Abstract
NEDD8 activating enzyme (NAE) has been identified as an essential regulator of the NEDD8 conjugation pathway, which controls the degradation of many proteins with important roles in cell-cycle progression, DNA damage, and stress responses. Here we report that MLN4924, a novel inhibitor of NAE, has potent activity in acute myeloid leukemia (AML) models. MLN4924 induced cell death in AML cell lines and primary patient specimens independent of Fms-like tyrosine kinase 3 expression and stromal-mediated survival signaling and led to the stabilization of key NAE targets, inhibition of nuclear factor-κB activity, DNA damage, and reactive oxygen species generation. Disruption of cellular redox status was shown to be a key event in MLN4924-induced apoptosis. Administration of MLN4924 to mice bearing AML xenografts led to stable disease regression and inhibition of NEDDylated cullins. Our findings indicate that MLN4924 is a highly promising novel agent that has advanced into clinical trials for the treatment of AML.
Introduction
Acute myeloid leukemia (AML) is a disease of the elderly, and the majority of newly diagnosed patients will be older than 60 years of age.1 Complete remission occurs in up to half of these patients. However, relapse is generally inevitable and prognosis is dismal. Although novel therapeutic approaches have the potential to improve outcomes for all patients with AML, more effective treatment strategies are urgently required for elderly patients who currently have a much poorer prognosis than younger patients with this disease. Preexisting myelodysplasia, unfavorable cytogenetics, treatment-related AML, and multidrug resistance are all more common in older patients.2 In addition, coexisting morbidities limit therapeutic options for many of these patients. Moreover, no standard induction approach exists for this group in part because of their poor representation in clinical studies. A recent study revealed that induction with low-dose cytarabine offered a survival advantage over supportive care for patients with good or intermediate prognosis cytogenetics (25% vs 10%, P = .004). However, no complete remissions were observed for those with poorer performance status and/or unfavorable cytogenetics on this study, thus highlighting the need for new therapies.3
The ubiquitin-proteasome system is responsible for the timed destruction of most intracellular proteins. NEDD8-activating enzyme (NAE) has been identified as an essential controller of the Nedd8 conjugation pathway, which regulates the activity of the cullin-dependent E3 ligases.4 The cullins control the ubiquitination and subsequent degradation of many proteins with important roles in cell-cycle progression, DNA damage, stress responses, and signal transduction.5-7 Considering that NEDD8 controls the homeostasis of proteins vitally important for the survival of AML cells,5-7 we evaluated the preclinical antileukemic activity of MLN4924, a novel first-in-class small molecule inhibitor of NAE.8
Methods
Cells and cell culture
HL-60 and HS-5 cells were obtained from ATCC. MV4-11, MOLM-13, and PL-21 cells were obtained from DSMZ. Primary human AML cells were isolated from the bone marrow of AML patients after obtaining informed consent in accordance with the Declaration of Helsinki. The University of Texas Health Science Center institutional review board approved the collection of peripheral blood and bone marrow specimens.
Chemicals and reagents
Reagents were obtained as follows: MLN4924 (Millennium Pharmaceuticals), antitubulin, antiactive caspase-3, antiphospho- and total IκBα, anti–CDT-1, anti-FLIP, anti–BCL-2, anti–BCL-xL, antiphospho- and total Chk1 antibodies (Cell Signaling), anti–NRF-2 antibody (Santa Cruz Biotechnology), and anti–β tubulin (Sigma-Aldrich).
Cell viability assay
Cells were plated in triplicate and treated with defined concentrations of MLN4924 for 72 hours. Viable cells were quantified using ATPLite according to the manufacturer's instructions (PerkinElmer Life and Analytical Sciences).8
Analysis of drug-induced apoptosis
Apoptosis was evaluated by propidium iodide/fluorescence-activated cell sorter (PI/FACS) analysis of sub-G0/G1 DNA content as previously described.9
Colony assays
AML cells were treated with MLN4924 for 24 hours, seeded in Methocult, and colonies were scored as previously described.10
Immunoblot analyses
Protein extracts were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis and transferred to nitrocellulose membranes as described previously.9 β-Tubulin documented equal loading.
Stable knockdown of FLT3 expression
SMARTVector lentiviral particles containing empty vector (control) and Fms-like tyrosine kinase 3 (FLT3) shRNA were used to infect MV4-11 cells according to the manufacturer's instructions (Dharmacon RNA Technologies).
Quantification of NFκB DNA-binding activity
DNA binding was quantified using the chemiluminescent nuclear factor-κB (NF-κB) p65 transcription factor kit according to the manufacturer's directions (Pierce Protein Research Products).
In vivo evaluation of MLN4924
HL-60 human leukemia cells were injected into the flanks of nude mice. After tumor growth reached 150 mm3, mice were randomly assigned to receive MLN4924 10, 30, 60, or 90 mg/kg twice a day (n = 10 per group), vehicle control (n = 10) for 21 days. Tumor growth and animal toxicity were assessed as previously described.11 The University of Texas Health Science Center institutional animal care and use committee approved the mouse studies.
Results and discussion
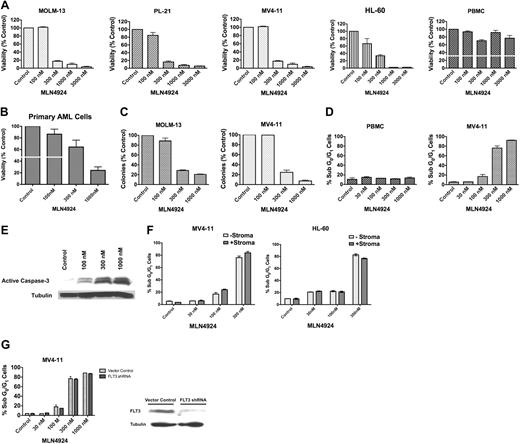
We first assessed the effects of MLN4924 treatment on the viability of AML cells. Nanomolar concentrations of MLN4924 (mean 50% inhibitory concentration = 211nM) selectively and potently inhibited the in vitro growth and survival of MOLM-13, PL-21, MV4-11, and HL-60 cells and primary AML cells from patients with different clinical and molecular features compared with peripheral blood mononuclear cells (PBMCs) from healthy donors (Figure 1A-B; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). MLN4924 treatment also dramatically disrupted the ability of AML cells to form colonies (Figure 1C) and led to a dose-dependent induction of apoptosis as evidenced by sub-G0/G1 DNA content and the processing of caspase-3 to its active form (Figure 1D-E).
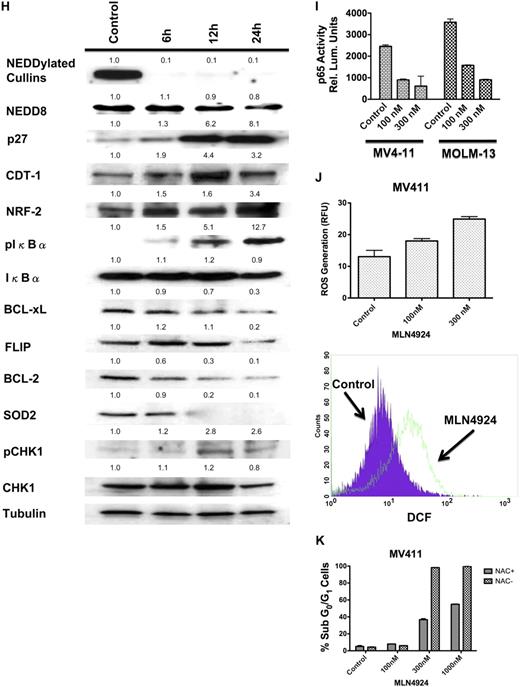
MLN4924 is highly active in preclinical AML models and primary AML cells. (A) MLN4924 potently reduces the viability of AML cells. MOLM-13, PL-21, MV411, and HL-60 cell lines and PBMCs from healthy donors were treated with the indicated concentrations of MLN4924 for 72 hours. Viability was assessed by the ATPLite assay. Results shown represent the mean of 3 experiments. Error bars represent SD. (B) MLN4924 has activity in primary AML cells. Primary cells were obtained from 5 AML patients. Of these patients, 1 had therapy-related AML and another had primary refractory AML indicating aggressive clinical disease. Primary cells were treated with MLN4924 for 72 hours. Viability was assessed by the ATPLite assay. (C) Dose-dependent inhibition of clonogenic survival after treatment with MLN4924. MOLM-13 and MV411 human AML cells were treated with the indicated concentrations of MLN4924 for 24 hours. Drug was washed away, cells were seeded in Methocult, and colonies were scored on day 10. n = 3; bars represent the mean ± SD. (D) Quantification of drug-induced apoptosis. MV411 cells and PBMCs from healthy donors were treated with the indicated concentrations of MLN4924 for 48 hours. Percentages of cells with subdiploid DNA were determined by PI/FACS. n = 3; bars represent the mean ± SD. (E) Activation of apoptosis after treatment with MLN4924. Cells were treated with the indicated concentrations of MLN4924 for 24 hours. Protein lysates were subjected to SDS-PAGE, blotted, and probed with an active caspase-3 specific antibody. Tubulin documented equal loading. (F) Effect of stromal coculture on the proapoptotic activity of MLN4924. MV411 and HL-60 cells were treated with the indicated concentrations of MLN4924 for 48 hours in the presence and absence of HS-5 bone marrow stromal cells. Percentages of apoptotic cells were determined by PI/FACS. n = 3; bars represent the mean ± SD. (G) Impact of FLT3 expression on the sensitivity of AML cells to MLN4924. MV411 cells with and without stable shRNA-mediated knockdown of FLT3 expression were treated with the indicated concentrations of MLN4924 for 48 hours. Percentages of apoptotic cells were determined by PI/FACS. n = 3; bars represent the mean ± SD. Immunoblotting was used to confirm knockdown of FLT3 expression. (H) Effects of MLN4924 on NEDDylated substrates and downstream effectors. Cells were treated with MLN4924 for 24 hours, lysed, subjected to SDS-PAGE, and probed with NEDD8, p27, CDT-1, NRF-2, pCHK1, total CHK1, pIκBα, total IκBα, BCL-2, BCL-xL, FLIP, and SOD2-specific antibodies. Tubulin documented equal loading. Densitometry analysis was carried out by quantifying the band density for each protein relative to the band density of tubulin using an Alpha Innotech FluorChem HD2 gel documentation system (Alpha Innotech). The densitometry values for all controls were normalized to 1.0. Densitometry values are indicated above each band and reflect the ratio of the change in protein expression from control levels for each respective protein. (I) Effects of MLN4924 on NFκB (p65) DNA-binding activity. Cells were treated with MLN4924 as indicated for 24 hours. Relative p65 DNA-binding activity was quantified using a chemiluminescent detection method. (J) Quantification of MLN4924-induced ROS generation. MV411 cells were treated with MLN4924 for 12 hours, and ROS production was evaluated by staining with dichlorofluorescein (DCF) followed by FACS analysis. n = 3; bars represent the mean ± SD. (K) Impact of antioxidant treatment on MLN4924-induced apoptosis. Drug-induced apoptosis was quantified after 48 hours of exposure to MLN4924 in the presence and absence of the antioxidant NAC. n = 3; bars represent the mean ± SD.
MLN4924 is highly active in preclinical AML models and primary AML cells. (A) MLN4924 potently reduces the viability of AML cells. MOLM-13, PL-21, MV411, and HL-60 cell lines and PBMCs from healthy donors were treated with the indicated concentrations of MLN4924 for 72 hours. Viability was assessed by the ATPLite assay. Results shown represent the mean of 3 experiments. Error bars represent SD. (B) MLN4924 has activity in primary AML cells. Primary cells were obtained from 5 AML patients. Of these patients, 1 had therapy-related AML and another had primary refractory AML indicating aggressive clinical disease. Primary cells were treated with MLN4924 for 72 hours. Viability was assessed by the ATPLite assay. (C) Dose-dependent inhibition of clonogenic survival after treatment with MLN4924. MOLM-13 and MV411 human AML cells were treated with the indicated concentrations of MLN4924 for 24 hours. Drug was washed away, cells were seeded in Methocult, and colonies were scored on day 10. n = 3; bars represent the mean ± SD. (D) Quantification of drug-induced apoptosis. MV411 cells and PBMCs from healthy donors were treated with the indicated concentrations of MLN4924 for 48 hours. Percentages of cells with subdiploid DNA were determined by PI/FACS. n = 3; bars represent the mean ± SD. (E) Activation of apoptosis after treatment with MLN4924. Cells were treated with the indicated concentrations of MLN4924 for 24 hours. Protein lysates were subjected to SDS-PAGE, blotted, and probed with an active caspase-3 specific antibody. Tubulin documented equal loading. (F) Effect of stromal coculture on the proapoptotic activity of MLN4924. MV411 and HL-60 cells were treated with the indicated concentrations of MLN4924 for 48 hours in the presence and absence of HS-5 bone marrow stromal cells. Percentages of apoptotic cells were determined by PI/FACS. n = 3; bars represent the mean ± SD. (G) Impact of FLT3 expression on the sensitivity of AML cells to MLN4924. MV411 cells with and without stable shRNA-mediated knockdown of FLT3 expression were treated with the indicated concentrations of MLN4924 for 48 hours. Percentages of apoptotic cells were determined by PI/FACS. n = 3; bars represent the mean ± SD. Immunoblotting was used to confirm knockdown of FLT3 expression. (H) Effects of MLN4924 on NEDDylated substrates and downstream effectors. Cells were treated with MLN4924 for 24 hours, lysed, subjected to SDS-PAGE, and probed with NEDD8, p27, CDT-1, NRF-2, pCHK1, total CHK1, pIκBα, total IκBα, BCL-2, BCL-xL, FLIP, and SOD2-specific antibodies. Tubulin documented equal loading. Densitometry analysis was carried out by quantifying the band density for each protein relative to the band density of tubulin using an Alpha Innotech FluorChem HD2 gel documentation system (Alpha Innotech). The densitometry values for all controls were normalized to 1.0. Densitometry values are indicated above each band and reflect the ratio of the change in protein expression from control levels for each respective protein. (I) Effects of MLN4924 on NFκB (p65) DNA-binding activity. Cells were treated with MLN4924 as indicated for 24 hours. Relative p65 DNA-binding activity was quantified using a chemiluminescent detection method. (J) Quantification of MLN4924-induced ROS generation. MV411 cells were treated with MLN4924 for 12 hours, and ROS production was evaluated by staining with dichlorofluorescein (DCF) followed by FACS analysis. n = 3; bars represent the mean ± SD. (K) Impact of antioxidant treatment on MLN4924-induced apoptosis. Drug-induced apoptosis was quantified after 48 hours of exposure to MLN4924 in the presence and absence of the antioxidant NAC. n = 3; bars represent the mean ± SD.
Drug resistance continues to be a major multifaceted problem that limits successful clinical outcomes for patients with AML. Stromal-mediated survival signaling has been proposed as an important mechanism of resistance to many classes of anticancer agents.12 Considering this, we investigated the impact of stromal interactions on the sensitivity of AML cells to MLN4924. Coculturing MV4-11 and HL-60 AML cells with HS-5 human stromal cells did not significantly impact the proapoptotic activity of MLN4924 (Figure 1F). This indicates that MLN4924 can overcome the survival advantage provided by stroma (supplemental Figure 1). Similarly, MV4-11 cells with and without stable shRNA-mediated knockdown of FLT3 expression responded equally to treatment with MLN4924. This suggests that MLN4924 may be an effective agent for patients with FLT3 internal tandem duplication and/or activating mutations in FLT3, which are typically associated with inferior outcomes to conventional induction therapy (Figure 1G).
We next investigated the effects of MLN4924 on the NEDDylation of cullins and the expression of cullin-dependent substrates in AML cells. Inhibition of NAE activity with MLN4924 produced a time-dependent decrease in the levels of NEDDylated cullins (Figure 1H), leading to stabilization of cullin-dependent substrates (p27, CDT-1, NRF-2, and phopsho-IκBα) and activation of the DNA damage sensor, CHK1 (Figure 1H).
The NF-κB transcription factors are constitutively active in many human cancers and regulate the transcription of several genes with fundamental roles in survival signaling and drug resistance.13-15 One potential underlying cause of constitutive NFκB activity is the inappropriate degradation of its inhibitor, IκBα.13,16 We hypothesized that MLN4924-mediated disruption of the NEDDylation of IκBα would lead to the inhibition of NFκB transcriptional activity. Treatment with MLN4924 resulted in a time-dependent decrease in the expression of the NFκB targets BCL-2, BCL-xL, FLIP, and superoxide dismutase 2 (SOD2). Accordingly, MLN4924 treatment also resulted in a significant reduction in the DNA-binding activity of the p65 subunit of NFκB (Figure 1I).
Many types of malignant cells generate significantly higher levels of reactive oxygen species (ROS) than their normal counterparts. This phenomenon can be therapeutically exploited to selectively kill cancer cells using agents that induce further ROS stress, which culminates in the induction of apoptosis.17 We hypothesized that the significant decrease in the expression of the antioxidant enzyme SOD2 induced by MLN4924 may result in increased ROS generation. Treatment with MLN4924 for 12 hours led to a significant, dose-dependent increase in ROS generation (Figure 1J). To test the contribution of MLN4924-induced ROS production to its anticancer mechanism of action, we evaluated the proapoptotic effects of MLN4924 in the presence and absence of the antioxidant N-acetylcysteine (NAC). NAC treatment significantly blunted the degree of apoptosis stimulated by MLN4924, indicating that ROS production is an important event in MLN4924-induced cell death.
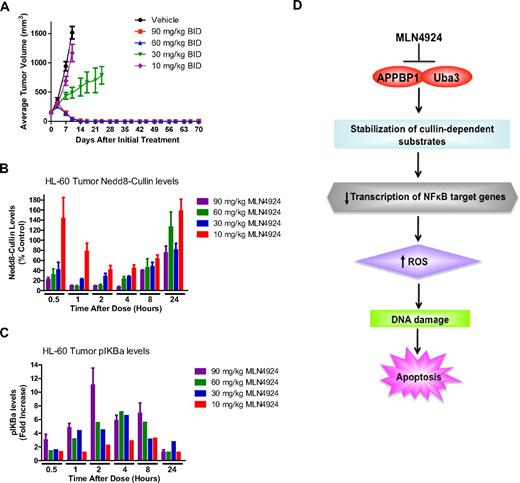
The in vivo anticancer activity of MLN4924 was evaluated by administering MLN4924 or vehicle control to mice implanted with HL-60 xenografts. MLN4924 treatment led to a dose-dependent decrease in disease burden, and 10 of 10 animals in the groups treated with 60 and 90 mg/kg experienced stable regressions (Figure 2A). Analysis of specimens collected from animals after administration of a single dose of MLN4924 demonstrated in vivo inhibition of cullin NEDDylation and accumulation of phospho-IκBα (Figure 2B-C). Our collective findings indicate that targeting NAE activity is a novel and highly effective approach for the treatment of AML that warrants further investigation in a clinical trial.
MLN4924 induces stable disease regression in an AML xenograft model. (A) Administration of MLN4924 to mice bearing HL-60 xenografts leads to a dose-dependent reduction in disease burden. Mice received vehicle control, 10, 30, 60, or 90 mg/kg MLN4924 twice a day for 21 days. Tumor volume was measured by calipers. n = 10 per group; bars represent the mean ± SD. (B) Effect of MLN4924 treatment on NEDDylation of cullins in vivo. Mice were administered a single dose of MLN4924, and the levels of NEDDylated cullins were quantified at the indicated time points (n = 3). (C) Dose-dependent stabilization of phospho-IκBα after treatment with MLN4924. Mice were given a single dose of MLN4924, and the levels of phospho-IκBα were quantified at the indicated time points after drug administration. (D) Schematic representation of the proposed mechanism of MLN4924-induced apoptosis in AML cells. MLN4924 inhibits the activity of NAE, leading to the abrogation of cullin NEDDylation, the stabilization of NEDD8-regulated substrates, increased ROS production, and DNA damage, which culminates in apoptosis.
MLN4924 induces stable disease regression in an AML xenograft model. (A) Administration of MLN4924 to mice bearing HL-60 xenografts leads to a dose-dependent reduction in disease burden. Mice received vehicle control, 10, 30, 60, or 90 mg/kg MLN4924 twice a day for 21 days. Tumor volume was measured by calipers. n = 10 per group; bars represent the mean ± SD. (B) Effect of MLN4924 treatment on NEDDylation of cullins in vivo. Mice were administered a single dose of MLN4924, and the levels of NEDDylated cullins were quantified at the indicated time points (n = 3). (C) Dose-dependent stabilization of phospho-IκBα after treatment with MLN4924. Mice were given a single dose of MLN4924, and the levels of phospho-IκBα were quantified at the indicated time points after drug administration. (D) Schematic representation of the proposed mechanism of MLN4924-induced apoptosis in AML cells. MLN4924 inhibits the activity of NAE, leading to the abrogation of cullin NEDDylation, the stabilization of NEDD8-regulated substrates, increased ROS production, and DNA damage, which culminates in apoptosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by LeukemiaTexas (J.S.C.), the Owens Biomedical Research Foundation (J.S.C.), and the AT&T Foundation (F.J.G.)
Authorship
Contribution: R.T.S. was involved in all aspects of the study, including experimental design, performing research, data analysis, and manuscript preparation; K.R.K., P.G.S., and J.J.G. provided intellectual input regarding experimental design and data interpretation, performed research, and were involved in the preparation of the manuscript; S.T.N. provided intellectual input and performed research; D.M., E.M., and K.O. performed research and contributed to data analysis; S.P., M.O., S.T.N., and F.J.G. provided intellectual input regarding experimental design, data interpretation, and manuscript preparation; and J.S.C. directed the study and was involved in all aspects of experimental design, data analysis/interpretation, and manuscript preparation.
Conflict-of-interest disclosure: P.G.S. and J.J.G. are employees of Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jennifer S. Carew, Institute for Drug Development, Cancer Therapy and Research Center at University of Texas Health Science Center, 14960 Omicron Dr, San Antonio, TX 78245; e-mail: carew@uthscsa.edu.