Abstract
A better understanding of apoptotic signaling in B-chronic lymphocytic leukemia (B-CLL) cells may help to define new therapeutic strategies. This study investigated endoplasmic reticulum (ER) stress signaling in spontaneous apoptosis of B-CLL cells and whether manipulating ER stress increases their apoptosis. Results show that a novel ER stress-triggered caspase cascade, initiated by caspase-4 and involving caspase-8 and -3, plays an important role in spontaneous B-CLL cell apoptosis. ER stress-induced apoptosis in B-CLL cells also involves CHOP/GADD153 up-regulation, increased JNK1/2 phosphorylation, and caspase-8–mediated cleavage of Bap31 to Bap20, known to propagate apoptotic signals from ER to mitochondria. In ex vivo B-CLL cells, some apoptotic events associated with mitochondrial pathway also occur, including mitochondrial cytochrome c release and caspase-9 processing. However, pharmacologic inhibition studies show that caspase-9 plays a minor role in B-CLL cell apoptosis. ER stress also triggers survival signals in B-CLL cells by increasing BiP/GRP78 expression. Manipulating ER signaling by siRNA down-regulation of BiP/GRP78 or treating B-CLL cells with 2 well-known ER stress-inducers, tunicamycin and thapsigargin, increases their apoptosis. Overall, our findings show that ER triggers an essential pathway for B-CLL cell apoptosis and suggest that genetic and pharmacologic manipulation of ER signaling could represent an important therapeutic strategy.
Introduction
B-chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of malignant B CD5+ cells, refractory to both physiologic and chemotherapy-induced apoptosis.1 Despite advances in treatments, B-CLL remains incurable, warranting further efforts to define novel therapeutic strategies.
Apoptosis-based anticancer therapies require an in-depth understanding of the apoptotic machinery components of malignant cells, not only apoptotic regulators but also apoptotic executioners, given that their expression levels and functional integrity strongly determine the efficacy of therapeutic treatments.2,3
Apoptosis is mediated by caspases, a conserved family of aspartate-specific cysteine proteases that are activated by several pathways.4 The intrinsic mitochondrial pathway relies on caspase-9 as initiator caspase and is controlled by Bcl-2 family members.5 The extrinsic pathway relies on caspase-8 and -10 as initiator caspases, is triggered by direct engagement of cell-surface death receptors, and can either converge on mitochondria or circumvent mitochondria to directly activate effector caspases.6 Recently, increasing evidence has identified another intrinsic apoptotic pathway regulated by the endoplasmic reticulum (ER).7,8 Besides controlling Ca2+ homeostasis and protein trafficking, ER senses stress stimuli that lead to unfolded protein accumulation, triggering a signaling cascade termed unfolded protein response (UPR).9 UPR is mediated by the activation of 3 ER transmembrane receptors, pancreatic ER kinase-like kinase, activating transcription factor 6, and inositol-requiring enzyme-1, which under physiologic conditions are maintained in an inactive state by the ER chaperone Binding Immunoglobulin Protein/Glucose-regulated protein78 (BiP/GRP78). UPR is initially a self-protective response aimed at restoring the normal ER function and maintaining cell survival, with BiP/GRP78 playing a key role as prosurvival factor.8,10 However, when ER stress is severe or prolonged, it overrides the salvage mechanisms of the initial UPR leading to apoptosis.7-9 Several mechanisms link ER stress to apoptosis, including a direct caspase activation, caspase-4 in humans11 and caspase-12 in mice,12 which can initiate a specific caspase cascade independent of mitochondria, and a cross-talk with the mitochondria mediated by various factors, including Ca2+, Bcl-2 family proteins, and stress-associated kinases.13-15 Because tumor cells can adapt to ER stress stimuli by triggering an altered protective UPR,16,17 specific targeting of the prosurvival UPR molecules or the use of agents able to induce an ER stress-mediated apoptosis may contrast tumor development and growth, thus defining new types of cancer therapy. In this context, ER has been proposed as a critical target for anticancer therapy in several tumor types, both solid and hematologic.8,16-19 Apoptosis induction mediated by ER stress-inducing agents is just beginning to be exploited also in B-CLL cells. Recent studies have shown that brefeldin A, an inhibitor of ER/Golgi protein transport, Tetrocarcin A, an antibiotic isolated from actinomycetes, and Xanthohumol, a plant-derived flavonoid, increase B-CLL cell apoptosis by different ER stress-mediated mechanisms.20-22 Considering that B-CLL cells resist chemostimulation of their mitochondrial apoptotic machinery because of high levels of antiapoptotic Bcl-2 family proteins23 and that the extrinsic apoptosis pathway is defective in these cells because of the low expression of the death receptors,24,25 an in-depth dissection of the apoptotic signaling pathways that, in B-CLL cells, are associated with ER stress may provide new important information about the therapeutic implications of ER in this leukemia.
Based on these considerations, we investigated ER stress signaling in spontaneous B-CLL cell apoptosis and whether genetic and pharmacologic manipulation of ER stress affects B-CLL cell survival. Results show that a novel ER stress-induced signaling, triggered by caspase-4 and involving caspase-8 and -3, plays an important role in spontaneous B-CLL cell apoptosis. ER stress also elicits survival signals in B-CLL cells undergoing spontaneous apoptosis, as suggested by the increased expression of the pro-survival UPR component BiP/GRP78. Furthermore, specific down-regulation by small-interfering RNA (siRNA) of BiP/GRP78, and also treating B-CLL cells with tunicamycin (an inhibitor of N-glycosylation) or thapsigargin (an inhibitor of ER Ca2+-ATPase) increases B-CLL cell apoptosis.
Methods
Patients
Twenty-three B-CLL patients entered this study. Diagnoses of B-CLL were based on Stanford criteria defined by the National Cancer Institute-sponsored Working Group,26 and clinical staging was based on the Binet classification.27 This study was approved by the Perugia University Institutional Review Board, and all patients signed informed consent in accordance with the Declaration of Helsinki.
B-CLL cell purification
Peripheral blood mononuclear cells were isolated from heparinized blood of B-CLL patients by Ficoll density-gradient centrifugation (Nycomed). Monocytes were removed by plastic adherence, and T cells by rosetting with sheep erythrocytes. All B-CLL samples contained more than 96% CD19+/CD5+ B-CLL cells, as assessed by flow cytometry (EPICS-XL-MCL; Beckman Coulter).
B-CLL clinical laboratory characteristics
To investigate VDJ rearrangement, genomic DNA was amplified using a mixture of sense primers annealing to the VH1 through VH6 FR1 and antisense primers complementary to the germline JH regions.28 For somatic hypermutation analysis, polymerase chain reaction products were gel purified (QIAGEN Gel Purification Kit; QIAGEN) and directly sequenced by the automatic DNA sequencer ABI PRISM 3130 (Applied Biosystems). The National Center for Biotechnology Information Ig Blast database and sequence alignment tool was used to identify and compare sequences with the most homologous germline VH gene family. VH gene sequences deviating more than 2% from the corresponding germline gene were defined mutated. CD38 surface expression was examined using double-immunofluorescence fluorescein isothiocyanate-CD19/phycoerythrin-CD38, and the percentage of CD38+ cells measured in the CD19+ fraction. B-CLL samples were considered CD38+ when the CD38+ cell percentage exceeded 20%. To determine ζ-chain–associated protein 70 (ZAP-70) expression, B-CLL cells were labeled with anti–CD19-Cy5, fixed and permeabilized with the Fix & Perm Kit, and then incubated with anti–ZAP-70 Alexa Fluor 488–conjugated monoclonal antibody (mAb; all from Caltag Laboratories). Flow cytometric analysis was performed following published methods,29 and the cutoff value was set at 20% of ZAP-70+ B-CLL cells.
Table 1 gives clinical laboratory characteristics and the Binet stage of B-CLL patients.
Patient characteristics
| Patient no. . | Binet stage . | Treatment status . | IgVH mutational status* . | ZAP-70 expression† . | CD38 expression‡ . |
|---|---|---|---|---|---|
| B-CLL1 | B | Untreated | Unmutated | + | − |
| B-CLL2 | B | Untreated | Unmutated | + | − |
| B-CLL3 | B | Untreated | Unmutated | + | − |
| B-CLL4 | A | Treated§ | Mutated | − | − |
| B-CLL5 | B | Treated§ | Unmutated | + | − |
| B-CLL6 | B | Untreated | Unmutated | + | + |
| B-CLL7 | B | Treated§ | Unmutated | + | − |
| B-CLL8 | A | Treated§ | Unmutated | + | − |
| B-CLL9 | A | Untreated | Mutated | + | − |
| B-CLL10 | A | Treated§ | Mutated | − | − |
| B-CLL11 | B | Untreated | Unmutated | + | − |
| B-CLL12 | A | Treated§ | Unmutated | + | − |
| B-CLL13 | A | Treated§ | ND | ND | ND |
| B-CLL14 | A | Treated§ | Mutated | + | − |
| B-CLL15 | B | Treated§ | ND | ND | ND |
| B-CLL16 | B | Treated§ | Unmutated | + | + |
| B-CLL17 | A | Untreated | Mutated | + | − |
| B-CLL18 | C | Treated§ | Unmutated | + | + |
| B-CLL19 | C | Treated§ | Unmutated | + | + |
| B-CLL20 | A | Treated§ | Mutated | − | − |
| B-CLL21 | A | Treated§ | Mutated | + | − |
| B-CLL22 | C | Treated§ | Mutated | + | + |
| B-CLL23 | B | Treated§ | Unmutated | − | − |
| Patient no. . | Binet stage . | Treatment status . | IgVH mutational status* . | ZAP-70 expression† . | CD38 expression‡ . |
|---|---|---|---|---|---|
| B-CLL1 | B | Untreated | Unmutated | + | − |
| B-CLL2 | B | Untreated | Unmutated | + | − |
| B-CLL3 | B | Untreated | Unmutated | + | − |
| B-CLL4 | A | Treated§ | Mutated | − | − |
| B-CLL5 | B | Treated§ | Unmutated | + | − |
| B-CLL6 | B | Untreated | Unmutated | + | + |
| B-CLL7 | B | Treated§ | Unmutated | + | − |
| B-CLL8 | A | Treated§ | Unmutated | + | − |
| B-CLL9 | A | Untreated | Mutated | + | − |
| B-CLL10 | A | Treated§ | Mutated | − | − |
| B-CLL11 | B | Untreated | Unmutated | + | − |
| B-CLL12 | A | Treated§ | Unmutated | + | − |
| B-CLL13 | A | Treated§ | ND | ND | ND |
| B-CLL14 | A | Treated§ | Mutated | + | − |
| B-CLL15 | B | Treated§ | ND | ND | ND |
| B-CLL16 | B | Treated§ | Unmutated | + | + |
| B-CLL17 | A | Untreated | Mutated | + | − |
| B-CLL18 | C | Treated§ | Unmutated | + | + |
| B-CLL19 | C | Treated§ | Unmutated | + | + |
| B-CLL20 | A | Treated§ | Mutated | − | − |
| B-CLL21 | A | Treated§ | Mutated | + | − |
| B-CLL22 | C | Treated§ | Mutated | + | + |
| B-CLL23 | B | Treated§ | Unmutated | − | − |
ND indicates not determined.
Mutated was defined as having a frequency of mutations > 2% from germline VH.
Positivity refers to detection of > 20% ZAP-70+/CD19+.
Positivity refers to detection of > 20% CD38+/CD19+.
Patients had not received treatment for at least 3 months before the study.
B-CLL cell culture
Freshly isolated B-CLL cells, resuspended at 2 × 106 cells/mL in complete medium consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Invitrogen), were incubated in culture tubes (3 mL/tube) at 37°C in a 5% CO2 atmosphere. In pharmacologic caspase inhibition experiments, B-CLL cells were cultured for 24 hours with the caspase-3 inhibitor z-DEVD-fmk, the caspase-8 inhibitor z-IETD-fmk, the caspase-9 inhibitor z-LEHD-fmk, the pan-caspase inhibitor z-VAD-fmk (all from Calbiochem), and the caspase-4 inhibitor z-LEVD-fmk (BioVision). In pharmacologic ER stress-induction experiments, B-CLL cells were cultured for 24 hours with tunicamycin or thapsigargin (both from BIOMOL Research Laboratories). Caspase inhibitors and ER stress-inducers were dissolved in dimethyl sulfoxide (DMSO) and further diluted in complete medium at the final concentrations used, which did not exceed 0.01% and did not affect B-CLL cell viability.
Apoptosis assays
Apoptosis was assessed by flow cytometric analysis (EPICS-XL-MCL) of annexin V-fluorescein isothiocyanate/propidium iodide (PI)-stained cells, and hypodiploid nuclei as previously reported.30 Annexin V/PI assay was performed using a commercial kit (Immunotech; Beckman Coulter) according to the manufacturer's instructions.
Whole-cell lysate extraction and subcellular fractionation
Whole-cell lysates were extracted by lysing cells in radioimmunoprecipitation assay buffer. Cytosolic and mitochondria-enriched fractions were prepared by resuspending cells in cytosol extraction buffer (75mM KCl, 1mM NaH2PO4, 8mM Na2HPO4, 250mM sucrose, 50 μg/mL digitonin, 1mM dithiothreitol, 0.1mM phenylmethanesulfonyl fluoride, 1mM benzaminidine, and a protease inhibitor cocktail [Sigma-Aldrich] added at 1:100 dilution). After 10-minute incubation on ice and centrifugation, the supernatants were recovered and used as cytosolic fraction, whereas the cell pellets were resuspended in mitochondrial lysis buffer (100mM NaCl, 10mM MgCl2, 2mM ethyleneglycoltetraacetic acid, 2mM ethylenediaminetetraacetic acid, 1% Igepal, 10% glycerol, 2mM phenylmethanesulfonyl fluoride, 10mM benzaminidine, and a protease inhibitor cocktail added at 1:100 dilution). After 10-minute incubation on ice and centrifugation, the supernatants were recovered and used as mitochondria-enriched fraction.
Western blot analysis
Equal amounts of proteins were separated by 10.0% to 15.0% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Protran, Schleicher & Schuell), which, after blocking, were incubated with the following primary Abs: mAb to caspase-4 (clone 4B9) from Medical & Biologic Laboratories; mAb to cytochrome c (clone 7H8.2C12) from BD Bioscences; mAb to CHOP/GADD153 (B-3) and polyclonal Abs to Bap31 (C-15), BiP/GRP78 (H-129), and Bax (N-20) from Santa Cruz Biotechnology; mAb to caspase-8 (clone 1C12), polyclonal Abs to caspase-3, PARP, phospho-SAPK/JNK (Thr183/Tyr185), and total SAPK/JNK from Cell Signaling Technology; mAbs to caspase-9 (clone 96-2-22) and Smac/DIABLO (clone 78-1-118) from Upstate Biotechnology; polyclonal Ab to Omi/HtrA2 from R&D Systems; mAb to cytochrome oxidase IV subunit II (COX IV; clone 12C4) from Invitrogen; and mAb to β-actin (clone AC-15) from Sigma-Aldrich. Signals were detected using appropriate horseradish peroxidase-conjugated secondary Abs and the enhanced chemiluminescence system (GE Healthcare). Densitometric analysis of CHOP/GADD153, BiP/GRP78 and Bap20 bands was performed using Quantity One software (Bio-Rad) and the relative densitometry units calculated relative to β-actin for each lane.
siRNA nucleofection
B-CLL cells (12 × 106), resuspended in 100 μL Cell Line Solution Kit V (Amaxa) with ON-TARGETplus SMARTpool siRNA to BiP/GRP78 (2μM), caspase-4 (0.5μM), or ON-TARGETplus siCONTROL nontargeting pool as negative control (Dharmacon RNA Technologies), were transfected with the Amaxa Nucleofector II device (program U-013), cultured in 12-well plates in complete medium for 72 hours, and then subjected to Western blot and apoptosis assays.
Statistical analysis
Statistical differences between mean values were evaluated using the Student t test. The minimal level of significance was P less than .05.
Results
Involvement of ER stress-associated signaling in spontaneous B-CLL cell apoptosis
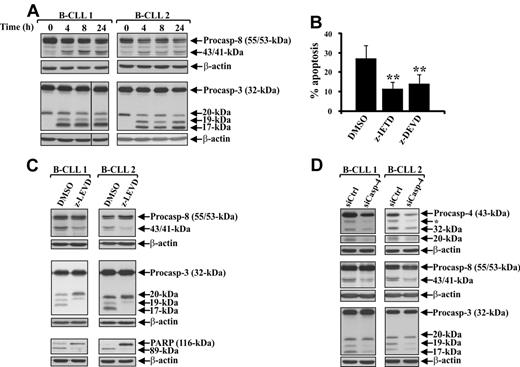
Several mechanisms have been proposed for linking ER stress to apoptosis, including a direct activation of ER-associated caspases, transcription factors, and kinases.11-15 It has been shown that caspases specifically involved in ER stress-induced apoptosis are caspase-4 in humans11 and caspase-12 in mice.12 To investigate the involvement of ER stress-associated signaling in spontaneous B-CLL cell apoptosis, we examined caspase-4 activation in fresh B-CLL cells and after 4-, 8-, and 24-hour culture in complete medium (patients 1-12). Western blot analysis, performed with an mAb able to detect both the proenzyme and the cleavage products, showed that fresh B-CLL cells from all patients expressed high levels of the 43-kDa procaspase-4, which from 4-hour culture onward was cleaved to its active forms, the 32-kDa intermediate and the 20-kDa large subunit (Figure 1A). To determine whether caspase-4 is required for spontaneous B-CLL cell apoptosis, fresh B-CLL cells were cultured for 24 hours with the caspase-4 inhibitor z-LEVD-fmk or DMSO as control, and apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei (patients 1-7). Parallel experiments using the pan-caspase inhibitor z-VAD-fmk were performed to evaluate the overall contribution of caspases in the spontaneous B-CLL cell apoptosis. Figure 1B shows that the mean percentage of hypodiploid nuclei, which was 26.8% plus or minus 7.0% after 24-hour culture in DMSO, was decreased by z-VAD-fmk to 3.9% plus or minus 1.7% (P < .01) and by z-LEVD-fmk to 17.2% plus or minus 5.2% (P < .01), suggesting that spontaneous B-CLL cell apoptosis is mainly caspase-dependent and that caspase-4 is one of the caspases required for spontaneous B-CLL cell apoptosis. To confirm caspase-4 requirement, we down-regulated caspase-4 expression by siRNA nucleofection (patients 1-7). Results demonstrate that B-CLL cells, 72 hours after transfection with caspase-4 siRNA, showed, with respect to control siRNA-transfected cells, a reduction of both caspase-4 proenzyme and its cleavage products (Figure 1C), which resulted in a reduced B-CLL cell apoptosis as indicated by the analysis of annexin V+ cells and hypodiploid nuclei (Figure 1D-E). We next examined ex vivo B-CLL cells (patients 1-12) for the expression of 2 important ER stress regulators, the ER stress-induced proapoptotic transcription factor C/EBP homologous protein/Growth Arrest and DNA Damage 153 (CHOP/GADD153)31 and the pro-survival UPR component BiP/GRP78.8,10 Figure 1F shows that fresh B-CLL cells constitutively expressed both proteins, with BiP/GRP78 expressed at higher levels than CHOP/GADD153, and that the expression of both proteins increased in B-CLL cells undergoing spontaneous apoptosis. It has been shown that another ER stress-induced proapoptotic pathway involves the c-Jun N-terminal protein kinase (JNK), which is activated downstream of the inositol-requiring enzyme-1-TRAF2-ASK1 branch of the UPR.13,15 Analysis of JNK1 (54-kDa)/JNK2 (46-kDa), performed in patients 1 to 12, showed that both isoforms were already phosphorylated in fresh B-CLL cells. JNK1/2 phosphorylation increased after 1 or 3 hours depending on the patients, but always declined after 6-hour culture (Figure 1G). Altogether, these results show that several proapoptotic molecules associated with ER stress apoptosis are activated in ex vivo B-CLL cells, and suggest that an ER stress-induced signaling is involved in spontaneous B-CLL cell apoptosis.
Involvement of ER stress-associated signaling in spontaneous B-CLL cell apoptosis. (A,F,G) Time-course analysis of caspase-4 proteolytic processing, CHOP/GADD153 and BiP/GRP78 expression, and JNK1/2 phosphorylation during spontaneous B-CLL cell apoptosis. Western blot analysis was performed in whole-cell lysates (25 μg for caspase-4, BiP/GRP78, and JNK1/2, and 40 μg for CHOP/GADD153) extracted from freshly isolated B-CLL cells and B-CLL cells cultured in complete medium for the indicated times (n = 12). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. In caspase-4 blots, separated panels are shown because long X-ray film exposure was necessary to detect the 20-kDa subunit. The asterisk in caspase-4 blots indicates bands derived from unknown cleavage. The density of the bands corresponding to CHOP/GADD153 and BiP/GRP78 was evaluated by densitometric analysis. Densitometry units (U) were calculated relative to β-actin. The data shown for patients 1 and 2 are representative of 12 patients. (B) Effect of pharmacologic caspase-4 inhibition on spontaneous B-CLL cell apoptosis. Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei in B-CLL cells cultured for 24 hours in complete medium with or without 50μM of the caspase-4 inhibitor z-LEVD-fmk, 50μM of the pan-caspase inhibitor z-VAD-fmk, or 0.005% DMSO as control (n = 7). Results are presented as the mean ± SD of all 7 patients examined. **P < .01 (each caspase inhibitor vs DMSO) according to Student t test. (C-E) Effect of caspase-4 down-regulation on spontaneous B-CLL cell apoptosis. Freshly isolated B-CLL cells were transfected with 0.5μM control nontargeting (siCtrl) or caspase-4 siRNA (siCasp-4) as described in “siRNA nucleofection” and then cultured in complete medium for 72 hours (n = 7). Caspase-4 expression (C) was analyzed by Western blot as described in panel A. Apoptosis was evaluated by flow cytometric analysis of annexin V/PI-stained cells (D) and hypodiploid nuclei (E). (D) Results are presented as the percentage of viable (annexin V−/PI−), early apoptotic (annexin V+/PI−), late apoptotic (annexin V+/PI+), and necrotic cells (annexin V−/PI+). (E) Percentage of hypodiploid nuclei. (C-E) Results shown for patients 1, 2, and 3 are representative of 7 patients.
Involvement of ER stress-associated signaling in spontaneous B-CLL cell apoptosis. (A,F,G) Time-course analysis of caspase-4 proteolytic processing, CHOP/GADD153 and BiP/GRP78 expression, and JNK1/2 phosphorylation during spontaneous B-CLL cell apoptosis. Western blot analysis was performed in whole-cell lysates (25 μg for caspase-4, BiP/GRP78, and JNK1/2, and 40 μg for CHOP/GADD153) extracted from freshly isolated B-CLL cells and B-CLL cells cultured in complete medium for the indicated times (n = 12). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. In caspase-4 blots, separated panels are shown because long X-ray film exposure was necessary to detect the 20-kDa subunit. The asterisk in caspase-4 blots indicates bands derived from unknown cleavage. The density of the bands corresponding to CHOP/GADD153 and BiP/GRP78 was evaluated by densitometric analysis. Densitometry units (U) were calculated relative to β-actin. The data shown for patients 1 and 2 are representative of 12 patients. (B) Effect of pharmacologic caspase-4 inhibition on spontaneous B-CLL cell apoptosis. Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei in B-CLL cells cultured for 24 hours in complete medium with or without 50μM of the caspase-4 inhibitor z-LEVD-fmk, 50μM of the pan-caspase inhibitor z-VAD-fmk, or 0.005% DMSO as control (n = 7). Results are presented as the mean ± SD of all 7 patients examined. **P < .01 (each caspase inhibitor vs DMSO) according to Student t test. (C-E) Effect of caspase-4 down-regulation on spontaneous B-CLL cell apoptosis. Freshly isolated B-CLL cells were transfected with 0.5μM control nontargeting (siCtrl) or caspase-4 siRNA (siCasp-4) as described in “siRNA nucleofection” and then cultured in complete medium for 72 hours (n = 7). Caspase-4 expression (C) was analyzed by Western blot as described in panel A. Apoptosis was evaluated by flow cytometric analysis of annexin V/PI-stained cells (D) and hypodiploid nuclei (E). (D) Results are presented as the percentage of viable (annexin V−/PI−), early apoptotic (annexin V+/PI−), late apoptotic (annexin V+/PI+), and necrotic cells (annexin V−/PI+). (E) Percentage of hypodiploid nuclei. (C-E) Results shown for patients 1, 2, and 3 are representative of 7 patients.
Caspase-4 acts upstream of caspase-8 and -3 processing during spontaneous B-CLL cell apoptosis
In some ER stress-induced apoptosis models, it has been shown that ER triggers its caspase cascade leading to apoptosis.32,33 Based on these observations, on the evidence that ER stress can activate caspase-8 independent of the death receptor pathway,34-36 and considering that caspase-3 is a converging point for several caspase pathways,4 we investigated whether in ex vivo B-CLL cells, caspase-8 and -3 are involved in their spontaneous apoptosis, and if so, whether caspase-4 acts upstream of their activation. Caspase-8 and -3 processing was examined in fresh B-CLL cells and after 4-, 8-, and 24-hour culture in complete medium (patients 1-12). Western blot analysis of caspase-8 showed that fresh B-CLL cells from all patients expressed high levels of both procaspase-8/a (55-kDa) and procaspase-8/b (53-kDa) isoforms and very low amounts of their 43/41-kDa intermediate cleavage products. Processing of caspase-8 to 43/41-kDa products increased from the 4-hour culture onward (Figure 2A). Western blot analysis of caspase-3 showed that fresh B-CLL cells from all patients expressed high levels of the 32-kDa procaspase-3 and lower levels of the inactive 20-kDa intermediate. Caspase-3 processing to the active 19- and 17-kDa subunits occurred at 4 hours and continued until 24 hours (Figure 2A). Contribution of caspase-8 and -3 for spontaneous B-CLL cell apoptosis was examined by analyzing hypodiploid DNA content in B-CLL cells cultured for 24 hours with the caspase-8 inhibitor z-IETD-fmk, the caspase-3 inhibitor z-DEVD-fmk, or DMSO as control (patients 1-7). Figure 2B shows that the mean percentage of hypodiploid nuclei of control B-CLL cells was significantly reduced by both z-IETD-fmk (11.0% ± 4.0% vs 26.8% ± 7.0%; P < .01) and z-DEVD-fmk (13.8% ± 4.9% vs 26.8% ± 7.0%; P < .01), suggesting that both caspase-8 and -3 play an important role in spontaneous B-CLL cell apoptosis. To examine whether caspase-4 acts upstream of caspase-8 and -3 activation, B-CLL cells were cultured for 24 hours with the caspase-4 inhibitor z-LEVD-fmk or DMSO as control, and their whole-cell lysates analyzed for caspase-8 and -3 cleavage (patients 1-6). Figure 2C shows that the cleavages of caspase-8 to its 43/41-kDa fragments and caspase-3 to its 19- and 17-kDa subunits were inhibited by z-LEVD-fmk. The inhibited caspase-3 processing corresponded to a reduced caspase-3 activity, as indicated by the evidence that degradation of PARP, a preferred caspase-3 substrate, to its 89-kDa inactive form occurring in control B-CLL cells, was also inhibited by z-LEVD-fmk (Figure 2C). These data suggest that caspase-4 is involved in the cleavage of caspase-8 and -3 and were confirmed by siRNA studies (patients 1-6). As shown in Figure 2D, caspase-4 down-regulation resulted in a reduced processing of both caspase-8 and -3. Altogether, these results suggest that, in ex vivo B-CLL cells, ER triggers through caspase-4 a caspase cascade involving caspase-8 and -3, which leads to spontaneous apoptosis of these cells.
Caspase-4 acts upstream of caspase-8 and -3 processing during spontaneous B-CLL cell apoptosis. (A) Time-course analysis of caspase-8 and -3 proteolytic processing during spontaneous B-CLL cell apoptosis. Western blot analysis was performed in 25 μg whole-cell lysates extracted from freshly isolated B-CLL cells and B-CLL cells cultured in complete medium for the indicated times (n = 12). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. Vertical lines inserted in caspase-3 blot indicate a repositioned gel lane. The data shown for patients 1 and 2 are representative of 12 patients. (B) Effect of pharmacologic inhibition of caspase-8 and -3 on spontaneous B-CLL cell apoptosis. Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei in B-CLL cells cultured for 24 hours in complete medium with 50μM of the caspase-8 inhibitor z-IETD-fmk, 50μM of the caspase-3 inhibitor z-DEVD-fmk, or 0.005% DMSO as control (n = 7). Results are the mean ± SD of all 7 patients examined. **P < .01 (each caspase inhibitor vs DMSO) according to Student t test. (C) Effect of pharmacologic caspase-4 inhibition on caspase-8 and -3 proteolytic processing and PARP degradation. Caspase-8 and -3 processing and PARP degradation were analyzed by Western blot in 25 μg whole-cell lysates extracted from B-CLL cells cultured for 24 hours in complete medium with 50μM of the caspase-4 inhibitor z-LEVD-fmk or 0.005% DMSO as control (n = 6). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. The data shown for patients 1 and 2 are representative of 6 patients. (D) Effect of caspase-4 down-regulation on caspase-8 and -3 proteolytic processing. Freshly isolated B-CLL cells were transfected with 0.5μM control nontargeting (siCtrl) or caspase-4 siRNA (siCasp-4) as described in “siRNA nucleofection” and then cultured in complete medium for 72 hours (n = 6). Caspase-4, -8, and -3 processing was analyzed by Western blot in 25 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. In caspase-4 blots, separated panels are shown because long X-ray film exposure was necessary to detect the 20-kDa subunit. The asterisk in caspase-4 blots indicates bands derived from unknown cleavage. Data shown for patients 1 and 2 are representative of 6 patients.
Caspase-4 acts upstream of caspase-8 and -3 processing during spontaneous B-CLL cell apoptosis. (A) Time-course analysis of caspase-8 and -3 proteolytic processing during spontaneous B-CLL cell apoptosis. Western blot analysis was performed in 25 μg whole-cell lysates extracted from freshly isolated B-CLL cells and B-CLL cells cultured in complete medium for the indicated times (n = 12). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. Vertical lines inserted in caspase-3 blot indicate a repositioned gel lane. The data shown for patients 1 and 2 are representative of 12 patients. (B) Effect of pharmacologic inhibition of caspase-8 and -3 on spontaneous B-CLL cell apoptosis. Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei in B-CLL cells cultured for 24 hours in complete medium with 50μM of the caspase-8 inhibitor z-IETD-fmk, 50μM of the caspase-3 inhibitor z-DEVD-fmk, or 0.005% DMSO as control (n = 7). Results are the mean ± SD of all 7 patients examined. **P < .01 (each caspase inhibitor vs DMSO) according to Student t test. (C) Effect of pharmacologic caspase-4 inhibition on caspase-8 and -3 proteolytic processing and PARP degradation. Caspase-8 and -3 processing and PARP degradation were analyzed by Western blot in 25 μg whole-cell lysates extracted from B-CLL cells cultured for 24 hours in complete medium with 50μM of the caspase-4 inhibitor z-LEVD-fmk or 0.005% DMSO as control (n = 6). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. The data shown for patients 1 and 2 are representative of 6 patients. (D) Effect of caspase-4 down-regulation on caspase-8 and -3 proteolytic processing. Freshly isolated B-CLL cells were transfected with 0.5μM control nontargeting (siCtrl) or caspase-4 siRNA (siCasp-4) as described in “siRNA nucleofection” and then cultured in complete medium for 72 hours (n = 6). Caspase-4, -8, and -3 processing was analyzed by Western blot in 25 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. In caspase-4 blots, separated panels are shown because long X-ray film exposure was necessary to detect the 20-kDa subunit. The asterisk in caspase-4 blots indicates bands derived from unknown cleavage. Data shown for patients 1 and 2 are representative of 6 patients.
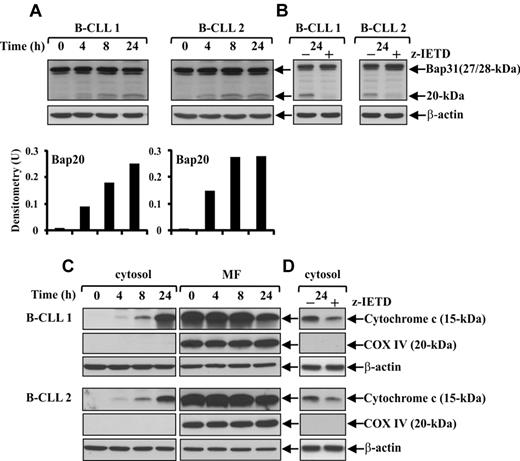
Caspase-8 is involved in Bap31 cleavage and mitochondrial cytochrome c release during spontaneous B-CLL cell apoptosis
Several lines of evidence suggest that ER stress, in parallel or alternatively to its specific caspase pathway, can trigger an apoptotic signaling that converges on mitochondria activating or amplifying a canonical mitochondrial pathway.14 In some apoptosis models based on ER-mitochondria interplay, it has been shown that caspase-8 is involved in coupling these 2 organelles, given its ability to cleave the ER membrane protein Bap31, which is a key regulator of protein trafficking and ER stress apoptosis,36,37 into the proapoptotic ER membrane-embedded fragment Bap20, which triggers various mitochondrial alterations.38 On this basis, we first investigated whether in B-CLL cells Bap31 protein was expressed and cleaved into Bap20, and if so, whether caspase-8 was required for this event. Bap31 analysis was performed in fresh B-CLL cells and after 4-, 8-, and 24-hour culture (patients 1-12) using an Ab directed to residues surrounding Asp164, the cleavage site by caspase-8.37 Results showed that B-CLL cells from all patients constitutively expressed sustained levels of the full-length Bap31, whose cleavage into Bap20 appeared after 4-hour culture and increased up to 24 hours (Figure 3A). Experiments performed culturing B-CLL cells for 24 hours with the caspase-8 inhibitor z-IETD-fmk or DMSO as control (patients 1-7) showed that Bap31 cleavage was almost completely prevented by this inhibitor suggesting that caspase-8 plays an important role in this event (Figure 3B).
Caspase-8 is involved in Bap31 cleavage and mitochondrial cytochrome c release during spontaneous B-CLL cell apoptosis. (A,C) Time-course analysis of Bap31 cleavage and mitochondrial cytochrome c release during spontaneous B-CLL cell apoptosis. Freshly isolated B-CLL cells were cultured for the indicated times in complete medium (n = 12). Bap31 cleavage (A) was analyzed by Western blot in 20 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. The density of the bands corresponding to Bap20 fragment was evaluated by densitometric analysis. Densitometry units (U) were calculated relative to β-actin. The release of cytochrome c from the mitochondria into the cytosol (C) was analyzed by Western blot in 20 μg of cytosol and mitochondria-enriched fraction (MF) extracts. The blots were reprobed with an anti-COX IV mAb to control the purity of cytosolic fraction and the loading of MF, and with an anti–β-actin mAb as a loading control for cytosolic extracts. (A,C) Data shown for patients 1 and 2 are representative of 12 patients. (B,D) Effect of pharmacologic caspase-8 inhibition on Bap31 cleavage and mitochondrial cytochrome c release. B-CLL cells were cultured for 24 hours in complete medium with 50μM of the caspase-8 inhibitor z-IETD-fmk or 0.005% DMSO as control (n = 7). Bap31 cleavage (B) was analyzed as described in panel A. Cytochrome c levels (D) were analyzed by Western blot in cytosolic fraction (20 μg). The blots were reprobed with an anti-COX IV mAb and an anti–β-actin mAb to control the purity and the loading of cytosolic fraction, respectively. (B,D) Data shown for patients 1 and 2 are representative of 7 patients.
Caspase-8 is involved in Bap31 cleavage and mitochondrial cytochrome c release during spontaneous B-CLL cell apoptosis. (A,C) Time-course analysis of Bap31 cleavage and mitochondrial cytochrome c release during spontaneous B-CLL cell apoptosis. Freshly isolated B-CLL cells were cultured for the indicated times in complete medium (n = 12). Bap31 cleavage (A) was analyzed by Western blot in 20 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. The density of the bands corresponding to Bap20 fragment was evaluated by densitometric analysis. Densitometry units (U) were calculated relative to β-actin. The release of cytochrome c from the mitochondria into the cytosol (C) was analyzed by Western blot in 20 μg of cytosol and mitochondria-enriched fraction (MF) extracts. The blots were reprobed with an anti-COX IV mAb to control the purity of cytosolic fraction and the loading of MF, and with an anti–β-actin mAb as a loading control for cytosolic extracts. (A,C) Data shown for patients 1 and 2 are representative of 12 patients. (B,D) Effect of pharmacologic caspase-8 inhibition on Bap31 cleavage and mitochondrial cytochrome c release. B-CLL cells were cultured for 24 hours in complete medium with 50μM of the caspase-8 inhibitor z-IETD-fmk or 0.005% DMSO as control (n = 7). Bap31 cleavage (B) was analyzed as described in panel A. Cytochrome c levels (D) were analyzed by Western blot in cytosolic fraction (20 μg). The blots were reprobed with an anti-COX IV mAb and an anti–β-actin mAb to control the purity and the loading of cytosolic fraction, respectively. (B,D) Data shown for patients 1 and 2 are representative of 7 patients.
To investigate whether in ex vivo B-CLL cells Bap31 cleavage was accompanied by mitochondrial alterations, we examined the release of cytochrome c into the cytosol. Western blot analysis of the cytosolic and mitochondria-enriched fractions extracted from fresh B-CLL cells and after 4-, 8-, and 24-hour culture in complete medium (patients 1-12) showed that, in fresh B-CLL cells, cytochrome c was exclusively present in the mitochondria-enriched fraction, whereas after 4-hour culture, low levels of cytochrome c were also detected in the cytosol and progressively increased up to 24 hours (Figure 3C). To determine the possible involvement of caspase-8 in mitochondrial cytochrome c release, we analyzed the effect of the caspase-8 inhibitor z-IETD-fmk on mitochondrial cytochrome c release after 24-hour culture (patients 1-7). Figure 3D shows that cytosolic cytochrome c accumulation detected in control B-CLL cells was reduced by z-IETD-fmk, suggesting that a caspase-8-mediated signaling leads to mitochondrial cytochrome c release.
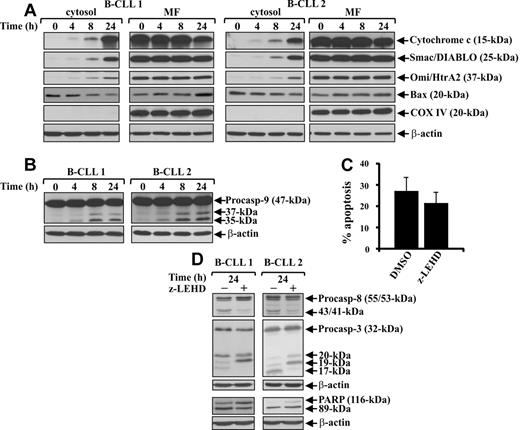
Ex vivo B-CLL cells show mitochondrial alterations but caspase-9 plays a minor role in their spontaneous apoptosis
Release of cytochrome c into the cytosol of ex vivo B-CLL cells suggests that the mitochondrial pathway might be activated during their spontaneous apoptosis. Therefore, we investigated other molecular events associated with mitochondrial pathway activation, such as the mitochondrial release of the apoptogenic proteins Smac/DIABLO and Omi/HtrA2, known to antagonize IAP-mediated inhibition of caspases,39 the translocation from cytosol to mitochondria of the proapoptotic Bcl-2 family member Bax, known to permeabilize the outer mitochondrial membrane,40 and the processing of caspase-9, known to initiate the cytochrome c–dependent caspase cascade.4 Western blot analysis of the cytosolic and mitochondria-enriched fractions extracted from the same cell populations examined for cytochrome c release (patients 1-12) showed that Smac/DIABLO and Omi/HtrA2 were also released into the cytosol with the same kinetics as cytochrome c. Whereas in fresh B-CLL cells, Smac/DIABLO and Omi/HtrA2 proteins were exclusively present in the mitochondria-enriched fraction, after 4-hour culture, low levels of these were also found in the cytosol and progressively increased up to 24 hours (Figure 4A). Analysis of Bax (patients 1-12) showed that, in fresh B-CLL cells, its expression was higher in cytosol than in mitochondria. After culture, there was a progressive reduction of Bax cytosolic levels accompanied by an increase in the mitochondrial levels, suggesting a translocation of Bax from cytosol to the mitochondria (Figure 4A).
Mitochondrial pathway is activated in ex vivo B-CLL cells, but caspase-9 plays a minor role in their spontaneous apoptosis. (A-B) Time-course analysis of mitochondrial apoptogenic protein release into the cytosol, Bax translocation to the mitochondria, and caspase-9 proteolytic processing during spontaneous B-CLL cell apoptosis. Freshly isolated B-CLL cells were cultured for the indicated times in complete medium (n = 12). The levels of cytochrome c, Smac/DIABLO, Omi/HtrA2, and Bax (A) were analyzed by Western blot in cytosol and mitochondria-enriched fraction (MF) extracts (20 μg). The blots were reprobed with an anti-COX IV mAb to control the purity of cytosolic fraction and the loading of MF, and with an anti–β-actin mAb to control the loading of cytosolic extracts. Caspase-9 proteolytic processing (B) was analyzed by Western blot in 25 μg whole-cell lysates, and protein loading was assessed reprobing the blots with an anti–β-actin mAb. (A-B) Data shown for patients 1 and 2 are representative of 12 patients. (C-D) Effect of pharmacologic caspase-9 inhibition on spontaneous B-CLL cell apoptosis, caspase-8 and -3 proteolytic processing, and PARP degradation. Freshly isolated B-CLL cells were cultured for 24 hours in complete medium with 50μM caspase-9 inhibitor z-LEHD-fmk or 0.005% DMSO as control (n = 7). Apoptosis (C) was evaluated by flow cytometric analysis of hypodiploid nuclei, and results are the mean ± SD of all 7 patients examined. The effect of z-LEHD-fmk on spontaneous B-CLL cell apoptosis is not significant. Caspase-3 and -8 processing and PARP degradation (D) were analyzed by Western blot in 25 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. The data shown for patients 1 and 2 are representative of 7 patients.
Mitochondrial pathway is activated in ex vivo B-CLL cells, but caspase-9 plays a minor role in their spontaneous apoptosis. (A-B) Time-course analysis of mitochondrial apoptogenic protein release into the cytosol, Bax translocation to the mitochondria, and caspase-9 proteolytic processing during spontaneous B-CLL cell apoptosis. Freshly isolated B-CLL cells were cultured for the indicated times in complete medium (n = 12). The levels of cytochrome c, Smac/DIABLO, Omi/HtrA2, and Bax (A) were analyzed by Western blot in cytosol and mitochondria-enriched fraction (MF) extracts (20 μg). The blots were reprobed with an anti-COX IV mAb to control the purity of cytosolic fraction and the loading of MF, and with an anti–β-actin mAb to control the loading of cytosolic extracts. Caspase-9 proteolytic processing (B) was analyzed by Western blot in 25 μg whole-cell lysates, and protein loading was assessed reprobing the blots with an anti–β-actin mAb. (A-B) Data shown for patients 1 and 2 are representative of 12 patients. (C-D) Effect of pharmacologic caspase-9 inhibition on spontaneous B-CLL cell apoptosis, caspase-8 and -3 proteolytic processing, and PARP degradation. Freshly isolated B-CLL cells were cultured for 24 hours in complete medium with 50μM caspase-9 inhibitor z-LEHD-fmk or 0.005% DMSO as control (n = 7). Apoptosis (C) was evaluated by flow cytometric analysis of hypodiploid nuclei, and results are the mean ± SD of all 7 patients examined. The effect of z-LEHD-fmk on spontaneous B-CLL cell apoptosis is not significant. Caspase-3 and -8 processing and PARP degradation (D) were analyzed by Western blot in 25 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. The data shown for patients 1 and 2 are representative of 7 patients.
Analysis of caspase-9 showed that fresh B-CLL cells from all patients examined (patients 1-12) expressed high levels of the 47-kDa proenzyme, which from 4-hour culture onward was processed to its active 35- and 37-kDa cleavage products (Figure 4B). These data indicate that caspase-9 cleavage occurs concomitantly to mitochondrial perturbations (Figure 4A-B). To examine whether caspase-9 is involved in spontaneous B-CLL cell apoptosis and plays a role in caspase cascade, we cultured B-CLL cells for 24 hours with the caspase-9 inhibitor z-LEHD-fmk or DMSO as control and examined its effect on hypodiploid DNA content and caspase-8 and -3 processing (patients 1-7). Results in Figure 4C show that the mean percentage of hypodiploid nuclei of control cells was not significantly decreased by z-LEHD-fmk (21.3% ± 5.4% vs 26.8% ± 7.0%; not significant). Furthermore, z-LEHD-fmk reduced caspase-8 processing but had only a modest inhibitory effect on caspase-3 processing, also demonstrated by the evidence that PARP degradation to its 89-kDa inactive form, observed in control cells, was only slightly reduced (Figure 4D). Altogether, these results show that several molecular events associated with mitochondrial pathway activation occur in ex vivo B-CLL cells, including caspase-9 processing, which however, does not play a significant role in the caspase cascade necessary for spontaneous B-CLL cell apoptosis.
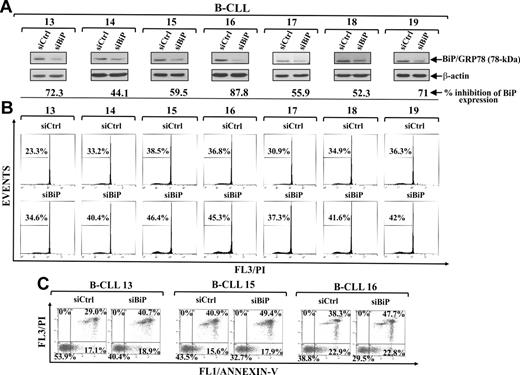
BiP/GRP78 down-regulation by siRNA increases spontaneous B-CLL cell apoptosis
Based on the antiapoptotic properties of BiP/GRP78 in ER stress apoptosis and its key role in cancer cell survival,8,41 and having shown that B-CLL cells constitutively express high levels of BiP/GRP78 protein, which increase during their spontaneous apoptosis, we investigated whether also in B-CLL cells, as observed in other tumor types,42,43 BiP/GRP78 mediates a cytoprotective response to ER stress. Therefore, we evaluated the effect of specific BiP/GRP78 down-regulation by siRNA nucleofection on B-CLL cell apoptosis (patients 13-19). Western blot analysis showed that, in BiP/GRP78 siRNA-transfected cells, a significant reduction of BiP/GRP78 was achieved in the different patient cells, ranging from 44.1% to 87.8% of the BiP/GRP78 levels detected in control siRNA-transfected cells (Figure 5A). In all patients examined, Bip/GRP78 down-regulation resulted in an increased B-CLL cell apoptosis as indicated by the increased percentage of hypodiploid nuclei (Figure 5B) and annexin V+ cells (Figure 5C and data not shown). These data indicate that BiP/GRP78 slows down the rate of spontaneous B-CLL cell apoptosis and suggest that BiP/GRP78 is involved in protecting B-CLL cells against ER stress-mediated apoptosis.
Effect of BiP/GRP78 down-regulation on spontaneous B-CLL cell apoptosis. (A-C) Freshly isolated B-CLL cells were transfected with 2.0μM control nontargeting (siCtrl) or BiP/GRP78 siRNA (siBiP) as described in “siRNA nucleofection” and then cultured in complete medium for 72 hours (n = 7). (A) BiP/GRP78 expression was analyzed by Western blot in 20 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. For each sample, blot lanes corresponding to siCtrl and siBiP were subjected to densitometric analysis and normalized to β-actin levels, and values under siBiP lane of each sample represent the percentage inhibition of BiP/GRP78 expression induced by siBiP compared with siCtrl. (B-C) Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei (B) and annexin V/PI-stained cells (C). (A-B) Results from all 7 patients examined are shown. (C) Results shown for patients 13, 15, and 16 are the percentage of viable (annexin V−/PI−), early apoptotic (annexin V+/PI−), late apoptotic (annexin V+/PI+), and necrotic cells (annexin V−/PI+).
Effect of BiP/GRP78 down-regulation on spontaneous B-CLL cell apoptosis. (A-C) Freshly isolated B-CLL cells were transfected with 2.0μM control nontargeting (siCtrl) or BiP/GRP78 siRNA (siBiP) as described in “siRNA nucleofection” and then cultured in complete medium for 72 hours (n = 7). (A) BiP/GRP78 expression was analyzed by Western blot in 20 μg whole-cell lysates, and protein loading was assessed by reprobing the blots with an anti–β-actin mAb. For each sample, blot lanes corresponding to siCtrl and siBiP were subjected to densitometric analysis and normalized to β-actin levels, and values under siBiP lane of each sample represent the percentage inhibition of BiP/GRP78 expression induced by siBiP compared with siCtrl. (B-C) Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei (B) and annexin V/PI-stained cells (C). (A-B) Results from all 7 patients examined are shown. (C) Results shown for patients 13, 15, and 16 are the percentage of viable (annexin V−/PI−), early apoptotic (annexin V+/PI−), late apoptotic (annexin V+/PI+), and necrotic cells (annexin V−/PI+).
Tunicamycin and thapsigargin accelerate spontaneous B-CLL cell apoptosis
Next, we examined whether B-CLL cells are susceptible to pharmacologic ER stress induction by tunicamycin, an inhibitor of protein glycosylation, and thapsigargin, a Ca2+-ATPase inhibitor. B-CLL cells were incubated for 24 hours with increasing concentrations of each agent or DMSO, and apoptosis was examined by analyzing hypodiploid nuclei and annexin V+ cells (patients 18-23). Results in Figure 6A show that both tunicamycin and thapsigargin increased spontaneous B-CLL cell apoptosis in a concentration-dependent manner, tunicamycin being more effective than thapsigargin. These results were confirmed by annexin V/PI staining (Figure 6B and data not shown).
Effect of tunicamycin and thapsigargin on spontaneous B-CLL cell apoptosis. (A-C) Freshly isolated B-CLL cells were cultured for 24 hours in complete medium with the indicated concentrations of tunicamycin (Tm), thapsigargin (Tg), or DMSO as control (n = 6). Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei (A) and annexin V/PI staining (B). (A) Data are mean ± SD of all 6 patients examined. *P < .05, **P < .01 (each agent vs DMSO) according to Student t test. (B) Results are the percentage of viable (annexin V−/PI−), early apoptotic (annexin V+/PI−), late apoptotic (annexin V+/PI+), and necrotic cells (annexin V−/PI+). (C) The expression of CHOP/GADD153 and BiP/GRP78, and the cleavages of caspase-3, -4, -8, and -9 and Bap31 were analyzed by Western blot (40 μg whole-cell lysates for CHOP/GADD153 and 20 μg for all other proteins). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. (B-C) Data shown for patient 21 are representative of 6 patients.
Effect of tunicamycin and thapsigargin on spontaneous B-CLL cell apoptosis. (A-C) Freshly isolated B-CLL cells were cultured for 24 hours in complete medium with the indicated concentrations of tunicamycin (Tm), thapsigargin (Tg), or DMSO as control (n = 6). Apoptosis was evaluated by flow cytometric analysis of hypodiploid nuclei (A) and annexin V/PI staining (B). (A) Data are mean ± SD of all 6 patients examined. *P < .05, **P < .01 (each agent vs DMSO) according to Student t test. (B) Results are the percentage of viable (annexin V−/PI−), early apoptotic (annexin V+/PI−), late apoptotic (annexin V+/PI+), and necrotic cells (annexin V−/PI+). (C) The expression of CHOP/GADD153 and BiP/GRP78, and the cleavages of caspase-3, -4, -8, and -9 and Bap31 were analyzed by Western blot (40 μg whole-cell lysates for CHOP/GADD153 and 20 μg for all other proteins). Protein loading was assessed by reprobing the blots with an anti–β-actin mAb. (B-C) Data shown for patient 21 are representative of 6 patients.
To evaluate the molecular events associated with tunicamycin- and thapsigargin-induced apoptosis in B-CLL cells, we analyzed B-CLL cells treated for 24 hours with 2.5μM tunicamycin, 2.5μM thapsigargin, or DMSO as control for the expression of CHOP/GADD153 and BiP/GRP78 and the cleavage of caspase-4, -8, and Bap31 (patients 18-23). Figure 6C shows that both ER stress-inducers increased CHOP/GADD153 and BiP/GRP78 expression as well as caspase-8 and Bap31 cleavage with respect to controls, but only tunicamycin increased caspase-4 cleavage. We then examined the effect of tunicamycin and thapsigargin on caspase-9 and -3 cleavage and observed that, whereas tunicamycin increased the cleavage of both caspases, thapsigargin increased only the cleavage of caspase-3 (Figure 6C).
Discussion
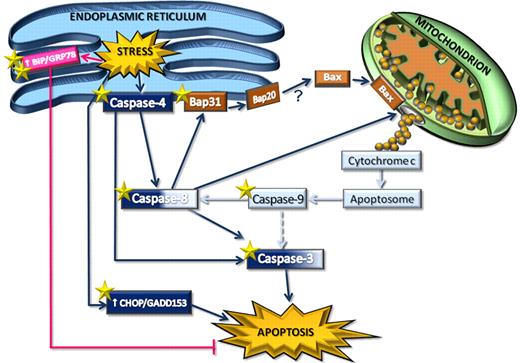
Increasing knowledge about the apoptotic pathways in B-CLL cells may help to design more effective therapies for this leukemia. In this study, we investigated ER stress signaling in spontaneous B-CLL cell apoptosis. We have shown, for the first time, that ex vivo B-CLL cells exhibit a functional ER stress-associated signaling, which plays an important role in their spontaneous apoptosis. This observation is supported by pharmacologic and genetic inhibition studies showing that caspase-4, which in humans has been specifically associated with ER stress-induced apoptosis,11 is required for spontaneous B-CLL cell apoptosis. Our results also demonstrate that, in ex vivo B-CLL cells, caspase-4 acts upstream of the activation of caspase-8 and -3, thus revealing in these cells the existence of a novel specific ER stress-triggered caspase cascade, initiated by caspase-4 and involving caspase-8 and -3, which plays an important role for their apoptosis (Figure 7).
Schematic representation of ER stress-triggered pathways in B-CLL cells, as potential therapeutic targets. ER stress induces activation of the ER membrane-resident caspase-4, which in turn activates caspase-8 and caspase-3, triggering a novel caspase pathway that leads to spontaneous B-CLL cell apoptosis. Caspase-8 connects ER and mitochondria. Indeed, it cleaves the ER membrane-embedded protein Bap31 into Bap20 and is involved in the release of cytochrome c from mitochondria into the cytosol. Caspase-8–driven cytochrome c release is accompanied by the Bax translocation from cytosol to mitochondria, but whether this latter event is caused by Bap20-dependent mechanisms remains to be elucidated (“?”). Cytochrome c release induces activation of caspase-9, which in turn contributes to cleave caspase-8 and, to a lesser extent (broken line), caspase-3. These results suggest that mitochondria-driven caspase-9 activation, converging on caspase-8, induces an amplification of the ER-triggered caspase cascade rather than an efficient mitochondrial pathway. Moreover, ER stress triggers increased expression of CHOP/GADD153, which also contributes to B-CLL cell apoptosis, and BiP/GRP78, which, on the contrary, triggers antiapoptotic signals in B-CLL cells. Both genetic and pharmacologic interventions increase B-CLL cell apoptosis. The star symbols indicate molecular targets for gene silencing (2 stars) and tunicamycin treatment (one star).
Schematic representation of ER stress-triggered pathways in B-CLL cells, as potential therapeutic targets. ER stress induces activation of the ER membrane-resident caspase-4, which in turn activates caspase-8 and caspase-3, triggering a novel caspase pathway that leads to spontaneous B-CLL cell apoptosis. Caspase-8 connects ER and mitochondria. Indeed, it cleaves the ER membrane-embedded protein Bap31 into Bap20 and is involved in the release of cytochrome c from mitochondria into the cytosol. Caspase-8–driven cytochrome c release is accompanied by the Bax translocation from cytosol to mitochondria, but whether this latter event is caused by Bap20-dependent mechanisms remains to be elucidated (“?”). Cytochrome c release induces activation of caspase-9, which in turn contributes to cleave caspase-8 and, to a lesser extent (broken line), caspase-3. These results suggest that mitochondria-driven caspase-9 activation, converging on caspase-8, induces an amplification of the ER-triggered caspase cascade rather than an efficient mitochondrial pathway. Moreover, ER stress triggers increased expression of CHOP/GADD153, which also contributes to B-CLL cell apoptosis, and BiP/GRP78, which, on the contrary, triggers antiapoptotic signals in B-CLL cells. Both genetic and pharmacologic interventions increase B-CLL cell apoptosis. The star symbols indicate molecular targets for gene silencing (2 stars) and tunicamycin treatment (one star).
In this study, we have shown that other molecular events associated with ER stress-induced apoptosis also occur in ex vivo B-CLL cells, such as the up-regulation of the proapoptotic transcription factor CHOP/GADD153,31 the increased phosphorylation of JNK1/2,15 and the cleavage of the ER stress regulator Bap3136,37 into the proapoptotic ER membrane-embedded Bap20 fragment (Figure 7), known to propagate apoptotic signals from the ER to mitochondria.38 Accumulating evidence has demonstrated that several proapoptotic molecules are involved in the ER-mitochondria interplay,13,14 with a key role played by caspase-8, which, activated by ER stress independent of the death receptor pathway,34-36 leads to mitochondrial cytochrome c release via Bap31 cleavage into Bap20.38 Consistent with this latter observation, we found that, in ex vivo B-CLL cells, pharmacologic caspase-8 inhibition almost completely prevents Bap31 cleavage into Bap20 and also reduces the mitochondrial cytochrome c release, suggesting a possible involvement of caspase-8-mediated cleavage of Bap31 in propagating proapoptotic signals from ER to mitochondria (Figure 7). However, further studies will be necessary to define the involvement of ER in activating mitochondria in B-CLL cells.
In this study, we have also shown that, in ex vivo B-CLL cells, several apoptotic events associated with the mitochondrial pathway are activated, such as the Bax translocation to mitochondria concomitant with the release of cytochrome c into the cytosol, and the caspase-9 processing (Figure 7), which are consistent with a previous report showing the formation of a functional apoptosome in B-CLL cell lysates.44 However, our results suggest that caspase-9 does not play a major role in spontaneous B-CLL cell apoptosis. Indeed, its pharmacologic inhibition prevents caspase-8 processing to a similar extent as the pharmacologic inhibition of caspase-4 but induces only a modest inhibitory effect on caspase-3 processing, suggesting that caspase-9 is less efficient than caspase-4 in activating the caspase cascade required for B-CLL cell apoptosis (Figure 7). These results, along with the evidence that, in ex vivo B-CLL cells, the apoptogenic proteins Smac/DIABLO and Omi/HtrA2, known to antagonize the c-IAP-mediated inhibition of caspases,39 are also released from mitochondria, lead us to hypothesize that, in these cells, the observed apoptotic mitochondrial-driven signals may serve to amplify the ER-triggered caspase cascade necessary for B-CLL cell apoptosis, rather than induce an efficient mitochondrial caspase cascade (Figure 7).
The evidence that, in ex vivo B-CLL cells, ER can trigger through caspase-4 its own caspase pathway, which leads to apoptosis of these cells, may have important therapeutic implications, given that B-CLL cells have a defective mitochondrial pathway, mainly because of the high expression of antiapoptotic proteins of the Bcl-2 family,23 and therefore are often resistant to conventional treatments that target this pathway. Consistent with these observations, a recent report showed that fludarabine-refractory B-CLL cells are efficiently killed in vitro, independent of their Bcl-2 overexpression, by targeting the ER-Golgi pathway with brefeldin A.20
We have shown that, in ex vivo B-CLL cells, ER stress, besides activating apoptotic pathways, also triggers survival signals by increasing the expression of the prosurvival UPR component and key regulator of cancer cell survival BiP/GRP78 (Figure 7). These results prompted us to investigate whether targeting BiP/GRP78 affects B-CLL cell survival. We have shown, for the first time, that down-regulation by siRNA of BiP/GRP78 increases B-CLL cell apoptosis, suggesting that this protein is involved in the resistance mechanisms of B-CLL cells to ER stress-induced apoptosis, as also observed in other tumor cells.42,43 These observations along with the evidence that B-CLL cells constitutively express high levels of BiP/GRP78 protein pose the question of whether alterations in BiP/GRP78 and/or in other protective UPR pathways may contribute to the anomalous B-CLL cell survival and B-CLL pathogenesis. In this context, although the pro-survival UPR components are established as critical for solid tumor progression and drug resistance,17,41-43,45 the role of BiP/GRP78 and UPR activation in hematologic cancers remains undefined. Regarding this, Ni et al46 recently discovered, in myeloid and lymphoblastic leukemia patients, a novel isoform of BiP/GRP78 with an important role in promoting cell leukemic survival under ER stress. Consistent with these studies, other authors have shown that primary Philadelphia chromosome-positive acute leukemia cells exhibit a constitutive high UPR activation that plays a significant antiapoptotic role, suggesting that UPR activation might be a common mechanism in both solid tumor and leukemia cells.47
Another important aspect of our study is that, in ex vivo B-CLL cells, ER apoptotic signaling is amenable to pharmacologic stimulation. Indeed, both tunicamycin and thapsigargin increase B-CLL cell apoptosis by increasing ER stress, as evidenced by CHOP/GADD153 and BiP/GRP78 up-regulation and the increased Bap31 cleavage. Their effects are concentration-dependent, with tunicamycin being more effective than thapsigargin, consistent with the evidence that tunicamycin activates more caspase pathways than thapsigargin. Whereas tunicamycin increases the processing of caspase-4, -8, -9, and-3, thapsigargin only increases caspase-8 and -3 processing. Considering the different action mechanisms of these 2 ER stress-inducers, tunicamycin, an N-glycosylation inhibitor, and thapsigargin, an ER Ca2+-ATPase inhibitor, it is probable that B-CLL cells are more susceptible to ER stress stimuli, which cause a protein accumulation, than to ER stress stimuli, which perturb Ca2+ homeostasis.
Overall, our study reveals a novel caspase pathway triggered by ER stress in B-CLL cells and identifies BiP/GRP78 as a new molecular target for increasing their apoptosis. These findings provide a framework for future clinical assessment of ER targeting strategies in B-CLL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Catherine Bennet Gillies for the excellent assistance in preparing the manuscript.
This work was supported by grants from the Fondazione Cassa di Risparmio di Perugia, Italy (2009; code 2009.020.0086; E.R.), the Associazione Italiana per la Ricerca sul Cancro (I.S.), and the Fondazione Cassa di Risparmio di Terni-Polo Didattico e Scientifico di Terni, Italy (2008-2009; P.M.).
Authorship
Contribution: E.R. conceived research, analyzed and interpreted data, and wrote the manuscript; R.S. and G.R. performed experiments and collected data; F.D.F performed experiments; M.D.I. and F.F. provided patient samples, patient clinical and laboratory data, and critical suggestions; K.F. assisted in cytofluorimetric analysis; A.B. collected data and assisted in statistical analysis; I.S. supervised the study and provided critical suggestions; and P.M. conceptualized the investigation and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emanuela Rosati, Department of Clinical and Experimental Medicine, General Pathology and Immunology Section, Via Enrico dal Pozzo, Padiglione W, 06126, Perugia, Italy; e-mail: erosati@unipg.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal