Abstract
Abstract 198
Idiopathic aplastic anemia (AA) is a syndrome characterized by pancytopenia and bone marrow (BM) hypoplasia, which are caused by the auto-immune destruction of hematopoietic stem cells (HSCs). However, little is known about the nature of HSCs that survive the occurrence of autoimmune insults and maintain hematopoiesis both during and after aplastic diathesis. While the severity of the autoimmunity is the major determinant of BM failure in patients with AA, some intrinsic genetic changes in HSCs could also be involved in the disease process, since the clonality of the residual, persistent hematopoiesis under the aplastic state has been well recognized.
To characterize the nature of the HSCs that support hematopoiesis in AA, peripheral blood (PB) specimens obtained from 317 patients with AA were subjected to the genome-wide analysis of genetic lesions using Affymetrix® 500K SNP arrays. For the normal controls, 1746 PB specimens from the Japan Marrow Donation Program (JMDP) were also analyzed. All specimens were genotyped for HLA-A, -B, -C, -DRB1, -DQB1 and -DPB1 alleles. In the eligible cases, PB leukocytes and CD34+ BM cells were also examined to determine their expression of HLA-A antigens using allele-specific monoclonal antibodies by flow cytometry (FCM). To identity the common HLA types associated with AA, a total of 6,629 registries from JMDP who had received allogeneic bone marrow transplantation between 1992 and 2008 were employed, where the HLA frequencies in AA (N=406) were compared with those among other hematopoietic disorders, in acute myeloid leukemia (N=1,822), acute lymphocytic leukemia (N=1,406), chronic myeloid leukemia (N=1,014), myelodysplastic syndromes (N=824), non-Hodgkin's lymphoma (N=565), and other neoplastic disorders (N=392).
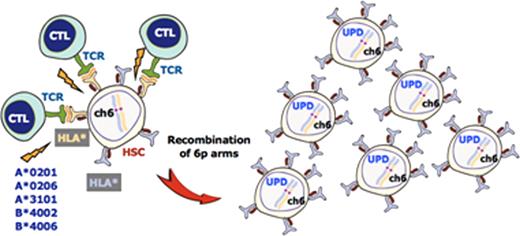
A number of genetic alterations were detected in our AA case series, among which the most conspicuous was acquired uniparental disomy (UPD), or the copy number-neutral loss of heterozygosity, involving the 6p arms (6pUPD). The 6pUPD was identified in 38 patients (12%) but not in any JMDP donor specimens, and it commonly affected the HLA locus, which was expected to result in the loss of one HLA haplotype. In fact, loss of HLA-A expression from the missing haplotype was confirmed by FCM in all 13 6pUPD-positive cases thus far tested, whereas the HLA-A expression from both haplotypes was preserved in the 58 samples without 6pUPD. The loss of HLA-A expression in the 6pUPD-positive cases was found in multiple lineages of leukocytes, including granulocytes, monocytes, B cells, BM CD34+ cells, and to a lesser extent in T cells. Of particular interest was the fact that the missing HLA haplotypes that were predicted from the SNP array data were extremely biased to particular class I HLA alleles, including HLA-A*02:01, HLA-A*02:06, A*31:01, B*40:02, and B*40:06. Moreover, when the frequencies of these alleles were compared among the 6,629 JMDP registries, they were shown to be strongly associated with AA in comparison to other non-significant HLA alleles, where the odds ratios for these alleles with regard to non-significant alleles as for the risk of the development of AA were 2.00 (95%CI; 1.52 – 2.62) for A*02:01, 2.37 (95%CI; 1.80 – 3.12) for A*02:06, 1.46 (95%CI; 1.06 – 2.02) for A*31:01, 2.07 (1.56 - 2.77) for B*40:02, and 2.67 (1.95 - 3.66) for B*40:06.
AA patients frequently show permissive hematopoiesis with 6pUPD, which is thought to develop due to the occurrence of auto-immune insult based on the Darwinian principle of “survival of the fittest” (Figure). The tight association of AA with particular class I antigens that are invariably missing in permissive hematopoiesis with 6pUPD strongly supports the hypothesis that the auto-immunity responsible for AA is primarily mediated by cytotoxic T cells which target a relatively limited species of auto-antigens presented on HSCs through these relevant HLAs. Our findings therefore also provide a solid basis for isolating the target auto-antigens responsible for the development of AA in the future studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal