Abstract
Essential survival signals within hematopoietic stem cell (HSC) and thymic niches are mediated by receptor tyrosine kinases, which can be reversibly inhibited using clinically available drugs. We studied whether sunitinib, a multityrosine kinase inhibitor that inhibits KIT, enhances engraftment after bone marrow transplantation (BMT) in mice. Sunitinib diminished hematopoietic progenitor cell numbers, and sunitinib enhanced marrow, peripheral myeloid, and lymphoid engraftment after BMT in Rag1−/− mice. Sunitinib augmented HSC engraftment because recipients displayed increased myeloid and lymphoid engraftment and because sunitinib-treated recipients of purified HSCs showed enhanced engraftment of secondary hosts. However, sunitinib preferentially augmented T-cell engraftment with lesser effects on myeloid and HSC engraftment. Consistent with this, sunitinib preferentially depleted the early thymic progenitor subset in the thymus. Sunitinib did not increase engraftment in mice with deficient KIT signaling, and the pattern of more potent effects on T cell compared with HSC engraftment observed in sunitinib-treated hosts was also observed after BMT into KITW/Wv mice. These results implicate KIT as a critical modulator of thymic niches. We conclude that transient, pharmacologic inhibition of KIT enhances accessibility of marrow and thymic niches, and provides a novel, noncytotoxic approach to accomplish engraftment after stem cell transplantation.
Introduction
Hematopoietic stem cell transplantation (HSCT) has experienced great strides since initial clinical trials of this procedure were initiated for leukemia in the 1960s.1 However, preparative regimen-associated toxicities2,3 and morbidity related to graft-versus-host disease remain barriers to progress. Improvements have been made in diminishing the intensity of preparative regimens, but essentially all regimens currently used to accomplish engraftment after HSCT incorporate irradiation and/or cytotoxic agents that carry risks for tissue damage, infection, and second malignancy. Risks associated with cytotoxic preparative regimens are especially pertinent when HSCT is undertaken for benign disease because short- and long-term procedure-related morbidity substantially increases the risk/benefit ratio for individual patients. The development of a targeted, nontoxic preparative regimen that could accomplish HSC engraftment would open new possibilities for transplantation of allogeneic or gene-modified autologous progenitors for benign disease.
Extensive work has demonstrated an essential role for KIT signaling in the HSC niche, and KIT is also expressed on early thymic progenitors (ETPs), the subset thought to represent the earliest T-cell progenitor within the thymus.4 Animals genetically deficient in KIT are receptive to HSC engraftment without conditioning,5,6 and KIT blockade via monoclonal antibody therapy created sufficient “space” within the HSC niche to permit mixed chimerism after HSCT in immunodeficient hosts.7 Pharmacologic inhibition of KIT is now readily available using a variety of receptor tyrosine kinase inhibitors approved for the treatment of malignancy.8–10 We sought to determine whether engraftment after HSCT could be accomplished in mice by modulating HSC and thymic progenitor niche accessibility via pharmacologic inhibition of KIT, in lieu of a cytotoxic preparative regimen. We found that administration of sunitinib monotherapy, a multityrosine kinase inhibitor with inhibitory effects on KIT, facilitated HSC engraftment and had an even greater impact on thymic and peripheral T-cell engraftment. This work provides proof of principle that targeted therapy using small molecules, which reversibly inhibit essential survival signals within the biologic niches, can modulate engraftment and raise the future prospect of targeted, noncytotoxic preparative regimens for HSCT.
Methods
Mice and BMT
C57Bl/6 CD45.1 or CD45.2 (B6, H-2b) mice were purchased from the Animal Production Unit of the National Cancer Institute. Rag1−/− (CD45.2), B6C3H.SWF1 (H-2b), and mice bearing nonsignaling mutations in the receptor tyrosine kinase KIT (WBB6F1/J-KITW/KITw−v, which we refer to as KITW/Wv; CD45.2) were purchased from The Jackson Laboratory. The Animal Care and Use Committee of the National Cancer Institute approved all experiments. Bone marrow (BM) was obtained from donor mice by passage of iced media (RPMI with 10% heat-inactivated fetal bovine serum, penicillin, streptomycin, l-glutamine, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, nonessential amino acids, sodium pyruvate, and β-mercaptoethanol) through the tibias and femurs, or by crushing vertebrae with a mortar and pestle. Red blood cells were depleted with ammonium chloride lysing buffer (Invitrogen); and where indicated, T-cell depletion was performed using anti-CD4, -CD8, and -Thy1.2 immunomagnetic selection or lineage depletion was performed using an antibody cocktail containing CD5, CD45R(B220), CD11b, Anti–Gr-1, (Ly-6G/C),7-4 and –Ter-119 followed by immunomagnetic selection according to the manufacturer's instructions (LD depletion column; Miltenyi Biotec). Recipient mice received vehicle or sunitinib (60 mg/kg, LC Laboratories) suspended in vehicle as previously described11 (Knowles Apothecary) and administered daily by oral gavage for 4 days, with the last dose administered at least 10 hours before transplantation. Previous work had established that 4 consecutive days of sunitinib at 60 mg/kg was well tolerated and effective at inhibiting KIT in murine cancer models,11 and pharmacologic data demonstrated that sunitinib would be cleared within 10 hours of the last dose,12 but pharmacodynamic inhibition of KIT would remain at that time point, thus providing a potential advantage to transferred marrow. Where indicated, recipient and control mice were irradiated using γ-irradiation with the doses indicated at a dose rate of 100 to 110 cGy/minute before transplantation.
Serial transplantation
Recipient CD45.2 Rag1−/− mice received vehicle or sunitinib for 4 days as described in “Mice and BMT.” BM was harvested from CD45.1 donor mice as described in “Mice and BMT” and electronically sorted as previously described.13 Donor-derived CD45.1 HSCs, defined as Lin−Sca-1+KIThiFlt3−, were transferred to the recipients via intravenous tail vein injection on day 0. Peripheral blood samples were harvested from these recipients on days 10 and 60 and analyzed by fluorescence-activated cell sorter (FACS). On day 60, recipients were killed and unfractionated BM was harvested as described in “Mice and BMT,” then transferred into lethally irradiated CD45.2 C57Bl/6 hosts. BM and spleens were harvested from the final recipients and analyzed by FACS.
Flow cytometry
At the time points noted after bone marrow transplantation (BMT), single-cell suspensions were made from splenocytes, thymi, peripheral blood, and BM (unilateral tibias and femurs) of recipient mice, red blood cells were lysed, and cells counted by a hemocytometer. Flow cytometry was performed using standard techniques. The following antibodies were used for staining and were conjugated to fluorescein isothiocyanate, phycoerythrin, phycoerythrin-Cy5, Pacific Blue, peridinin chlorophyll protein-Cy5.5, allophycocyanin, allophycocyanin-Alexa Fluor 750, APC-Alexa Fluor 780, and biotin: anti-CD8α(53-6.7), CD117(2B8), CD25 (7D4), CD45.1(A20), CD45.2(104), CD3ϵ(145-2C11), CD4(LT34), Gr-1(RB6-8C5), Sca-1(Ly6A/E)(7D), CD11b(M1/70), CD135(Flt-3)(A2F10.1), CD45R/B220(RA3-6B2), I-A/I-E(2G9), Ter119(Ly-76), NK1.1(PK136), TCRβ(H57-597), TCRγδ(GL3), CD19(1D3), and CD8β(H35-17.2) (BD Biosciences PharMingen); anti-CD117(2B8), CD44(IM7), CD45.1(A20), CD45.2(104), CD3ϵ(145-2C11), CD11b(M1/70), Sca-1(Ly6A/E)(7D), CD4(LT34), CD135(Flt-3)(A2F10.1), CD45R/B220(RA3-6B2), TCRβ(H57-597), TCRγδ(GL3), CD8b(H35-17.2), CD127 (IL7Rα, A7R34), and CD150 (SLAM 9D1) (eBioscience); and anti-CD45R/B220(RA3-6B2) (Invitrogen). Lineage cocktails were composed of anti-Ter119, CD45R/B220(RA3-6B2), TCRβ(H57-597), CD3ϵ(145-2C11), TCRγδ(GL3) CD8α(53-6.7), CD8β(H35-17.2), CD11b(M1/70), NK1.1(PK136), CD19(1D3), CD11c(HL3), Ly-6G, and Gr-1(RB6-8C5). Isotype controls were used to define background staining. Four-color flow cytometry was performed using a dual-laser FACSCalibur (BD Biosciences), and 5- to 7-color flow cytometry was performed using the FACSAria (BD Biosciences). Fluorescence data were collected from viable cells based on forward and side scatter intensity and analyzed using FlowJo software (TreeStar).
Immunoprecipitation and Western blot
BM cells were harvested from untreated mice or, where indicated, from mice treated with either sunitinib or vehicle for 4 days. Cells were treated ex vivo with sunitinib (100nM; LC Laboratories) or vehicle for 2 hours, and then stimulated with 250 ng/mL recombinant mouse stem cell factor (R&D Systems) for 30 minutes. Cells were then lysed with lysis buffer (40mM Tris-Cl, 280mM NaCl, 20% glycerol, 2% NP-40, 4mM ethylenediaminetetraacetic acid, 80nM NaF) containing phosphatase inhibitor (0.1 μg/mL phenylmethylsulfonyl fluoride) and Protease Inhibitor Cocktail Tablets (Roche Molecular Biochemicals). Protein concentration in lysates was determined using the BCA assay kit according to the manufacturer's instructions (Pierce Chemical). A total of 1 mg of protein from each sample was immunoprecipitated overnight at 4°C with anti-KIT antibody (2B8; eBioscience), and protein A–agarose immunoprecipitation reagent (sc-2001; Santa Cruz Biotechnology) immunocomplexes were washed in lysis buffer containing inhibitors. Proteins were eluted by boiling in sodium dodecyl sulfate sample buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose. Membranes were probed with an antiphosphotyrosine antibody (sc-508; Santa Cruz Biotechnology) and then stripped and reprobed with anti-KIT antibody (2B8).
Statistical analysis
Data were analyzed using Prism (GraphPad Software). Groups were compared using Mann-Whitney test, with P values less than .05 considered significant. Percentage donor and recipient chimerism was determined by expression of CD45.1 (donor) versus CD45.2 (recipient) on at least 50 000 cells evaluated by flow cytometry.
Results
Sunitinib-mediated KIT inhibition diminishes hematopoietic progenitors
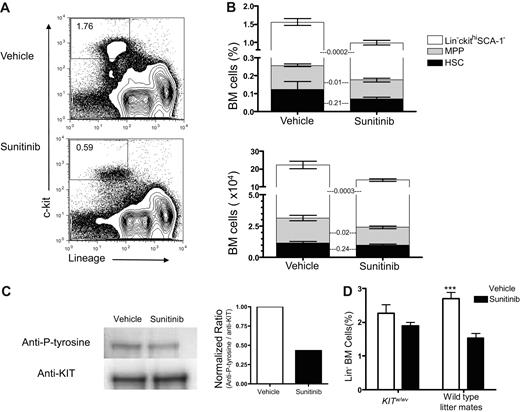
Sunitinib inhibits several receptor tyrosine kinases, including Flt3, VEGFR1, VEGFR2, VEGFR3, PDGFRα, PDGFRβ, RET, CSF1R, and KIT.14,15 KIT signaling plays a primary role in maintaining the HSC niche and is required for stem cell renewal.16 KIT is also expressed on ETPs, thought to be the earliest intrathymic T-cell progenitors.4 Previous work had established that 60 mg/kg of sunitinib was well tolerated and effective at inhibiting KIT in murine models.11 We sought to determine the effects of sunitinib therapy on early hematopoietic and thymic progenitors, as well as on marrow and thymic engraftment after BMT in mice. After 4 daily doses of sunitinib monotherapy, the percentages and absolute numbers of Lin−KIThi BM progenitors were significantly reduced (dot plots from a representative mouse in Figure 1A and the sum total of the stacked bar graphs in Figure 1B: top, P < .001; bottom, P < .05), predominantly because of reductions in committed Lin−KIThiSca-1− progenitors and SCA-1hi multipotent progenitors (MPPs). The percentages and absolute numbers of HSCs (LSKFlt3−) were also decreased, but these changes were not statistically significant (Figure 1B). Although sunitinib inhibits Flt3 signaling, similar reductions in Flt3+ versus Flt3− subgroups within the SCA-1− population were observed; and although sunitinib recipients trended toward slightly lower peripheral blood leukocyte numbers, only peripheral blood monocytes and basophils were significantly reduced after 4 days of therapy (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Sunitinib treatment inhibits KIT signaling in the BM and decreases BM progenitors. (A) Representative flow cytometry plots showing diminished frequencies of Lin−KIThi marrow progenitors in WT mice 4 hours after the fourth daily dose of vehicle (top panel) or sunitinib (bottom panel). (B) Frequency (top panel) and absolute numbers (bottom panel) of total marrow Lin−KIThi cells (represented by the total of all 3 stacked bar graphs) are reduced after sunitinib therapy administered as in panel A (mean percentage ± SEM: 1.55 ± 0.1 after sunitinib vs 1.03 ± 0.08 after vehicle, P < .001; and mean absolute number ± SEM: 19.2 ± 0.75 × 104 after sunitinib vs 11.42 ± 0.74 × 104 after vehicle, P < .001). Within the Lin−KIThi population, frequency and absolute numbers of MPPs and Lin−KIThiSCA-1− progenitors are significantly reduced by sunitinib, whereas changes in HSC frequency and absolute numbers are not significant (P values shown). Data represent pooled results from 4 independent experiments; N = 18 to 20/group. (C) BM cells were harvested from untreated WT mice and then treated with sunitinib (100nM) or vehicle for 2 hours followed by recombinant mouse stem cell factor (250 ng/mL) for 30 minutes; then immunoprecipitation for KIT was performed and blotted with antiphosphotyrosine (left). Densitometric normalized ratios of phosphotyrosine/KIT are shown (right). This experiment was repeated 3 times with similar results. (D) KITW/Wv recipients and WT littermates received sunitinib or vehicle as described in panel A. Differences between sunitinib- and vehicle-treated KITW/Wv mice were insignificant (P = .18), whereas sunitinib significantly decreased the number of Lin−BM progenitors in wild-type littermate controls (P < .001); n = 10 mice per group. Similar results were seen in 2 different experiments. ***P < .001.
Sunitinib treatment inhibits KIT signaling in the BM and decreases BM progenitors. (A) Representative flow cytometry plots showing diminished frequencies of Lin−KIThi marrow progenitors in WT mice 4 hours after the fourth daily dose of vehicle (top panel) or sunitinib (bottom panel). (B) Frequency (top panel) and absolute numbers (bottom panel) of total marrow Lin−KIThi cells (represented by the total of all 3 stacked bar graphs) are reduced after sunitinib therapy administered as in panel A (mean percentage ± SEM: 1.55 ± 0.1 after sunitinib vs 1.03 ± 0.08 after vehicle, P < .001; and mean absolute number ± SEM: 19.2 ± 0.75 × 104 after sunitinib vs 11.42 ± 0.74 × 104 after vehicle, P < .001). Within the Lin−KIThi population, frequency and absolute numbers of MPPs and Lin−KIThiSCA-1− progenitors are significantly reduced by sunitinib, whereas changes in HSC frequency and absolute numbers are not significant (P values shown). Data represent pooled results from 4 independent experiments; N = 18 to 20/group. (C) BM cells were harvested from untreated WT mice and then treated with sunitinib (100nM) or vehicle for 2 hours followed by recombinant mouse stem cell factor (250 ng/mL) for 30 minutes; then immunoprecipitation for KIT was performed and blotted with antiphosphotyrosine (left). Densitometric normalized ratios of phosphotyrosine/KIT are shown (right). This experiment was repeated 3 times with similar results. (D) KITW/Wv recipients and WT littermates received sunitinib or vehicle as described in panel A. Differences between sunitinib- and vehicle-treated KITW/Wv mice were insignificant (P = .18), whereas sunitinib significantly decreased the number of Lin−BM progenitors in wild-type littermate controls (P < .001); n = 10 mice per group. Similar results were seen in 2 different experiments. ***P < .001.
We also evaluated the capacity for marrow to respond to stem cell factor, the ligand for KIT, after sunitinib therapy. Consistent with known effects of this agent, we observed that sunitinib treatment of unfractionated BM ex vivo diminished KIT phosphorylation after exposure to stem cell factor (Figure 1C). Interestingly, we did not observe complete inhibition of KIT phosphorylation after ex vivo exposure to sunitinib, and we could not detect diminished KIT phosphorylation after in vivo exposure to stem cell factor in BM cells harvested from sunitinib-treated animals (data not shown).
Because sunitinib did not completely inhibit KIT phosphorylation ex vivo and because we wanted to determine whether the effects observed were KIT dependent, we analyzed the effects of sunitinib on KITW/Wv mice. Because KITW/Wv mice are KIT deficient, it was not possible to enumerate HSC, LSK, and MPP specific subsets in these mice before and after sunitinib therapy because HSCs, LSKs, and MPPs are all defined as being KIThi. However, we clearly observed effects of sunitinib on Lin− BM progenitors in wild-type (WT) mice (P < .001), whereas no significant effects on this population were observed in KITW/Wv mice (Figure 1D). Together, the data confirm that sunitinib therapy inhibits KIT signaling in BM progenitor cells and reduces marrow progenitors in animals dependent on KIT signaling for hematopoiesis. Further, they implicate KIT as the primary target of sunitinib responsible for the hematopoietic effects observed because no significant effect was seen in animals genetically deficient in KIT signaling.
Sunitinib monotherapy enhances HSC engraftment in immunodeficient hosts
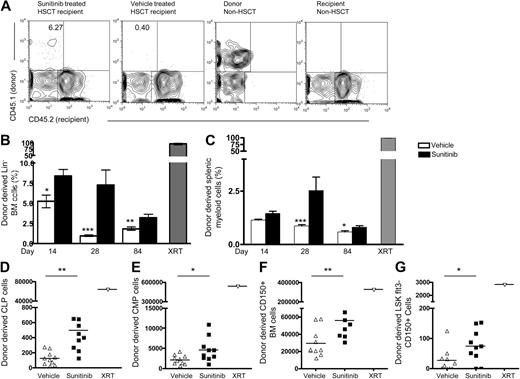
Previous work demonstrated that mice with mutations in KIT signaling are receptive to engraftment5 and that monoclonal antibody-mediated blockade of KIT enhanced engraftment after HSCT in Rag1−/− recipients.7 We therefore sought to determine whether sunitinib induced changes within the marrow stem cell and progenitor pool enhance engraftment after BMT. Rag1−/− mice received sunitinib or vehicle before administration of T cell–depleted congenic BM. As early as day 14 after BMT, sunitinib-treated Rag1−/− recipients showed increased donor chimerism in the Lin− BM compartment, compared with vehicle controls. The magnitude of the differences observed diminished with time, but the differences between sunitinib- and vehicle-treated animals remained significant as late as day 84 after BMT (Figure 2A-B). Sunitinib recipients also showed increased levels of splenic myeloid engraftment on days 28 and 84 after BMT, a peripheral cell subset demonstrated to be a surrogate for BM HSC chimerism17 (Figure 2C), and increased levels of common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs) on day 28 after BMT (Figure 2D-E). Self-renewing long-term HSCs (LT-HSCs) have been recently defined using SLAM family markers,18 such as CD150. We evaluated the effects of sunitinib therapy on day 28 engraftment of total CD150+ BM cells (Figure 2F) and LSK Flt3− CD150+ LT-HSCs and saw similar increases in engraftment as observed in other HSC subsets (Figure 2G).
Sunitinib enhances marrow and peripheral myeloid engraftment after BMT into Rag1−/− recipients. CD45.2+Rag1−/− recipients received daily doses of sunitinib or vehicle on days −3 through 0, then 5 × 106 TCD BM cells from WT CD45.1+C57Bl/6 mice on days 0 and 1. (A) Representative flow cytometry plots of gated Lin− marrow on day 28 show increased donor-derived progenitors in sunitinib recipients. (B-C) Mean donor-derived marrow Lin− cell frequencies and splenic myeloid cells from groups of Rag1−/− recipients treated as in panel A and studied at serial time points. (B) Sunitinib-treated recipients showed significantly increased proportions of donor-derived Lin− marrow progenitors compared with vehicle controls at all time points. (C) Significantly increased donor-derived CD3−B220−CD11b+ myeloid splenocytes on day 28 and 84 and significantly increased donor-derived CLPs (D), CMPs (E), CD150+ BM cells (F), and LSKFlt3−CD150+ BM cells (G) on day 28. *P < .05. **P < .005. ***P < .001. Radiation therapy composed Rag1−/− recipients conditioned with 1000 cGY before BMT as controls. (B-C) Day 14, n = 4 or 5 mice/group; day 28, n = 8 to 13 mice/group; day 84, n = 4 to 15 mice/group. Data represent pooled results from 2 independent experiments. Experiments were repeated 2 to 5 times with similar results. (D-F) n = 10 mice/group; only day 28 data are available for the cell subsets.
Sunitinib enhances marrow and peripheral myeloid engraftment after BMT into Rag1−/− recipients. CD45.2+Rag1−/− recipients received daily doses of sunitinib or vehicle on days −3 through 0, then 5 × 106 TCD BM cells from WT CD45.1+C57Bl/6 mice on days 0 and 1. (A) Representative flow cytometry plots of gated Lin− marrow on day 28 show increased donor-derived progenitors in sunitinib recipients. (B-C) Mean donor-derived marrow Lin− cell frequencies and splenic myeloid cells from groups of Rag1−/− recipients treated as in panel A and studied at serial time points. (B) Sunitinib-treated recipients showed significantly increased proportions of donor-derived Lin− marrow progenitors compared with vehicle controls at all time points. (C) Significantly increased donor-derived CD3−B220−CD11b+ myeloid splenocytes on day 28 and 84 and significantly increased donor-derived CLPs (D), CMPs (E), CD150+ BM cells (F), and LSKFlt3−CD150+ BM cells (G) on day 28. *P < .05. **P < .005. ***P < .001. Radiation therapy composed Rag1−/− recipients conditioned with 1000 cGY before BMT as controls. (B-C) Day 14, n = 4 or 5 mice/group; day 28, n = 8 to 13 mice/group; day 84, n = 4 to 15 mice/group. Data represent pooled results from 2 independent experiments. Experiments were repeated 2 to 5 times with similar results. (D-F) n = 10 mice/group; only day 28 data are available for the cell subsets.
To investigate whether sunitinib-mediated effects on marrow homing could be responsible for the findings observed, we investigated the ability of cells transferred from sunitinib- versus vehicle-treated recipients to home to the BM. A total of 7 × 105 Lin− CD45.1 BM cells were transferred to CD45.2+Rag1−/− recipients; 15 hours after the transfer, the recipients were killed and their BM was harvested for FACS analysis. We saw no difference in the absolute number of donor cells homing to the recipient marrow (supplemental Figure 2A); however, the percentage of donor BM cells was higher in sunitinib-treated recipients (supplemental Figure 2B), consistent with sunitinib-mediated decreases in the size of the recipient marrow progenitor pool (Figure 1). Thus, we saw no evidence for modulation of marrow homing by sunitinib therapy.
Together, the results supported sunitinib-mediated enhancement of HSC engraftment because engraftment involved multiple lineages and was long-lasting, but we sought to further address this issue by administering electronically sorted populations of congenic HSCs (KIT+Sca-1+Lin−Flt3−) to Rag1−/− mice with or without sunitinib therapy. We used donor Gr-1+ peripheral blood cells as a marker to follow engraftment over time via peripheral blood and avoid killing the animals so that they could be used as marrow donors in a later experiment. Analysis of Gr-1+ peripheral blood cells on day 25 demonstrated significantly increased engraftment in recipients treated with sunitinib compared with those receiving vehicle (Figure 3A). The difference was nonsignificant at day 60, but nonetheless subsequent serial transfer of marrow from animals receiving the purified HSCs into lethally irradiated CD45.2 C57Bl/6 hosts demonstrated that marrow from sunitinib-treated recipients mediated enhanced engraftment of CD45.1 cells derived from the original HSC inoculum. Indeed, serial transfer of marrow harvested from sunitinib- versus vehicle-treated hosts showed significantly increased engraftment of BM progenitors, HSCs, splenic B and T cells, but no significant increase in splenic myeloid engraftment in secondary recipients (Figure 3C). Together, these data demonstrate that sunitinib monotherapy administered to immunodeficient Rag1−/− mice before HSCT enhances BM engraftment, with the greatest effect on short-term marrow progenitors and significant but lesser effects on HSC engraftment.
Enhanced engraftment by sunitinib is transferable to secondary recipients. CD45.1−CD45.2+Rag1−/− recipients received sunitinib or vehicle as described in Figure 1A, followed by transfer of 3.3 × 104 electronically sorted HSCs from CD45.1+CD45.2− C57Bl/6 mice on day 0. (A) Sunitinib recipients showed an increased percentage of donor-derived Gr-1 peripheral blood cells compared with vehicle recipients on day 25; N = 4 mice/group. (B) Sixty days after BMT, whole BM from recipients described in panel A was transferred to lethally irradiated CD45.1−CD45.2+ C57Bl/6 mice. (C) Frequencies of CD45.1+ cells in the recipients of the secondary transplantation are shown 7 months after the secondary transplantation: n = 8 for sunitinib group; n = 5 for vehicle group. Similar results were seen on day 32 and day 63 after the secondary transplantation. *P < .05. **P < .001.
Enhanced engraftment by sunitinib is transferable to secondary recipients. CD45.1−CD45.2+Rag1−/− recipients received sunitinib or vehicle as described in Figure 1A, followed by transfer of 3.3 × 104 electronically sorted HSCs from CD45.1+CD45.2− C57Bl/6 mice on day 0. (A) Sunitinib recipients showed an increased percentage of donor-derived Gr-1 peripheral blood cells compared with vehicle recipients on day 25; N = 4 mice/group. (B) Sixty days after BMT, whole BM from recipients described in panel A was transferred to lethally irradiated CD45.1−CD45.2+ C57Bl/6 mice. (C) Frequencies of CD45.1+ cells in the recipients of the secondary transplantation are shown 7 months after the secondary transplantation: n = 8 for sunitinib group; n = 5 for vehicle group. Similar results were seen on day 32 and day 63 after the secondary transplantation. *P < .05. **P < .001.
Sunitinib diminishes thymic progenitors and enhances thymic engraftment after BMT
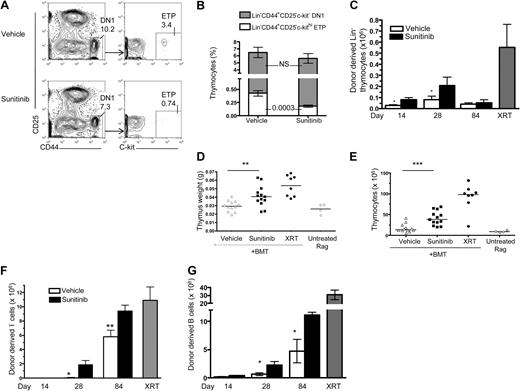
In addition to effects on early marrow progenitors, both KIT and Flt3 also provide essential survival signals during early thymopoieis. Therefore, we specifically investigated the effects of sunitinib therapy on thymic progenitor populations. Thymic size was not affected by short-term sunitinib therapy as administered here, but percentages of thymic DN1 cells (Lin−CD44+CD25−) were reduced by sunitinib, with preferential reductions in KIThi ETPs within this population (Figure 4A-B). This finding could result from decreased input from marrow progenitors and/or from direct effects on the KIThi thymocytes but would be expected to enhance access to thymic niches and facilitate thymic engraftment after BMT. We therefore analyzed thymic engraftment in the thymi of sunitinib- versus vehicle-treated Rag1−/− recipient mice after BMT. As early as day 14 after BMT, sunitinib recipients showed higher absolute numbers of donor-derived Lin− thymocytes than vehicle-treated recipients (Figure 4C); and on day 28 after BMT, thymi from sunitinib-treated recipients showed increased weight (Figure 4D), cellularity (Figure 4E), and numbers of donor-derived thymocytes (84.22 ± 2.37 vs 54.37 ± 7.25 × 106 in sunitinib vs vehicle controls; P < .001). Enhanced thymic engraftment was not maintained long term, however, as the differences observed on day 84 were not significant. In the periphery, sunitinib recipients showed enhanced donor lymphoid chimerism in the spleen and higher numbers of donor-derived mature B and T lymphocytes than animals treated with vehicle before transplantation (Figure 4F-G). Interestingly, donor-derived peripheral T-cell engraftment after sunitinib monotherapy was essentially equal to that accomplished after ablative radiotherapy. In contrast, the effects on B-cell engraftment were not as substantial as on T-cell engraftment because they did not reach the same numbers as irradiated controls. The effects on both peripheral B and T cells were long lasting with continued significant differences on day 84. Thus, sunitinib dramatically enhances T-cell regeneration after BMT for immunodeficiency, and the degree of donor T-cell chimerism accomplished with sunitinib monotherapy is substantially greater than the degree of sunitinib induced myeloid chimerism or B-cell chimerism. However, the immunodeficient mice used in these experiments provide a potent selection advantage for engrafting T-cell populations that confound our ability to determine whether these findings relate to more marked effects of sunitinib therapy on thymic engraftment compared with HSC engraftment and/or is a result of the immunodeficient model system used here.
Sunitinib treatment decreases thymic progenitors and enhances thymic and peripheral lymphoid donor chimerism after BMT. (A-B) WT mice received sunitinib or vehicle as described in Figure 1A, and thymi were analyzed on day 4. (A) Representative flow cytometry plot showing diminished frequencies of DN1 thymocytes and thymic ETPs in sunitinib- (bottom panel) versus vehicle- (top panel) treated mice. (B) Sunitinib did not significantly decrease the percentage of CD44+CD25− DN1 thymocytes (represented by total of stacked bar graphs), but it did induce a selective, significant decline of the KIT+ ETP (white stacked bar graph) within the DN1 population. Data represent pooled results from 4 independent experiments; n = 20 mice/group. (C-F) Rag1−/− recipients received sunitinib or vehicle for 4 days, followed by transfer of 5 × 106 TCD congenic BM cells from WT C57Bl/6 mice on days 0 and 1. On day 28 after BMT, sunitinib recipients showed increased numbers of donor-derived Lin− thymocytes (C), increased thymic weight (D), increased total thymocyte numbers (E), increased numbers of donor-derived splenic CD3+ (F), and splenic B cells (CD3−B220+MHCII; G) compared with vehicle controls. Radiation therapy composed Rag1−/− recipients conditioned with 1000 cGY before BMT as controls. Data represent pooled results from 3 independent experiments. In scatterplots, each shape represents a mouse from day 28 of panels C and F. Day 14, n = 4 or 5 mice/group; day 28, n = 8 to 13 mice/group; day 84, n = 4 to 15 mice/group. Data represent pooled results from 2 independent experiments. Experiments were repeated 2 to 5 times with similar results. *P < .05. **P < .005. ***P < .001.
Sunitinib treatment decreases thymic progenitors and enhances thymic and peripheral lymphoid donor chimerism after BMT. (A-B) WT mice received sunitinib or vehicle as described in Figure 1A, and thymi were analyzed on day 4. (A) Representative flow cytometry plot showing diminished frequencies of DN1 thymocytes and thymic ETPs in sunitinib- (bottom panel) versus vehicle- (top panel) treated mice. (B) Sunitinib did not significantly decrease the percentage of CD44+CD25− DN1 thymocytes (represented by total of stacked bar graphs), but it did induce a selective, significant decline of the KIT+ ETP (white stacked bar graph) within the DN1 population. Data represent pooled results from 4 independent experiments; n = 20 mice/group. (C-F) Rag1−/− recipients received sunitinib or vehicle for 4 days, followed by transfer of 5 × 106 TCD congenic BM cells from WT C57Bl/6 mice on days 0 and 1. On day 28 after BMT, sunitinib recipients showed increased numbers of donor-derived Lin− thymocytes (C), increased thymic weight (D), increased total thymocyte numbers (E), increased numbers of donor-derived splenic CD3+ (F), and splenic B cells (CD3−B220+MHCII; G) compared with vehicle controls. Radiation therapy composed Rag1−/− recipients conditioned with 1000 cGY before BMT as controls. Data represent pooled results from 3 independent experiments. In scatterplots, each shape represents a mouse from day 28 of panels C and F. Day 14, n = 4 or 5 mice/group; day 28, n = 8 to 13 mice/group; day 84, n = 4 to 15 mice/group. Data represent pooled results from 2 independent experiments. Experiments were repeated 2 to 5 times with similar results. *P < .05. **P < .005. ***P < .001.
Sunitinib enhances marrow engraftment in immunocompetent hosts with or without irradiation
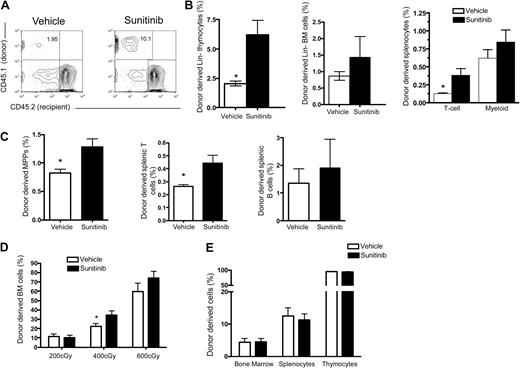
To determine whether sunitinib enhanced myeloid and/or lymphoid engraftment after BMT in immunocompetent recipients, where donor lymphocytes would not experience a competitive advantage, marrow from CD45.1 C57Bl/6 donors was transferred into vehicle- or sunitinib-treated CD45.2 C57Bl/6 recipients. We observed no difference in thymic size between groups in these experiments. However, sunitinib-treated recipients showed significantly increased proportions of donor-derived Lin− thymocytes (Figure 5A-B), and donor-derived peripheral T cells, with trends toward increased BM and peripheral myeloid engraftment that did not reach statistical significance (Figure 5B). Similarly, sunitinib monotherapy significantly increased BM multipotent progenitor (P = .03) and peripheral T-cell (P = .03) engraftment after transfer of parental C57BL/6 (H-2b) BM into C57BL/6 × C3H.SW (H-2b) (F1) recipients mismatched for minor histocompatibility antigens (Figure 5C) but did not enhance myeloid or B-cell engraftment in this setting. Importantly, sunitinib does not enhance engraftment in parent into F1 transplantations with a major mismatch (C57BL/6 [H-2b] marrow into C57BL/6 × C3H.HeN [H-2b × H-2k] F1 recipients) presumably because of hybrid resistance, nor did sunitinib mediate engraftment across minor histocompatibility barriers C57BL/6 (H-2b) marrow into C3H.SW(H-2b) recipients. Thus, sunitinib does not overcome immunologic barriers to engraftment after HSCT.
Sunitinib enhances engraftment in immunocompetent hosts but does not enhance engraftment in KITW/Wv mice. (A-B) C57Bl/6 CD45.2 recipients received 4 daily doses of sunitinib or vehicle followed by transfer of 5 × 106 congenic CD45.1 TCD BM cells on days 0 and 1. Organs were harvested and analyzed by FACS on day 28. (A) Representative flow cytometry plots of Lin−-gated thymocytes from vehicle- (left) versus sunitinib- (right) treated recipients. (B) Sunitinib recipients showed significantly increased Lin− thymic and splenic T-cell engraftment compared with vehicle controls. (C) C57BL/6 × C3H.SW (H-2b) (F1) recipients received 4 daily doses of sunitinib or vehicle followed by transfer of 5 × 106 congenic CD45.1 TCD BM cells on days 0 and 1. Organs were harvested and analyzed by FACS on day 28. Sunitinib significantly increased BM multipotent progenitor (P = .03) and peripheral T-cell (P = .03) engraftment; n = 4 mice per group. (D) C57Bl/6 CD45.2 recipients received 4 daily doses of sunitinib or vehicle followed by total body irradiation with the doses indicated on day 0 and transfer of 5 × 106 congenic CD45.1 TCD BM cells on days 0 and 1. On day 28, sunitinib-treated mice show increased BM engraftment compared with vehicle controls. This difference was significant at 400 cGy; n = 5 mice/group. (E) KITW/Wv recipients received 4 daily doses of sunitinib or vehicle followed by transfer of 5 × 106 CD45.1 TCD BM cells on days 0 and 1. Sunitinib did not significantly increase the frequency of BM cells (P = .93), splenocytes (P = .70), or thymocytes (P = .69) 28 days after BMT. Data represent pooled results from 2 independent experiments; n = 13 or 14 mice/group. *P < .05. ***P < .001.
Sunitinib enhances engraftment in immunocompetent hosts but does not enhance engraftment in KITW/Wv mice. (A-B) C57Bl/6 CD45.2 recipients received 4 daily doses of sunitinib or vehicle followed by transfer of 5 × 106 congenic CD45.1 TCD BM cells on days 0 and 1. Organs were harvested and analyzed by FACS on day 28. (A) Representative flow cytometry plots of Lin−-gated thymocytes from vehicle- (left) versus sunitinib- (right) treated recipients. (B) Sunitinib recipients showed significantly increased Lin− thymic and splenic T-cell engraftment compared with vehicle controls. (C) C57BL/6 × C3H.SW (H-2b) (F1) recipients received 4 daily doses of sunitinib or vehicle followed by transfer of 5 × 106 congenic CD45.1 TCD BM cells on days 0 and 1. Organs were harvested and analyzed by FACS on day 28. Sunitinib significantly increased BM multipotent progenitor (P = .03) and peripheral T-cell (P = .03) engraftment; n = 4 mice per group. (D) C57Bl/6 CD45.2 recipients received 4 daily doses of sunitinib or vehicle followed by total body irradiation with the doses indicated on day 0 and transfer of 5 × 106 congenic CD45.1 TCD BM cells on days 0 and 1. On day 28, sunitinib-treated mice show increased BM engraftment compared with vehicle controls. This difference was significant at 400 cGy; n = 5 mice/group. (E) KITW/Wv recipients received 4 daily doses of sunitinib or vehicle followed by transfer of 5 × 106 CD45.1 TCD BM cells on days 0 and 1. Sunitinib did not significantly increase the frequency of BM cells (P = .93), splenocytes (P = .70), or thymocytes (P = .69) 28 days after BMT. Data represent pooled results from 2 independent experiments; n = 13 or 14 mice/group. *P < .05. ***P < .001.
Sunitinib monotherapy enhanced myeloid engraftment and more potently enhanced lymphoid engraftment in immunodeficient hosts, and enhanced lymphoid engraftment in immunocompetent hosts, but chimerism levels remained low. To determine whether sunitinib could enhance myeloid and/or lymphoid engraftment in conjunction with low-dose irradiation, we compared engraftment rates in animals receiving congenic marrow after treatment with 200, 400, or 600 cGy of irradiation with or without sunitinib. We observed significant increases in marrow (P = .047), peripheral T cell (P = .002), peripheral B cell (P = .012), and splenic myeloid cell (P = .012) engraftment when sunitinib was combined with 400 cGy (Figure 5D). Interestingly, even 200 cGy of irradiation alone induced substantial thymic engraftment, but these effects were not further enhanced by sunitinib (data not shown).
Sunitinib therapy does not augment engraftment in hosts with defective KIT signaling
To investigate the mechanism by which sunitinib enhances engraftment after BMT, T cell–depleted (TCD) BM from CD45.1 C57B1/6 donors was transferred into vehicle- or sunitinib-treated CD45.2 KITW/Wv recipients. Previous work has shown that KITW/Wv mice are receptive to HSC engraftment without conditioning resulting from mutations in KIT signaling.5,6 KITW/Wv show incomplete HSC engraftment after transfer of TCD marrow, which is not increased by sunitinib therapy (Figure 5E). Interestingly however, these mice experienced almost complete donor chimerism in the thymus, despite less than 10% donor chimerism in the BM. Therefore, genetic mutations in KIT signaling induce results similar to that seen with sunitinib therapy, namely, substantial thymic engraftment and significant but more modest effects on HSC engraftment. Together, these results suggest that the effects of sunitinib are primarily related to blockade of KIT signaling, as opposed to the numerous other tyrosine kinases inhibited by this agent, because no further increase in engraftment was observed when sunitinib was administered to KITW/Wv hosts. Furthermore, the fact that increased thymic compared with marrow engraftment is observed both with mutant KIT and sunitinib is consistent with a model wherein KIT-mediated survival signals play essential roles within the thymic niche.
Discussion
This work provides proof of principle that administration of a tyrosine kinase inhibitor that inhibits KIT signaling for a period exceeding its pharmacokinetic half-life can provide an advantage to transplanted BM, resulting in enhanced engraftment. We demonstrate that sunitinib-mediated tyrosine kinase inhibition decreases hematopoietic and thymic progenitors, allowing for dramatic increases in lymphoid engraftment and significant increases in myeloid engraftment in Rag1−/− immunodeficient recipients. T-cell engraftment was also enhanced by sunitinib after transfer of congenic marrow and parental marrow into F1 immunocompetent recipients, although the effects were less potent and nonsignificant for myeloid engraftment. We further demonstrate that these effects of sunitinib are probably the result of KIT inhibition because mice deficient in KIT do not show increased engraftment when treated with sunitinib. Given that current models implicate an essential role for KIT signaling in the HSC niche,7,19 these results are not unexpected.
However, these studies provide the unexpected observation that sunitinib enhances thymic and T-cell engraftment to a greater extent than myeloid engraftment. This enhancement is probably not the result of differential effects on marrow myeloid versus lymphoid progenitors because short-term studies show similar engraftment rates by CLPs and CMPs with sunitinib. Rather, the enhanced effects of sunitinib on T-cell engraftment probably reflect potent modulation of thymic niches by KIT inhibition, thus giving donor thymic progenitors a competitive advantage during early thympoiesis and allowing donor-derived T cells to effectively seed the periphery. The lesser effects of sunitinib in enhancing peripheral B-cell engraftment are also consistent with a potent effect of sunitinib on thymic engraftment. Given that sunitinib also inhibits Flt3 signaling, and Flt3 ligand plays a role in early thymopoiesis, the specific tyrosine kinase (TK) inhibition profile of sunitinib could contribute to these results. However, only a small subset of ETPs express Flt3, and Flt3−/− mice undergo normal thymopoiesis.4 Rather, we propose that this may illustrate the essential role that KIT plays in early thymopoiesis. KIT expression is a hallmark of the ETP thymic subset,4 and the data shown here that sunitinib therapy selectively and efficiently depletes ETPs are consistent with KIT, providing an essential survival signal for this population. ETPs are posited to be the critical T-cell progenitor population within the thymus,20 and the potent decrease in ETP numbers induced by sunitinib (Figure 4A) would be expected to preferentially enhance T-cell, compared with myeloid and B-cell, engraftment. Although the high level T-cell engraftment observed in Rag1−/− hosts treated with sunitinib is largely related to the void within the T-cell compartment, we also saw increased T-cell engraftment compared with B-cell or myeloid engraftment in immunocompetent hosts treated with sunitinib (Figure 5B right panel). Furthermore, the studies in KITW/Wv mice also showed a similar pattern of increased thymic and peripheral T-cell engraftment compared with myeloid engraftment (Figure 5E), although KITW/Wv presumably have normal Flt3 signaling. Together, the data are consistent with ETP KIT signaling, playing an essential role within the thymic niche, and suggest that preparative regimens that selectively target KIT signals will preferentially enhance T-cell engraftment to a greater extent than myeloid or B-cell engraftment.
With regard to the potential for clinical translation of this approach for augmenting engraftment after HSCT, several points should be noted. Most importantly, although sunitinib-mediated TK inhibition enhanced HSC, myeloid and thymic engraftment of histocompatible BM in immunodeficient hosts, myeloid engraftment was generally low and immunocompetent hosts did not demonstrate enhanced myeloid engraftment with sunitinib therapy. These results are probably the result of weak effects of sunitinib on HSC engraftment, and the reasons for this remain unclear. The results could be the result of incomplete HSC KIT inhibition with sunitinib as administered here because we observed only approximately 50% inhibition of KIT signaling in BM treated with sunitinib ex vivo (Figure 1C) and the degree of KIT inhibition observed in marrow progenitors after sunitinib therapy in vivo was below detection levels (data not shown). If incomplete KIT inhibition is the cause, then regimens incorporating alternative doses and schedules of this or another KIT inhibitor could increase the efficiency of KIT inhibition and HSC and myeloid engraftment beyond that observed here. However, myelosuppression is not a major dose-limiting toxicity of any of the KIT TK inhibitors currently on the market,9,10,21 suggesting that these drugs in bioactive doses do not completely inhibit stem cell function over a prolonged period. Furthermore, similar low levels of engraftment were observed with anti-KIT monoclonal antibody therapy,7 suggesting that low-level engraftment may be a general property of preparative regimens based solely on KIT inhibition.
Potent effects on progenitor engraftment with lesser effects on HSC engraftment would explain the relatively low levels of myeloid engraftment observed and the diminished effects observed over time. Some data demonstrating increased engraftment of CD150+ LT-HSCs (Figure 2F-G), combined with the fact that Lin− BM engraftment in sunitinib- versus vehicle-treated animals remained significant even at late time points, such as 84 days after BMT (Figure 2B) and the enhanced engraftment seen in the serial transplantation (Figure 3B-C) are consistent with sunitinib-mediated enhancement of true self-renewing HSC engraftment. However, we did see diminished engraftment percentages at later compared with earlier time points (Figure 2B), consistent with a model wherein hematopoietic progenitors efficiently engraft early after sunitinib monotherapy, but their relative contributions diminish over the weeks after engraftment. Indeed, the data demonstrating that sunitinib depletes MPPs more efficiently than HSCs (Figure 1B) are consistent with more effective engraftment of MPPs than HSCs with this approach. KITW/Wv mice show increased engraftment over time as the competitive disadvantage is permanent in this system whereas it is transient with sunitinib; thus, we would not expect engraftment to increase over time.
We therefore propose that KIT-based targeting does not efficiently modulate the LT-HSC niche, which is composed of a nonsynchronized, largely quiescent population of cells. Rather, transient inhibition of KIT-dependent cycling and/or KIT-mediated trophic signals probably disadvantages only a fraction of the quiescent HSC pool at any given time. This is in contrast to a tumor population, such as gastrointestinal stromal tumors with mutated KIT, where constitutive KIT activation in all cells results in oncogene addiction and substantial cell death after transient exposure to a KIT inhibitor, such as sunitinib.21 Given that short-term pharmacologic inhibition of KIT with tyrosine kinase inhibitors is essentially devoid of toxicity, multiple rounds of KIT inhibition followed by HSCT could potentially overcome this limitation if continued low-level engraftment occurred with minimal regimen-related toxicity. Furthermore, modulating the dose and schedule of this regimen could improve rates of engraftment but is beyond the scope of this report.
Despite the limitations on HSC engraftment observed, even low-level chimerism, as accomplished here, may be clinically significant because mixed chimerism after HSCT of allogeneic or genetically modified autologous cells is potentially curative for some benign diseases, especially if the underlying defect results in a competitive disadvantage to resident hematopoietic or lymphoid populations. This is clearly evidenced when HSCT is undertaken for severe combined immunodeficiency both in the clinical setting and in the models presented here, where near normalization of the peripheral lymphoid pool is observed.22 Indeed, the potent effects of sunitinib on T-cell engraftment probably persist in the long term because homeostatic cycling of mature T cells provides the primary source for peripheral T-cell renewal in vivo. This is clinically relevant to transplantation for immunodeficiency, where replacement of the T-cell pool with minimal toxicity is desired. Similarly, transplantation for systemic metabolic disorders,23 hemoglobinopathies, such as thalassemia24 and sickle cell anemia,25 can also reap substantial benefits even in the face of low-level HSC chimerism. Furthermore, immunomodulatory effects of low-level engraftment are significant because mixed chimerism appears to decrease the risk for graft-versus-host disease26 and microchimerism achieved through HSCT can induce immune tolerance when used as a prelude to solid organ transplantation.27–29 For conditions where higher levels of chimerism are necessary for clinical benefit, the data presented here also demonstrate that administration of a targeted TK inhibitor may allow one to lower the dose of a cytotoxic agent, such as irradiation, and still accomplish significant engraftment, an approach that could diminish toxicity.
Finally, it is important to note that sunitinib, as administered here, did not overcome immunologic rejection either in a minor mismatch model or hybrid resistance in the parent into the F1 model. Thus, although this approach provides a mechanism to increase “marrow” and “thymic” space, clinical application across allogeneic barriers in an outbred population would require incorporation of antirejection therapies to accomplish engraftment. This could potentially also be accomplished, however, with noncytotoxic therapies, such as rapamycin, which has been shown to prevent graft rejection in nonmyeloablative model systems for benign disease.30,31
In conclusion, biomedical research has seen an explosion in the number of newly available targeted therapies, largely driven by the development of new drugs for cancer. This opens new opportunities to precisely modulate a variety of essential physiologic pathways for the treatment of benign disease as well. Promising results have been demonstrated using targeted therapies for autoimmune diseases32 and in treatment of benign tumors,33 but more new applications for targeted therapies in benign disease will result from the plethora of small molecules becoming available. In the context of HSCT, benign diseases favor less intense conditioning regimens because stable mixed chimerism is often sufficient for clinical benefit and the risks associated with cytotoxic preparative regimens are more difficult to justify. We demonstrate here that an entirely noncytotoxic approach, which reversibly inhibits KIT signaling, can achieve HSC engraftment by modulating accessibility of BM and thymic niches. Future studies are needed to evaluate the effectiveness of this approach as a prelude to genetically modified autologous HSCs or in combination with antirejection therapy in a minor mismatch setting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Dan Fowler for his careful review of the manuscript, the Experimental Transplantation and Immunology Branch Flow Cytometry Core for support of our studies, and Alan Chiet for expert compounding of sunitinib administered in these experiments.
This work was supported by the National Institutes of Health (Intramural Research Program).
National Institutes of Health
Authorship
Contribution: A.C.K. and N.M.F. designed experiments, performed the research, analyzed data, and wrote the manuscript; M.G. provided intellectual input and designed experiments; J.L.M. and S.D. performed the experiments; and C.L.M. provided intellectual input and research support and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Natasha M. Fewkes, 10-CRC 1W-3750, 10 Center Dr, MSC 1104, Bethesda, MD 20892; e-mail: fewkesnm@mail.nih.gov.
References
Author notes
N.M.F. and A.C.K. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal