Abstract
Kinetics of hematopoietic recovery driven by different types of human stem and progenitor cells after transplantation are not fully understood. Short-term repopulating cells (STRCs) dominate early hematopoiesis after transplantation. STRCs are highly enriched in adult mobilized peripheral blood compared with cord blood, but the length of their contribution to hematopoiesis remains unclear. To understand posttransplantation durability and lineage contribution of STRCs, we compared repopulation kinetics of mobilized peripheral blood (high STRC content) with cord blood transplants (low STRC content) in long-lived NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (IL2RG−/−) mice. This comparison demonstrates that quantitative contribution of human STRCs to hematopoiesis is restricted to the first 5 months after transplantation. The ratio of STRCs to long-term repopulating cells dramatically changes during ontogeny. This model enables to precisely determine early and late engraftment kinetics of defined human repopulating cell types and to preclinically assess the engraftment kinetics of engineered stem cell transplants.
Introduction
Swift hematopoietic recovery is important to minimize posttransplant toxicity. Comparative transplantation studies of human hematopoietic cells in fetal sheep1 and immunodeficient mice2-4 as well as retroviral marking studies in autografted patients5 have demonstrated the importance of short-term repopulating cells (STRCs) in early hematopoiesis. We have previously identified 2 distinct classes of human STRCs in NOD.Cg-PrkdcscidB2mtm1Unc/J (B2m−/−) mice: myeloid-restricted STRC-M generating myeloid progeny for up to 3 weeks after transplantation, and lymphomyeloid STRC-ML with a more protracted activity. STRCs from different hematopoietic sources display an intrinsically fixed differentiation program6 with 7- to 12-fold enrichment in human mobilized peripheral blood (mPB) compared with bone marrow (BM) and umbilical cord blood (CB).4 However, because of the short life span of B2m−/− mice,7 the durability of contribution of human STRCs and long-term repopulating cells (LTRCs) to hematopoiesis could not be addressed experimentally and remained unknown. Recently, an improved immunodeficient mouse strain was developed that combines interleukin-2 receptor common γ-chain deficiency on NOD/SCID background (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ [IL2RG−/−]). IL2RG−/− mice allow very efficient human hematopoietic engraftment8 and have a comparable life span to wild-type, thus permitting long-term repopulation studies of human hematopoiesis beyond 6 months of observation time.9 To understand the relative contribution of STRCs and LTRCs to hematopoiesis over time, we compared the lineage differentiation, peripheralization, and long-term kinetics of human mPB (high STRC content) and CB transplants (low STRC content) in IL2RG−/− mice.
Methods
Human cells
Human granulocyte colony-stimulating factor–mobilized mPB was collected according to protocols approved by the Institutional Review Board of the Freiburg University Hospital. CB was obtained from full-term delivered infants with parental consent in accordance with the principles of the Declaration of Helsinki. After Ficoll-Hypaque density gradient centrifugation (PAA Laboratories), CD34+ cells were magnetically isolated (Miltenyi Biotec) and cryopreserved in Dulbecco modified Eagle medium (Invitrogen) containing 30% fetal calf serum (FCS; StemCell Technologies) and 15% dimethyl sulfoxide (Sigma-Aldrich). Purity ranged from 71.1% plus or minus 9.05% for CB and 92.1% plus or minus 5.4% for mPB CD34+ cells. Before transplantation, cells were thawed, washed, and resuspended in Dulbecco modified Eagle medium containing 10% FCS and 1% glutamine (Invitrogen).
Xenotransplantation
NOD.Cg-PrkdcscidB2mtm1Unc/J (B2m−/−) and NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (IL2RG−/−) mice were bred and housed at the German Cancer Research Center pathogen-free animal facility according to applicable laws and regulations. After transplantation, 0.5 mL/L Baytril (10%, BayerVital) was added to the drinking water.
CB CD34+ cells (5 × 104 or 2 × 105) or mPB CD34+ cells (6 × 105 or 2 × 106) were injected intravenously into irradiated (325 cGy) 8- to 10-week-old mice. Three, 8, and 12 weeks and then every 8th week after transplantation BM was aspirated and PB was collected. After lysis of erythrocytes (0.15M ammonium chloride), cells were washed in Hanks balanced salt solution (Sigma-Aldrich) containing 2% FCS. After Fc-receptor blocking (anti–mouse CD16/CD32), cells were stained with anti–human CD45/71, CD19/20, CD15/66b, CD33, CD34, and glycophorin-A (BD Biosciences). Positive engraftment was defined as more than 10 human CD45/71+ cells per 2 × 104 living cells analyzed at LSRII (BD Biosciences).
Statistical analyses
Significant differences between groups were assessed using Student t test (P < .05). LTRC frequencies were calculated using Poisson statistics (L-Calc, StemCell Technologies).
Results and discussion
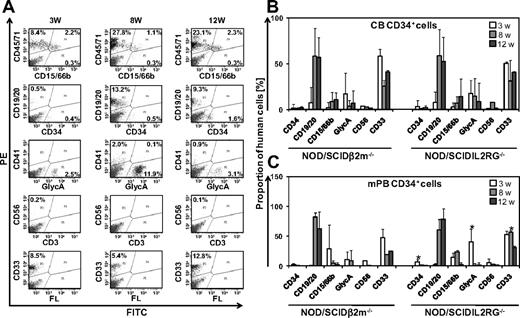
To investigate whether IL2RG−/− mice allow efficient engraftment of human STRCs, we compared early repopulating activity and differentiation of CD34+ CB and mPB cells in IL2RG−/− to the established STRC assay in B2m−/− mice.4 Human cells engrafted in all transplanted mice. At 3 to 12 weeks after transplantation, proportions of human CD45/71+ cells detected in the BM of B2m−/− mice varied from 0.1% to 40.7% after mPB and from 0.1% to 68.8% after CB transplantation. Engraftment levels of human CD45/71+ cells in BM of IL2RG−/− mice ranged from 0.2% to 93.7% after mPB transplantation and 0.1% to 82.2% after CB transplantation, indicating that early human hematopoietic reconstitution was at least equally efficient compared with B2m−/− mice. Human CD34+ CB and mPB reliably generated myeloid (CD15/66b+, CD33+, glycophorin-A+), B-lymphoid (CD19/20+), and CD34+ cells in the BM of both mouse strains (Figure 1A-C). Predominantly myeloid cell engraftment was observed 3 weeks after transplantation, indicating STRC-M activity.4 High levels of B-lymphoid cells were detected 8 to 12 weeks after transplantation, demonstrating contribution of STRC-ML to hematopoiesis.4 Lineage distribution and engraftment of human transplants were similar in B2m−/− and IL2RG−/− mice, only differing by a 3- to 4-fold larger proportion of erythroid, CD34+, and myeloid CD33+ cells in IL2RG−/− mice (Figure 1B-C). Taken together, our results demonstrate that IL2RG−/− mice are well-suited hosts for human STRC engraftment.
Human STRC-M and STRC-ML efficiently engraft in IL2RG−/− mice. (A) Representative fluorescence-activated cell sorter profiles of human hematopoietic lineages in BM of IL2RG−/− mouse at 3, 8, and 12 weeks after transplantation of 2 × 105 CD34+ cells. Percentages in fluorescence-activated cell sorter plots refer to lineage marker-positive cells of 2 × 104 total BM cells analyzed. (B) Human CD34+ CB cells show similar multilineage differentiation patterns in the BM of IL2RG−/− mice (n = 10) and B2m−/− mice (n = 10) 3, 8, and 12 weeks after transplantation. (C) In IL2RG−/− mice (n = 12), a larger proportion of CD34+ and GlycA+ cells were detected at 3 weeks and CD33+ cells at 8 weeks after transplantation of human mPB CD34+ cells compared with B2m−/− mice (n = 6). GlycA indicates glycophorin-A; w, weeks; PE, phycoerythrin; and FITC, fluorescein isothiocyanate. Error bars represent SD. *Significant difference (P < .05).
Human STRC-M and STRC-ML efficiently engraft in IL2RG−/− mice. (A) Representative fluorescence-activated cell sorter profiles of human hematopoietic lineages in BM of IL2RG−/− mouse at 3, 8, and 12 weeks after transplantation of 2 × 105 CD34+ cells. Percentages in fluorescence-activated cell sorter plots refer to lineage marker-positive cells of 2 × 104 total BM cells analyzed. (B) Human CD34+ CB cells show similar multilineage differentiation patterns in the BM of IL2RG−/− mice (n = 10) and B2m−/− mice (n = 10) 3, 8, and 12 weeks after transplantation. (C) In IL2RG−/− mice (n = 12), a larger proportion of CD34+ and GlycA+ cells were detected at 3 weeks and CD33+ cells at 8 weeks after transplantation of human mPB CD34+ cells compared with B2m−/− mice (n = 6). GlycA indicates glycophorin-A; w, weeks; PE, phycoerythrin; and FITC, fluorescein isothiocyanate. Error bars represent SD. *Significant difference (P < .05).
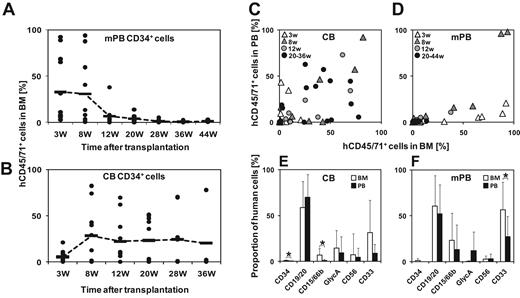
We have previously shown that mPB is highly enriched in STRCs, whereas CB contains a higher number of LTRCs.4 We hypothesized that long-term engraftment kinetics of human CD45/71+ cells from both hematopoietic cell sources should be different. To assess the relative hematopoietic contribution of human STRCs and LTRCs over time, we compared long-term engraftment kinetics of human mPB with those of CB transplants in IL2RG−/− mice. Serial analysis of BM aspirates for up to 44 weeks after transplantation revealed largely different engraftment kinetics. mPB transplants generated maximum progeny starting in week 3 for up to 8 weeks after transplantation. Subsequently, human engraftment gradually declined to low levels at 20 to 28 weeks after transplantation (Figure 2A). In contrast, CB transplants produced maximal engraftment 8 weeks after transplantation that remained stable for the whole observation period (Figure 2B). The frequency of LTRCs in mPB transplants was calculated using Poisson statistics on the number of positive and negative mice. The value obtained was 1 per 4.1 × 105. As all mice were positive after CB transplantation, only the lower limit of the LTRC frequency could be calculated. It was at least 12-fold higher than in mPB (at least 1 per 3.0 × 104 CB cells). Kinetics of mPB transplants (high STRC content) compared with CB transplants (low STRC content) demonstrated that quantitative contribution of STRC-ML to hematopoiesis is transient and restricted to the first 5 months after transplantation (Figure 2A-B).
Deficient peripheralization and different repopulation kinetics of human transplants in IL2RG−/− mice. (A) Very low engraftment levels of human cells at 28 weeks after mPB transplantation indicate quantitative contribution of STRC-ML to hematopoiesis only for the first 5 months (n = 12). Horizontal bars indicate mean values. Data were pooled from 3 independent experiments. (B) LTRCs contribute efficiently to stable long-term engraftment in the BM of IL2RG−/− mice after CB transplantation (n = 10). Horizontal bars represent mean values. Data were pooled from 3 independent experiments. (C) Similar proportions of human CD45/71+ cells in PB and BM of IL2RG−/− mice transplanted with human CD34+ CB cells (n = 10). (D) Proportion of human CD45/71+ cells in murine BM at 3 weeks after transplantation of human CD34+ mPB cells is significantly higher compared with murine PB (n = 12). (E-F) Deficient peripheralization of human myeloid cells after transplantation of human CB (E) and mPB (F) CD34+ cells. GlycA indicates glycophorin-A; and w, weeks. Error bars represent SD. *Significant difference (P < .05).
Deficient peripheralization and different repopulation kinetics of human transplants in IL2RG−/− mice. (A) Very low engraftment levels of human cells at 28 weeks after mPB transplantation indicate quantitative contribution of STRC-ML to hematopoiesis only for the first 5 months (n = 12). Horizontal bars indicate mean values. Data were pooled from 3 independent experiments. (B) LTRCs contribute efficiently to stable long-term engraftment in the BM of IL2RG−/− mice after CB transplantation (n = 10). Horizontal bars represent mean values. Data were pooled from 3 independent experiments. (C) Similar proportions of human CD45/71+ cells in PB and BM of IL2RG−/− mice transplanted with human CD34+ CB cells (n = 10). (D) Proportion of human CD45/71+ cells in murine BM at 3 weeks after transplantation of human CD34+ mPB cells is significantly higher compared with murine PB (n = 12). (E-F) Deficient peripheralization of human myeloid cells after transplantation of human CB (E) and mPB (F) CD34+ cells. GlycA indicates glycophorin-A; and w, weeks. Error bars represent SD. *Significant difference (P < .05).
We then asked whether human blood cells peripheralize into the PB of IL2RG−/− mice. Numbers and lineage differentiation of engrafted cells were compared in BM and PB of IL2RG−/− mice transplanted with either human CD34+ CB (n = 10) or mPB cells (n = 12). In most mice, human cells were reliably detectable in the periphery after CB but not after mPB transplantation (Figure 2C-D). The full lineage spectrum of human cells could only be observed in murine BM. Human CD15/66b+ cells derived from both cell sources did not efficiently peripheralize in IL2RG−/− mice. Almost all human cells detected in PB were B lymphocytes (Figure 2E-F).
Taken together, our data provide direct in vivo evidence that hematopoietic activity of human STRCs is transient and restricted to the first 5 months after transplantation. Our results suggest that long-term repopulation studies of human transplants in IL2RG−/− mice can accurately predict clinical results5 if they are observed for at least 6 months after transplantation to specifically address LTRC activity. Our findings confirm the hypothesis of a biphasic nature of engraftment generated in gene-marking studies in humans and nonhuman primates.5,10,11 In autografted CML patients, gene-marked mPB cells only contribute to hematopoietic reconstitution during the first 2 to 4 months after transplantation.5 Gene-marked LTRC clones in autografted monkeys were not detectable before 2 months after transplantation.11 Interestingly, studies in syngeneic murine hosts have concluded that a minimum of 16 weeks, optimally 6 months, is required to assess long-term posttransplant hematopoiesis.12-14 Quantification of human hematopoietic cells by IL2RG−/− mouse transplantation could allow to more closely predict the speed and durability of hematopoietic reconstitution of ex vivo engineered clinical hematopoietic transplants.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Wei Wang for invaluable critical comments and discussion and Daniela Belle for the excellent technical assistance.
This work was supported by grants from the German Cancer Aid (Deutsche Krebshilfe, project 107217).
Authorship
Contribution: O.Z. and C.R.B. designed and performed research, analyzed data, and wrote the manuscript; F.H. helped to analyze the data; S.F. performed transplants; M.S. designed research and analyzed data; C.v.K. designed research and wrote the manuscript; and H.G. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christof von Kalle, National Center for Tumor Diseases, German Cancer Research Center, Im Neuenheimer Feld 350, Heidelberg, Germany; e-mail: christof.kalle@nct-heidelberg.de.
References
Author notes
O.Z. and C.R.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal