Abstract
A major limitation for adenoviral transduction in vivo is the profound liver tropism of adenovirus type 5 (Ad5). Recently, we demonstrated that coagulation factor X (FX) binds to Ad5-hexon protein at high affinity to mediate hepatocyte transduction after intravascular delivery. We developed novel genetically FX-binding ablated Ad5 vectors with lower liver transduction. Here, we demonstrate that FX-binding ablated Ad5 predominantly localize to the liver and spleen 1 hour after injection; however, they had highly reduced liver transduction in both control and macrophage-depleted mice compared with Ad5. At high doses in macrophage-depleted mice, FX-binding ablated vectors transduced the spleen more efficiently than Ad5. Immunohistochemical studies demonstrated transgene colocalization with CD11c+, ER-TR7+, and MAdCAM-1+ cells in the splenic marginal zone. Systemic inflammatory profiles were broadly similar between FX-binding ablated Ad5 and Ad5 at low and intermediate doses, although higher levels of several inflammatory proteins were observed at the highest dose of FX-binding ablated Ad5. Subsequently, we generated a FX-binding ablated virus containing a high affinity Ad35 fiber that mediated a significant improvement in lung/liver ratio in macrophage-depleted CD46+ mice compared with controls. Therefore, this study documents the biodistribution and reports the retargeting capacity of FX binding-ablated Ad5 vectors in vitro and in vivo.
Introduction
Of the 54 different adenoviral serotypes isolated to date, adenovirus serotype 5 (Ad5) has been the most commonly used vector in gene therapy clinical trials. This is, in part, due to clear advantages over alternate strategies including the relatively easy manipulation of its viral genome and feasible scale-up production to high titers (up to 1013 viral particles (vp)/mL). Nevertheless, Ad5 presents 2 substantial limitations that have required attention to optimize the use of Ad5 in gene therapy. These include the observation that the majority of the human population has pre-existing neutralizing antibodies against Ad51-3 and the profound liver tropism observed for Ad5 after intravascular delivery.4,5 For this reason, fundamental aspects of Ad5 biology need to be further studied to provide safer and target-specific Ad5 gene therapy vectors. The mechanism of Ad5-mediated gene transfer has now been relatively well characterized. In vitro studies have shown that Ad5 and those Ads from subspecies A, C, D, E, and F may use the coxsackievirus and Ad receptor (CAR) as a primary binding receptor.6-9 This interaction occurs via the fiber knob domain with subsequent interaction of the Ad5 penton base with cellular integrins (αvβ3 and αvβ5), mediating capsid internalization.10,11 Although CAR and integrin-binding ablated mutant Ad vectors show a substantial reduction in transduction in vitro, these vectors still predominantly transduce hepatocytes in vivo after intravascular administration.12,13
Injection of Ad5 into the bloodstream leads to a complex series of interactions that impact on the resulting biodistribution and tropism of the virus. It has been demonstrated that Ad5 vectors can interact with a variety of blood cells including neutrophils,14 platelets,15 red blood cells,2,16-18 macrophages (including Kupffer cells in the liver19 and macrophages in the spleen20 ), as well as circulating soluble factors including complement factors,21,22 neutralizing antibodies3 and coagulation factors.23-27 A number of recent studies have focused on the role of coagulation factors in defining the tropism of adenoviruses in vivo.24,26,27 Originally, factor (F)IX and complement 4 binding protein (C4BP) were shown to interact with fiber knob to bridging the Ad capsid to the hepatocyte cell surface.23 Subsequently it was demonstrated that vitamin K–dependent coagulation factors FVII, FIX, FX and protein C all possessed the ability to enhance adenoviral tropism in vitro at physiologic levels.24 Using a warfarin-pretreatment model, we demonstrated that FX was the only coagulation factor able to rescue liver gene transfer in warfarin-treated mice.25,26
We recently demonstrated that FX binds at high affinity (∼ 2nM) to the Ad5 hexon protein through interaction with the hyper-variable regions (HVRs) on the Ad5 hexon and that this interaction mediates hepatocyte transduction in vivo.26 The γ-carboxyglutamic acid (Gla) domain of FX binds to the hexon protein through HVRs and the serine protease domain of FX bridges the capsid to cell receptors.26 Heparan sulfate proteoglycans have been shown to have a role on in vivo Ad5 liver transduction,23 thus suggesting its presence on hepatocytes cell surface. Using 23Å cryo-electron microscopy resolution and modeling based on existing crystallographic data, we identified the contact regions between the FX Gla domain and Ad5-hexon.27 We used this model to engineer novel Ad5 vectors with refined mutations in the Ad5 hexon and assessed their effect on FX binding and FX-mediated gene transfer in vitro and in vivo at low dose (1 × 1010 vp/mouse). We showed that HVR5 and, more importantly, HVR7 manipulation reduced FX binding as evidenced by surface plasmon resonance (SPR) studies and reduced in vitro and in vivo gene transfer to liver.27
Here, we performed detailed in vivo studies at increasing doses of mutant Ad5 vectors in mice, because liver transduction is a nonlinear process, involving vector entrapment, macrophage scavenging and hepatocyte transduction.5,19,20 We report the detailed liver and spleen uptake of mutant viruses at increasing doses and demonstrate the substantial effect of FX-binding ablation on biodistribution and gene transfer from Ad in vivo. In addition, we provide a detailed study of the inflammatory profiles of these vectors and the utility of FX-binding ablated Ad5 vectors for retargeting in vivo through incorporating a high affinity CD46 interacting fiber.28
Methods
Vector generation and amplification
Ad5 and hexon-modified Ad vectors were generated by homologous recombination as previously described.27 Ad5-CTL, Ad5-KO1, Ad5-PD1, and Ad5-KO1PD1 were kindly provided by Sue Stevenson29 and Ad35Luc by Jerome Custers (Crucell, The Netherlands). The Ad35 fiber (F35++), including 2 amino acid changes (D207G and T245A), was cloned into pAd5CMVlacZ-HVR5*7*E451Q backbone. See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for the detailed cloning strategy. Adenoviruses were amplified in our laboratory following the previously described methodology.27 Adenoviral particle titers were calculated by determining protein concentrations using the micro-bicinchoninic acid (BCA) Protein Assay kit (Thermo Scientific) followed by titer calculations using the formula 1 μg protein = 4 × 109 vp.30
In vivo methods
All animal procedures were approved by the United Kingdom Home Office. Male MF1 outbred mice aged between 7 and 9 weeks (Harlan) were used for in vivo experiments. CD46 transgenic mice were obtained as previously described.31 Clodronate liposomes (http://clodronateliposomes.org; 200 μL) were injected into the tail vein 48 hours prior to adenovirus administration as previously described.23 X-bp treated animals were injected with 4.8 mg/Kg X-bp 30 minutes before injection of the virus as previously described.26 Adenoviral titers used in this experiment were: Ad5: 3.2 × 1012 vp/mL; 1.9 × 1011 plaque-forming units (pfu)/mL, vp/pfu: 16.9; Ad5-HVR5*7*E451Q: 4.3 × 1012 vp/mL; 1.3 × 1011 pfu/mL; vp/pfu: 34.4; Ad5-HVR5*7*E451Q/F35++: 1.2 × 1012 vp/mL; 2.5 × 1010 pfu/mL; vp/pfu: 47.2. All animals experiments were performed with a minimum n = 5. Statistical analysis was generated using the unpaired Student t test with statistical significance accepted at P < .05.
β-Galactosidase transduction profiles in liver and spleen
β-Galactosidase transgene expression was analyzed using β-Gal enzyme-linked immunosorbent assay (ELISA) kit (Roche) according to the manufacturer's instructions. Liver lobes and spleens were fixed in 2% paraformaldehyde for 16 hours at 4°C and stained for β-galactosidase activity in 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) staining solution [0.1 M phosphate buffer, pH 7.3, 2 mM MgCl2, 5 mM K3F3(CN)6, 5 mM K4Fe(CN6)6, and 1 mg/mL X-gal] at 37°C overnight.
Viral genome content of liver and spleens
DNA was extracted from tissues using the QIAamp DNA mini kit (QIAGEN) following the manufacturer's instructions. DNA was quantified using Nanodrop spectophotometer (ThermoScientific) and 100 ng of DNA containing viral genomes were quantified using SyBR green real-time polymerase chain reaction (PCR; 7900HT Sequence Detection System; Applied Biosystems) using a concentration of 0.2 μM hexon primers.27
Immunohistochemistry in liver and spleen
Paraformaldehyde fixed, paraffin tissue sections (3 μm) were deparaffinized and rehydrated through an alcohol gradient before incubation with rabbit anti–β-galactosidase antibody (8.4 μg/mL; MP Biomedicals) or matched rabbit IgG nonimmune control (8.4 μg/mL; Invitrogen) then detected with biotinylated universal secondary antibody (7.5 μg/mL, Vector), ABC kit (Vector), and standard diaminobenzidine (DAB) staining and counterstained with hematoxylin or nuclear fast red. For frozen sections, spleens were embedded in Optimal Cutting Temperature medium (O.C.T.)-containing cryomolds (Tissue-Tek), and frozen at −80°C immediately after necropsy. To assess the effect of clodronate liposomes on various splenocyte cell populations, 6 μm spleen sections from phosphate-buffered saline (PBS) control groups −/+ clodronate treatment were analyzed by immunohistochemistry using antibodies for the following markers: F4/80, MARCO, CD169, SIGNR1, CD11b, CD11c, and B220 (see Table 1). Isotype matched controls were used at the same final concentrations (Table 1). Viral transgene expression (Escherichia coli β-galactosidase) was detected 48 hours after injection using a rabbit anti–β-galactosidase polyclonal antibody (MP Biomedicals LLC) in combination with SIGNR1, CD11b, CD11c, B220, reticular fibroblast marker (ER-TR7), and MAdCAM-1. Frozen sections were fixed using either ice-cold acetone or 4% paraformaldehyde, sections rinsed in PBS and blocked for 30 minutes in 10% normal goat serum (Vector Laboratories). Primary antibodies were incubated simultaneously for 1 hour at room temperature. Secondary antibodies were diluted 1:500-1:750 in PBS + Tween 0.05% with 2% normal goat serum and incubated for 1 hour at room temperature. Slides were mounted with ProLong Gold+DAPI (Invitrogen). Immunofluorescence was captured using an Olympus BX60 fluorescence microscope (10× objective) and images acquired using Cell software (Olympus). Monochrome images (1200 pix × 1600 pix = 885 × 1180 μm with a 10× objective) were acquired through fluorescence filters for FITC and TRITC using an Olympus BX60 fluorescence microscope (10× objective) at room temperature. Images were acquired and merged using Cell software (Olympus). Images were processed to reduce background using ImageJ (National Institutes of Health). Adjustments were applied equally to all compared images.
Antibodies used for IHC-Fr
| Antibody specificity . | Isotype . | Fixation . | Clone . | Final conc. . | Source . | Secondary antibody . |
|---|---|---|---|---|---|---|
| β-galactosidase (Escherichia coli) | rabbit IgG1 | Acetone/4% PFA | 8.3 μg/mL | MP Biomedical | Gt α-Rb IgG Alexa488 | |
| B220 (CD45R) | rat IgG2a, κ | 4% PFA | RA3–6B2 | 1.25 μg/mL | eBiosciences | Gt α-Rt IgG Alexa488/546 |
| CD11b | rat IgG2b, κ | Acetone | M1/70 | 0. 5 μg/mL | Abcam | Gt α-Rt IgG Alexa488/546 |
| CD11c (αXβ2 integrin) | Ar hamster IgG | Acetone | N418 | 2.5 μg/mL | Abcam | Gt α-Ham IgG Alexa488/546 |
| CD169 (Sialoadhesin) | rat IgG2a | 4% PFA | Moma-1 | 0. 5 μg/mL | Bachem | Gt α-Rt IgG Alexa488 |
| CD209b (SIGNR1) | rat IgM | 4% PFA | ER-TR9 | 4 μg/mL | Abcam | Gt α-Rt IgM Alexa488/594 |
| F4/80 (MΦ) | rat IgG2a | 4% PFA | CI:A3–1 | 20 μg/mL | Abcam | Gt α-Rt IgG Alexa488 |
| MAdCAM-1 (mucosal vascular addressin-1) | rat IgG2a, κ | Acetone | MECA-367 | 10 μg/mL | eBiosciences | Gt α-Rt IgG Alexa488/546 |
| MARCO (SR-A) | rat IgG1 | 4% PFA | ED31 | 2 μg/mL | Bachem | Gt α-Rt IgG Alexa488 |
| Reticular fibroblast and fibres Ab | rat IgG2a | 4% PFA | ER-TR7 | 1 μg/mL | Abcam | Gt α-Rt IgG Alexa488 |
| Antibody specificity . | Isotype . | Fixation . | Clone . | Final conc. . | Source . | Secondary antibody . |
|---|---|---|---|---|---|---|
| β-galactosidase (Escherichia coli) | rabbit IgG1 | Acetone/4% PFA | 8.3 μg/mL | MP Biomedical | Gt α-Rb IgG Alexa488 | |
| B220 (CD45R) | rat IgG2a, κ | 4% PFA | RA3–6B2 | 1.25 μg/mL | eBiosciences | Gt α-Rt IgG Alexa488/546 |
| CD11b | rat IgG2b, κ | Acetone | M1/70 | 0. 5 μg/mL | Abcam | Gt α-Rt IgG Alexa488/546 |
| CD11c (αXβ2 integrin) | Ar hamster IgG | Acetone | N418 | 2.5 μg/mL | Abcam | Gt α-Ham IgG Alexa488/546 |
| CD169 (Sialoadhesin) | rat IgG2a | 4% PFA | Moma-1 | 0. 5 μg/mL | Bachem | Gt α-Rt IgG Alexa488 |
| CD209b (SIGNR1) | rat IgM | 4% PFA | ER-TR9 | 4 μg/mL | Abcam | Gt α-Rt IgM Alexa488/594 |
| F4/80 (MΦ) | rat IgG2a | 4% PFA | CI:A3–1 | 20 μg/mL | Abcam | Gt α-Rt IgG Alexa488 |
| MAdCAM-1 (mucosal vascular addressin-1) | rat IgG2a, κ | Acetone | MECA-367 | 10 μg/mL | eBiosciences | Gt α-Rt IgG Alexa488/546 |
| MARCO (SR-A) | rat IgG1 | 4% PFA | ED31 | 2 μg/mL | Bachem | Gt α-Rt IgG Alexa488 |
| Reticular fibroblast and fibres Ab | rat IgG2a | 4% PFA | ER-TR7 | 1 μg/mL | Abcam | Gt α-Rt IgG Alexa488 |
Antibodies used in this study are listed alphabetically. Isotype controls were as follows: rabbit IgG1 (Vector Laboratories); rat IgG2a (Abcam); rat IgG2b (Abcam); Armenian hamster IgG (eBiosciences); rat IgM (Abcam); and rat IgG1 (Vector Laboratories).
IHC-Fr indicates immunohistochemistry frozen; CD, cluster of differentiation molecule; MΦ, macrophage; SIGNR1, specific ICAM3 grabbing nonintegrin related-1; SR-A; scavenging receptor A; Ab, antibody; IgG, immunoglobulin G; Ar Ham, Armenian hamster; IgM, immunoglobulin M; PFA, paraformaldehyde; Gt, goat; Rb, rabbit; and Rt, rat.
Cytokine and chemokine analysis
See supplemental Methods.
SPR analysis
Binding analysis was performed using a Biacore T100 (GE Healthcare). FX (455 RU) and CD46 (542 RU) were covalently immobilized onto the flowcell of a CM5 biosensor chip by amine coupling according to the manufacturer's instructions. Subtracted sensorgrams were generated by subtracting the signal from a surface subjected to a blank amine immobilization. Virus (1011 VP/mL) in 10 mM HEPES pH 7.4; 150 mM NaCl; 5 mM CaCl2; 0.05% Tween 20 was passed over the chip at a flow rate of 30 μL/minute.
Results
We recently generated a series of hexon-modified Ad5 vectors with point mutations or selected HVR loop “swaps” in HVR5 and HVR7 for the respective regions of the Ad26 hexon, that do not bind FX.27 Table 2 describes the hexon modifications incorporated. All hexon-modified Ad5 vectors including HVR swaps and incorporating several mutations displayed reduced liver transduction after intravenous delivery of 1 × 1010 vp per mouse.27 In addition, at the same dose, intramuscular injection (tibialis anterior) of Ad5 and Ad5-HVR5*7*E451Q showed no difference on transduction profiles in mice (supplemental Figure 1). As defined previously, liver transduction is a nonlinear and highly dose-dependent process.5 Therefore, we sought to further evaluate liver transduction under defined conditions.
Hexon modifications introduced in FX-binding ablated Ad5 vectors
| Virus nomenclature . | Promoter and transgene . | Hexon modification . |
|---|---|---|
| Ad5 | CMVlacZ | Wild-type Ad5 |
| Ad5-HVR5(Ad26) | CMVlacZ | Ad5HVR5 swapped for Ad26 |
| Ad5-HVR7(Ad26) | CMVlacZ | Ad5HVR7 swapped for Ad26 |
| Ad5-HVR5+7(Ad26) | CMVlacZ | Ad5HVR5+7 swapped for Ad26 |
| Ad5-HVR5* | CMVlacZ | HVR5 (T270P and E271G) |
| Ad5-HVR7* | CMVlacZ | HVR7 (I421G, T423N, E424S, L426Y) |
| Ad5-E451Q | CMVlacZ | HVR7(E451Q) |
| Ad5-HVR5*7*E451Q | CMVlacZ | HVR5 (T270P and E271G) and HVR7 (I421G, T423N, E424S, L426Y and E451Q) |
| Virus nomenclature . | Promoter and transgene . | Hexon modification . |
|---|---|---|
| Ad5 | CMVlacZ | Wild-type Ad5 |
| Ad5-HVR5(Ad26) | CMVlacZ | Ad5HVR5 swapped for Ad26 |
| Ad5-HVR7(Ad26) | CMVlacZ | Ad5HVR7 swapped for Ad26 |
| Ad5-HVR5+7(Ad26) | CMVlacZ | Ad5HVR5+7 swapped for Ad26 |
| Ad5-HVR5* | CMVlacZ | HVR5 (T270P and E271G) |
| Ad5-HVR7* | CMVlacZ | HVR7 (I421G, T423N, E424S, L426Y) |
| Ad5-E451Q | CMVlacZ | HVR7(E451Q) |
| Ad5-HVR5*7*E451Q | CMVlacZ | HVR5 (T270P and E271G) and HVR7 (I421G, T423N, E424S, L426Y and E451Q) |
Liver transduction by hexon-modified vectors in macrophage-depleted mice after high-dose viral administration
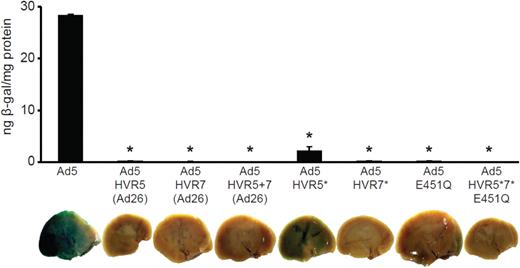
To characterize the effect of Ad5-hexon modification on liver gene transfer, we studied the effect of high-dose viral administration in macrophage-depleted mice. Liver sinusoidal macrophages (Kupffer cells) efficiently clear intravenously administered Ad reducing liver gene transfer.5 Intravenous injection of clodronate liposomes has been shown to deplete Kupffer cells as well as macrophage subpopulations in the spleen, lung, lymph nodes, joints, and testis.19,32 Macrophage-depleted MF1 mice were intravenously injected with 1 × 1011 vp/mouse and killed 48 hours later. Although Ad5 efficiently transduced the liver, no detectable liver transduction was observed for Ad5-HVR5(Ad26), Ad5-HVR7(Ad26), or Ad5-HVR5 + 7(Ad26) at high doses (Figure 1). Consistent with previous results,27 a low level of liver transduction was observed in mice injected with Ad5-HVR5* (Figure 1). However, no liver transduction was observed in mice injected with Ad5-HVR7*, the Ad5-E451Q point mutant or Ad5-HVR5*7*E451Q, indicating that point mutations introduced in HVR7 have a substantial impact on liver transduction.27 Therefore, Ad5-HVR7(Ad26) and Ad5-HVR5*7*E451Q were selected for further detailed studies.
Liver transduction of hexon modified Ad5 vectors using 1 × 1011 vp per mouse. β-galactosidase transgene expression of Ad5 and hexon modified adenoviral vectors after high-dose administration of Ad to macrophage-depleted mice. Animals were killed 48 hours after injection and livers harvested for analysis. (*P < .0001 vs Ad5, n = 5).
Liver transduction of hexon modified Ad5 vectors using 1 × 1011 vp per mouse. β-galactosidase transgene expression of Ad5 and hexon modified adenoviral vectors after high-dose administration of Ad to macrophage-depleted mice. Animals were killed 48 hours after injection and livers harvested for analysis. (*P < .0001 vs Ad5, n = 5).
Biodistribution of FX-binding ablated Ad5 vectors at early (1 hour) and late (48 hours) time points
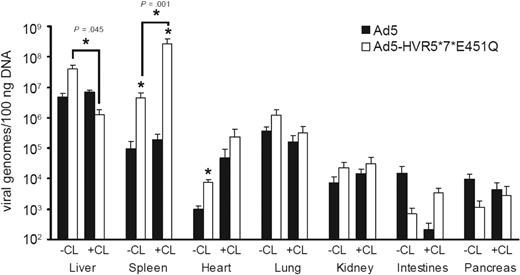
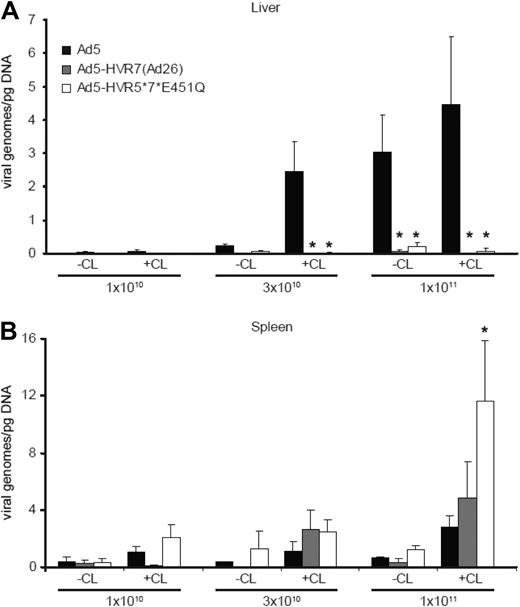
Toxicity and hence safety and efficacy of Ad vectors in the setting of intravenous delivery are dictated by early biodistribution and subsequent gene transfer. We therefore performed a series of experiments quantifying viral genomes in various tissues at an early time point after injection (1 hour) with Ad5 or Ad5-HVR5*7*E451Q. Macrophage-depleted mice treated with clodronate liposomes (CL+) or control mice (CL−) were injected intravenously with high-dose Ad (1 × 1011 vp/mouse) and killed 1 hour after injection (Figure 2). Ad5 levels in the liver and spleen at 1 hour after injection were unaffected by macrophage depletion (Figure 2). However, Ad5 levels in the liver were substantially higher than in the spleen. For FX-binding ablated Ad5-HVR5*7*E451Q, a different distribution profile was observed. After macrophage depletion, levels of Ad5-HVR5*7*E451Q in the liver were significantly reduced, but substantially increased in the spleen (Figure 2). Of note, levels of Ad5-HVR5*7*E451Q in the spleen were substantially higher than Ad5 in both control and macrophage-depleted mice (Figure 2). No statistically significant differences in viral genome content were observed between Ad5 and Ad5-HVR5*7*E451Q in the lung (Figure 2). Although we observed higher levels of FX-binding ablated Ad genomes in the hearts of control mice, the levels were not significantly different to macrophage-depleted mice (Figure 2). In comparison to liver and spleen, lower levels were detected in kidney, pancreas and intestines in control and macrophage-depleted mice administered with Ad5 or Ad5-HVR5*7*E451Q (Figure 2). To further define the biodistribution of these vectors at late time points, we analyzed the viral genome content in the liver and spleen 48 hours after injection with increasing doses of Ad, using titers both higher and lower than the reported nonlinear dose range for Ad5 in mice.5 For Ad5, as expected, we observed an increase in liver genome accumulation with increasing doses (Figure 3A). However, liver levels of Ad5-HVR7(Ad26) and Ad5-HVR5*7*E451Q were significantly lower at all doses compared with Ad5 (Figure 3A). Although macrophage depletion increased the quantity of Ad genomes in the liver after Ad5 administration, we observed no effect on the quantity of liver genomes for either Ad5-HVR7(Ad26) and Ad5-HVR5*7*E451Q (Figure 3A). We next assessed splenic uptake of each virus. Interestingly, significantly higher levels of viral genomes were detected in spleens from mice injected with Ad5-HVR5*7*E451Q compared with Ad5 at the highest dose (Figure 3B). This phenomenon was, however, only observed in macrophage-depleted mice administered with a high dose of virus (Figure 3B), suggesting that the macrophage-mediated clearance of viral particles significantly reduces splenic accumulation of intravenously administered Ad regardless of FX-hexon interaction. We also observed an increase in splenic uptake of Ad5-HVR7(Ad26) although these levels were not statistically different (P = .36) from those observed for Ad5 (Figure 3B).
Viral genome content of control and FX-binding ablated Ad vectors at high dose at an early time point. Viral genome content of liver, spleen, heart, lung, kidney, intestines and pancreas tissue 1 hour after the administration of 1 × 1011 vp of Ad5 and Ad5-HVR5*7*E451Q in control (CL−) or macrophage-depleted mice (CL+). DNA was extracted from all tissues and viral genomes quantified using quantitative PCR. (*P < .05 vs Ad5, n = 5).
Viral genome content of control and FX-binding ablated Ad vectors at high dose at an early time point. Viral genome content of liver, spleen, heart, lung, kidney, intestines and pancreas tissue 1 hour after the administration of 1 × 1011 vp of Ad5 and Ad5-HVR5*7*E451Q in control (CL−) or macrophage-depleted mice (CL+). DNA was extracted from all tissues and viral genomes quantified using quantitative PCR. (*P < .05 vs Ad5, n = 5).
Viral genome content of control and FX-binding ablated Ad vectors 48 hours after administration. Viral genome content of liver (A) and spleen (B) from control (CL−) or macrophage-depleted (CL+) mice 48 hours after injection with increasing doses of Ad5, Ad5-HVR7(Ad26), or Ad5-HVR5*7*E451Q. Ad genomes were detected in liver and spleen after DNA extraction and analysis performed by quantitative PCR. (*P < .05 vs Ad5, n = 5).
Viral genome content of control and FX-binding ablated Ad vectors 48 hours after administration. Viral genome content of liver (A) and spleen (B) from control (CL−) or macrophage-depleted (CL+) mice 48 hours after injection with increasing doses of Ad5, Ad5-HVR7(Ad26), or Ad5-HVR5*7*E451Q. Ad genomes were detected in liver and spleen after DNA extraction and analysis performed by quantitative PCR. (*P < .05 vs Ad5, n = 5).
Effect of macrophage depletion on transduction profiles of FX-binding ablated Ad5 vectors
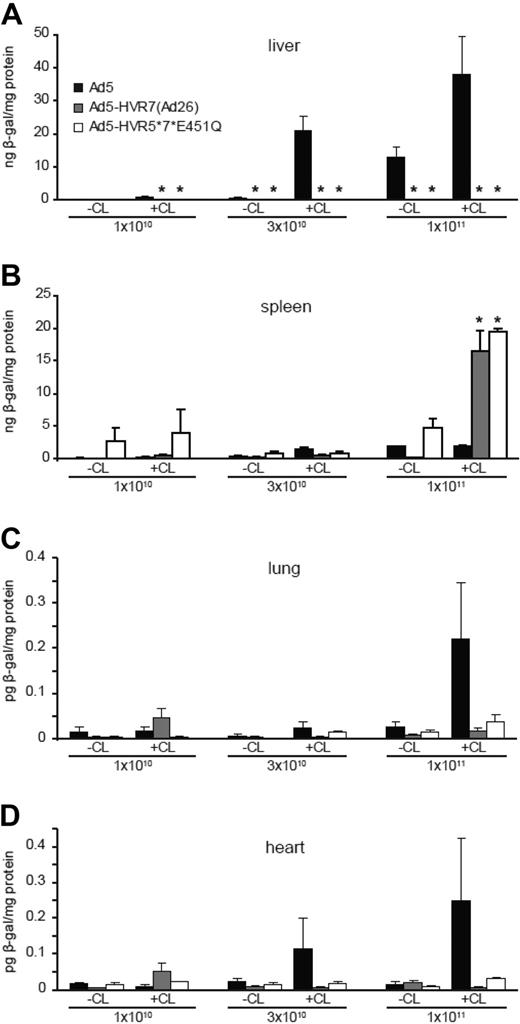
We next quantified the level of gene transfer at 48 hours. As expected, with Ad5-HVR7(Ad26) and Ad5-HVR5*7*E451Q we observed significantly reduced liver transduction at all doses compared with Ad5, regardless of macrophage depletion (Figure 4A and supplemental Figure 2A). Consistent with the viral genome profiles in these animals (Figure 3A-B), higher levels of spleen transduction were observed in mice injected with the highest dose of FX-binding ablated Ad compared with Ad5 (Figure 4B). This increase was only statistically significant in macrophage-depleted mice (Figure 4B and supplemental Figure 2B). Although the increase in the spleen levels of Ad5-HVR7(Ad26) viral genomes were not significantly different, significantly higher levels of splenic transduction were observed. Liver and spleen transduction profiles were corroborated by staining in whole tissues (supplemental Figure 2A-B). Compared with Ad5, lower levels of lung and heart transduction were also observed in mice administered with Ad5-HVR7(Ad26) and Ad5-HVR5*7*E451Q at high doses in macrophage-depleted mice, although the values obtained were substantially lower than those observed for liver and spleen (Figure 4C-D). Collectively, these results indicate that these vectors have inherent splenic tropism at high doses in line with the results presented in Figures 2 and 3.
Transduction profiles of Ad5, Ad5-HVR7(Ad26), and Ad5-HVR5*7*E451Q in control (CL−) or macrophage-depleted (CL+) mice. β-galactosidase expression was quantified by ELISA and normalized to total protein content. Animals were killed 48 hours after injection and liver and spleen were harvested for analysis. Liver (A), spleen (B), lung (C), and heart (D) transduction profile at increasing doses (1 × 1010, 3 × 1010, and 1 × 1011 vp/mouse; *P < .05 vs Ad5, n = 5).
Transduction profiles of Ad5, Ad5-HVR7(Ad26), and Ad5-HVR5*7*E451Q in control (CL−) or macrophage-depleted (CL+) mice. β-galactosidase expression was quantified by ELISA and normalized to total protein content. Animals were killed 48 hours after injection and liver and spleen were harvested for analysis. Liver (A), spleen (B), lung (C), and heart (D) transduction profile at increasing doses (1 × 1010, 3 × 1010, and 1 × 1011 vp/mouse; *P < .05 vs Ad5, n = 5).
To further evaluate the effect of FX binding ablation on Ad5 gene transfer in other species, we also analyzed the transduction and viral genome accumulation of intravenously administered FX binding-ablated Ad5 vectors in Brown Norway rats. Transduction and viral genome accumulation data showed Ad5-HVR5*7*E451Q presented a similar biodistribution profile in Brown Norway rats to that observed in MF1 mice, with a significant increase in spleen transduction in the presence of macrophage depletion and ablated liver transduction and viral genome accumulation at the 48-hour time point (supplemental Figure 3).
Localization of transgene expression in liver and spleen
To visualize the cell types transduced by Ad5-HVR5*7*E451Q, immunohistochemical analysis of β-galactosidase expression was carried out in livers and spleens of macrophage-depleted or control mice 48 hours after injection at increasing doses (1 × 1010, 3 × 1010, and 1 × 1011 vp) of Ad5 or Ad5-HVR5*7*E451Q. Significant levels of hepatocyte transduction were observed in liver sections from mice injected with Ad5, regardless of macrophage depletion (Figure 5). Conversely, no hepatocyte transduction was observed in liver sections from mice injected with Ad5-HVR5*7*E451Q (Figure 5). We did not observe any transduced cells in any liver section analyzed from any of the Ad5-HVR5*7*E451Q-injected animals under any condition (Figure 5). In the spleen, low levels of transduction of marginal zone cells were observed in sections from mice injected with Ad5, although only at the highest dose of vector (Figure 5). Low levels of marginal zone cell transduction were also observed in spleen sections from mice injected with Ad5-HVR5*7*E451Q (Figure 5). However, in comparison to Ad5, higher levels of marginal zone cell transduction were observed in spleen sections from macrophage-depleted mice injected with Ad5-HVR5*7*E451Q at the highest dose (Figure 5), consistent with the results presented above. The transduced cells appeared to localize entirely around the white pulp in the marginal zone. To identify the virus transgene-expressing cell type in the spleen, we first characterized the effect of clodronate liposome pre-treatment on a number of relevant cell populations within the marginal zone of the spleen (F4/80, MARCO, CD169, SIGNR1, CD11b, CD11c, B220, ER-TR7, MAdCAM-1) in PBS-treated animals (supplemental Figure 4). Importantly, we also included structural components (reticular fibroblasts and sinus lining endothelial cells) that are known to be localized within this region of the spleen. The markers that remained detectable within the marginal zone after clodronate treatment (and were therefore available to interact with virus) were selected for colocalization studies. We observed limited or low level colocalization of β-galactosidase expression with SIGNR1+, CD11b+ and B220+ cell types (Figure 6). Moreover, we detected clear colocalization of β-galactosidase expression with CD11c+ cells and with structural components of the marginal zone, the reticular fibroblasts (ER-TR7) and MAdCAM-1+ sinus lining endothelial cells (Figure 6).
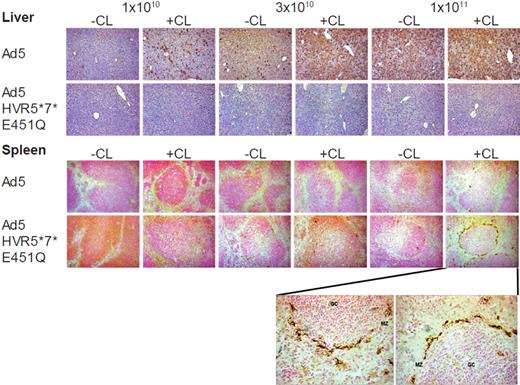
Immunohistochemical analysis of liver and spleen sections. Immunohistochemistry of liver (top panel) and spleen (bottom panel) from macrophage-depleted (CL+) or control mice (CL−) 48 hours after the intravascular administration of 1 × 1010, 3 × 1010, and 1 × 1011 vp of Ad5 or Ad5-HVR5*7*E451Q at low magnification (10×). Liver transduction was only observed for Ad5, while only Ad5-HVR5*7*E451Q efficiently transduced the spleen at high dose in macrophage-depleted mice. High power magnification (40×) of the spleen section at 1 × 1011 vp of Ad5-HVR5*7*E451Q in macrophage-depleted mice is represented below spleen panel. MZ indicates marginal zone; and GC, germinal center.
Immunohistochemical analysis of liver and spleen sections. Immunohistochemistry of liver (top panel) and spleen (bottom panel) from macrophage-depleted (CL+) or control mice (CL−) 48 hours after the intravascular administration of 1 × 1010, 3 × 1010, and 1 × 1011 vp of Ad5 or Ad5-HVR5*7*E451Q at low magnification (10×). Liver transduction was only observed for Ad5, while only Ad5-HVR5*7*E451Q efficiently transduced the spleen at high dose in macrophage-depleted mice. High power magnification (40×) of the spleen section at 1 × 1011 vp of Ad5-HVR5*7*E451Q in macrophage-depleted mice is represented below spleen panel. MZ indicates marginal zone; and GC, germinal center.
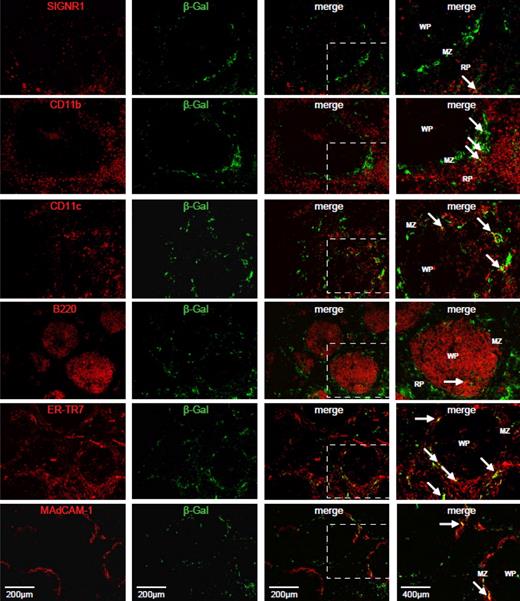
Colocalization of viral transgene expression with spleen cell markers after treatment with clodronate liposomes. Frozen spleen sections (6 μm) were stained with antibodies for SIGNR1, CD11b, CD11c, B220, ER-TR7, and MAdCAM-1 in combination with an antibody to viral transgene, β-galactosidase (Table 1). Single stained and merged images are presented. An enlarged portion of each merged image is displayed in the far right panel, highlighted by a white box. White arrows indicate regions of colocalization (in yellow). WP indicates White pulp; MZ, Marginal zone, and RP, Red pulp. Images are representative of multiple fields of view from 2-4 different animals.
Colocalization of viral transgene expression with spleen cell markers after treatment with clodronate liposomes. Frozen spleen sections (6 μm) were stained with antibodies for SIGNR1, CD11b, CD11c, B220, ER-TR7, and MAdCAM-1 in combination with an antibody to viral transgene, β-galactosidase (Table 1). Single stained and merged images are presented. An enlarged portion of each merged image is displayed in the far right panel, highlighted by a white box. White arrows indicate regions of colocalization (in yellow). WP indicates White pulp; MZ, Marginal zone, and RP, Red pulp. Images are representative of multiple fields of view from 2-4 different animals.
In addition to hexon modification, we analyzed the effect of other capsid modifications on spleen transduction. CAR-binding ablated and integrin-binding ablated Ad5 vectors produced significantly reduced spleen transduction in MF1 mice pre-injected with FX binding protein (X-bp) in the presence of macrophage depletion. Although significant lower levels of transduction were observed in the spleen, CAR-binding ablation, integrin-binding ablation or the combination of both modifications in the parental Ad5 vector were not able to completely preclude spleen transduction after intravenous adenoviral delivery (supplemental Figure 5).
Inflammatory profiles in mice injected with Ad5 and FX-binding ablated vectors
Ad capsid proteins induce innate toxicity after intravascular delivery that elicits the release of different cytokines and chemokines. To analyze the systemic toxicity associated with FX-binding ablated Ad vectors, serum was obtained from mice injected with Ad5 or Ad5-HVR5*7*E451Q 1 hour or 6 hours after administration. Cytokine and chemokine analysis was carried out using a multiplex panel. Serum cytokine/chemokine profiles showed that levels of most inflammatory factors were not significantly different between Ad5 and Ad5-HVR5*7*E451Q after administration of low and intermediate doses (supplemental Table 1). However, at the highest dose we observed an increase in the levels of most cytokines/chemokines on the panel for both Ad5 and Ad5-HVR5*7*E451Q. Comparing Ad5 versus Ad5-HVR5*7*E451Q, we observed a significant increase in interleukin-12 (IL-12), monokine-induced by interferon γ (MIG), and 10-kDa interferon γ–induced protein (IP-10) levels in control and macrophage depleted mice 6 hours after administration of Ad5-HVR5*7*E451Q (P < .05; supplemental Figure 6). IL-12, MIG and IP-10 levels were lower in macrophage-depleted mice for both vectors although Ad5-HVR5*7*E451Q induced significantly higher levels of these cytokines and chemokines in both control and macrophage-depleted mice (P < .05; supplemental Figure 6). Indeed, we observed higher levels of monocyte chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1α (MIP-1α) (P < .05) with Ad5-HVR5*7*E451Q, but only in macrophage-depleted mice compared with Ad5 (supplemental Figure 6A-B). IL-6 and interferon γ (IFN-γ) cytokines levels have been reported to increase after intravascular delivery of Ad5.33 In this study, IL-6 levels were similar for Ad5 and Ad5-HVR5*7*E451Q in control mice. However, we observed a significant decrease in IL-6 levels for Ad5-HVR5*7*E451Q in macrophage-depleted mice compared with control mice (supplemental Figure 6C). In addition, IFN-γ levels did not change between vectors at the highest dose (supplemental Figure 6D). Although we observed significant differences in serum cytokine/chemokine profiles between Ad5 and Ad5-HVR5*7*E451Q, we cannot predict what the associated toxicity for FX-binding ablated Ad in humans will be because the dose that produced changes in inflammatory profiles in mice (4 × 1012 vp/Kg) is significantly higher than doses used in clinical trials.34-37
Construction and analysis of a FX-binding ablated Ad5 vector carrying a high affinity fiber from Ad35
To assess the retargeting ability of Ad5-HVR5*7*E451Q, we genetically exchanged the Ad5 fiber for the Ad35 fiber (Ad35++) generating Ad5-HVR5*7*E451Q/F35++. The F35++ gene includes 2 amino acid changes previously described,28 which confers 60× higher affinity to CD46 compared with Ad35. To analyze the binding to FX and CD46, we performed SPR experiments using different Ad5 vectors. SPR analysis of the interaction of whole virus to FX immobilized on the sensorchip showed Ad5 could only bind to FX, Ad35Luc to both FX and CD46 while Ad5-HVR5*7*E451Q was not able to bind to FX or CD46 (Figure 7A). Having demonstrated that the F35++ fiber conferred the ability to bind CD46 (Figure 7A), we performed cell binding and transduction experiments in vitro in the presence or absence of FX. In these experiments, we used a cell line stably transfected to express CD46 (CHO-BC1) and a control cell line that does not express this receptor (CHO-WTR). Ad5 bound and transduced both cell types but only in the presence of FX (Figure 7B and supplemental Figure 7A-B) while Ad5-HVR5*7*E451Q was not able to bind or transduce either cell type under these conditions (Figure 7B and supplemental Figure 7A-B). Of note, Ad5-HVR5*7*E451Q/F35++ was able to transduce CHO-BC1 cells in the absence of FX, showing clear retargeting to CD46 (Figure 7B and supplemental Figure 7A-B). To evaluate the potential retargeting of Ad5-HVR5*7*E451Q/F35++ in vivo, we injected Ad5-HVR5*7*E451Q or Ad5-HVR5*7*E451Q/F35++ into CD46 transgenic mice intravenously in the presence or absence of macrophage depletion. Transduction profiles at 48 hours showed Ad5-HVR5*7*E451Q/F35++ transduced the lung in macrophage-depleted mice as previously described,31 precluding liver transduction and significantly reducing the levels of spleen transduction compared with Ad5-HVR5*7*E451Q (Figure 7C). In macrophage-depleted CD46 transgenic mice, the lung/liver ratio for Ad5-HVR5*7*E451Q/F35++ was ∼ 800 (compared with a ratio of ∼ 0.05 for Ad5-HVR5*7*E451Q) showing an approximated 16 000-fold increase in lung transduction compared with Ad5-HVR5*7*E451Q. Although we did not observe increased lung transduction in the absence of clodronate liposomes after administration of Ad5-HVR5*7*E451Q, lower levels of spleen and basal liver transduction were observed (Figure 7C). Collectively, these data provide proof of concept for retargeting of FX-binding ablated Ad5 vectors. Nevertheless, strategies will be required to ablate macrophage uptake and fully exploit the retargeting potential of these vectors.
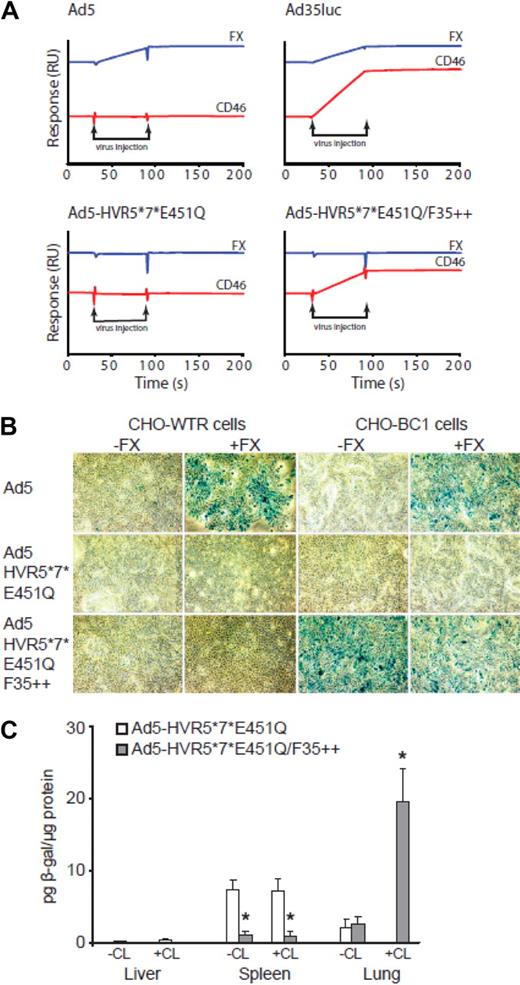
Retargeting of Ad5-HVR5*7*E451Q/F35++ to CD46 in vitro and in vivo. (A) Binding analysis was performed by surface plasmon resonance. Flow cell (Fc)1: blank immobilization; Fc2: 542RU CD46; and Fc4 455RU FX were prepared by amine coupling. Viruses at a concentration of 1011 VP/mL were injected across all 4 flow cells at a flow rate of 30 μL/minute in 10 mM HEPES pH 7.4; 150 mM NaCl; 5 mM CaCl2; 0.005% Tween 20. Representative subtracted sensorgrams (Fc2-Fc1; Fc3-Fc1; Fc4-Fc1) for each virus show the relative change in response (RU). The sensorgrams are off set for easier visualization. (B) Xgal staining of CHO-WTR and CHO-BC1 cells using 1000 viral particles of Ad5, Ad5-HVR5*7*E451Q, and Ad5-HVR5*7*E451Q/F35++ vectors in the presence or absence of FX (*P < .05 vs PBS control). (C) Analysis of β-gal transgene expression in the liver, spleen and lung tissue after the intravascular administration of 1 × 1011 vp into CD46 transgenic mice in the presence or absence of macrophage depletion. (*P < .05 vs Ad5-HVR5*7*E451Q, n = 5).
Retargeting of Ad5-HVR5*7*E451Q/F35++ to CD46 in vitro and in vivo. (A) Binding analysis was performed by surface plasmon resonance. Flow cell (Fc)1: blank immobilization; Fc2: 542RU CD46; and Fc4 455RU FX were prepared by amine coupling. Viruses at a concentration of 1011 VP/mL were injected across all 4 flow cells at a flow rate of 30 μL/minute in 10 mM HEPES pH 7.4; 150 mM NaCl; 5 mM CaCl2; 0.005% Tween 20. Representative subtracted sensorgrams (Fc2-Fc1; Fc3-Fc1; Fc4-Fc1) for each virus show the relative change in response (RU). The sensorgrams are off set for easier visualization. (B) Xgal staining of CHO-WTR and CHO-BC1 cells using 1000 viral particles of Ad5, Ad5-HVR5*7*E451Q, and Ad5-HVR5*7*E451Q/F35++ vectors in the presence or absence of FX (*P < .05 vs PBS control). (C) Analysis of β-gal transgene expression in the liver, spleen and lung tissue after the intravascular administration of 1 × 1011 vp into CD46 transgenic mice in the presence or absence of macrophage depletion. (*P < .05 vs Ad5-HVR5*7*E451Q, n = 5).
Discussion
Over the past 20 years, virologists and gene therapists alike have sought to elucidate the basic mechanisms used by Ad5 to transduce cells in vivo. Here, we show that vectors ablated for FX-binding display altered biodistribution, tropism and inflammatory profiles compared with parental Ad5 after systemic administration. As previously demonstrated with Ad5,5 the dose of vector delivered intravenously can significantly affect liver uptake. In this study, we observed that at all doses studied the levels of liver gene transfer for FX-binding ablated Ad vectors were substantially lower compared with Ad5 in control or macrophage-depleted mice. However, significant differences in splenic uptake and transduction were found. Interestingly, macrophage depletion promoted viral genome accumulation in the spleen after systemic administration of FX-binding ablated vectors at the highest dose used. Despite these substantial differences in biodistribution and tropism, inflammatory profiles were largely unaffected compared with parental Ad5 at low and intermediate doses.
We have previously shown using SPR analysis that neither Ad5-HVR7(Ad26) or Ad5-HVR5*7*E451Q bind to FX27 and we have now further shown purified hexon from these viruses has also undetectable binding to FX (supplemental Figure 8). It is clear from this work and from our previous studies that modulation of HVR7 is required to fully ablate FX binding. Even at substantial viral doses (1 × 1011 vp/mouse), we failed to detect any liver transduction at the cellular level. Therefore, as a vector system to preclude liver transduction, vectors based on the above documented mutations will show considerable promise for liver detargeting purposes. However, our results show that Ad5 vectors devoid of FX-binding still accumulate within macrophages. Previous studies have shown that there is significantly higher accumulation of Ad5 in macrophages of warfarinized mice at early time points (eg, 1 hour) after administration.22 Compared with parental Ad5, we observed a potential increase in viral genome accumulation in the liver 1 hour after intravenous delivery of FX-binding ablated Ad5. Although this increase was not statistically significant, it suggests that there may be increased uptake of FX-binding ablated Ad5 vectors by Kupffer cells and therefore warrants further investigation.
Importantly, we show that FX-binding ablation affected not only Ad5 biodistribution and liver/spleen transduction but also the transduction of other organs. Of note, lung and heart transduction by FX-binding ablated Ad5 vectors was substantially lower than parental Ad5 at high doses in macrophage-depleted mice (Figure 4C-D). Our data show that although viral genome accumulation in these organs at early time points is not affected by FX-binding ablation, FX-binding may promote adenoviral transduction of certain lung or heart cell populations at the 48-hour time point.
Despite the highly efficient liver detargeting and the lack of transduction in other tissues (lung and heart), we observed important biodistribution and tropism differences for the spleen that may impact on the design and utility of FX-binding ablated vectors. Increased transduction of the spleen by FX-binding ablated vectors only occurs at high dose and only when macrophages are depleted. Of note, the levels of cytokines/chemokines were substantially increased at high dose regardless of macrophage depletion. Our results suggest that FX-binding ablated vectors induce a more robust Th1 immune response than parental Ad5. Macrophage depletion attenuates this response (supplemental Figure 6), indicating that these inflammatory mediators may in part be secreted by macrophages or their secretion induced by macrophage-derived mediators. It is difficult to judge whether the increased Th1 response to FX-ablated vectors would be relevant in the clinical context, as the majority of clinical trials use much lower relative doses of Ad vectors (between 2.5-7.5 × 1010 vp/Kg,34-37 compared with our highest dose of 4 × 1012 vp/kg). In our study, we found no differences in spleen transduction or inflammatory responses for the majority of cytokines/chemokines between FX-binding ablated vectors and parental Ad5 at the low or intermediate doses (4 × 1011 vp/Kg or 1.2 × 1012 vp/Kg, respectively). Previous studies have shown that Ad5 can activate macrophages and dendritic cells.38 In addition, Di Paolo and collaborators showed that metallophilic and marginal zone macrophages (CD169+ and MARCO+ cells) colocalise with Ad vectors in the spleen at early time points after intravascular administration.20 We found significantly greater numbers of β-gal positive cells in the splenic marginal zones after intravenous delivery of FX-binding ablated Ad vectors, but only in macrophage-depleted mice. A number of cell types are known to reside in the marginal zone including metallophilic macrophages, marginal zone macrophages, dendritic cells, fibroblasts, marginal zone B cells and lymphocytes.39 Immunohistochemistry studies showed that the specific transduced cell types found in the marginal zone of the spleen particularly colocalized with CD11c+, ER-TR7+, and MAdCAM-1+. Although we attempted to fully characterize the spleen cell population transduced by Ad5-HVR5*7*E451Q using an extensive range of cell surface markers, the majority of β-gal+ cells did not colocalize with single markers, indicating a complex cellular interaction in the spleen mediated by Ad5-HVR5*7*E451Q. Of note, our results indicate that Ad5-HVR5*7*E451Q use FX-independent pathways to transduce splenic cells, perhaps via cell surface interactions with fiber, penton or unmodified hexon regions. Importantly, we found a significant reduction in spleen transduction using CAR-binding or integrin-binding ablated Ad5 vectors. However, these modifications, alone or in combination, did not completely preclude spleen transduction by Ad5 that suggests multiple mechanisms facilitate spleen uptake and transduction.
Retargeting strategies for Ad5 have been extensively investigated over the past few years (for review see Mebius39 and Alemany40 ). However, most retargeting strategies have not been entirely successful due to the high affinity of the FX-Ad5 complex for hepatocytes.26 The potential for detargeting via FX modulation has been demonstrated by a recent study using an oncolytic version of the HVR5-modified Ad5 vector Ad-GL-HB.41 The authors showed reduced liver transduction but no decrease in the number of liver viral genomes 5 days after delivery. Although we observed significantly reduced IL-6 induction after administration of Ad5-HVR5*7*E451Q to macrophage-depleted mice, in the Shashkova study IL-6 levels remained high after Ad-GL-HB administration even after viral predosing.41 These differences could be explained by the fact that the HVR5*7*E451Q mutations completely abolish the interaction with FX27 (supplemental Figure 8) while Ad-GL-HB still is able to bind FX, albeit at a greatly reduced level, and transduces the liver.42 Here, we provided a genetic retargeting strategy exchanging the Ad5 fiber for the Ad35++ fiber in Ad5-HVR5*7*E451Q and showed efficient retargeting to CD46 in vitro without binding to coagulation FX. In vivo, we observed lung retargeting of Ad5-HVR5*7*E451Q/F35++ in macrophage-depleted CD46 transgenic mice with a lung/liver ratio ∼16 000 times higher than Ad5-HVR5*7*E451Q thereby illustrating the potential of these vectors in future retargeting strategies. However, the retargeting to the lung was only achieved in the presence of macrophage depletion. Additional strategies are still required to manipulate the uptake of Ad5 by macrophages. Therefore, our results indicate that modifications in HVR7 or point mutations included in both HVR5 + 7 have the potential to improve current tumor targeting strategies by increasing liver detargeting and warrant further investigation.
In summary, the present study investigated the biodistribution and tropism of FX-binding ablated Ad5 vectors after intravascular administration. We show that Ad5 vectors devoid of FX binding are not able to transduce hepatocytes regardless of dose or macrophage depletion. Crucially, dosing experiments revealed differences in Ad5 uptake and transduction between FX-binding ablated Ads and parental Ad5, with increased spleen transduction by FX-binding ablated Ads observed only at the highest dose in macrophage-depleted mice. Low levels of transduction observed in other organs indicate a clear splenic tropism for these modified vectors. Therefore, FX-binding ablated Ad vectors in combination with retargeting strategies, offer a new platform for gene therapy applications where evasion of liver gene transfer is required.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nicola Britton and Gregor Aitchison at the British Heart Foundation Glasgow Cardiovascular Research Center (BHF GCRC) for technical assistance, Sue Stevenson for providing the Ad5-CTL, Ad5-KO1, Ad5-PD1, and Ad5-KO1PD1 viruses, and Jerome Custers (Crucell) for the Ad35Luc vector. We thank Paul Garside and James M. Brewer for helpful discussions on spleen immunohistochemistry.
This work was supported by the Biotechnology and Biological Sciences Research Council and British Heart Foundation (A.H.B.). A.M.M. holds a BHF intermediate fellowship. A.L.P. holds a personal research fellowship from the Royal Society of Edinburgh Scotland Foundation.
Authorship
Contribution: R.A., A.C.B., L.C., R.A.M., S.N.W., S.M.K.B., J.A.G., J.H.M., A.L.P., and A.M.M. performed research; R.A., L.C., S.A.N., J.H.M., and A.H.B. analyzed data; H.W., A.L., and N.v.R. provided essential reagents; R.A. and A.H.B. wrote the paper and made the figures with S.N.W., A.C.B., L.C., S.A.N., and A.H.B., and all authors edited and agreed; and A.H.B. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew H. Baker, British Heart Foundation Glasgow Cardiovascular Research Centre, Division of Cardiovascular and Medical Sciences, 126 University Pl, University of Glasgow, Glasgow, G12 8TA, United Kingdom; e-mail: ab11f@clinmed.gla.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal