Umbilical cord blood grafts are increasingly used as sources of hematopoietic stem cells in adults. Data regarding the outcome of this approach in adults are consistent with delayed and insufficient immune reconstitution resulting in high infection-related morbidity and mortality. Using cytomegalovirus (CMV)–specific immunity as a paradigm, we evaluated the status, mechanism, and clinical implications of immune reconstitution in adults with hematologic malignancies undergoing unrelated double unit cord blood transplantation. Our data indicate that CD8+ T cells capable of secreting interferon-γ (IFN-γ) in a CMV-specific enzyme-linked immunosorbent spot (ELISpot) assay are detectable at 8 weeks after transplantation, before reconstitution of thymopoiesis, but fail to clear CMV viremia. Clearance of CMV viremia occurs later and depends on the recovery of CD4+CD45RA+ T cells, reconstitution of thymopoiesis, and attainment of T-cell receptor rearrangement excision circle (TREC) levels of 2000 or more copies/μg DNA. In addition, overall survival was significantly higher in patients who displayed thymic regeneration and attainment of TREC levels of 2000 or more copies/μg DNA (P = .005). These results indicate that reconstitution of thymopoiesis is critical for long-term clinical outcome in adult recipients of umbilical cord blood transplant. The trial was prospectively registered at http://www.clinicaltrials.gov (NCT00133367).
Introduction
Umbilical cord blood (UCB) is an alternative to bone marrow or peripherally mobilized hematopoietic stem cells for transplantation, and its use in adults with hematopoietic malignancies is increasing.1,,–4 The low number of hematopoietic stem cells in a single cord blood unit has been a limiting factor for the use of cord blood in adult recipients in the past, but outcomes have improved by the use of 2 cord blood units in each adult recipient.5,–7 Despite appropriate CD34+ cell numbers per kilogram of body weight after transplantation, immune recovery remains delayed and insufficient, and infection-related morbidity remains a leading cause of mortality in this group of patients.5,6,8,9
Among subsets of cell populations involved in the generation of productive immune responses, T helper CD4+ and T effector CD8+ T cells have a central role.10 Delayed recovery of both these cell populations after UCB transplantation has been reported. Recovery of cellular populations after UCB transplantation follows different kinetics from those observed if an adult stem cell product is used.11,,,,–16 Although after UCB transplantation B cells display a rapid and enhanced expansion above normal levels, CD8+ and more strikingly CD4+ T cells recover slowly over a year or longer. In contrast, after conventional transplantation, CD4+ and CD8+ cell recovery occurs within 6 to 9 months, whereas B-cell recovery remains delayed for 12 to 18 months after transplantation. Antigen-specific responses to viruses and fungi are impaired in UCB recipients, suggesting a selective impairment in T effector CD8+ function.17,,,–21 In addition, quantitative or qualitative defects of CD4+ cells may contribute to or cause CD8+ T effector cell defects because of deprivation of appropriate help.22,23
Recipient thymic activity represents a significant parameter that correlates with the functional recovery of the immune system after stem cell transplantation. Quantitative assessment of T-cell receptor rearrangement excision circles (TRECs), represents an informative surrogate marker for recipient thymic T-cell neogenesis.24 Measurable deficiencies of TRECs correlate with clinical morbidity and mortality from infection, especially early after transplantation.25
Studies on immune reconstitution in adult recipients of umbilical cord blood are limited, because this approach has only recently been optimized and broadly adopted in this patient population (reviewed in Brown and Boussiotis26 ). Moreover, the current reports are based on mixed populations of patients receiving various conditioning and immunosuppressive regimens.2,6,11,27 Prior studies have either failed to detect TRECs in adult cord blood recipients11 or have reported their recovery very late after transplantation.13 In contrast, studies in pediatric populations have reported no differences in the recovery of T-cell neogenesis between bone marrow and cord blood recipients.12 The clinical implications of thymic recovery, including pathogen-specific immunity and overall survival, in adult recipients of umbilical cord blood have not been examined.
In the present study, we examined the quantitative and qualitative recovery of the T-cell immune response in adult recipients of double cord blood unit transplant from a single cancer center treated with one protocol of pretransplantation conditioning and posttransplantation immunosuppression. We used cytomegalovirus (CMV)–specific immunity as a model to evaluate the functional role of the T-cell component transferred from the graft and the T-cell component generated by thymic T-cell neogenesis in the adult recipient. Our data demonstrate that although naive cord blood T cells transferred to adult UCB recipients could initiate immune responses to CMV and become CMV-specific effectors as early as 8 weeks after transplantation, they were functionally incompetent and failed to clear CMV viremia. Clearance of CMV viremia correlated with recovery of CD4+CD45RA+ T cells and was dependent on T-cell neogenesis in the host as determined by quantitative assessment of TREC numbers. Thymic regeneration and detection of TREC also had significant implications on overall survival (OS), indicating that T-cell neogenesis is critical for the long-term clinical outcome in adult recipients of umbilical cord blood transplant.
Methods
This research protocol was approved by the Institutional Review Board of the Dana-Farber/Harvard Cancer Center. Written informed consent was obtained from all patients for the correlative laboratory study of immune reconstitution before enrollment and participation in accordance with the Declaration of Helsinki. The trial was prospectively registered at http://www.clinicaltrials.gov (NCT00133367).
Patients
Patients with hematologic malignancies were eligible for inclusion in the study if they lacked a 6/6 or 5/6 HLA A–, HLA B–, and HLA DRB1–matched related donor, a 10/10 HLA A–, HLA B–, HLA C–, HLA DRB1–, and HLA DQ–matched unrelated donor. Eligibility was based on previously established criteria.5
Selection of UCB units
UCB units were obtained from national and international cord blood banks. Both units were required to be a 4/6 or greater HLA A, HLA B, and HLA DRB1 allele–level match with each other and the patient.
Treatment
Patients received the following conditioning regimen before UCB transplantation: fludarabine 30 mg/m2 per day from day −8 through day −3 (total dose of 180 mg/m2), melphalan 100 mg/m2 per day on day −2 only, and rabbit antithymocyte globulin 1.5 mg/kg per day on days −7, −5, −3, and −1. Prophylaxis for graft-versus-host disease (GVHD) consisted of tacrolimus starting on day −3 at a dose of 0.05 mg/kg orally. A loading dose of sirolimus (12 mg) was given on day −3, with subsequent daily doses of 4 mg/day with a target blood level of 3 to 12 ng/mL. In the absence of GVHD, tacrolimus and sirolimus were tapered from day +100 through +180. Patients received filgrastim at 5 μg/kg per day from day +5 until an absolute neutrophil count higher than 2.0 × 109 cells/L was reached for 2 consecutive days.
Immunophenotyping
Peripheral blood mononuclear cells (PBMCs) were obtained immediately before transplantation (before administration of conditioning chemotherapy) and at 4 weeks, 8 weeks, 100 days, 6 months, and 12 months after transplantation for staining with fluorescence-conjugated monoclonal antibodies for lineage-specific markers and analysis using a BD FACSCanto flow cytometer (BD Biosciences).
Monitoring for CMV infection
Before transplantation, recipient and donor CMV serology was assessed using enzyme-linked immunoassay for immunoglobulin M (IgM) and IgG antibodies for CMV late antigens.28 Monitoring of CMV viremia after transplantation was performed weekly by polymerase chain reaction starting from day +15. All transplantation patients received CMV-safe blood products.
IFN-γ ELISpot assay
Evaluation of CMV-specific effectors was performed on patient blood samples submitted immediately before transplantation and at 8 weeks, 100 days, 6 months, and 1 year after transplantation. PBMCs were isolated using Ficoll-Paque Plus (GE Healthcare). Interferon-γ (IFN-γ) secretion from CMV-specific T cells was evaluated using the BD enzyme-linked immunosorbent spot (ELISpot) IFN-γ kit (BD Biosciences) according to the manufacturer's instructions and as previously described.29 A total of 4 × 105 PBMCs were added to each well of the ELISpot plate in culture media containing RPMI 1640 with 10% fetal calf serum (Invitrogen). To stimulate CMV-specific IFN-γ production, 9 μg of a cell lysate from CMV (strain AD-169)–infected MRC-5 cells (Microbix Biosystems Inc) was added to duplicate wells. As a negative control, 9 μg of uninfected MRC-5 cell lysate was added to 2 additional wells. ELISpot plates were analyzed using a CTL-Immunospot Analyzer and ImmunoSpot 3.2 analysis software (Cellular Technology Ltd). Cultures incubated with CMV lysates were scored as positive if the number of resulting spots was 3 standard deviations above the control.
TREC analysis
TREC analysis was performed according to a previously described protocol.30 DNA was isolated from PBMCs using the QIAamp DNA Mini Kit (QIAGEN). Quantitation of signal-joint TCR excision circle (sjTREC) DNA was performed by Taqman real-time polymerase chain reaction using Rotor-Gene 6000 thermal cycler (Corbett Life Science). Quantitation of the sjTREC copy number for each patient sample was performed using a standard curve prepared with 10-fold dilutions of a plasmid containing the sjTREC sequence (kindly provided by Dr Daniel Douek, National Institute of Allergy and Infectious Diseases).
Statistical analysis
The Wilcoxon rank-sum test was used to test differences between continuous variables, whereas the Fisher exact test was used for categoric measures. The Jonckheere-Terpstra test was used to test a trend of ordered categoric variable in Table 4. Correlation between continuous variables was assessed using Spearman rank test. Overall survival (OS) was defined as the time from transplantation to death from any cause, whereas progression-free survival (PFS) was defined as the time from transplantation to progression or death from any cause. Univariable and multivariable Cox regression analyses were performed for OS and PFS. CMV serostatus, ELISpot status, TREC level (categorized as ≥ 2000 vs < 2000 copies/μg DNA), and phenotypic markers were included as time-varying covariates. Age, sex mismatch (between a patient and 2 cords), and risk group were adjusted for multivariable models.31 Association between acute or chronic GVHD and viremia was not assessed because only one patient developed acute GVHD and 3 patients developed chronic GVHD in this patient cohort. Phenotypic markers were measured at pre-established time points: before transplantation, 4 weeks, 8 weeks, 100 days, 6 months, and 1 year after transplantation. For the purpose of the statistical analysis, the status of CMV viremia was considered before transplantation and within the following intervals: 0 to 8 weeks, 8 weeks to 100 days, 100 days to 6 months, and 6 months to 1 year. All phenotypic markers, analyzed as continuous variables, were log10 transformed in the Cox regression model. All P values were based on 2-sided tests and were computed using SAS 9.2 software (SAS Institute).
Results
Characteristics of patients and umbilical cord blood units
A total of 32 patients with hematologic malignancies were enrolled in the study. Four patients were excluded from analysis because of death before day 100, and 1 patient was excluded because of insufficient number of samples after transplantation. Thus, the results presented in this report are based on 27 patients. The CMV serostatus before transplantation was available in 25 of the 27 patients and 16 patients (64%) were found to have detectable anti-CMV antibody titers (Table 1). Cellular characteristics were determined for each of the UCB units (Table 1).
Baseline characteristics of patients and cord blood units
| Patients . | No. . |
|---|---|
| No. of patients | 27 |
| Median age, y (range) | 48 (19-67) |
| Male, percentage | 52 |
| Days to engraftment, n = 23 | |
| ANC more than 500 × 109/L, median (range) | 20 (13-35) |
| Platelet count more than 20 × 109/L, median (range) | 42 (25-162) |
| Pretransplantation CMV antibody titer, n = 25 (%) | |
| Positive | 16 (64) |
| Negative | 9 (36) |
| Cord blood units | |
| Total nucleated cell dose, ×107/kg, median (range) | |
| Donor 1 | 2.75 (1.87-67.00) |
| Donor 2 | 2.33 (1.81-3.94) |
| CD34+ cell dose, ×105/kg, median (range) | |
| Donor 1 | 7.20 (0.10-25.60) |
| Donor 2 | 3.80 (1.90-14.40) |
| Patients . | No. . |
|---|---|
| No. of patients | 27 |
| Median age, y (range) | 48 (19-67) |
| Male, percentage | 52 |
| Days to engraftment, n = 23 | |
| ANC more than 500 × 109/L, median (range) | 20 (13-35) |
| Platelet count more than 20 × 109/L, median (range) | 42 (25-162) |
| Pretransplantation CMV antibody titer, n = 25 (%) | |
| Positive | 16 (64) |
| Negative | 9 (36) |
| Cord blood units | |
| Total nucleated cell dose, ×107/kg, median (range) | |
| Donor 1 | 2.75 (1.87-67.00) |
| Donor 2 | 2.33 (1.81-3.94) |
| CD34+ cell dose, ×105/kg, median (range) | |
| Donor 1 | 7.20 (0.10-25.60) |
| Donor 2 | 3.80 (1.90-14.40) |
For the 27 patients who were included in this study, the median time to neutrophil and platelet engraftment was 20 and 42 days, respectively. One patient developed acute GVHD and 3 patients developed chronic GVHD. Eleven patients had infectious complications, 1 of whom had CMV pneumonitis; 12 patients relapsed during the study. A total of 11 patients died and causes of death were relapse (n = 5), posttransplantation lymphoproliferative disorder (PTLD; n = 3), sepsis/multisystem organ failure (MSOF; n = 1), donor-related leukemia (n = 1), and trauma (n = 1). Detailed patient characteristics and clinical outcome of the entire clinical trial will be reported elsewhere.
Reconstitution of lymphoid and myeloid subpopulations displays distinct kinetics
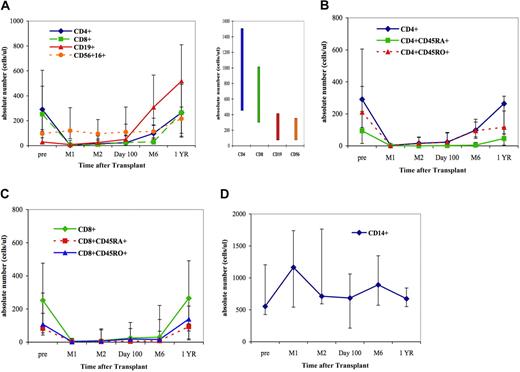
Before transplantation, all peripheral blood leukocyte (PBL) subpopulations were at or below the lower limit of normal values with the exception of CD14+ monocytes (Figure 1). Immediately after transplantation, the median values of CD4+ and CD8+ T cells fell dramatically (Figure 1A). A slight increase in CD4+ T-cell numbers was seen at 6 months after transplantation (Figure 1A-B). Neither CD4+CD45RA+ nor CD4+CD45RO+ T cells reached normal median values within 1 year after transplantation (Figure 1B). Subsets of CD8+ T cells were substantially depressed through 6 months but reached normal levels by 1 year after transplantation (Figure 1A). Conversely to CD4+ and CD8+ T cells, the numbers of CD56+16+ natural killer (NK) cells remained stable and within normal limits throughout the first year (Figure 1A). The median value of CD14+ monocytes also remained within normal limits throughout the study (Figure 1D). CD19+ B cells were no longer measurable at 4 weeks after transplantation. However, beginning after day 100 and continuing through 1 year, there was a marked increase of CD19+ B cells to levels that exceeded the upper limit of normal values (Figure 1A).
Reconstitution of various cell populations after double UCB transplantation. (A) T-cell, B-cell, and NK cell reconstitution. The bar graph in the right panel indicates the normal range for each of the lymphocyte subsets. (B) CD4+ T-cell subsets. (C) CD8+ T-cell subsets. (D) Monocytes. The 25th and 75th percentiles are denoted by the error bars; solid symbols (■, ♦, ▲, ●) denote median values.
Reconstitution of various cell populations after double UCB transplantation. (A) T-cell, B-cell, and NK cell reconstitution. The bar graph in the right panel indicates the normal range for each of the lymphocyte subsets. (B) CD4+ T-cell subsets. (C) CD8+ T-cell subsets. (D) Monocytes. The 25th and 75th percentiles are denoted by the error bars; solid symbols (■, ♦, ▲, ●) denote median values.
Active generation of new T cells and restoration of TREC values occurs 6 months after transplantation
To further examine immune reconstitution, we evaluated recovery of thymic function by assessing T-cell receptor excision circles (TRECs). During the rearrangement of T-cell receptor gene segments during thymic maturation, small sequences of excised DNA are produced. These episomal fragments, or TRECs, are characteristic of recent thymic emigrants produced during thymopoiesis.30 TRECs are therefore useful for evaluating the de novo production of T cells originating from newly engrafted stem cells.
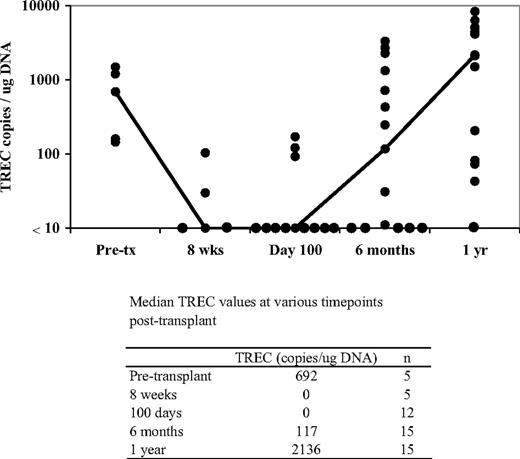
Before transplantation, median TREC values were slightly below the lowest level of normal range in this heavily pretreated patient population (Figure 2). After transplantation, median TREC values fell substantially and were below the limit of detection through 100 days. In contrast, a marked increase in TRECs was observed at 6 months that continued to show substantial gains through 1 year after transplantation. This increase in TRECs temporally paralleled the rise of T-cell populations, displaying a moderate increase in CD4+ and a slight increase in CD8+ subsets (Figure 1B-C).
Reconstitution of TREC values at various time points after transplantation. The line connects the median values for each of the individual time points. Each dark circle represents the TREC value from 1 patient. TREC concentrations were determined from DNA isolated from total PBLs. The limit of detection of the TREC assay was 10 copies/μg DNA.
Reconstitution of TREC values at various time points after transplantation. The line connects the median values for each of the individual time points. Each dark circle represents the TREC value from 1 patient. TREC concentrations were determined from DNA isolated from total PBLs. The limit of detection of the TREC assay was 10 copies/μg DNA.
CMV-specific effectors are detected before thymic regeneration in UCB recipients
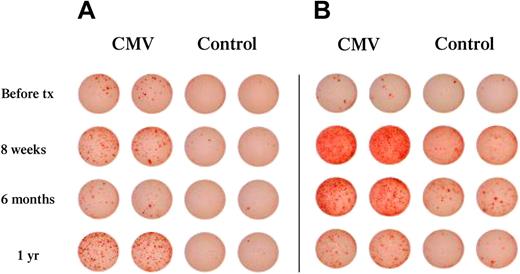
To evaluate functional immune reconstitution in vivo, we used as a model the assessment of immunity against CMV, a virus that causes serious clinical consequences because of its reactivation in patients undergoing hematopoietic stem cell (HSC) transplantation.18,19,21 To determine the ability of UCB recipients to mount immune responses against CMV, we assessed the development of functional CMV effectors by CMV-specific IFN-γ ELISpot. For this purpose, we used total PBMCs because, by including both antigen-presenting cells and T cells, this cell population represents the most biologically relevant population to evaluate for its capacity to mount CMV-specific responses in vivo. As shown in Figure 3, CMV effectors were detected as early as 8 weeks after transplantation. This result demonstrates that patients can develop functional CMV-specific T effector cells at this early period after transplantation, during which there is no evidence of thymic T-cell neogenesis, as documented by the absence of detectable TRECs (Figure 2). These observations are consistent with the hypothesis that these CMV-specific effectors are generated from naive T cells transferred to the patient in the UCB grafts.
Development of CMV-specific T effector cells is detected in patients with or without detectable CMV viremia. Total PBLs were collected at the indicated time points after transplantation and were assessed for the presence of IFN-γ–producing effectors in response to CMV, as described in “IFN-γ ELISpot assay.” Cultures incubated with CMV lysates were scored as positive if the number of resulting spots was 3 standard deviations above the control. (A) Representative patient with detectable CMV effectors and CMV viremia. (B) Representative patient with detectable CMV effectors without detectable viremia.
Development of CMV-specific T effector cells is detected in patients with or without detectable CMV viremia. Total PBLs were collected at the indicated time points after transplantation and were assessed for the presence of IFN-γ–producing effectors in response to CMV, as described in “IFN-γ ELISpot assay.” Cultures incubated with CMV lysates were scored as positive if the number of resulting spots was 3 standard deviations above the control. (A) Representative patient with detectable CMV effectors and CMV viremia. (B) Representative patient with detectable CMV effectors without detectable viremia.
Because cellular reconstitution occurred gradually after transplantation and various leukocyte populations displayed distinct kinetics, we sought to determine whether the generation of CMV-specific effectors was associated with the presence of certain leukocyte subsets. Statistical analysis revealed that, by 6 months after transplantation, CMV-specific T effector cells positively correlated with the numbers of the CD3+ T cells (r = 0.57; P = .01), CD8+ T cells (r = 0.53; P = .02), and CD8+CD45RA+ T cells (r = 0.59; P = .01; Table 2). Interestingly, detection of CMV-specific T effector cells inversely correlated with the numbers of CD20+ B cells 100 days after transplantation (r = −0.51; P = .04; Table 2), when B-cell numbers started to display a marked increase that exceeded normal B-cell values. Although no statistically significant correlation was detected with the numbers of the NK cells, it is possible that NK cells may be a source of IFN-γ detected in ELISpot assay.
Detection of CMV-specific effectors by ELISpot after UCB transplantation depends on the reconstitution of certain leukocyte subsets
| . | n . | Correlation coefficient, r . | P . |
|---|---|---|---|
| CD3* | 19 | 0.57 | .01 |
| CD8* | 19 | 0.53 | .02 |
| CD8CD45RA* | 19 | 0.59 | .01 |
| CD20† | 16 | −0.51 | .04 |
| . | n . | Correlation coefficient, r . | P . |
|---|---|---|---|
| CD3* | 19 | 0.57 | .01 |
| CD8* | 19 | 0.53 | .02 |
| CD8CD45RA* | 19 | 0.59 | .01 |
| CD20† | 16 | −0.51 | .04 |
Six months after transplantation.
One hundred days after transplantation.
We further assessed CMV-specific effectors with regard to the corresponding TREC values for each patient. We detected a significant association between the presence of TRECs and responses of CMV-specific T effector cells at 6 months after transplantation (median = 720 copies/μg DNA [range = 0-3333 copies/μg DNA] for positive [n = 9] and median = 0 copies/μg DNA [range = 0-248 copies/μg DNA] for negative [n = 6]; P = .02). No association was detected at earlier time intervals, consistent with our findings that median TREC values were below the level of detection at those time intervals. These results indicate that CMV-specific effectors could be generated despite the absence of thymic function at those early time intervals after UCB transplantation, supporting the conclusion that these CMV-specific effectors are generated from naive T cells transferred with the UCB units.
The absence of CMV viremia depends on reconstitution of certain leukocyte subpopulations and thymic regeneration
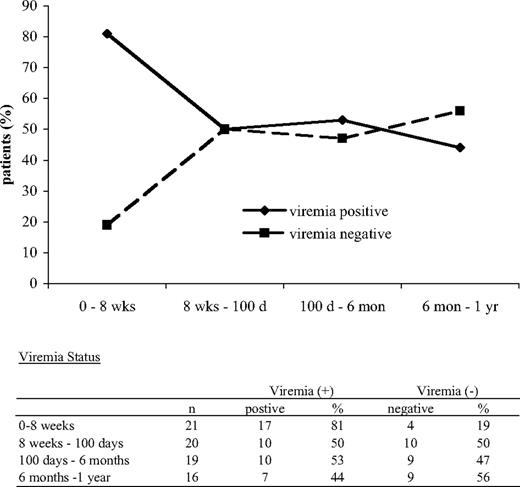
It is well documented that UCB recipients display a high incidence of persistent CMV viremia.18,19 Our studies showed that CMV-specific effectors were detected in CMV-seropositive patients who developed detectable CMV viremia after transplantation (Figure 3A) but also in patients in whom CMV viremia was never detectable (Figure 3B). Moreover, we did not observe statistically significant correlation between the detection of CMV effectors and absence of CMV viremia. For these reasons, we sought to determine which components of immune reconstitution may play a role in regulating the capacity of CMV effectors to control CMV viremia, which is the most clinically relevant end point of their function. We focused these studies in the patient group that was at risk for developing CMV reactivation after transplantation. Before transplantation, 16 patients had detectable anti-CMV antibody titers (Table 1) and therefore were at risk for developing CMV reactivation after transplantation. However, anti-CMV antibody titers may often show false-negative results in this heavily pretreated patient population at the time of screening before transplantation. Moreover, the risk of CMV infection after UCB transplantation is extremely remote. For these reasons, 5 patients who were tested negative for anti-CMV antibody titers before transplantation but developed CMV viremia after transplantation were also included in the population at risk (n = 21). First, we investigated the proportion of patients with CMV viremia at various time intervals after transplantation. During the 1-year follow-up, there was a decrease in the proportion of patients with CMV viremia and a concomitant increase in the number of patients who became negative for CMV viremia (Figure 4).
The proportion of patients with CMV viremia declines, whereas the proportion of patients without CMV viremia increases after transplantation. Patients at risk for CMV (n = 21) were analyzed at various time intervals after transplantation. This patient population included 16 patients who had positive anti-CMV antibody titers before transplantation and 5 patients who were negative for anti-CMV antibody titers but developed CMV viremia after transplantation. The proportion of patients with and without viremia among the tested population at risk is shown for each indicated time interval.
The proportion of patients with CMV viremia declines, whereas the proportion of patients without CMV viremia increases after transplantation. Patients at risk for CMV (n = 21) were analyzed at various time intervals after transplantation. This patient population included 16 patients who had positive anti-CMV antibody titers before transplantation and 5 patients who were negative for anti-CMV antibody titers but developed CMV viremia after transplantation. The proportion of patients with and without viremia among the tested population at risk is shown for each indicated time interval.
The interval of 0 to 8 weeks after transplantation displayed the highest proportion of patients with CMV viremia. During that interval, none of the factors tested was associated with the absence of viremia. During the interval of 100 days to 6 months after transplantation, factors significantly associated with absence of viremia included the number of total PBLs and the CD14+ cells. As shown in Table 3, PBLs were significantly higher in patients without CMV viremia (median = 1365 [range = 390-3040], compared with patients with CMV viremia [median = 425; range = 210-1596]; P = .04). CD14+ cells were also significantly higher in patients without viremia compared with patients who developed CMV viremia (median = 1359 vs 740, respectively; P = .05). During the interval of 6 months to 1 year after transplantation, CD4+CD45RA+ T cells were significantly higher in patients without viremia compared with those with CMV viremia (P = .04), although the sample size at this time point was very small. Thus, although generation of CMV effectors after UCB transplantation depends on the presence of CD8+ T cells (Table 2), the functional capacity of these effectors in vivo, as determined by the absence CMV viremia, depends on the presence of CD4+CD45RA+ T cells (Table 3).
Absence of CMV viremia after UCB transplantation depends on the reconstitution of certain leukocyte subsets
| . | Viremia (−) . | Viremia (+) . | P . | ||
|---|---|---|---|---|---|
| No. . | Median (range) . | No. . | Median (range) . | ||
| PBL* | 8 | 1365 (390-3040) | 9 | 425 (210-1596) | .04 |
| CD14* | 8 | 1359 (897-4039) | 8 | 740 (77-4218) | .05 |
| CD4/CD45RA† | 5 | 139 (8-455) | 5 | 2 (2-87) | .04 |
| . | Viremia (−) . | Viremia (+) . | P . | ||
|---|---|---|---|---|---|
| No. . | Median (range) . | No. . | Median (range) . | ||
| PBL* | 8 | 1365 (390-3040) | 9 | 425 (210-1596) | .04 |
| CD14* | 8 | 1359 (897-4039) | 8 | 740 (77-4218) | .05 |
| CD4/CD45RA† | 5 | 139 (8-455) | 5 | 2 (2-87) | .04 |
Six months after transplantation.
One year after transplantation.
Because thymic regeneration was a critical event of immune reconstitution that occurred during the first year after transplantation in our patients, we examined whether the ability of UCB recipients to generate new, postthymic T cells may be directly related to the efficacy of the CMV effectors in clearing CMV viremia in vivo. We categorized our patients into 3 groups: (1) no viremia, (2) clearance of viremia (patients with CMV viremia, who subsequently cleared the virus), and (3) persistent viremia. We evaluated viremia status in each group with regard to the TREC values. Using as a cutoff concentration that of 2000 copies/μg DNA, which has been indicated as the lower limit of the normal range for TREC values in adults,32 we observed that patients with TREC values of 2000 or more copies/μg DNA were more likely to display either no viremia or clearance of viremia compared with patients with TREC values less than 2000 copies/μg (100% vs 56% vs 44%, respectively [P = .05]; Table 4). Although the sample size is small, these results suggest that thymic reconstitution and generation of new T cells in adult patients after UCB transplantation is associated with clinically significant, functional implications.
TREC copy number versus absence of viremia in patients at risk for CMV reactivation
| . | No viremia . | Clearance of viremia . | Persistent viremia . | P . |
|---|---|---|---|---|
| TREC 2000 or more copies/μg | 1 (100) | 5 (56) | 1 (14) | .05 |
| TREC less than 2000 copies/μg | 0 (0) | 4 (44) | 6 (86) | |
| Total | 1 (100) | 9 (100) | 7 (100) |
| . | No viremia . | Clearance of viremia . | Persistent viremia . | P . |
|---|---|---|---|---|
| TREC 2000 or more copies/μg | 1 (100) | 5 (56) | 1 (14) | .05 |
| TREC less than 2000 copies/μg | 0 (0) | 4 (44) | 6 (86) | |
| Total | 1 (100) | 9 (100) | 7 (100) |
Data are stated as number (%).
Thymic regeneration and reconstitution of T-cell immunity are associated with improved OS
Our data demonstrated that reconstitution of lymphocyte subpopulations and restoration of T-cell thymopoiesis significantly associated with reconstitution of CMV-specific T effector cell immunity. Because we used CMV immunity as a paradigm for immune reconstitution after UCB transplantation, we examined whether reconstitution of CMV-specific immunity and parameters of cellular T-cell immunity may be directly linked to distinct outcomes of OS and PFS. Univariable analysis (Table 5) demonstrated that improved OS was significantly associated with the ability of patients to develop CMV-specific responses as determined by ELISpot assay (P = .03), with overall recovery of PBLs (P = .04), and with reconstitution of several T-lymphocyte subsets critical for immune function: CD3+ (P = .01), CD4+ (P = .02), CD4+/CD45RO+ (P = .01), CD8+ (P = .03), CD8+/CD45RA+ (P = .03), and CD8+/CD45RO+ (P = .05). Improved PFS was also significantly associated with the ability of the patients to develop CMV-specific responses as determined by ELISpot assay (P = .02) and with the values of CD56+/CD16+ NK cells (P = .02). Similar results regarding the role of these variables on OS and PFS were obtained using multivariable analysis except that PBL, CD8+/CD45RA+, and CD8+/CD45RO+ in OS did not reach statistical significance (Table 5). All of these variables were included as time-varying covariates in the models. Supplemental Tables 1 and 2 provide the univariable and multivariable models for OS and PFS with all of the parameters examined (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Univariable and multivariable models for overall survival and progression-free survival
| Variable . | No. . | Univariable model . | Multivariable model* . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| Overall survival | |||||||
| Viremia (pos vs neg) | 27 | 3.72 | 0.91-15.06 | .07 | 3.22 | 0.77-13.44 | .11 |
| ELISpot | 25 | 0.41 | 0.18-0.94 | .03 | 0.29 | 0.11-0.81 | .02 |
| PBL | 27 | 0.14 | 0.02-0.96 | .04 | 0.17 | 0.02-1.39 | .10 |
| CD3 | 27 | 0.20 | 0.06-0.71 | .01 | 0.19 | 0.04-0.84 | .03 |
| CD4 | 27 | 0.21 | 0.06-0.76 | .02 | 0.20 | 0.05-0.92 | .04 |
| CD4+/CD45RO+ | 27 | 0.17 | 0.04-0.65 | .01 | 0.18 | 0.04-0.82 | .03 |
| CD8 | 27 | 0.32 | 0.12-0.87 | .03 | 0.33 | 0.12-0.94 | .04 |
| CD8+/CD45RA+ | 27 | 0.30 | 0.10-0.88 | .03 | 0.35 | 0.12-1.05 | .06 |
| CD8+/CD45RO+ | 27 | 0.36 | 0.13-1.01 | .05 | 0.35 | 0.12-1.03 | .06 |
| TREC,† 2000 or more vs less than 2000 | 22 | < 0.001 | 0.00 | .005 | |||
| Progression-free survival | |||||||
| ELISpot | 25 | 0.54 | 0.33-0.90 | .02 | 0.50 | 0.28-0.89 | .02 |
| CD56+/CD16+ | 27 | 0.35 | 0.15-0.82 | .02 | 0.32 | 0.13-0.81 | .02 |
| Variable . | No. . | Univariable model . | Multivariable model* . | ||||
|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||
| Overall survival | |||||||
| Viremia (pos vs neg) | 27 | 3.72 | 0.91-15.06 | .07 | 3.22 | 0.77-13.44 | .11 |
| ELISpot | 25 | 0.41 | 0.18-0.94 | .03 | 0.29 | 0.11-0.81 | .02 |
| PBL | 27 | 0.14 | 0.02-0.96 | .04 | 0.17 | 0.02-1.39 | .10 |
| CD3 | 27 | 0.20 | 0.06-0.71 | .01 | 0.19 | 0.04-0.84 | .03 |
| CD4 | 27 | 0.21 | 0.06-0.76 | .02 | 0.20 | 0.05-0.92 | .04 |
| CD4+/CD45RO+ | 27 | 0.17 | 0.04-0.65 | .01 | 0.18 | 0.04-0.82 | .03 |
| CD8 | 27 | 0.32 | 0.12-0.87 | .03 | 0.33 | 0.12-0.94 | .04 |
| CD8+/CD45RA+ | 27 | 0.30 | 0.10-0.88 | .03 | 0.35 | 0.12-1.05 | .06 |
| CD8+/CD45RO+ | 27 | 0.36 | 0.13-1.01 | .05 | 0.35 | 0.12-1.03 | .06 |
| TREC,† 2000 or more vs less than 2000 | 22 | < 0.001 | 0.00 | .005 | |||
| Progression-free survival | |||||||
| ELISpot | 25 | 0.54 | 0.33-0.90 | .02 | 0.50 | 0.28-0.89 | .02 |
| CD56+/CD16+ | 27 | 0.35 | 0.15-0.82 | .02 | 0.32 | 0.13-0.81 | .02 |
HR indicates hazard ratio for 1 unit change on log10 scale (except viremia and TREC); pos, positive; and neg, negative.
Adjusted for patient's age, sex mismatch between patient and cords, and risk status at time of transplantation.
Because of the small sample size, the multivariable result is not shown. All patients who died had TREC level less than 2000. The upper 95% CI is not estimable.
Given that reconstitution of functional CMV-specific immunity and absence of viremia were significantly associated with TREC recovery, we hypothesized that the reconstitution of thymopoiesis may also imply a successful immune reconstitution and an improved capacity for generation of immune responses against other pathogens or tumor antigens. Consistent with this hypothesis, our data showed that the main causes of death in our patients were relapse (n = 5), PTLD (n = 3), and sepsis/MSOF (n = 1). Assessment of OS showed that patients whose TREC levels were 2000 or more copies/μg DNA by 1 year after transplantation had a significantly improved OS (P = .005) compared with patients whose TREC value was less than 2000, when included as a time-varying covariate in the univariable model (Table 5).
Discussion
Despite improvements in hematologic recovery observed with the use of 2 partially matched UCB units in adults, immune reconstitution after UCB transplantation remains problematic.5,–7,33 The reasons for the impaired immune reconstitution in UCB recipients remain poorly understood. A major mechanism of the impaired immune reconstitution involves the numbers and properties of T cells in the UCB grafts.22,23,34,–36 It has been proposed that T cells transferred with the UCB are low in numbers and naive, and for this reason UCB grafts are associated with reduced incidence of GVHD but also reduced protection against pathogens, resulting in higher infection-related morbidity and mortality that is predominantly related to fungal infections, CMV, adenovirus, and Epstein-Barr virus.17,20,37,38
We examined the reconstitution of T-cell immunity after double UCB transplantation in adults and assessed its functional significance in the context of CMV reactivation, as a paradigm of clinically relevant functional immunity. Our studies showed the same pattern of reconstitution kinetics of various cell populations as reported previously.11,13 In contrast to the previous reports that did not detect recovery of thymopoiesis in adult UCB recipients within 1 year after transplantation,11,13 our data revealed a clear recovery of T-cell neogenesis by 6 months, resulting in median TREC values within normal limits at 1 year after transplantation. We extended our observations beyond quantitative assessment and we evaluated the components of immune reconstitution that contribute to the recovery of functional immunity using CMV-specific responses and clearance of CMV viremia. Our data showed that CMV-specific effectors, assessed by IFN-γ ELISpot, were detectable as early as 8 weeks after transplantation, before recovery of thymopoiesis. However, these cells failed to clear CMV, as indicated by the lack of statistically significant correlation with clearance of viremia, and the high proportion of patients displaying viremia during this time interval. In contrast, clearance of CMV viremia occurred later, and was dependent on the recovery of the CD4+CD45RA+ T cells and the reconstitution of thymopoiesis. Strikingly, patients with evidence of thymic regeneration and attainment of TREC values of 2000 or more copies/μg DNA had not only higher frequency of CMV viremia clearance but also improved overall survival (P = .005) compared with patients who had no evidence of thymic regeneration. Although weaknesses of the study may include the relatively small number of patients with more than one primary end point assessed, the differences are large and the significance of these observations relies on the fact that these data were generated from adult recipients of double UCB transplantation from a single cancer center, treated with one protocol of pretransplantation conditioning and posttransplantation immunosuppression. These results provide the first observation of the significant role of thymic reconstitution in the clinical outcome of UCB transplantation in adults.
T-cell reconstitution after allogeneic stem cell transplantation involves a peripheral and a central mechanism: (1) peripheral expansion of mature T cells transferred with the graft and (2) thymic selection and generation of donor-derived postthymic T cells. Consistent with our findings, previous studies39,–41 have reported that CMV-specific T cells can be identified from day 0 to 8 weeks after HSC transplantation, indicating that such effectors are detected before reconstitution of thymic function and originate from peripheral expansion of mature T cells transferred with the graft. Because UCB T cells are naive and do not contain pathogen-specific effectors, in contrast to T cells transferred from adult HSC donors, our data indicate that the CMV-specific effectors detected in our patients were generated from naive cord blood T cells after exposure to the CMV antigens in the host. Despite the presence of CMV effectors, clearance of CMV viremia did not occur in all patients, indicating that additional components of the immune system are required for reconstitution of the effector function.
Immediately after UCB transplantation, donor T cells undergo peripheral, antigen-driven proliferation in the absence of thymopoiesis, resulting in the expansion of a limited number of T-cell clones with a restricted repertoire. Evidence of thymopoiesis in adult UCB recipients was observed starting at 18 months after transplantation, but T-cell repertoire diversity recovered to normal levels in 3 to 4 years after transplantation.13 Our studies showed that patients with impaired thymic reconstitution not only had higher frequency of CMV viremia but also reduced overall survival. These events may be related to the restricted repertoire and the impaired ability of these patients to mount immune responses against pathogens and against tumor antigens, as previously proposed for pediatric patients undergoing UCB transplantation.42 Notably, 9 of the 11 deaths in our patient group were due to disease relapse (n = 5), PTLD (n = 3), a condition directly related to impaired Epstein-Barr virus–specific immunity, and sepsis/MSOF (n = 1).
We detected CMV-specific effectors in CMV-seropositive patients who developed CMV viremia but also in patients who did not develop CMV viremia after transplantation. Detection of CMV effectors without detectable CMV viremia was previously observed after HSC transplantation and was attributed to occult CMV reactivation.39,43 These observations raise intriguing questions about the mechanisms that may regulate the ability of CMV effectors to successfully fight the virus and clear CMV viremia in some but not all the patients. We determined that patients with higher levels of CD3+ and CD8+ T cells in general, and the CD8+CD45RA+ subset in particular, had higher numbers of IFN-γ–producing CMV effectors at 6 months after transplantation. However, clearance of CMV viremia required reconstitution of CD4+CD45RA+ T cells. Consistent with these findings, Roux et al have reported that the numbers of CD4+CD45RA+ naive T cells at 6 months after transplantation with T-depleted bone marrow are directly related to the diversification of the T-cell repertoire and the capacity to respond to vaccination.44
Our data indicated that CD8+ T-cell numbers reach the lower limit of normal values at 1 year after transplantation, whereas CD4+ T cells require a longer period of reconstitution, consistent with previous reports.11,13 CD4+ T cells play a crucial role in providing sufficient help to CD8+ T cells during the primary immune response.10 Thus, this unbalanced restoration of the T-cell subpopulations in adult UCB recipients results in an inverted CD4+/CD8+ ratio that has critical implications for CMV-specific immunity and potentially to immunity against other antigens. In support of this, an efficient immune response to CMV after allogeneic HSC or kidney transplantation is reliant upon a synchronous increase in both the CD4+ and CD8+ T-cell populations.41,45,46
TRECs have been reliably detected as early as 100 days after HSC transplantation.24,25,47 However, in reports of UCB transplantation in adults, the appearance of TRECs is substantially delayed, remaining undetectable during the first year and recovering at low levels starting at 18 months after transplantation.11,13 This delayed kinetics in the recovery of thymic function in adults is in contrast to that in pediatric patients, in which the mean TREC levels are within normal limits at 1 year after transplantation.11,13 We were able to detect TRECs in 5 patients by 100 days, although detection became increasingly more frequent at 6 months and 1 year after transplantation. The levels of detection (3191 copies/μg of DNA) reported in pediatric patients at 1 year after transplantation12 were present at this time point only in 6 of 15 patients in our group, consistent with the well-known inverse relationship between age and T-cell recovery in patients who undergo transplantation.24,25 However, the speed of TREC reconstitution in our study was faster than in other adult UCB recipients reported previously. A significant difference between our study and the previous reports was the use of 2 UCB products in all our patients, whereas only a single UCB product was used in the previous 2 trials.11,13 Human UCB is enriched in endothelial precursor cells that can populate and sustain thymopoiesis in a human thymic graft transplanted into immunodeficient mice.48 Thymic epithelial cells produce interleukin-7, which is a critical growth factor for thymopoiesis and potentially regulates T-cell neogenesis via additional mechanisms.49 Thus, the infusion of 2 UCB units in transplant recipients may have a cell dosage effect on thymic regeneration similar to that seen with improved rates of hematologic engraftment in double versus single UCB recipients.
In conclusion, our present results showed that OS, as well as the ability to clear CMV viremia in adult patients undergoing umbilical cord transplantation, strongly depends on the reconstitution of thymopoiesis. Specific approaches to protect thymic function and to promote thymic regeneration by targeting thymic epithelial cells may be highly beneficial in this particular group of patients.
An Inside Blood analysis of this article appears at the front of the issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health: CA123855, AI43552, CA104596, HL087870 (V.A.B.), T32CA081156 (J.B.), AI029530 and CA142106 (H.T.K., K.S.), and CA142106 (J.H.A.).
National Institutes of Health
Authorship
Contribution: J.A.B. performed experiments, analyzed results, and generated the figures and paper; K.S. and H.T.K. performed the statistical analysis of the data; S.M., C.R., M.H., and J.R. generated flow cytometric data; D.L., K.B., C.C., V.H., G.K., P.A., J.K., E. Alyea, S.M., E. Attar, B.D., T.S., R.S., J.R., and J.H.A. provided patient care; V.A.B. designed the research and was responsible for the project and the preparation of the paper; J.B., K.S., H.T.K., C.C., and V.A.B. performed data analyses; and all authors reviewed the paper and had access to the primary clinical trial data.
Conflict-of-interest disclosure: T.S. and R.S. received consultancy income from Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Vassiliki A. Boussiotis, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; e-mail: vboussio@bidmc.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal