Abstract
Fatty acid metabolism governs multiple intracellular signaling pathways in many cell types, but its role in hematopoietic stem cells (HSCs) is largely unknown. Herein, we establish a critical role for 12/15-lipoxygenase (12/15-LOX)–mediated unsaturated fatty acid metabolism in HSC function. HSCs from 12/15-LOX–deficient mice are severely compromised in their capacity to reconstitute the hematopoietic compartment in competitive and serial reconstitution assays. Furthermore, we demonstrate that 12/15-LOX is required for the maintenance of long-term HSC quiescence and number. The defect in HSCs is cell-autonomous and associated with a selective reduction in 12/15-LOX–mediated generation of bioactive lipid mediators and reactive oxygen species and with a decrease in canonical Wnt signaling as measured by nuclear β-catenin staining. These results have implications for development, aging, and transformation of the hematopoietic compartment.
Introduction
Hematopoiesis is the process whereby the blood cell lineages are generated from a multipotent hematopoietic stem cell (HSC). HSCs possess the unique ability to self-renew but also differentiate to give rise to mature blood cells. Long-term HSCs (LT-HSCs) are the most primitive HSCs and can fully reconstitute the hematopoietic compartment of lethally irradiated animals. They reside in a hypoxic niche and are generally quiescent.1,2 In comparison, short-term HSCs (ST-HSCs) are temporally limited in their ability to reconstitute lethally irradiated animals and more actively cycle. HSCs are regulated through their environmental niche, cytokine signaling, and the orchestrated activities of various transcription factors.3 However, there is a relative paucity of information about the signal transduction events that regulate HSC function. In particular, the effects of fatty acid (FA) metabolism and lipid mediators on HSC function are not well understood.
The function of HSCs is dependent on their unique capacity to both differentiate and self-renew, which is related to their proliferative capacity.4,5 Highly proliferative HSCs are less efficient at reconstituting lethally irradiated mice.6 Thus, HSCs that lack cell-cycle inhibitors required to maintain quiescence, such as p21cip/WAF, are defective.7 HSC function is also regulated by transcription factors including Gfi-1, Bmi, Hox4b, and PU.1.8-11 Furthermore, canonical Wnt signaling was shown to regulate HSC function by maintaining quiescence and initiating a self-renewal program of gene transcription.12-14
12/15-Lipoxygenase (12/15-LOX) is a lipid-peroxidizing enzyme that mediates unsaturated FA metabolism. 12/15-LOX introduces molecular oxygen into arachidonic acid (AA) and linoleic acid to produce bioactive labile lipid intermediates such as 12(S)-hydroperoxyeicosatetraenoic acid, 15(S)-hydroperoxyeicosatetraenoic acid, and 13(S)-hydroperoxyoctadecadienoic acid. These intermediates are rapidly reduced by glutathione reductase, which releases reactive oxygen species (ROS) and produces additional bioactive lipid metabolites, including 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE), 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE), 13(S)-hydroxyoctadecadienoic (13(S)-HODE), lipoxins, and hepoxilins.15 Eicosanoid products activate multiple signaling pathways and transcription factors such as peroxisome proliferator–activated receptor-γ, interferon consensus sequence-binding protein/interferon regulatory factor-8 (ICSBP/IRF-8), and nuclear factor-κB to modulate gene transcription.16,17 These lipid mediators have pleiotropic affects on myriad cell types,18 but our knowledge of their role in HSCs is limited.19,20 Notably, in leukemia that results from abnormal hematopoiesis, lipid mediators are reduced.21-23
Previously, we showed that 12/15-LOX is a novel suppressor of myeloproliferative disease (MPD).24 Although less than 15% of 12/15-LOX–deficient mice (Alox15 mice) develop a severe MPD over the course of a year, the majority (> 85%) of Alox15 mice remain asymptomatic and exhibit only modest splenomegaly, basophilia, and an increased percentage of granulocytes in blood and spleen. Our previous studies defined 12/15-LOX as a novel mediator upstream of phosphatidylinositol 3-kinase and the transcription factor ICSBP/IRF-8 in the effected myeloid population. These studies indicate that 12/15-LOX acts as a suppressor of leukemogenesis.
Importantly, 12/15-LOX-regulated pathways have been implicated in hematopoietic cell differentiation. Aging HSCs, which exhibit reduced capacity to self-renew, have reduced ICSBP/IRF-8 levels.25 In addition, HSC function is partly controlled by ROS-dependent regulation of the p38 mitogen-activated protein kinase (MAPK) pathway.26 Moreover, 12(S)-HETE and 15(S)-HETE can affect development of human CD34+ hematopoietic progenitors in culture.19 However, a role for 12/15-LOX per se has not been established in hematopoietic development and HSC function.
Here we report that Alox15 mice exhibit a severe primary defect in HSC function. 12/15-LOX is required for maintenance of LT-HSC quiescence and number. The defect in Alox15 HSC function is associated with a selective reduction in 12/15-LOX–dependent generation of lipid metabolites and ROS, and with dysregulation of canonical Wnt signaling, which is required for normal HSC self-renewal. These data establish that 12/15-LOX is critical for normal HSC function.
Methods
Mice
C57BL/6 (B6) and Alox15 mice, backcrossed 11 generations, were purchased from The Jackson Laboratory. Mice were housed and bred in The Wistar Institute. Mice were used between 8 and 10 weeks of age before the onset of MPD except where explicitly stated. Congenic B6.SJL mice were obtained from Taconic Farms or the National Cancer Institute. All animal procedures were approved by The Wistar Institute Institutional Animal Care and Use Committee.
Hematologic analysis
Whole blood obtained via submandibular bleeding into ethylenediaminetetraacetic acid anticoagulant tubes was analyzed on an Advia2120 hematology analyzer in the mouse mode (Siemens Healthcare Diagnostics).
Antibodies
Single-cell suspensions were prepared from thymus, spleen, and bone marrow (BM) isolated from long bones. Red blood cells (RBCs) were lysed using ammonium chloride except for erythroid progenitor analysis. The following antibodies and reagents for flow cytometry were obtained from the following sources: c-Kit (Invitrogen); CD34, CD45.1 (Biolegend); CD3, immunoglobulin M (IgM), Gr-1, streptavidin conjugates (BD Biosciences); all others (eBioscience). Differentiated cells were depleted with antibodies against Gr-1, B220, CD3, interleukin-7 receptor-α (IL-7Rα), Ter119, NK1.1, Mac1, and CD11c; anti–IL-7Rα was omitted when analyzing common lymphoid progenitor (CLP), early thymic progenitor (ETP), and double-negative (DN) thymocytes DN2 and DN3. Cells were analyzed by flow cytometry on FACSCalibur or LSR II using FlowJo software (Tree Star). Cell-cycle analysis was performed using Hoechst 33342 and PyroninY (Sigma-Aldrich) as described.27 Lin−cKit+ cells were obtained using MACS separation kit according to the manufacturer's instructions (Miltenyi Biotec). Nuclear extracts were prepared using Nucbuster Kit (EMD Biosciences) from Lin−cKit+ cells from pooled mice. Lysates were normalized to total protein using Bradford assay (Pierce) and resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and immunoblotted with antibodies for Retinoblastoma protein (Santa Cruz Biotechnology) and β-catenin (BD Biosciences). Peroxidase-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories.
BM reconstitution and 5-fluorouracil assays
Recipient mice were irradiated with 1000 rad of γ-irradiation from a Cesium Mark irradiator (J.L. Sheppard and Associates). Mice received transplants of 1.8 × 106 (9:1) or 2 × 105 (1:1) BM cells from B6 or Alox15 age-matched mice mixed with 2 × 105 competitor BM cells, or with 1 × 106 (2:1) B6 or Alox15 embryonic day 14.5 (E14.5) fetal liver cells mixed with 0.5 × 106 competitor BM cells for competitive reconstitution assays and 1 × 106 BM cells for noncompetitive and serial reconstitution assays. Mice were injected intraperitoneally with 200 mg/kg 5-fluorouracil (5-FU; Roche).
Product quantification
ROS levels was analyzed on Lin−Sca1+cKit+ (LSK) cells isolated by cell sorting. LSK cells were stimulated with 10μM AA (Cayman Chemical) and loaded with 10μM 5-(and-6)-chloromethyl-21,71-dichlorodihydro-fluorescein diacetateacetylester (CM-H2DCFDA) (Invitrogen) for 30 minutes at 37°C and analyzed by flow cytometry. For lipid analysis, BM or LSK isolated by cell sorting were stimulated for 30 minutes with 10μM AA, and supernatants were extracted and analyzed by stable isotope dilution normal phase chiral liquid chromatography coupled with electron capture atmospheric pressure chemical ionization/mass spectrometry as described.24
Cell sorting and quantitative polymerase chain reaction
BM cells from mice were pooled, stained, and sorted on an Aria cell sorter at the University of Pennsylvania or The Wistar Institute flow cytometry facility. mRNA was isolated using RNeasy microRNA kit (QIAGEN), treated with turbo DNase (Ambion), cDNA synthesized using reverse transcriptase and quantitative real-time polymerase chain reaction (PCR) was performed with SYBR Green Master Mix on ABI 7000 cycler (Applied Biosystems) and normalized to glyceraldehyde-3-phosphate dehydrogenase levels. Primer sequences were designed using Primer Express (Applied Biosystems).
Immunofluorescence
The 50 000 B6 and Alox15 LSK cells isolated by cell sorting were adhered to glass slides by cytospin. Cells were fixed and made permeable using methanol. Slides were blocked with 1% bovine serum albumin/phosphate-buffered saline and stained with either β-catenin–fluorescein isothiocyanate (FITC) or FITC-conjugated isotype control (BD Biosciences). Slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted using Slow Fade (Invitrogen). The 8 separate fields, more than 545 cells in total, were analyzed in each of 2 independent experiments using a Nikon E600 upright microscope (Nikon Instruments Inc) under 40×/.95 NA magnification. Images were captured with SPOT RT slider digital camera (Diagnostic Instruments) with Image Pro Plus Version 4.0 and processed with Image Pro Plus Version 7.0 (Media Cybernetics).
Statistical analysis
One-way ANOVA or t tests were applied using GraphPad Prism software or Microsoft Excel. Results in which P was less than .05 were considered statistically significant for all tests. All error bars represent mean (± SEM).
Results
Alox15 mice exhibit multiple cell-autonomous hematopoietic defects
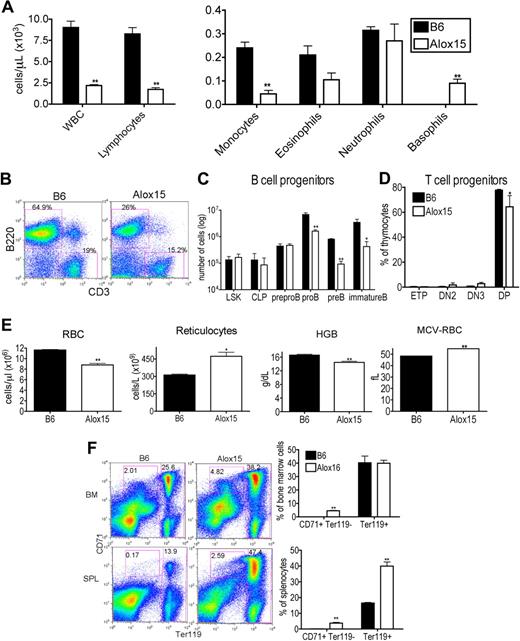
To establish the impact of 12/15-LOX deletion on the hematopoietic compartment, we performed hematologic analysis of peripheral blood from “asymptomatic” 12- to 15-week-old Alox15 and B6 mice. Alox15 mice exhibited a lower white blood cell (WBC) count, attributable to reduced lymphocytes and monocytes. Although eosinophil and neutrophil numbers were similar, they accounted for a greater percentage of total cells in Alox15 mice. The absolute number of basophils in asymptomatic Alox15 mice was increased as noted previously24 (Figure 1A). These defects were also evident in younger (6 weeks) mice and in older (26-30 weeks) mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Alox15 mice exhibit multiple hematopoietic defects. (A) Reduction in WBC, lymphocytes, and monocytes and an increase in basophils in 12- to 15-week-old wild-type Alox15 compared with B6 mice as an average (± SEM) of n = 4. (B-C) Defective B-cell development in Alox15 mice. (B) Splenocytes were isolated and stained for B cells (B220+) and T cells (CD3+). The percentage of B cells and T cells is indicated as the percentage of total cells for a representative experiment of n = 5. (C) BM cells were stained for B-cell progenitors and analyzed by flow cytometry: LSK, CLP (Lin−IL-7Rα+cKitloSca1lo), pre-pro-B (B220+CD43+HSA−CD19−), pro-B (B220+CD43+HSA+CD19+), pre-B (B220+CD43−IgM−CD19+), and immature B (B220+CD43−IgM+CD19+) cells as an average (± SEM) of n = 3. (D) Defect in Alox15 T-cell development. Thymocytes were stained for T-cell progenitors and analyzed by flow cytometry: ETP (Lin−CD25−cKit+), DN2 (Lin−CD25+cKit+), DN3 (Lin−CD25+cKit−), and double positive (CD4+CD8+) as an average (± SEM) of n = 3. (E) Hematologic analysis of peripheral blood from 12- to15-week-old B6 and Alox15 mice. The number of RBC, reticulocytes, hemoglobin (HGB), and the mean cell volume of the RBCs (MCV-RBC) in B6 and Alox15 is depicted as the average (± SEM) of n = 4. (F) Stress erythropoiesis in Alox15 mice demonstrated by expansion of erythroid progenitors. Proerythrocytes (CD71+Ter119lo) and erythrocytes (Ter119hi) in BM and spleen are indicated as a percentage of total cells for a representative experiment and a summary of 4 experiments; *P < .05, **P < .01 compared with wild-type.
Alox15 mice exhibit multiple hematopoietic defects. (A) Reduction in WBC, lymphocytes, and monocytes and an increase in basophils in 12- to 15-week-old wild-type Alox15 compared with B6 mice as an average (± SEM) of n = 4. (B-C) Defective B-cell development in Alox15 mice. (B) Splenocytes were isolated and stained for B cells (B220+) and T cells (CD3+). The percentage of B cells and T cells is indicated as the percentage of total cells for a representative experiment of n = 5. (C) BM cells were stained for B-cell progenitors and analyzed by flow cytometry: LSK, CLP (Lin−IL-7Rα+cKitloSca1lo), pre-pro-B (B220+CD43+HSA−CD19−), pro-B (B220+CD43+HSA+CD19+), pre-B (B220+CD43−IgM−CD19+), and immature B (B220+CD43−IgM+CD19+) cells as an average (± SEM) of n = 3. (D) Defect in Alox15 T-cell development. Thymocytes were stained for T-cell progenitors and analyzed by flow cytometry: ETP (Lin−CD25−cKit+), DN2 (Lin−CD25+cKit+), DN3 (Lin−CD25+cKit−), and double positive (CD4+CD8+) as an average (± SEM) of n = 3. (E) Hematologic analysis of peripheral blood from 12- to15-week-old B6 and Alox15 mice. The number of RBC, reticulocytes, hemoglobin (HGB), and the mean cell volume of the RBCs (MCV-RBC) in B6 and Alox15 is depicted as the average (± SEM) of n = 4. (F) Stress erythropoiesis in Alox15 mice demonstrated by expansion of erythroid progenitors. Proerythrocytes (CD71+Ter119lo) and erythrocytes (Ter119hi) in BM and spleen are indicated as a percentage of total cells for a representative experiment and a summary of 4 experiments; *P < .05, **P < .01 compared with wild-type.
To dissect the reduction in lymphocytes in Alox15 mice, we examined lymphoid development in BM and thymus. The reduction in lymphocytes was mainly attributed to a reduction in B cells (Figure 1B). Moreover, B-cell development was defective in Alox15 mice. Although the absolute number of earliest B lineage progenitors, including LSK, CLP, and pre-pro-B cells was similar between wild-type and Alox15 BM, the number of pro-B, pre-B, and immature B cells was reduced in Alox15 BM (Figure 1C). We observed a less dramatic but significant defect in T-cell development. Double positive thymocytes were decreased in Alox15 compared with B6 mice, but ETP, DN2, and DN3 were similar (Figure 1D).
As 12/15-LOX regulates erythroid development in rabbits and humans, we also investigated whether 12/15-LOX regulates murine erythroid development. Alox15 mice exhibited a decrease in RBC number at all ages examined (6-30 weeks), and a concomitant increase in reticulocyte number at 12 to 15 weeks of age (Figure 1E and supplemental Figure 1). Moreover, hemoglobin was decreased, whereas the mean cell volume of the RBCs was increased, indicating that Alox15 mice developed a macrocytic anemia (Figure 1E). Despite the decrease in mature RBC, Alox15 erythroid progenitors were expanded, particularly in the spleen, the major site of stress erythropoiesis in mice (Figure 1F). These data are consistent with the reported expansion of splenic red pulp in Alox15 mice.24 Thus, Alox15 mice have a defect in late-stage erythroid differentiation in concordance with previous observations that a functional homolog of 12/15-LOX, 15-LOX, and its specific products, 15(S)-HETE and 13(S)-HODE, in other species regulate reticulocyte maturation by modulating membrane degradation.28,29
We also determined the WBC subsets of the Alox15 mice that become moribund over the course of a year.24 This analysis indicated that neutrophils in the blood were increased and there was a severe decrease in RBC and hematocrit as the Alox15 mice progressed from the asymptomatic syndrome to the more aggressive disease (supplemental Figure 1).
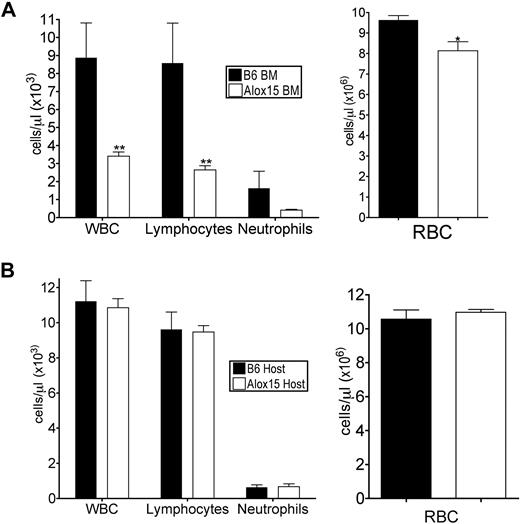
To determine whether the defects in Alox15 mice were cell-autonomous, we performed adoptive transfer studies to define whether the phenotype was inherent to BM-derived cells and/or dependent on the host environment. B6 or Alox15 donor BM cells were engrafted into lethally irradiated congenic wild-type recipients. At 16 weeks after engraftment, recipient mice reconstituted with Alox15 cells had decreased total WBC, lymphocytes, and RBC in peripheral blood compared with mice reconstituted with B6 BM (Figure 2A), analogous to the defects observed in cells from Alox15 mice. Conversely, when congenic wild-type (B6.SJL) BM was used to reconstitute B6 or Alox15 mice, there were no differences in total or differential WBCs (Figure 2B). These data indicate that 12/15-LOX regulates hematopoiesis in a cell-autonomous manner.
Defects in Alox15 hematopoiesis are cell-autonomous. (A) Assessment of hematopoietic reconstitution of lethally irradiated B6.SJL mice 16 weeks after engraftment with 1 × 106 B6 or Alox15 BM cells (n = 6). (B) Hematopoietic reconstitution of lethally irradiated B6 and Alox15 mice 16 weeks after engraftment of 1 × 106 B6.SJL BM cells (n = 3); *P < .05, * *P < .01 compared with wild-type. All error bars represent the average (± SEM).
Defects in Alox15 hematopoiesis are cell-autonomous. (A) Assessment of hematopoietic reconstitution of lethally irradiated B6.SJL mice 16 weeks after engraftment with 1 × 106 B6 or Alox15 BM cells (n = 6). (B) Hematopoietic reconstitution of lethally irradiated B6 and Alox15 mice 16 weeks after engraftment of 1 × 106 B6.SJL BM cells (n = 3); *P < .05, * *P < .01 compared with wild-type. All error bars represent the average (± SEM).
Alox15 mice exhibit a primary defect in HSC function
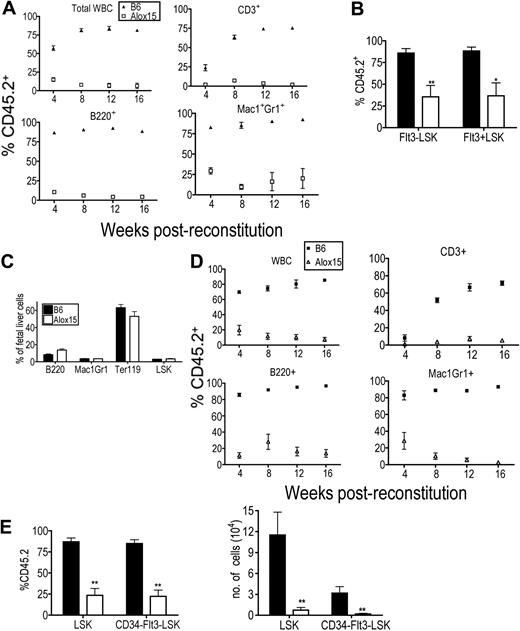
Because Alox15 mice exhibited multiple hematopoietic defects, we hypothesized that there was a defect in Alox15 HSC function. We first established that 12/15-LOX was expressed in HSCs (supplemental Figure 2), that Alox15 LSK cells were present in similar numbers in B6 and Alox15 BM (Figure 1C) and that B6 and Alox15 LSK cells homed to the BM with comparable efficiency (supplemental Figure 2). We then tested the functionality of the HSCs in reconstitution assays. BM cells from age-matched B6 or Alox15 mice (CD45.2) were mixed 9:1 with wild-type competitor B6.SJL BM cells (CD45.1) and engrafted into lethally irradiated B6.SJL recipient mice. Reconstitutions were monitored by bleeding recipient mice every 4 weeks and analyzing the frequency of CD45.2 cells, which were derived from Alox15 or B6 control BM cells, among the total CD45+ population. At all time points after reconstitution, the contribution of Alox15-derived total CD45+ cells, T cells, B cells, and granulocytes were dramatically reduced compared with the contribution of B6 donor-derived cells (Figure 3A). After 16 weeks, BM was isolated and analyzed for progenitor subsets. Alox15 HSCs were at a profound disadvantage compared with B6-derived HSC under competitive conditions as evidenced by a marked reduction in the proportion of Alox15-derived HSCs compared with B6-derived HSCs in recipient mice (Figure 3B). Additional competitive reconstitution assays demonstrated that limiting numbers of Alox15 BM resulted in a further decrease in Alox15-derived HSC during competitive reconstitution assays (supplemental Figure 3).
Alox15 mice exhibit primary defect in HSC function. (A) The hematopoietic compartment derived from Alox15 BM in competitive reconstitution assay is defective. Lethally irradiated congenic B6.SJL mice were reconstituted with a 9:1 ratio of B6 (▴) or Alox15 (□) (CD45.2, test) BM cells to B6.SJL (CD45.1, competitor) BM cells. Reconstitution was monitored by quantifying the percentage of donor-derived CD45.2+ blood cells in recipients using flow cytometry at the indicated times. The percentage of CD45.2+ total leukocytes (CD45+ cells), CD3+ cells, B220+ cells, and Mac1+Gr1+ is depicted; **P < .01. (B) Reduction in Alox15 LT-HSC following during competitive reconstitution. At 16 weeks after engraftment, the percentage of B6 and Alox15 CD45.2 donor-derived Flt3−LSK and Flt3+LSK in 9:1 competitively reconstituted mice was determined by flow cytometry (n = 6). (C) Hematopoietic subsets in B6 and Alox15 E14.5 fetal livers analyzed by flow cytometry. (D-E) HSC-derived from Alox15 fetal livers are functionally defective. Analysis of hematopoietic reconstitution of lethally irradiated congenic mice at indicated times after engraftment of B6 or Alox15 E14.5 fetal liver cells mixed 2:1 with congenic competitor BM cells. (D) CD45.2+ WBC subsets were determined by flow cytometry. (E) Indicated progenitor populations in BM harvested 16 weeks after transfer were analyzed for CD45.2+ cells. Both percentage and absolute number of B6 and Alox15-derived cells are shown (n = 5); *P < .05, **P < .01. All error bars represent mean (± SEM).
Alox15 mice exhibit primary defect in HSC function. (A) The hematopoietic compartment derived from Alox15 BM in competitive reconstitution assay is defective. Lethally irradiated congenic B6.SJL mice were reconstituted with a 9:1 ratio of B6 (▴) or Alox15 (□) (CD45.2, test) BM cells to B6.SJL (CD45.1, competitor) BM cells. Reconstitution was monitored by quantifying the percentage of donor-derived CD45.2+ blood cells in recipients using flow cytometry at the indicated times. The percentage of CD45.2+ total leukocytes (CD45+ cells), CD3+ cells, B220+ cells, and Mac1+Gr1+ is depicted; **P < .01. (B) Reduction in Alox15 LT-HSC following during competitive reconstitution. At 16 weeks after engraftment, the percentage of B6 and Alox15 CD45.2 donor-derived Flt3−LSK and Flt3+LSK in 9:1 competitively reconstituted mice was determined by flow cytometry (n = 6). (C) Hematopoietic subsets in B6 and Alox15 E14.5 fetal livers analyzed by flow cytometry. (D-E) HSC-derived from Alox15 fetal livers are functionally defective. Analysis of hematopoietic reconstitution of lethally irradiated congenic mice at indicated times after engraftment of B6 or Alox15 E14.5 fetal liver cells mixed 2:1 with congenic competitor BM cells. (D) CD45.2+ WBC subsets were determined by flow cytometry. (E) Indicated progenitor populations in BM harvested 16 weeks after transfer were analyzed for CD45.2+ cells. Both percentage and absolute number of B6 and Alox15-derived cells are shown (n = 5); *P < .05, **P < .01. All error bars represent mean (± SEM).
As the competitor cells provided a virtually normal hematopoietic compartment, these data indicate that the defect in Alox15 HSCs was primary rather than secondary to abnormalities in hematopoietic cells at later stages of differentiation. Nonetheless, to formally exclude the possibility that the defect in Alox15 HSCs was secondary to later hematopoietic defects, we repeated the competitive reconstitution assays with E14.5 fetal liver cells. Importantly, we found that Alox15 E14.5 fetal livers appeared normal based on flow cytometric analysis of hematopoietic subsets, hematoxylin and eosin staining, and by differentiation in methylcellulose assays (Figure 3C and supplemental Figure 4). In addition, these cells were mixed 2:1 with normal congenic BM to ensure readout of fetal liver-derived HSC function in the context of a normal hematopoietic compartment. Even under these conditions of competitive reconstitution, the contribution of Alox15-derived cells to the blood cell populations was significantly reduced compared with B6 at all times after transfer into lethally irradiated congenic recipient mice (Figure 3D). Moreover, Alox15-derived LSK and CD34−Flt3−LSK cells in the BM were significantly reduced by percentage and number compared with the corresponding populations in wild-type BM (Figure 3E). These data effectively demonstrate that the defect in Alox15 HSCs is not secondary to defects in mature hematopoietic subsets. Rather, 12/15-LOX directly regulates HSC function.
12/15-LOX regulates number, proliferation, and function of LT-HSCs
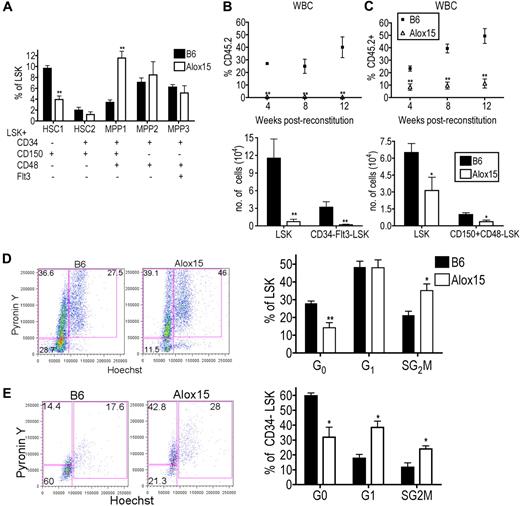
We further characterized the distribution of HSCs within the LSK population between B6 and Alox15.1,30 Interestingly, Alox15 BM had a decreased percentage of primitive dormant LT-HSC (CD34−CD150+CD48−Flt3−LSK) and an increased percentage of more differentiated multipotent progenitor (MPP1; CD34+CD150+CD48+Flt3−LSK) compared with B6 BM (Figure 4A). The LT-HSC population is highly enriched for HSC function and quiescent cells, whereas the more differentiated cells are highly proliferative and have decreased capacity to self-renew. These data suggest that 12/15-LOX regulates the most primitive LT-HSCs.
12/15-LOX regulates proliferation and function of LT-HSC. (A) Flow cytometric analysis of phenotypic subsets demonstrated depletion of the most primitive HSC phenotype and a concomitant increase in a more mature MPP phenotype in Alox15 compared with B6 LSK (n = 5). (B-C) 12/15-LOX regulates the function of LT-HSC. Lethally irradiated congenic mice were reconstituted with 500 CD34−Flt3−LSK (B) or 500 CD150+CD48−LSK (C) from B6 or Alox15 BM mixed with 2 × 105 competitor cells. (Top) Reconstitution was analyzed by flow cytometry of CD45.2+ of total CD45+ WBCs. (Bottom) Analysis at 12 weeks after BM transfer for CD45.2+ LSK and CD34−Flt3−LSK (B) or CD150+CD48−LSK (C) by flow cytometric analysis of n = 8 in 2 independent experiments. (D-E) Increased proliferation of Alox15 compared with B6 determined by flow cytometric analysis by incorporation of Hoechst 33342 and PyroninY into DNA and RNA, respectively, of cells in the total LSK population (D) and CD34−LSK–gated population (E). Shown are a representative flow cytometric analysis and a summary of 4 independent experiments; *P < .05, **P < .01. All error bars represent the average (± SEM).
12/15-LOX regulates proliferation and function of LT-HSC. (A) Flow cytometric analysis of phenotypic subsets demonstrated depletion of the most primitive HSC phenotype and a concomitant increase in a more mature MPP phenotype in Alox15 compared with B6 LSK (n = 5). (B-C) 12/15-LOX regulates the function of LT-HSC. Lethally irradiated congenic mice were reconstituted with 500 CD34−Flt3−LSK (B) or 500 CD150+CD48−LSK (C) from B6 or Alox15 BM mixed with 2 × 105 competitor cells. (Top) Reconstitution was analyzed by flow cytometry of CD45.2+ of total CD45+ WBCs. (Bottom) Analysis at 12 weeks after BM transfer for CD45.2+ LSK and CD34−Flt3−LSK (B) or CD150+CD48−LSK (C) by flow cytometric analysis of n = 8 in 2 independent experiments. (D-E) Increased proliferation of Alox15 compared with B6 determined by flow cytometric analysis by incorporation of Hoechst 33342 and PyroninY into DNA and RNA, respectively, of cells in the total LSK population (D) and CD34−LSK–gated population (E). Shown are a representative flow cytometric analysis and a summary of 4 independent experiments; *P < .05, **P < .01. All error bars represent the average (± SEM).
To determine whether the defect in Alox15 HSCs in the BM competitive reconstitution assays was because of 12/15-LOX regulation of the absolute number of LT-HSCs or the function of LT-HSCs, we performed competitive reconstitution assays using equal numbers of purified LT-HSCs. Five hundred CD34−Flt3−LSK cells, purified by cell sorting from B6 or Alox15 BM, were mixed with competitor BM cells and injected into lethally irradiated recipient mice. At each time point after reconstitution, there was a significant decrease in the percentage of Alox15-derived blood cells (Figure 4B). Analysis of BM at 12 weeks after reconstitution showed a decrease in the absolute number of Alox15-derived LSK and CD34−Flt3−LSK cells compared with B6 (Figure 4B). Similarly, Alox15 LT-HSCs isolated by cell sorting using different markers, CD150+CD48−LSK, were also defective during competitive reconstitution assays compared with B6 (Figure 4C). These data demonstrate that 12/15-LOX regulates the function of LT-HSC.
To determine the mechanism whereby 12/15-LOX regulates LT-HSC, we compared the rates of cell death and proliferation in Alox15 and B6 HSC. The extent of apoptosis ex vivo and in vitro was comparable between B6 and Alox15 LSK cells (supplemental Figure 5). However, Alox15 LSK cells contained an increased percentage of cycling cells compared with wild-type LSK cells (Figure 4D). This difference was most dramatic in the CD34−LSK subset in which there was a decrease in the percentage of Alox15 cells in G0 (31.9% vs 59.7%), but increased percentage of cells in G1 (38.5% vs 17.3%) and SG2M (24.0% vs 11.6%) phases (Figure 4E). Therefore, 12/15-LOX regulates the number, proliferation, and function of primitive LT-HSCs. Moreover, the decrease in quiescent LT-HSCs suggests a mechanism underlying the decrease in the number of LT-HSCs in Alox15 mice. As HSCs proliferate, they may differentiate rather than self-renew, leading to depletion of the population. To formally test this hypothesis, we next tested whether 12/15-LOX regulates self-renewal.
Defective maintenance of Alox15 HSC numbers
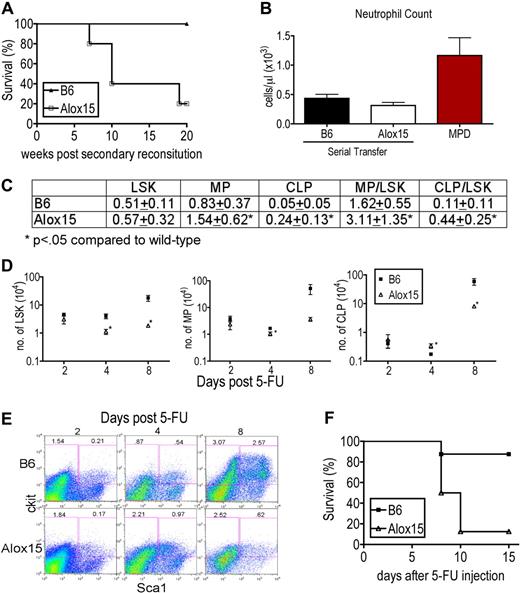
We tested whether 12/15-LOX regulates self-renewal capacity using 2 approaches. In the first, we compared the self-renewal capacity of Alox15 and B6 HSCs in serial transfer assays in the absence of competitors. Alox15 BM was able to reconstitute lethally irradiated primary recipient mice but recapitulated the defects in Alox15 mice, compared with the hematopoietic profile of primary recipients of B6 BM (Figure 2A). However, when BM cells from primary recipients were serially transferred, there was a marked reduction in the survival of secondary recipients of Alox15 BM compared with B6 (Figure 5A). The morbidity was not because of development of MPD (Figure 5B). These data demonstrate that Alox15 BM indeed contains decreased numbers of LT-HSCs and that Alox15 BM has a decreased capacity to reconstitute secondary irradiated recipients compared with B6.
12/15-LOX maintains the number of LT-HSC. (A) Kaplan-Meier plot of secondary recipients of B6 and Alox15 BM. BM cells from lethally irradiated primary recipients were harvested 16 weeks after engraftment of B6 and Alox15 BM cells and transferred into secondary lethally irradiated congenic recipients, and survival was monitored (n = 5). (B) Neutrophils in the blood of secondary recipients at 15 weeks after secondary reconstitution compared with mice with MPD eliminating MPD as the cause of morbidity in Alox15 secondary recipients. (C) Increased ratio of MP (Lin−Sca1−cKit+) to LSK and CLP to LSK quantified by flow cytometry day 4 after treatment with 200 mg/kg 5-FU indicate a defect in maintenance of LSK numbers in Alox15 mice compared with B6 (n = 9). (D-E) Kinetics of expansion of LSK and progenitor populations in 5-FU–treated B6 and Alox15 mice, demonstrating loss of the LSK subset in Alox15 mice by day 8. (D) Number of LSK, MP, and CLP in Alox15 mice after treatment with 5-FU. (E) Representative flow cytometric analyses at times indicated of gated Lin− cells with percentage of progenitor and LSK subsets among total cells shown. (F) Kaplan-Meier plot demonstrating decreased survival of 5-FU–treated Alox15 mice compared with B6 (n = 8).
12/15-LOX maintains the number of LT-HSC. (A) Kaplan-Meier plot of secondary recipients of B6 and Alox15 BM. BM cells from lethally irradiated primary recipients were harvested 16 weeks after engraftment of B6 and Alox15 BM cells and transferred into secondary lethally irradiated congenic recipients, and survival was monitored (n = 5). (B) Neutrophils in the blood of secondary recipients at 15 weeks after secondary reconstitution compared with mice with MPD eliminating MPD as the cause of morbidity in Alox15 secondary recipients. (C) Increased ratio of MP (Lin−Sca1−cKit+) to LSK and CLP to LSK quantified by flow cytometry day 4 after treatment with 200 mg/kg 5-FU indicate a defect in maintenance of LSK numbers in Alox15 mice compared with B6 (n = 9). (D-E) Kinetics of expansion of LSK and progenitor populations in 5-FU–treated B6 and Alox15 mice, demonstrating loss of the LSK subset in Alox15 mice by day 8. (D) Number of LSK, MP, and CLP in Alox15 mice after treatment with 5-FU. (E) Representative flow cytometric analyses at times indicated of gated Lin− cells with percentage of progenitor and LSK subsets among total cells shown. (F) Kaplan-Meier plot demonstrating decreased survival of 5-FU–treated Alox15 mice compared with B6 (n = 8).
In the second approach, we compared functionality of B6 and Alox15 HSCs after treatment with 5-FU (200 mg/kg intraperitoneally) to stimulate division and differentiation of HSCs resulting in repletion of the hematopoietic compartment. HSC self-renewal capacity was assessed by determining the ratio of LSK to myeloid progenitors (MP) and CLP at day 4 after injection, when the HSCs are expected to be cycling to replenish the hematopoietic compartment. If the ratio of CLP/LSK or MP/LSK is increased in 5-FU–treated Alox15 BM compared with 5-FU–treated wild-type controls, it would indicate a preference for Alox15 HSC to differentiate rather than to self-renew, whereas a reduced ratio indicates a preference for Alox15 HSCs to self-renew rather than differentiate. On day 4 after treatment with 5-FU, Alox15 BM contained a greater percentage of differentiated cells compared with wild-type BM, including an increase in the percentage of MP and increased percentage and number of CLP (Figure 5C-D). Moreover, the ratio of MP/LSK, and CLP/LSK were increased compared with wild-type, demonstrating a decreased capacity for Alox15 HSC to self-renew and an asymmetric division skewed toward differentiation (Figure 5C). B6 and Alox15 LSK cells displayed similar resistance to 5-FU per se because at day 2 after treatment with 5-FU, there was no difference in the number or percentage of LSK, MP, or CLP. Furthermore, by day 8 after treatment with 5-FU, the LSK subset in B6 had expanded, whereas Alox15 exhibited a marked reduction in number and percentage of LSK. (Figure 5D-E). The reduction in LSK cells in BM of 5-FU–treated Alox15 mice on day 8 appeared to be because of exhaustion of the capacity of HSC to self-renew as required to maintain the populations of LSK cells and progenitors. 5-FU–treated Alox15 mice also exhibited decreased survival; 7 of 8 (87.5%) Alox15 mice succumbed to treatment compared with 1 of 8 (12.5%) B6 mice (Figure 5F). The cause of death of the 5-FU–treated Alox15 mice appeared to be severe anemia, as the hematocrit at day 8 after treatment was 14.52% (± 4.81%) for Alox15 mice versus 32.53% (± 8.08%) for B6 mice. The findings of increased proliferation but decreased number of LT-HSC in Alox15 mice, decreased survival of recipients that received serially transferred Alox15 BM and the increased ratios of Alox15 progenitor/LSK during 5-FU assays, when taken together, demonstrate that 12/15-LOX regulates the size of the LT-HSC population possibly by regulating self-renewal.
Reduction of 12/15-LOX–dependent generation of lipid mediators and ROS in Alox15 LSK
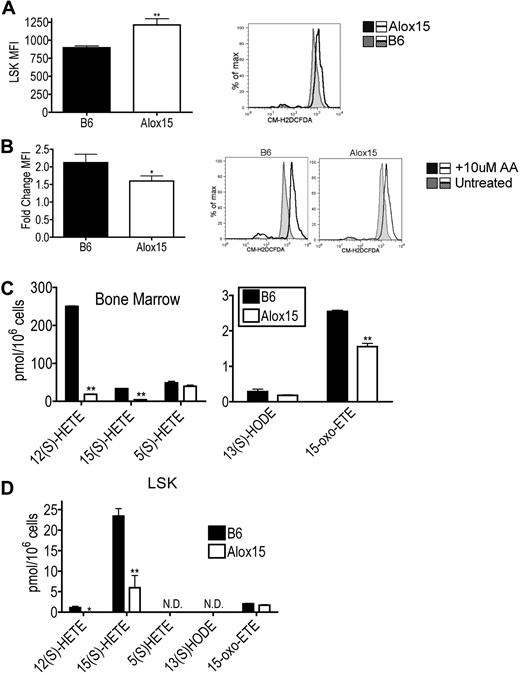
To determine which products of 12/15-LOX activity mediate HSC function, we compared levels of ROS and lipid metabolites in B6 and Alox15 progenitor cells. Purified Alox15 LSK cells isolated by cell sorting exhibited increased basal ROS levels ex vivo using the oxygen-sensitive CM-H2DCFDA probe compared with B6 (Figure 6A). Because ROS levels are known to be decreased in LT-HSCs,31 the increase in basal ROS levels is likely a reflection of the decreased numbers of LT-HSCs within the Alox15 LSK cells (Figure 6A). Interestingly, however, wild-type LSK cells exhibited a greater fold increase in ROS compared with Alox15 after loading with exogenous AA indicating that 12/15-LOX–mediated ROS production is in fact defective in Alox15 LSK cells (Figure 6B). Hence 12/15-LOX–mediated ROS production may contribute to 12/15-LOX signaling in HSCs.
Reduction in select 12/15-LOX–generated metabolites in Alox15 HSC. (A) Increased basal ROS in Alox15 HSC. B6 and Alox15 LSK were isolated by cell sorting and analyzed for ROS formation using the ROS sensitive indicator CM-H2DCFDA and flow cytometry. Shown are a summary of mean fluorescence intensity (MFI) of n = 5 and a representative flow cytometric analysis. Shaded histogram represents B6 and open histogram represents Alox15. (B) B6 and Alox15 LSK were stimulated with 10μM AA before measuring ROS. Shown are a summary of n = 4 as fold increase of mean fluorescence intensity compared with unstimulated cells and representative flow cytometric analyses. Shaded histogram represents unstimulated and open histogram represents stimulated. (C-D) Lipid product formation in B6 and Alox15 BM (C) and LSK (D) cells after stimulation with 10μM AA. N.D. indicates not detectable; *P < .05, **P < .01 compared with wild-type. All error bars represent the average (± SEM).
Reduction in select 12/15-LOX–generated metabolites in Alox15 HSC. (A) Increased basal ROS in Alox15 HSC. B6 and Alox15 LSK were isolated by cell sorting and analyzed for ROS formation using the ROS sensitive indicator CM-H2DCFDA and flow cytometry. Shown are a summary of mean fluorescence intensity (MFI) of n = 5 and a representative flow cytometric analysis. Shaded histogram represents B6 and open histogram represents Alox15. (B) B6 and Alox15 LSK were stimulated with 10μM AA before measuring ROS. Shown are a summary of n = 4 as fold increase of mean fluorescence intensity compared with unstimulated cells and representative flow cytometric analyses. Shaded histogram represents unstimulated and open histogram represents stimulated. (C-D) Lipid product formation in B6 and Alox15 BM (C) and LSK (D) cells after stimulation with 10μM AA. N.D. indicates not detectable; *P < .05, **P < .01 compared with wild-type. All error bars represent the average (± SEM).
We also compared the generation of 12/15-LOX–mediated lipid metabolites between B6 and Alox15 BM and LSK cells. Both Alox15 BM and LSK cells exhibited decreased 12(S)-HETE and 15(S)-HETE production compared with B6 (Figure 6C-D). These differences were specific for 12/15-LOX products as there were no differences in the 5-LOX product 5(S)-HETE in Alox15 BM. Interestingly, Alox15 BM also exhibited decreased levels of the novel recently described 15(S)-HETE derivative 15-oxo-eicosatetraenoic acid, reported to inhibit proliferation in human umbilical vein endothelial cells.32 12/15-LOX lipid product signaling by 12(S)-HETE and 15(S)-HETE likely at least in part mediate the effects of 12/15-LOX on HSC function.
12/15-LOX regulates gene expression and canonical Wnt signaling in LSK cells
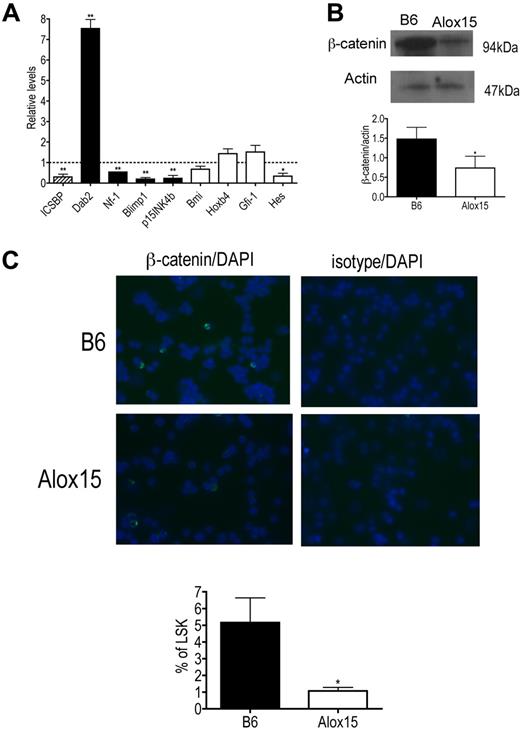
To determine the mechanism whereby 12/15-LOX regulates HSC function, we compared gene expression in B6 and Alox15 LSK cells isolated by cell sorting. Specifically, we compared gene expression of ICSBP/IRF-8 and its downstream targets because we previously reported that 12/15-LOX regulates ICSBP/IRF-8 in other cell types17,24 and because ICSBP/IRF-8 gene expression is also reduced in aging stem cells and its targets, including Dab2, are involved in signaling pathways that govern HSC function.25,33 Interestingly, we found that gene expression of ICSBP/IRF-8 was decreased and furthermore, that expression of ICSBP/IRF-8 target genes were dysregulated in Alox15 LSK cells compared with B6 (Figure 7A). ICSBP/IRF-8 is known to negatively regulate Dab2 and positively regulate Nf-1, Blimp1, and p15INK4b.34-36 Consistent with this, Dab2 mRNA was increased, whereas Nf-1, Blimp1, and p15INK4b mRNA were decreased in Alox15 LSK cells compared with wild-type LSK cells (Figure 7A).
Alterations in ICSBP/IRF-8 and ICSBP/IRF-8 target gene expression and canonical Wnt signaling in Alox15 HSC. (A) Quantitative PCR of mRNA of ICSBP/IRF-8 (▨), ICSBP/IRF-8-regulated genes (■), and genes known to regulate HSC self-renewal but not regulated by ICSBP/IRF-8 (□) in sorted B6 and Alox15 LSK presented as average fold change from B6 (n = 3). (B-C) Decreased canonical Wnt signaling in Alox15 HSC. (B) Immunoblot (top) and densitometric quantification (bottom) of β-catenin, a downstream mediator of canonical Wnt signaling, in lysates of B6 and Alox15 Lin−cKit+ cells are shown (n = 4). (C) Decreased percentage of Alox15 LSK expressing β-catenin in the nucleus determined by immunofluorescence of sorted cells. Representative images of B6 and Alox15 LSK β-catenin or isotype-matched control (FITC) staining and DAPI (Blue) are shown at an original magnification ×40, with a summary of results; *P < .05, **P < .01 compared with wild-type. All error bars represent the average (± SEM).
Alterations in ICSBP/IRF-8 and ICSBP/IRF-8 target gene expression and canonical Wnt signaling in Alox15 HSC. (A) Quantitative PCR of mRNA of ICSBP/IRF-8 (▨), ICSBP/IRF-8-regulated genes (■), and genes known to regulate HSC self-renewal but not regulated by ICSBP/IRF-8 (□) in sorted B6 and Alox15 LSK presented as average fold change from B6 (n = 3). (B-C) Decreased canonical Wnt signaling in Alox15 HSC. (B) Immunoblot (top) and densitometric quantification (bottom) of β-catenin, a downstream mediator of canonical Wnt signaling, in lysates of B6 and Alox15 Lin−cKit+ cells are shown (n = 4). (C) Decreased percentage of Alox15 LSK expressing β-catenin in the nucleus determined by immunofluorescence of sorted cells. Representative images of B6 and Alox15 LSK β-catenin or isotype-matched control (FITC) staining and DAPI (Blue) are shown at an original magnification ×40, with a summary of results; *P < .05, **P < .01 compared with wild-type. All error bars represent the average (± SEM).
We also analyzed expression of other genes that regulate HSC function not believed to be directly regulated by ICSBP/IRF-8 including Hes1, Bmi, Hoxb4, and Gfi-1. Of these genes, only Hes1 was significantly decreased in Alox15 compared with wild-type LSK cells (Figure 7A). Because Hes1 is also decreased when canonical Wnt signaling is inhibited13 and because the ICSBP/IRF-8 target Dab2, which was increased at the mRNA and protein levels (Figure 7A and data not shown), negatively regulates canonical Wnt signaling by inhibiting disshelved and stabilizing axin,33,37 we investigated whether 12/15-LOX regulates canonical Wnt signaling by measuring β-catenin levels in wild-type and Alox15 progenitors. Indeed, β-catenin protein was decreased in total lysates of Alox15 Lin−cKit+ progenitors compared with wild-type progenitors (Figure 7B). Moreover, using immunofluorescence we compared the incidence of nuclear localization of β-catenin in B6 and Alox15 LSK isolated by cell sorting. The incidence of nuclear localization of β-catenin determined by overlap between β-catenin staining (FITC) and nuclear DAPI staining, was reduced in Alox15 compared with B6 LSK cells (Figure 7C). These data implicate 12/15-LOX as a novel regulator of canonical Wnt signaling. Canonical Wnt signaling is known to regulate HSC quiescence and self-renewal,12-14 and therefore the dysregulation of the Wnt signaling pathway including β-catenin is likely to be at least one mechanism underlying the reduced quiescence and function of Alox15 HSCs.
Discussion
This study establishes the importance of unsaturated FA metabolism in hematopoiesis by demonstrating that Alox15 mice have a cell-autonomous defect in hematopoietic development. Depletion of 12/15-LOX results in a primary defect in HSC function. 12/15-LOX directly regulates the number, proliferation, and function of LT-HSCs. The defects in Alox15 HSCs are associated with a selective decrease in 12/15-LOX-mediated ROS and lipid metabolites and may be at least partly because of disruption of canonical Wnt signaling.
The decrease in the number of LT-HSCs in Alox15 mice may be attributed to a decrease in self-renewal capacity. Alox15 HSCs showed a bias toward differentiation rather than self-renewal during the 5-FU assays as evidenced by increased numbers of Alox15 CLP and an increased ratio of progenitors to LSK at day 4 compared with B6. Alternatively, the already reduced number of Alox15 LT-HSCs or possible defects in later stages of hematopoietic differentiation, such as in ST-HSCs, may contribute to the decreased function of Alox15 HSCs observed during serial reconstitution and 5-FU assays resulting in exhaustion of the LT-HSC population rather than a defect in self-renewal per se. However, competitive reconstitution assays in which the presence of wild-type competitor BM cells provide a relatively stable BM environment, demonstrate that the defect in Alox15 LT-HSC function represents a primary defect in this population. Taken together, these data demonstrate that 12/15-LOX intrinsically regulates the maintenance of LT-HSCs possibly through self-renewal.
ROS, which are functional by-products of 12/15-LOX activity,15 are known to regulate HSC function. Because LT-HSCs exhibit decreased ROS,31 the finding that Alox15 LSK cells exhibit increased basal levels of ROS may in part be attributed to the reduction in LT-HSC among Alox15 LSK cells. However, as the majority of LSK cells are not LT-HSCs, the increased basal ROS levels in Alox15 HSCs may be determined by other oxidases, such as nicotinamide adenine dinucleotide phosphate oxidase.38 In any case, increased levels of ROS may contribute to the defect in HSC function.26,31,39,40 Moreover, ROS are known to regulate cell-cycle progression41 and may in fact contribute to the increased cycling in Alox15 LSK cells. However, we have demonstrated that 12/15-LOX–mediated ROS production is defective in Alox15 LSK and hence may contribute to 12/15-LOX signaling in HSCs.
Deficiency in 12/15-LOX also resulted in a marked reduction in the generation of select 12/15-LOX lipid mediators by hematopoietic progenitors. 12(S)-HETE and 15(S)-HETE were decreased in Alox15 LSK and BM compared with B6. Lipid metabolites of 12/15-LOX may regulate HSC function intrinsically either individually or in combination and may act on multiple signal transduction pathways including activation of protein kinase C by 12(S)-HETE.15 Some 12/15-LOX products function by incorporation into cell membranes, which can alter phosphoinositol42 and receptor signaling.43 Moreover, 12/15-LOX products directly influence gene transcription by binding and activating transcription factors such as peroxisome proliferator–activated receptor-γ.16 Recent studies have shown that the recently described 15(S)-HETE derivative 15-oxo-eicosatetraenoic acid inhibits human umbilical vein endothelial cell proliferation.32 Further studies are required to determine how 12/15-LOX lipid products regulate HSC proliferation and function.
Interestingly, FA metabolism mediated by other enzymes that also target AA as their substrate have been implicated in normal and leukemic HSC function. The cyclooxygenase product prostaglandin E2 is known to signal extrinsically through its receptor on HSC to promote self-renewal by enhancing canonical Wnt signaling and β-catenin accumulation.44,45 Prostaglandin E2 has also been shown to regulate HSC progression into the cell cycle.46 In addition, it was recently reported that 5-LOX–deficient mice are protected from breakpoint cluster region (BCR) gene with the Abl tyrosine kinase BCR-ABL–induced chronic myelogenous leukemia (CML) through inhibition of leukemic stem cell differentiation. In this study, we demonstrate a seemingly opposing role for 12/15-LOX because 12/15-LOX promotes self-renewal of normal HSC and suppresses development of myeloid leukemia.24 Because cyclooxygenase, 5-LOX, and 12/15-LOX share AA as a substrate, it will be interesting in the future to investigate whether substrate availability or substrate rediversion may contribute to the phenotypes observed in the HSC compartment in the absence of 12/15-LOX or 5-LOX.
12/15-LOX regulation of canonical Wnt signaling is specific as we have shown that other pathways known to regulate HSC function are similar including Hoxb4 and Bmi (Figure 7A). Although it would be ideal to restore β-catenin role in Alox15 HSC function, in spite of numerous attempts using a variety of established approaches, Alox15 cells failed to survive transfer into lethally irradiated mice after retroviral infection under conditions in which cells derived from transduced wild-type cells could readily be recovered from recipient mice (data not shown). Furthermore, we do not exclude the possibility that 12/15-LOX regulates other pathways to regulate HSC function.
HSC function and hematopoietic differentiation are related and disruptions in HSC function can lead to the development of leukemia; for example, in the floxed PTEN mouse.47 Alox15 mice develop a CML-like MPD in 15% of the mice over the course of a year. It is possible that the disruption in HSC function contributes to the development of MPD in Alox15 mice.24 Dysregulation of ICSBP and decreased 12/15-LOX activity, particularly levels of 12(S)-HETE, are also associated with human CML.21,23 The contribution of decreased lipid mediators to the leukemic state remains largely unknown, although eicosanoid products may induce apoptosis of leukemic cell lines.48,49
In summary, we have found that 12/15-LOX–mediated FA metabolism regulates the asymmetric division of HSC by promoting quiescence and maintenance of LT-HSCs. In the absence of 12/15-LOX, HSCs exhibit increased proliferation and a bias in their asymmetric division toward differentiation rather than self-renewal. The depletion of 12/15-LOX products is associated with a defect canonical Wnt signaling. As we have shown that FA metabolism plays an important role in mediating HSC function, understanding the mechanisms whereby FA metabolism regulates HSCs may have implications for hematopoiesis, leukemia, and hematopoietic aging.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Warren Pear, Martin Carroll, and Melissa Middleton for scientific discussions. We acknowledge the generous technical support of The Wistar Institute flow cytometry, microscopy, and animal facilities, University of Pennsylvania flow cytometry facility, and Children's Hospital of Philadelphia histology and pathology facilities.
This work was supported in part by National Institutes of Health Training grants (T32GM07229 and T32CA09171, RO1CA091016, and P30CA10815) and by the Pennsylvania Department of Health and Ludwig Institute for Cancer Research. M. Kinder and C.W. are PhD candidates at University of Pennsylvania and this work is submitted in partial fulfillment of the requirement for PhD.
National Institutes of Health
Authorship
Contribution: M. Kinder designed and performed experiments, collected and interpreted data, and wrote the manuscript; C.W. and S.G.S. performed, collected, and interpreted lipid and cell analysis data, respectively; M. Kundu reviewed and interpreted pathologic specimens; L.Z. aided in design and execution of reconstitution experiments; I.A.B. provided reagents and interpreted lipid data; and E.P. aided in experimental design and data interpretation and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M. Kundu is Department of Pathology, St Jude Children's Research Hospital, Memphis, TN.
Correspondence: Ellen Puré, The Wistar Institute, 3601 Spruce St, Philadelphia, PA 19104; e-mail: pure@wistar.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal