activated αIIbβ3 were less, and the sustained time of activation was shorter than platelets. From these observations, we assume that αIIbβ3 in CMK cells under our experimental conditions may not be fully activated. It is possible that this is the reason why α-actinin knockdown further enhances integrin activation in CMK cells. Thus, the α-actinin binding to the β3 tail may keep αIIbβ3 in a low-affinity state, and it is possible that the dissociation of α-actinin from the β3 tail may lead to the easy accessibility of talin to the β3 tail by inside-out signaling. Like β3 integrin, β2 integrins can change affinity on a subsecond time scale.1 Recent studies have shown that intermediate-affinity lymphocyte function-associated antigen-1 bound to α-actinin, whereas high-affinity lymphocyte function antigen-1 bound to talin.17,18 These data show that α-actinin and talin regulate integrin activation differently.
α-Actinin colocalizes with actin and stabilizes the actin filament web in nonmuscle cells.13 It has been suggested that most α-actinin forms a bridge between the actin filaments and that there is some α-actinin associated with integrin at the end of actin filaments.14 Indeed, we have confirmed that most α-actinin was not associated with αIIbβ3, as evidenced by the presence of large amounts of α-actinin in the supernatant even in immunoprecipitates of αIIbβ3 from resting platelets (data not shown). In the fibroblast, the phosphorylation of α-actinin reduces its affinity for actin and prevents its localization to focal adhesion plaques.42 Another report showed that the phosphorylation of α-actinin might serve to modulate the coupling/uncoupling of integrins to the cytoskeleton in platelets.23 Outside-in signaling involving interaction of αIIbβ3 with its immobilized ligand fibrinogen triggers tyrosine phosphorylation and cytoskeletal reorganization in platelets.20 Pathologic shear forces, such as those encountered in stenotic midsized coronary and cerebral arteries, directly affect ligand-dependent αIIbβ3 outside-in signaling, and a recent study showed that pathologic shear stress induced dissociation of α-actinin from the β3 tail, which is αIIbβ3 dependent.43 Our observations described here are clearly different phenomena from those induced by outside-in signaling; an αIIbβ3-specific antagonist, FK633, did not inhibit the dissociation, and dephosphorylation was induced even in thrombasthenic platelets. Thus, both inside-out signaling and outside-in signaling regulate the dissociation of α-actinin from αIIbβ3.
Integrin function is a complex process regulated by the balance of positive and negative regulatory proteins. Several factors have been identified as positive and negative regulators of αIIbβ3.44,45 Platelet agonists, such as thrombin and ADP, induce αIIbβ3 activation, whereas various vasodilators released from the endothelial cells keep αIIbβ3 inactive in circulating blood. In addition to platelet agonists and vasodilators, the balance of positive and negative regulatory proteins in IAC may regulate αIIbβ3 activation. Our observations described here suggest that α-actinin may act as a negative regulator in resting platelets. Although further work is needed to determine each specific role of α-actinin in platelets, the α-actinin–integrin interaction might be added to the discussion of new potential targets for atherothrombotic disease therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan, from the Ministry of Health, Labor and Welfare in Japan; and “Academic Frontier” Project in Japan.
Authorship
Contribution: S.T. designed and performed experiments, analyzed data, and wrote the paper; T.N., T.K., and K.K. helped perform parts of the experiments; H.K. and S.H. provided feedback and experimental advice; and Y.K. and Y.T. supervised the project and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seiji Tadokoro, Department of Hematology and Oncology, Osaka University Graduate School of Medicine C9, 2-2 Yamada-oka, Suita Osaka 565-0871, Japan; e-mail: tadokoro@hp-blood.med.osaka-u.ac.jp.