Abstract
Factor VIII (FVIII)–specific memory B cells are essential components for regulating anamnestic antibody responses against FVIII in hemophilia A with FVIII inhibitors. We asked how stimulation and inhibition of FVIII-specific memory B cells by low and high concentrations of FVIII, respectively, are affected by concurrent activation of the innate immune system. Using CD138− spleen cells from hemophilic mice treated with FVIII to study restimulation and differentiation of memory B cells in vitro, we tested modulating activities of agonists for Toll-like receptors (TLRs) 2, 3, 4, 5, 7, and 9. Ligands for TLR7 and 9 were most effective. They not only amplified FVIII-specific memory responses in the presence of stimulating concentrations of FVIII, but also countered inhibition in the presence of inhibitory concentrations of FVIII. Notably, CpG oligodeoxynucleotide (CpG-ODN), a ligand for TLR9, expressed biphasic effects. It amplified memory responses at low concentrations and inhibited memory responses at high concentrations, both in vitro and in vivo. Both stimulatory and inhibitory activities of CpG-ODN resulted from specific interactions with TLR9. Despite their strong immunomodulatory effects in the presence of FVIII, ligands for TLR induced negligible restimulation in the absence of FVIII in vitro and no restimulation in the absence of FVIII in vivo.
Introduction
Memory B cells are fundamentally important for maintaining immunologic memory to ensure long-lasting protection against invading pathogens, such as viruses and bacteria.1 They are also involved in long-term maintenance of immunopathologic conditions, such as chronic antibody-dependent immunologic disorders.2 Memory B cells have the unique capacity to rapidly differentiate into antibody-secreting plasma cells (ASCs) upon re-exposure to their specific antigen, thereby replenishing the pool of plasma cells.1 They also act as efficient antigen-presenting cells for the restimulation of CD4+ T cells because they express high-affinity antigen receptors as well as MHC class II and costimulatory molecules.3
We and others have demonstrated the presence of factor VIII (FVIII)–specific memory B cells in the circulation of patients with hemophilia A and FVIII inhibitors.4,5 van Helden et al5 reported the disappearance of memory B cells from the circulation of patients during successful immune tolerance-induction therapy. These findings are in line with the assumption that FVIII-specific memory B cells are important for the stimulation of anamnestic antibody responses against FVIII. Little information is available on the regulation of these cells in patients with hemophilia A. Their low frequency in the circulation (0.07%-0.35% of total immunoglobulin G [IgG] memory B cells4,5 ) and the inaccessibility of their major residencies (peripheral lymphoid organs, such as the spleen6 ) are important obstacles in studying these cells in patients with hemophilia A and FVIII inhibitors. Therefore, we used the E17 hemophilic mouse model to obtain a better understanding of the regulation of these cells. Previously, we established a spleen cell culture system that enabled us to study the function and regulation of FVIII-specific memory B cells.7,8 We demonstrated that the differentiation of FVIII-specific memory B cells into ASCs depends on the presence of activated T cells and certain costimulatory interactions.7 Furthermore, we showed that the differentiation of memory B cells is sensitive to increasing doses of FVIII. Concentrations of human FVIII in the range of 0.1-100 ng/mL (corresponding to approximately 0.001-1 U/mL) restimulate memory B cells and induce their differentiation into ASCs. Higher doses of human FVIII, however, inhibit FVIII-specific memory B-cell responses.8 Based on these previous results, we then asked how stimulation and inhibition of FVIII-specific memory B cells by low and high concentrations of human FVIII, respectively, are influenced by concurrent activation of the innate immune system. The innate immune system becomes activated as a first line of defense against invading pathogens during natural microbial infections and primes the adaptive immune system to generate antigen-specific immune responses.9,10 Cells of the innate immune system express various pattern-recognition receptors that recognize conserved structures of pathogens, so-called pathogen-associated molecular patterns.11 Several classes of pattern-recognition receptors have been identified, such as Toll-like receptors (TLRs), retinoic acid–inducible gene-I–like receptors, and C-type lectin receptors or nucleotide oligomerization domain protein-like receptors.11,12 We were particularly interested in the activities of agonists for TLR, which represent the best characterized group of innate immune receptors with respect to known ligands, downstream signaling pathways, and functional relevance.13-16
So far, 12 different TLRs have been identified in mammals.15 They are differentially expressed on many cell types of hematopoietic and nonhematopoietic origin,13-15 either at the cell surface (TLR1, TLR2, TLR4, TLR5, and TLR6) or in endolysosomal compartments (TLR3, TLR7, TLR8, and TLR9). Upon activation, TLR expressed at the cell surface can enter the endocytic pathway. The distinct localization of TLR is associated with the specific nature of their stimulatory ligands. TLRs expressed on the cell surface primarily sense microbial membrane molecules, such as lipopeptides, peptidoglycans, lipopolysaccharide (LPS), and flagellin (TLR1, TLR2, TLR4, TLR5, and TLR6). TLR expressed intracellularly recognize microbial nucleic acids, such as CpG-containing DNA sequences (TLR9), viral single-stranded RNA (TLR7 and TLR8), or double-stranded RNA (TLR3). A growing body of evidence suggests that TLR agonists modify the restimulation of memory B cells and their differentiation into ASCs.17-19 Moreover, it was postulated that memory B cells can be activated by TLR agonists to differentiate into ASCs in the complete absence of antigen.17,20 Based on these findings, it is important to understand how stimulation and inhibition of FVIII-specific memory responses by different concentrations of FVIII are affected by agonists for TLR. Furthermore, one could speculate that FVIII-specific memory B cells in patients might become activated during infections or vaccinations in the complete absence of any replacement therapy with FVIII-containing products. A better understanding of how TLR agonists affect FVIII-specific memory responses is therefore of utmost importance in designing new treatments for patients with hemophilia and FVIII inhibitors.
Methods
Hemophilic mice
Our colony of hemophilic E17 mice (characterized by a targeted disruption of exon 17 of the FVIII gene) was established with a breeding pair from the original colony21,22 and crossed into the C57BL/6J background.23 All mice were male and aged 8-12 weeks at the beginning of the experiments. All studies were carried out in accordance with Austrian federal law (Act BG 501/1989) regulating animal experimentation.
Treatment with human FVIII and TLR ligands
If not stated otherwise, hemophilic E17 mice received 4 intravenous doses of 200 ng of recombinant human FVIII (approximately 8 μg/kg corresponding to approximately 80 U/kg FVIII), diluted in 200 μL of Dulbecco phosphate-buffered saline (DPBS; Sigma-Aldrich), at weekly intervals. The recombinant human FVIII used throughout the studies was albumin-free bulk material obtained from Baxter BioScience.
TLR ligands were reconstituted according to the manufacturer's instructions and administered either together with FVIII or prior to FVIII in a volume of 200 μL, diluted in DPBS as indicated.
Sampling of tissue and blood
Tissue and blood samples were collected 7 days after the last dose of FVIII, if not otherwise indicated. All invasive procedures were carried out under anesthesia with pentobarbital (Nembutal; Richter Pharm). Blood samples were obtained by cardiac puncture or tail snipping. The samples obtained from individual mice were added to 0.1M sodium citrate at a 4:1 (vol/vol) ratio. Plasma was separated by centrifugation and stored at −20°C until further analysis.
Preparation of spleen cells
Spleen cells were prepared as previously described.24
Restimulation of memory B cells in vitro
Restimulation of memory B cells in vitro was studied as previously described.7,8 Briefly, spleen cells obtained from E17 hemophilic mice treated with 4 intravenous doses of 200 ng of human FVIII (∼ 8 μg/kg corresponding to ∼ 80 U/kg FVIII), then were isolated and depleted of CD138+ cells (CD138 is a marker for ASC). Remaining CD138− spleen cells were cultured for 6 days (if not otherwise stated). Different concentrations of human FVIII were added to the cultures at day 0. TLR ligands were added together with FVIII at day 0 or at later time points, as indicated. After 6 days of culture, newly formed ASCs were detected by enzyme-linked immunospot (ELISPOT) assays as previously described.7,8
Restimulation of FVIII-specific memory responses in vivo
Restimulation after priming with a single dose of human FVIII.
Hemophilic mice received an initial dose of 200 ng intravenous human FVIII (∼ 8 μg/kg corresponding to ∼ 80 U/kg FVIII) on day 1. One week after the first dose of FVIII, mice were treated with either phosphate-buffered saline (PBS) buffer (negative control) or with different doses of TLR ligands. On the following day, mice received either PBS buffer (negative control), FVIII only (200 or 1000 ng; both doses have previously been shown to stimulate FVIII-specific antibody responses24,25 ), or FVIII at a dose of 200 ng together with different doses of TLR ligand. This dosing schedule was chosen based on results we published previously, which demonstrated that a single intravenous dose of 200 ng of human FVIII does not induce antibodies, but primes the immune system for further FVIII treatment.24 A second intravenous dose of 200 ng of human FVIII induces detectable FVIII-specific antibodies in the circulation that further increase in titers after 3 doses.24 By choosing the second FVIII dose for the potential modulation by TLR ligands, we had the possibility to detect both potential amplifying and potential inhibiting effects induced by TLR ligands.
Restimulation after transfer of FVIII-specific memory cells into naive mice, as described.7,8
Spleen cells, isolated from hemophilic mice treated with 4 doses of 200 ng of human FVIII (∼ 8 μg/kg corresponding to ∼ 80 U/kg FVIII), were depleted of CD138+ ASCs. A total of 107 CD138− spleen cells were intravenously injected into naive hemophilic mice. One day after cell transfer, mice were injected with a single intravenous dose of PBS buffer (negative control), with a combination of intravenous FVIII and intraperitoneal ligands for TLR (or PBS for negative control) or with intraperitoneal TLR ligands only.
TLR ligands
The following ligands were used: Zymosan for TLR2 (0.1-10 000 ng/mL); Poly I:C for TLR3 (1-50 000 ng/mL), LPS for TLR4 (0.1-10 000 ng/mL), Flagellin for TLR5 (0.01-1000 ng/mL), Loxoribine and Imiquimod for TLR7 (1-50 000 ng/mL), CpG-ODN (CpG-B, ODN1826, sequence: 5′-tcc atg acg ttc ctg acg tt-3′) for TLR9 (0.1-10 000 ng/mL). All TLR ligands were obtained from InvivoGen.
The following ligands served as controls: ODN2088 (CpG-DNA, sequence: 5′-tcc tgg cgg gga agt-3′) as inhibitor for TLR9 (100-10 000 ng/mL; InvivoGen), ODN1826-control (GpC-DNA, sequence: 5′-tcc atg agc ttc ctg agc tt-3′) as negative control for ODN1826 (0.1-10 000 ng/mL; InvivoGen) for TLR9.
Detection of anti-FVIII antibodies in blood plasma
Titers of total anti-FVIII antibodies in blood plasma were measured by enzyme-linked immunosorbent assay (ELISA) as previously described.24
Analysis of cytokines in cell-culture supernatants
Murine interleukin-4 (IL-4), IL-6, IL-10, and interferon-γ (IFN-γ) were detected in cell culture supernatants using a Bio-Plex Mouse Cytokine 9-Plex Assay (Bio-Rad Laboratories).
Statistical analysis
Results presented in Figure 4B were compared using an unpaired 2-tailed Student t test. A difference between 2 groups was considered to be significant if P < .05.
Statistical analyses of antibody titers presented in Figure 7C were performed with SAS version 9.1.3 of the SAS System for Linux(SAS Institute Inc). The null hypotheses of no differences were tested against their 2-sided alternatives at a level of 5% statistical significance. Log2-transformed titers were assumed to follow a negative binomial distribution.26 As the data were collected longitudinally (ie, over time), a repeated-measures analysis was performed using the SAS procedure GENMOD.
Results
Agonists for TLR7 and 9 are most effective in modulating FVIII-specific memory responses in vitro
We identified TLR agonists that modulate the stimulation or inhibition of FVIII-specific memory responses by screening ligands for TLRs 2, 3, 4, 5, 7, and 9 over a range of different concentrations using the previously described CD138− spleen cell culture system.7,8 CD138− spleen cells obtained from E17 hemophilic mice treated with 4 doses of human FVIII were restimulated in vitro, and the impact of TLR stimulation on the differentiation of memory B cells into anti-FVIII ASCs was analyzed by ELISPOT. TLR ligands were added to the cultures on day 0 in the complete absence of human FVIII as well as in the presence of stimulatory (10 ng/mL) or inhibitory (1 and 20 μg/mL) concentrations of human FVIII. Our results indicated that most TLR agonists tested modulated both the restimulation and inhibition of FVIII-specific memory B cells, to some degree. However, agonists for TLR7 (Loxoribine and Imiquimod) and TLR9 (CpG-ODN) were most effective (Table 1). At optimal concentrations, agonists for TLR7 and TLR9 amplified the restimulation of FVIII-specific memory B cells induced by low concentrations (10 ng/mL) of human FVIII considerably and countered the inhibition of memory responses induced by high concentrations (1 and 20 μg/mL) of human FVIII (Table 1). In conclusion, all TLR ligands affected FVIII-specific memory responses, but TLR7 and TLR9 ligands were most effective.
Screening of TLR ligands
| TLR ligand . | Conc. tested, ng/mL . | Conc. at max. stimulation, ng/mL . | FVIII . | No. of experiments . | |||
|---|---|---|---|---|---|---|---|
| 0 ng/mL . | 10 ng/mL . | 1000 ng/mL . | 20 000 ng/mL . | ||||
| Control | 0 (0-1) | 100 | 7 (3-10) | 0 (0-2) | 10 | ||
| Zymosan/TLR2 | 0.1-10 000 | 10 000 | 1 (1) | 71 (21-121) | 9 (5-13) | 3 (1-5) | 2 |
| Poly (I:C)/TLR3 | 1-50 000 | 1000-10 000 | 1 (1) | 263 (227-298) | 31 (28-34) | 2 (1-2) | 2 |
| LPS/TLR4 | 0.1-10 000 | 1-100 | 2 (1-3) | 199 (144-292) | 87 (42-131) | 2 (1-3) | 2-3 |
| Flagellin/TLR5 | 0.01-1000 | 1-1000 | 1 (1) | 91 (35-146) | 53 (11-94) | 2 (1-3) | 2 |
| Loxoribine/TLR7 | 1-50 000 | 10 000-50 000 | 9 (2-12) | 478 (196-1023) | 212 (187-260) | 57 (12-112) | 3-5 |
| Imiquimod/TLR7 | 1-50 000 | 100-1000 | 2 (1-3) | 794 (205-1118) | 174 (126-220) | 38 (19-59) | 3-4 |
| CpG-ODN/TLR9 | 0.1-10 000 | 100 | 3 (0-6) | 676 (132-1884) | 198 (44-320) | 135 (24-194) | 4-5 |
| TLR ligand . | Conc. tested, ng/mL . | Conc. at max. stimulation, ng/mL . | FVIII . | No. of experiments . | |||
|---|---|---|---|---|---|---|---|
| 0 ng/mL . | 10 ng/mL . | 1000 ng/mL . | 20 000 ng/mL . | ||||
| Control | 0 (0-1) | 100 | 7 (3-10) | 0 (0-2) | 10 | ||
| Zymosan/TLR2 | 0.1-10 000 | 10 000 | 1 (1) | 71 (21-121) | 9 (5-13) | 3 (1-5) | 2 |
| Poly (I:C)/TLR3 | 1-50 000 | 1000-10 000 | 1 (1) | 263 (227-298) | 31 (28-34) | 2 (1-2) | 2 |
| LPS/TLR4 | 0.1-10 000 | 1-100 | 2 (1-3) | 199 (144-292) | 87 (42-131) | 2 (1-3) | 2-3 |
| Flagellin/TLR5 | 0.01-1000 | 1-1000 | 1 (1) | 91 (35-146) | 53 (11-94) | 2 (1-3) | 2 |
| Loxoribine/TLR7 | 1-50 000 | 10 000-50 000 | 9 (2-12) | 478 (196-1023) | 212 (187-260) | 57 (12-112) | 3-5 |
| Imiquimod/TLR7 | 1-50 000 | 100-1000 | 2 (1-3) | 794 (205-1118) | 174 (126-220) | 38 (19-59) | 3-4 |
| CpG-ODN/TLR9 | 0.1-10 000 | 100 | 3 (0-6) | 676 (132-1884) | 198 (44-320) | 135 (24-194) | 4-5 |
CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated in vitro with FVIII at the concentrations indicated in the presence of TLR ligands in the range of concentrations shown. Newly formed anti-FVIII ASCs were detected by ELISPOT assay after 6 days of culture.
All results were normalized to compare experiments done on different days. Results obtained in differentiation cultures containing 10 ng/mL FVIII only were always set as 100%. The results shown represent the highest response obtained for each ligand tested. This highest response is expressed as the mean value and range of response, in percent, compared with the response after stimulation with 10 ng/mL FVIII.
Poly I:C indicates polyinosinic:polycytidylic acid; Conc., concentration; and max., maximum.
CpG-ODN induces both stimulatory and inhibitory effects on FVIII-specific memory responses
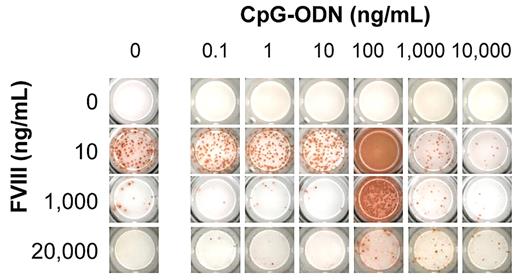
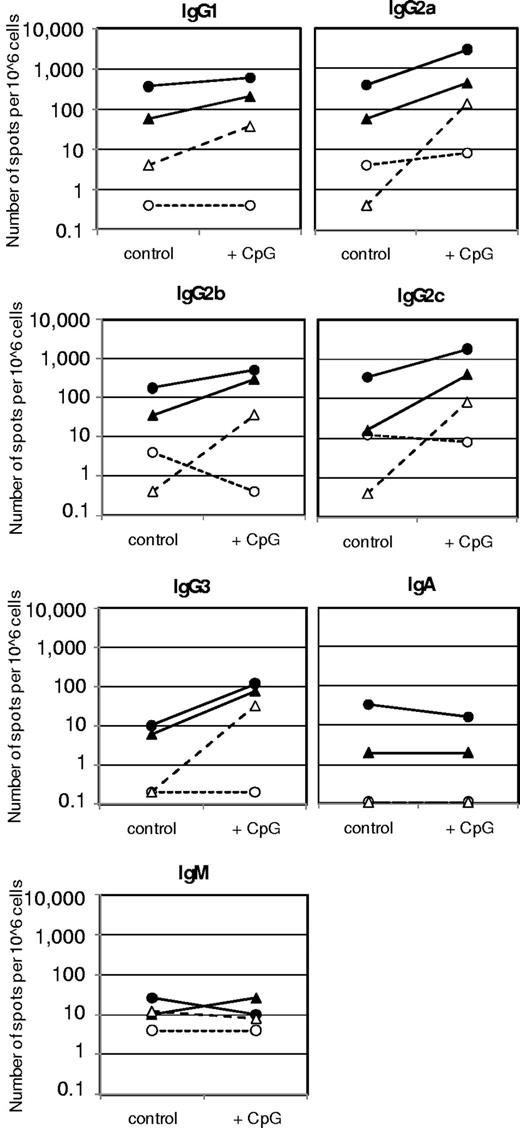
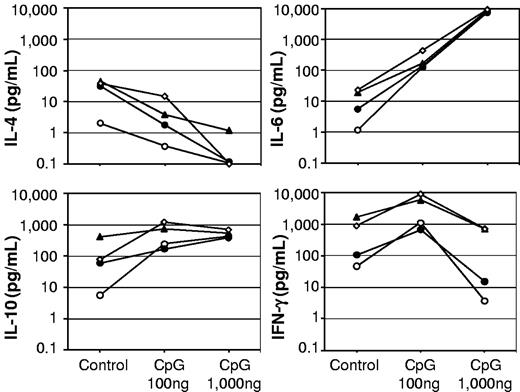
When we studied the modulatory effects of CpG-ODN at different concentrations, it appeared that CpG-ODN expressed a biphasic effect. At 100 ng/mL, CpG-ODN amplified the memory response at stimulatory concentrations (10 ng/mL) of human FVIII and countered the inhibition at inhibiting concentrations of human FVIII (1 and 20 μg/mL; Figures 1 and 2). Analysis of antibody isotypes and IgG subclasses of antibodies secreted by FVIII-specific ASCs showed that CpG-ODN at 100 ng/mL amplified the differentiation of memory B cells into ASCs of all IgG subclasses, but did not stimulate or even down-regulate the differentiation into ASCs of immunoglobulin M (IgM) and immunoglobulin A (IgA) isotypes (Figure 2). The analysis of T-cell cytokines secreted into cell culture supernatants during differentiation of memory B cells into anti-FVIII ASCs revealed a down-regulation of IL-4 and an up-regulation of IFN-γ (Figure 3), which supports the idea that CpG-ODN polarizes FVIII-specific memory responses toward T helper cell type 1 (Th1)–driven responses. Furthermore, IL-6 and IL-10 were up-regulated.
Screening panel for in vitro restimulation of FVIII-specific memory B cells in the presence of FVIII and CpG. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated with stimulating (10 ng/mL) and inhibiting (1 and 20 μg/mL) concentrations of FVIII in the presence of CpG-ODN. Newly formed anti-FVIII ASC were detected by ELISPOT assay after 6 days of culture. Each spot represents one anti-FVIII ASC. Concentrations of FVIII and CpG-ODN are indicated. A representative ELISPOT is presented.
Screening panel for in vitro restimulation of FVIII-specific memory B cells in the presence of FVIII and CpG. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated with stimulating (10 ng/mL) and inhibiting (1 and 20 μg/mL) concentrations of FVIII in the presence of CpG-ODN. Newly formed anti-FVIII ASC were detected by ELISPOT assay after 6 days of culture. Each spot represents one anti-FVIII ASC. Concentrations of FVIII and CpG-ODN are indicated. A representative ELISPOT is presented.
Ig isotypes and IgG subclasses of anti-FVIII ASC in vitro differentiated in the presence of FVIII and 100 μg/mL CpG-ODN. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated with stimulating (10 ng/mL) and inhibiting (1 and 20 μg/mL) concentrations of FVIII in the presence of medium (control) or 100 ng/mL CpG-ODN (CpG). Newly formed anti-FVIII ASC were detected by ELISPOT assay after 6 days of culture. Arithmetic means of a representative experiment are presented. Medium control without FVIII (○); 10 ng/mL FVIII (●); 1 μg/mL FVIII (▴); and 20 μg/mL FVIII (▵).
Ig isotypes and IgG subclasses of anti-FVIII ASC in vitro differentiated in the presence of FVIII and 100 μg/mL CpG-ODN. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated with stimulating (10 ng/mL) and inhibiting (1 and 20 μg/mL) concentrations of FVIII in the presence of medium (control) or 100 ng/mL CpG-ODN (CpG). Newly formed anti-FVIII ASC were detected by ELISPOT assay after 6 days of culture. Arithmetic means of a representative experiment are presented. Medium control without FVIII (○); 10 ng/mL FVIII (●); 1 μg/mL FVIII (▴); and 20 μg/mL FVIII (▵).
Cytokine release into culture supernatants during in vitro differentiation of FVIII-specific memory B cells into anti-FVIII ASC. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated with stimulating (10 ng/mL) and inhibiting (1 and 20 μg/mL) concentrations of FVIII in the presence of medium (control), 100 ng/mL, or 1000 ng/mL CpG-ODN. Culture supernatants were taken after 6 days of culture and analyzed for cytokines. Arithmetic means of 2-5 experiments are presented. Medium control without FVIII (○); 10 ng/mL FVIII (●); 1 μg/mL FVIII (▴); and 20 μg/mL FVIII (◇).
Cytokine release into culture supernatants during in vitro differentiation of FVIII-specific memory B cells into anti-FVIII ASC. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated with stimulating (10 ng/mL) and inhibiting (1 and 20 μg/mL) concentrations of FVIII in the presence of medium (control), 100 ng/mL, or 1000 ng/mL CpG-ODN. Culture supernatants were taken after 6 days of culture and analyzed for cytokines. Arithmetic means of 2-5 experiments are presented. Medium control without FVIII (○); 10 ng/mL FVIII (●); 1 μg/mL FVIII (▴); and 20 μg/mL FVIII (◇).
CpG-ODN lost its stimulatory potential at high concentrations (1000 and 10 000 ng/mL). It inhibited FVIII-specific memory responses at stimulatory concentrations (10 ng/mL) of human FVIII and was no longer able to counter the inhibition of memory responses at inhibitory concentrations (1 and 20 μg/mL) of human FVIII (Figure 1). Analysis of cytokines secreted into cell culture supernatants during differentiation into ASCs revealed a down-regulation of IL-4, a down-regulation of IFN-γ, no change in IL-10, and an up-regulation of IL-6 when effects of 1000 ng/mL CpG-ODN were compared with those of 100 ng/mL CpG-ODN (Figure 3). Thus, CpG-ODN seemed to exert a dual function, either stimulatory or inhibitory, depending on the concentration applied.
Both positive and negative immunomodulatory effects of CpG-ODN are because of specific interactions with TLR9
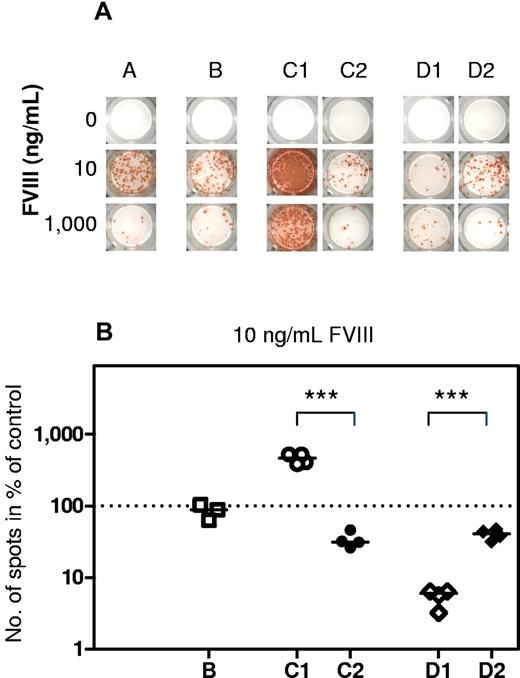
The question arose whether inhibitory effects of high concentrations of CpG-ODN were because of specific interactions with TLR9 or caused by unspecific (eg, toxic) effects. To address this question, we used a TLR9-blocking agent that prevented the binding of CpG-ODN to TLR9. If inhibitory effects of high-dose CpG-ODN were because of specific interactions with TLR9, the addition of a TLR9-blocking agent should prevent these effects. Our results indicated that this was, indeed, the case. The addition of the TLR9-blocking agent prevented both the amplifying effect of low-dose and the inhibitory effect of high-dose CpG-ODN. Furthermore, a negative control DNA (GpC-DNA) did not show any modulating activities (Figure 4A-B). The control DNA had a similar sequence to CpG-ODN, but the CpG motifs were replaced by GpC sequences, which do not have agonistic activity for TLR9. Thus, the biphasic immunomodulatory effect of CpG-ODN was because of specific interactions with TLR9.
Both stimulatory and inhibitory activities of CpG-ODN are caused by specific interactions with TLR9. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng FVIII and restimulated in vitro with stimulating (10 ng/mL) and inhibiting (1000 ng/mL) concentrations of FVIII in the presence of CpG-ODN or controls. Newly formed anti-FVIII ASC were detected by ELISPOT assay after 6 days of culture. (A) Representative ELISPOT assay. Each spot represents one anti-FVIII ASC. Cells were differentiated in the presence of no FVIII (0), 10 ng/mL FVIII (10), or 1000 ng/mL FVIII (1000). The differentiation cultures were supplemented with (A) medium only; (B) 100 ng/mL GpC-ODN (negative control of CpG-ODN); (C1) 100 ng/mL CpG-ODN only; (C2) 100 ng/mL CpG-ODN together with TLR9-blocking agent; (D1) 1000 ng/mL CpG-ODN only; and (D2) 1000 ng/mL CpG-ODN together with TLR9-blocking agent. (B) Quantitative evaluation of results presented in 4A for 10 ng/mL FVIII. Results of cultures differentiated in the presence of FVIII only (A) were set to 100% and presented as a dotted line. (B) One hundred ng/mL GpC-ODN (negative control of CpG-ODN); (C1) 100 ng/mL CpG-ODN only; (C2) 100 ng/mL CpG-ODN together with TLR9-blocking agent; (D1) 1000 ng/mL CpG-ODN only; and (D2) 1000 ng/mL CpG-ODN together with TLR9-blocking agent. Results of individual ELISPOT analyses and the median of all individual results for each group are presented. ***P < .001.
Both stimulatory and inhibitory activities of CpG-ODN are caused by specific interactions with TLR9. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng FVIII and restimulated in vitro with stimulating (10 ng/mL) and inhibiting (1000 ng/mL) concentrations of FVIII in the presence of CpG-ODN or controls. Newly formed anti-FVIII ASC were detected by ELISPOT assay after 6 days of culture. (A) Representative ELISPOT assay. Each spot represents one anti-FVIII ASC. Cells were differentiated in the presence of no FVIII (0), 10 ng/mL FVIII (10), or 1000 ng/mL FVIII (1000). The differentiation cultures were supplemented with (A) medium only; (B) 100 ng/mL GpC-ODN (negative control of CpG-ODN); (C1) 100 ng/mL CpG-ODN only; (C2) 100 ng/mL CpG-ODN together with TLR9-blocking agent; (D1) 1000 ng/mL CpG-ODN only; and (D2) 1000 ng/mL CpG-ODN together with TLR9-blocking agent. (B) Quantitative evaluation of results presented in 4A for 10 ng/mL FVIII. Results of cultures differentiated in the presence of FVIII only (A) were set to 100% and presented as a dotted line. (B) One hundred ng/mL GpC-ODN (negative control of CpG-ODN); (C1) 100 ng/mL CpG-ODN only; (C2) 100 ng/mL CpG-ODN together with TLR9-blocking agent; (D1) 1000 ng/mL CpG-ODN only; and (D2) 1000 ng/mL CpG-ODN together with TLR9-blocking agent. Results of individual ELISPOT analyses and the median of all individual results for each group are presented. ***P < .001.
CpG-ODN rescues FVIII-specific memory responses suppressed by inhibitory concentrations of human FVIII, even when added 24 hours after FVIII
We showed that CpG-ODN at concentrations of 100 ng/mL countered the inhibition of FVIII-specific memory responses by inhibitory concentrations (1 and 20 μg/mL) of human FVIII. The question arose if CpG-ODN could rescue FVIII-specific memory responses only when added together with inhibitory concentrations of human FVIII or, also, when added at later time points. Results presented in Figure 5 demonstrate that CpG-ODN could counter the inhibition of FVIII-specific memory responses, both when added together with inhibitory concentrations (20 μg/mL) of FVIII and when added 24 hours after the same concentration of FVIII. CpG-ODN could rescue FVIII-specific memory responses, to some degree, even when added 48 hours after the addition of inhibitory concentrations of FVIII. However, no rescue was possible at later time points. For comparison, we tested the ability of CpG-ODN to amplify FVIII-specific memory responses by administering it together with, or after stimulation of CD138− spleen cell cultures with FVIII at 10 ng/mL. Our results indicate that CpG-ODN amplified the response at all time points investigated. However, the absolute number of FVIII-specific ASCs decreased between days 6 and 11 after the initiation of memory B-cell restimulation, which was probably because of the limited viability of ASCs differentiated from FVIII-specific memory B cells in vitro and reflects the limit of the in vitro system (Figure 5). In conclusion, CpG-ODN, at 100 ng/mL, abrogated the inhibitory effect of inhibitory concentrations (20 μg/mL) of FVIII, even when added up to 2 days after the addition of FVIII and amplified the stimulatory effect of low concentrations (10 ng/mL) of FVIII at all time points investigated.
Immunomodulatory activity of CpG-ODN in vitro when added with a time delay. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and in vitro restimulated with either an inhibiting concentration (20 μg/mL) of FVIII (A and B) or a stimulating concentration (10 ng/mL) of FVIII (C and D). Then, 100 ng/mL CpG-ODN or medium were added either together with FVIII on day 0 or at different times (days 1-5) after FVIII. Newly differentiated anti-FVIII ASCs were analyzed by ELISPOT assay 6 days after the addition of CpG-ODN. All results were normalized in relation to results obtained with 10 ng/mL FVIII without CpG-ODN (0/6), which was set to 100%. Arithmetic means of a representative experiment are presented. (A) Twenty μg/mL FVIII without CpG-ODN; (B) 20 μg/mL FVIII + 100 ng/mL CpG-ODN; (C) 10 ng/mL FVIII without CpG-ODN; and (D) 10 ng/mL FVIII + 100 ng/mL CpG-ODN.
Immunomodulatory activity of CpG-ODN in vitro when added with a time delay. CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and in vitro restimulated with either an inhibiting concentration (20 μg/mL) of FVIII (A and B) or a stimulating concentration (10 ng/mL) of FVIII (C and D). Then, 100 ng/mL CpG-ODN or medium were added either together with FVIII on day 0 or at different times (days 1-5) after FVIII. Newly differentiated anti-FVIII ASCs were analyzed by ELISPOT assay 6 days after the addition of CpG-ODN. All results were normalized in relation to results obtained with 10 ng/mL FVIII without CpG-ODN (0/6), which was set to 100%. Arithmetic means of a representative experiment are presented. (A) Twenty μg/mL FVIII without CpG-ODN; (B) 20 μg/mL FVIII + 100 ng/mL CpG-ODN; (C) 10 ng/mL FVIII without CpG-ODN; and (D) 10 ng/mL FVIII + 100 ng/mL CpG-ODN.
Modulation of FVIII-specific antibody responses by CpG-ODN in vivo
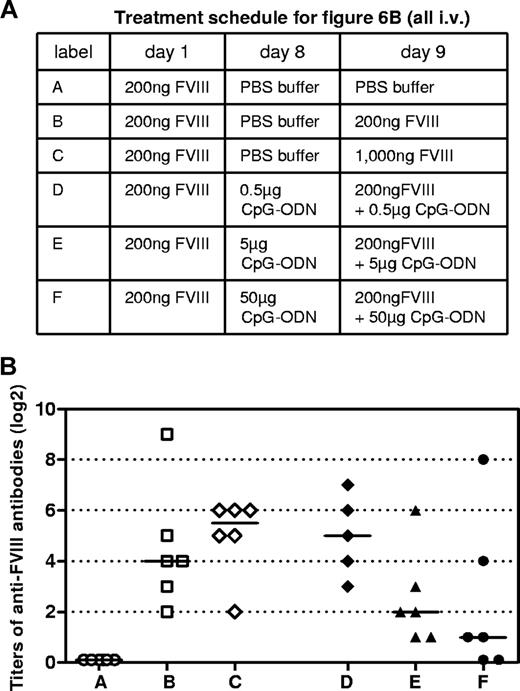
Based on the data obtained in vitro, we asked whether the positive and negative regulatory effects of CpG-ODN on FVIII-specific immune responses would also be observed in vivo. Hemophilic mice were treated intravenously with 1 dose of 200 ng of human FVIII, which does not induce detectable levels of circulating anti-FVIII antibodies, but primes the immune system for further exposure to FVIII.24 One week after the first dose of FVIII, mice were treated with either PBS buffer (negative control) or with different doses of CpG-ODN (0.5, 5, or 50 μg). On the following day, mice received either PBS buffer (negative control), FVIII only (200 or 1000 ng, with both doses have been previously shown to stimulate FVIII-specific antibody responses), or FVIII at a dose of 200 ng, together with different doses of CpG-ODN. Both intraperitoneal and intravenous applications of CpG-ODN were tested. Mice boosted with 200 ng of FVIII only developed detectable levels of circulating anti-FVIII antibodies. The antibody response was amplified when mice were boosted with 1000 ng of FVIII (Figure 6B). No modulation of anti-FVIII antibody responses was observed when CpG-ODN was given intraperitoneally (data not shown). However, a dose-dependent immunomodulatory effect became apparent when CpG-ODN was given intravenously, and 50 μg of CpG-ODN inhibited FVIII-specific antibody responses in 4 of 6 hemophilic mice treated. In addition, 5 μg of CpG-ODN still inhibited FVIII-specific antibody responses, but the inhibitory effect was less pronounced than after 50 μg. Lower doses (0.5 μg) of CpG-ODN slightly amplified the FVIII-specific antibody response (Figure 6B). However, this amplification did not reach statistical significance (compare rows B and D in Figure 6B). Doses of CpG-ODN of 0.05 and 0.005 μg did not induce any immunomodulatory effect (data not shown). These results demonstrate that the inhibitory effect of high-dose CpG-ODN on FVIII-specific antibody responses can be clearly seen not only in vitro, but also in vivo. However, the stimulatory effect of low-dose CpG-ODN seemed less pronounced in vivo than that observed in vitro (compare Figures 1 and 6B). The lack of statistically significant amplification of FVIII-specific antibody responses by low doses of CpG-ODN in vivo could have been because of a rapid degradation of CpG-ODN after intravenous application, which has been previously described.27
Modulation of anti-FVIII immune response by CpG-ODN in vivo. Hemophilic mice were treated intravenously with 1 dose of 200 ng of FVIII on day 1 to prime the immune system for further exposure to FVIII. On day 8, mice received either buffer or CpG-ODN to stimulate the innate immune system. On day 9, mice received either PBS buffer, FVIII, or FVIII together with different doses of CpG-ODN. On day 16, blood samples were taken for the analysis of circulating anti-FVIII antibodies. (A) Treatment schedule. (B) Titers of anti-FVIII antibodies together with medians for each group. Each point represents the results of an individual mouse. Results presented were confirmed in 2 independent experiments.
Modulation of anti-FVIII immune response by CpG-ODN in vivo. Hemophilic mice were treated intravenously with 1 dose of 200 ng of FVIII on day 1 to prime the immune system for further exposure to FVIII. On day 8, mice received either buffer or CpG-ODN to stimulate the innate immune system. On day 9, mice received either PBS buffer, FVIII, or FVIII together with different doses of CpG-ODN. On day 16, blood samples were taken for the analysis of circulating anti-FVIII antibodies. (A) Treatment schedule. (B) Titers of anti-FVIII antibodies together with medians for each group. Each point represents the results of an individual mouse. Results presented were confirmed in 2 independent experiments.
CpG-ODN in the absence of FVIII does not induce differentiation of FVIII-specific memory B cells
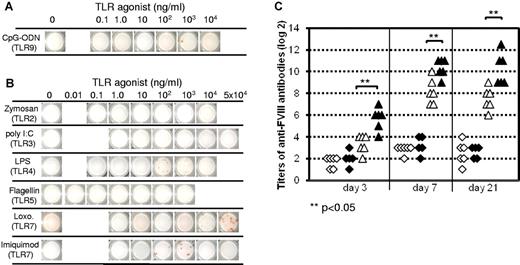
Previous reports suggested that memory B cells become activated to differentiate into ASCs by TLR agonists in the absence of antigen.17,20 Based on these results, one could speculate that FVIII-specific memory B cells in patients might become activated during viral or bacterial infections in the absence of any replacement therapy with FVIII-containing products. Therefore, we asked whether FVIII-specific memory B cells could be restimulated by CpG-ODN in the absence of FVIII. We tested a range of concentrations of CpG-ODN using the CD138− spleen cell-culture system. We did not see an induction of memory B-cell differentiation at any of the concentrations tested (Figure 7A). In some experiments, we observed a few spots in the ELISPOT assay. However, this was also occasionally seen in the medium controls. Therefore, we conclude that CpG-ODN either does not stimulate the differentiation of FVIII-specific memory B cells in the absence of FVIII or only stimulates the differentiation to a limited extent that cannot be clearly differentiated from the background signals.
Restimulation of FVIII-specific memory responses by TLR agonists in the absence of FVIII in vitro and in vivo. (A-B) CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated in vitro with CpG-ODN (A) or ligands for TLRs 2, 3, 4, 5, and 7 (B) in the absence of FVIII (Loxo. = loxoribine). Newly formed anti-FVIII ASCs were detected by ELISPOT assay after 6 days of culture. Each spot represents one anti-FVIII ASC. Concentrations of CpG-ODN (A) and ligands for TLRs 2, 3, 4, 5, and 7 (B) are indicated. Representative ELISPOTs are presented. (C) CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and intravenously injected into naive hemophilic mice. One day after cell transfer, mice were injected with a single dose of PBS buffer (◇), PBS + 1000 μg of loxoribine (♦), 200 ng of FVIII (▵), or 200 ng of FVIII + 1000 μg of loxoribine (▴). TLR ligands were given intraperitoneally, PBS buffer and FVIII were given intravenously and blood samples were taken 3, 7, and 21 days after application, for the analysis of circulating anti-FVIII antibodies. Each point represents the results of an individual mouse. **P < .05.
Restimulation of FVIII-specific memory responses by TLR agonists in the absence of FVIII in vitro and in vivo. (A-B) CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and restimulated in vitro with CpG-ODN (A) or ligands for TLRs 2, 3, 4, 5, and 7 (B) in the absence of FVIII (Loxo. = loxoribine). Newly formed anti-FVIII ASCs were detected by ELISPOT assay after 6 days of culture. Each spot represents one anti-FVIII ASC. Concentrations of CpG-ODN (A) and ligands for TLRs 2, 3, 4, 5, and 7 (B) are indicated. Representative ELISPOTs are presented. (C) CD138− spleen cells were obtained from hemophilic mice treated with 4 weekly doses of 200 ng of FVIII and intravenously injected into naive hemophilic mice. One day after cell transfer, mice were injected with a single dose of PBS buffer (◇), PBS + 1000 μg of loxoribine (♦), 200 ng of FVIII (▵), or 200 ng of FVIII + 1000 μg of loxoribine (▴). TLR ligands were given intraperitoneally, PBS buffer and FVIII were given intravenously and blood samples were taken 3, 7, and 21 days after application, for the analysis of circulating anti-FVIII antibodies. Each point represents the results of an individual mouse. **P < .05.
We next asked whether other TLR ligands could restimulate FVIII-specific memory B cells in the absence of FVIII. Our results indicate that only ligands for TLR4 and TLR7 induced weak positive signals, and this was only occasionally (Figure 7B). We then asked whether we would observe any restimulation of FVIII-specific memory B cells by TLR agonists in the absence of FVIII in vivo. We used 2 different methods for this purpose. In the first set of experiments, we treated hemophilic mice with 1 dose of 200 ng of human FVIII to prime FVIII-specific antibody responses and treated them with different doses of TLR agonists 1 week after dosing with FVIII. We did not see any detectable levels of anti-FVIII antibodies in the circulation (data not shown). In the second set of experiments, we treated hemophilic mice with 4 intravenous doses of 200 ng of human FVIII and isolated CD138− spleen cells containing FVIII-specific memory cells as previously described.7,8 We transferred the CD138− spleen cells into naïve hemophilic mice and treated mice 1 day after transfer with either of the following: PBS buffer (negative control), 200 ng of human FVIII only, loxoribine (TLR7 agonists that had induced weak effects in vitro) only, 200 ng of human FVIII plus loxoribine. We measured anti-FVIII antibodies in the circulation 3, 7, and 21 days after treatment. The presence of anti-FVIII antibodies in the circulation indicated the restimulation and differentiation of transferred FVIII-specific memory B cells into anti-FVIII ASCs. Loxoribine amplified the restimulation of FVIII-specific memory responses in the presence of FVIII, but did not stimulate any memory responses in the absence of FVIII (Figure 7C). In conclusion, in vivo FVIII-specific memory B cells were not restimulated either by CpG-ODN or by any other TLR ligand tested in the absence of FVIII.
Discussion
Restimulation of FVIII-specific memory B cells is sensitive to increasing doses of FVIII. Low doses restimulate memory B cells and induce their differentiation into anti-FVIII ASC, but high doses inhibit restimulation.8 Here, we asked how stimulation and inhibition of FVIII-specific memory B-cell responses by FVIII are modulated by concurrent activation of the innate immune system through TLR agonists. Initial screening of agonists for TLRs 2, 3, 4, 5, 7, and 9 indicated that all agonists induced some degree of amplification of FVIII-specific memory responses in the presence of stimulating concentrations of human FVIII. Amplification could be because of either direct effects by stimulating TLR expressed on memory B cells or indirect effects by stimulating TLR expressed on other immune cells, such as macrophages, dendritic cells, or lymphocytes. TLR activation initiates intracellular signaling pathways16,28 that induce the expression of genes encoding proinflammatory cytokines (eg, IL-1, IL-6, tumor necrosis factor-α, and IL-12) or type I interferons. Both were shown to either directly or indirectly affect B-cell responses.29-31 Moreover, TLR signaling induces the up-regulation of maturation markers and costimulatory molecules, such as CD80, CD83, and CD86 on dendritic cells,32 which could amplify the stimulation of CD4+ T cells required for the induction of memory B-cell differentiation. In view of these findings, it is not surprising that all TLR agonists tested modulate FVIII-specific memory responses, to some degree.
Agonists for TLR7 and 9 induced the strongest modulation. They amplified the restimulation of FVIII-specific memory B cells in the presence of stimulatory concentrations of human FVIII and countered the inhibition in the presence of inhibitory concentrations of human FVIII. TLR7 and 9 are expressed in a range of different murine immune cells, for example, dendritic cells, macrophages, naive B cells and memory B cells.33,34 Therefore, agonists for TLR7 and 9 could act on FVIII-specific memory B cells directly and indirectly. Their indirect action could be via triggering TLR7 and 9 expressed in dendritic cells or macrophages, thereby inducing the release of proinflammatory cytokines and type I interferon.
When we tested different concentrations of CpG-ODN, we observed biphasic and concentration-dependent effects. Whereas 100 ng/mL amplified the memory response, 1000 ng/mL and 10 000 ng/mL inhibited the response. Both stimulation and inhibition of memory responses were because of specific interactions with TLR9. Whereas the inhibitory effects of high-dose CpG-ODN could be clearly demonstrated both in vitro and in vivo, the stimulatory effect of low doses of CpG-ODN in vivo was rather weak, compared with the effect seen in vitro. In fact, the stimulatory effect in vivo did not reach statistical significance. This might be because of rapid degradation of CpG-ODN or ineffective delivery into intracellular compartments of TLR9-expressing cells in vivo, which has been previously described.27 Previous studies have shown that CpG-ODN and antigen (human FVIII in our study) need to be delivered to the same antigen-presenting cell to express the full stimulatory activity. Different strategies for in vivo codelivery of antigen and CpG-ODN were developed,27,35 but it would have been beyond the scope of this study to develop such codelivery systems for FVIII and CpG-ODN.
The amplification of memory B-cell restimulation in vitro was associated with a down-regulation of IL-4 and an up-regulation of IFN-γ in cell-culture supernatants, which suggests a predominant amplification of a Th1-type immune response by CpG-ODN and confirms data published by other groups using different systems.35
The inhibitory effect of high-dose CpG-ODN raises a question about the mechanism responsible for this effect. Recent data suggested that CpG-ODN given systemically at high doses induces the enzyme indoleamine 2,3-dioxygenase (IDO) in immune cells, particularly in dendritic cells.36-38 IDO expresses immunosuppressive activities caused by both tryptophan deprivation and the production of kynurenines, which act on IDO− dendritic cells and render an otherwise stimulatory dendritic cell capable of regulatory effects.39 IDO+ dendritic cells could induce regulatory T cells that would prevent the activation of CD4+ T cells required for the restimulation of FVIII-specific memory B cells. Our in vitro data show that high-dose CpG-ODN induced a reduction of IFN-γ release into culture supernatants, which indicates a decreased activation of Th1 cells and hints at an inhibition of memory B-cell differentiation because of an inhibition of CD4+ T-cell activation. In addition, negative regulators of TLR signaling could contribute to the inhibitory effects observed with high-dose CpG-ODN. A range of intracellular-signaling molecules have been described as negative regulators of TLR signaling40,41 and could therefore contribute to the negative regulatory effects of high doses of CpG-ODN. However, our in vitro cytokine release data revealed an amplification of IL-6 release in cultures supplemented with inhibitory concentrations of CpG-ODN. IL-6 is one of the major indicators of the stimulation of TLR-triggered signal-transduction pathways, which would argue against an inhibition of these pathways by negative regulators.
Alternatively, inhibition of immune responses could be mediated by the induction of IL-10, an immunoregulatory cytokine that has been shown to limit CpG responses.42 However, our in vitro cytokine-release data show an increase in IL-10 release at stimulatory concentrations of CpG-ODN, but no further increase at inhibitory concentrations of CpG-ODN. Therefore, at least in vitro, it seems unlikely that IL-10 is involved in the inhibitory effect of high-dose CpG-ODN.
The exact mechanisms responsible for the inhibition of FVIII-specific memory responses by high-dose CpG-ODN are currently being investigated.
CpG-ODN at stimulatory concentration of 100 ng/mL not only amplified the restimulation of FVIII-specific memory B cells in the presence of stimulatory concentrations of human FVIII, but also countered the inhibition caused by high concentrations of FVIII. The exact mechanisms responsible for the inhibitory effect of high concentrations of FVIII have not been comprehensively studied. However, we previously reported that a pan-caspase inhibitor prevented the inhibitory effects, which indicates that inhibition involves the induction of apoptosis.8 The question arises whether triggering of TLR9 by CpG-ODN might generate survival signals for FVIII-specific memory B cells that prevent the induction of apoptosis by high concentrations of FVIII. CpG-ODN has been shown to protect B cells, macrophages, and plasmacytoid dendritic cells against apoptosis.43,44 Recently, Kuo et al demonstrated that CpG-ODN up-regulates Hsp90-β in a TLR9/MyD88/phosphatidylinositol 3-kinase–dependent pathway. Furthermore, they provided evidence that CpG-ODN induces its antiapoptotic effect by stimulating the binding of Hsp90-β to B-cell lymphoma-2 (Bcl-2), thereby increasing the antiapoptotic activity of B-cell lymphoma-2, namely, the inhibition of cytochrome c release and the prevention of caspase-3 activation.45 These findings could explain why CpG-ODN counters the inhibition of FVIII-specific memory B-cell differentiation by high concentrations of FVIII. In conclusion, one would expect that an inhibition of memory B-cell differentiation by high doses of FVIII in vivo could be countered by microbial infections that would trigger TLR9 in these cells. However, the outcome of such a scenario would probably depend on the strength of signals that are induced by high-dose FVIII on the one hand and TLR9 agonists on the other hand.
Despite its strong immunostimulatory activity in the presence of FVIII, CpG-ODN induced little restimulation in the absence of FVIII. The number of anti-FVIII ASCs after restimulation of CD138− spleen cell cultures with stimulatory concentrations of CpG-ODN in the absence of FVIII in vitro was similar to, or slightly above, the background of negative control cultures. Furthermore, we never observed any restimulation of FVIII-specific memory responses in the absence of FVIII in vivo, either after intravenous or after intraperitoneal application of CpG-ODN. For comparison, we tested agonists for TLRs 2, 3, 4, 5, and 7 in vitro and agonists for TLR 7 in vivo and came to similar conclusions. Although we observed weak effects in the absence of FVIII when we tested agonists for TLR4 and 7 in vitro, we never observed any in vivo effects in the absence of FVIII. These results contrast with the findings of Bernasconi et al in the human system.17 The authors demonstrated that human memory B cells differentiate into plasma cells in response to polyclonal stimuli, such as bystander T-cell help or CpG-ODN in vitro. Furthermore, they showed that antibodies to recall antigens are produced in vivo, even years after antigenic stimulation. Based on their results, the authors hypothesized that quiescent memory B cells are periodically activated by TLR agonists or bystander T-cell help to undergo self-renewal and differentiate into ASCs in the absence of antigen. However, our results agree with recent results reported by Benson et al, who demonstrated that murine memory B cells neither clonally expand nor differentiate into ASCs in response to inflammatory stimuli, such as TLR agonists, polyclonal T-cell activation, protein vaccination, or even acute vaccinia virus infection in the absence of a specific antigen in vivo.46 Several additional studies in mice and humans provided data that could be interpreted either in favor of47 or against48-50 the idea that memory B cells respond to bystander inflammatory signals in the absence of the specific antigen. Clearly, our data do not support this theory. However, we cannot exclude the possibility of other inflammatory signals, which were not included in our study, that would be able to restimulate FVIII-specific memory B cells in the absence of FVIII.
Summarizing our data, we conclude that TLR agonists, in particular agonists for TLR7 and 9, can modulate the outcome of an anamnestic antibody response against FVIII. Depending on the actual conditions, this modulation can cause amplification or inhibition of the antibody response. However, it is difficult to predict whether natural infections would induce local concentrations of TLR agonists sufficient to induce inhibition of antibody responses.
Furthermore, we conclude that, at least in the murine system, it is unlikely that FVIII-specific memory B cells are restimulated by TLR agonists in the absence of FVIII. Future studies will show if this conclusion can be extended to the human immune system in patients where it would be relevant for both natural infections and vaccinations.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Elisabeth Hopfner, Nidha Abrar, Monika Grewal, and Markus Weiller for technical assistance and to John-Philip Lawo for statistical analysis. We also thank Elise Langdon-Neuner for editing the manuscript and Pauline van Helden for critical discussion.
This study was supported by Baxter BioScience.
Authorship
Contribution: P.A. designed research, did most in vitro analysis, analyzed and interpreted data, and wrote the manuscript; C.K.B. designed research and analyzed and interpreted data; A.G.P. designed research and analyzed and interpreted data; R.U.A. designed, supervised, and performed animal experiments; H.P.S. interpreted data; and B.M.R. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: P.A., A.G.P., R.U.A., H.P.S., and B.M.R. are employees of Baxter BioScience. C.K.B. was an employee of Baxter BioScience at the time when she contributed to this study.
The current address of C.K.B. is Blood Center of Wisconsin, Blood Research Institute, Milwaukee, WI.
Correspondence: Birgit M. Reipert, Baxter BioScience, Industriestrasse 72, A-1220 Vienna, Austria; e-mail: birgit_reipert@baxter.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal