Abstract
Platelets are classified as terminally differentiated cells that are incapable of cellular division. However, we observe that anucleate human platelets, either maintained in suspension culture or captured in microdrops, give rise to new cell bodies packed with respiring mitochondria and α-granules. Platelet progeny formation also occurs in whole blood cultures. Newly formed platelets are structurally indistinguishable from normal platelets, are able to adhere and spread on extracellular matrix, and display normal signal-dependent expression of surface P-selectin and annexin V. Platelet progeny formation is accompanied by increases in biomass, cellular protein levels, and protein synthesis in expanding populations. Platelet numbers also increase during ex vivo storage. These observations indicate that platelets have a previously unrecognized capacity for producing functional progeny, which involves a form of cell division that does not require a nucleus. Because this new function of platelets occurs outside of the bone marrow milieu, it raises the possibility that thrombopoiesis continues in the bloodstream.
Introduction
After they are shed from the cytoplasm of megakaryocytes,1 platelets circulate in the bloodstream for 9 to 11 days. There is no evidence that these anucleate cytoplasts undergo cellular division, but recent studies by our group and others have identified unexpected cellular functions of platelets,2-4 including the capacity to process pre-mRNA2-5 and translate mRNA into protein.6-10 Platelets also continue to synthesize protein for several days when they are stored ex vivo.11 These findings indicate that, despite their terminally differentiated state, platelets are biosynthetically more sophisticated than previously thought.12 They also suggest that platelets may be able to adjust their phenotypic composition in response to environmental cues.
Here we show that platelets give rise to new cells that are structurally and functionally similar to their parent counterparts. The formation of platelet progeny is associated with increases in platelet biomass, protein synthetic events, and total intracellular protein. This heretofore undescribed proliferative capacity of platelets may have significant consequences for normal and pathologic thrombopoiesis in humans in addition to having clinical implications for transfusion medicine.
Methods
Platelet isolation and culture
All studies were approved by the University of Utah Institutional Review Board committee (no. 392). Leukocyte-depleted platelets were isolated as previously described.2,5 Washed platelets were resuspended at 100 000/μL in serum-free M199 medium, placed in round-bottom polypropylene tubes (BD Biosciences), and cultured in a 37°C humidified incubator under gentle rotation (MacsMix, slow, 45° angle; Miltenyi). The same suspension culture conditions were also used for the whole blood studies shown in Figure 2A.
Stored platelets were obtained from the ARUP Blood Transfusion Services at the University of Utah or the Institute of Transfusion Medicine at the University of Greifswald. The apheresed platelets were immediately placed in standardized platelet bags and stored under constant agitation in a climate-controlled chamber (Melco Engineering Corp) that was maintained between 20°C and 24°C. On select days, samples of the ex vivo aged platelets were removed under sterile conditions, gently washed, and subsequently resuspended in culture media as described in the previous paragraph.
For the cell fusion experiments shown in Figure 1D, platelets from the same donor were incubated either with 1μM CFDA-SE (Vybrant CFDA SE Cell Tracer Kit, V12883; Invitrogen) or with 1μM CellTrace Far red DDAO-SE (C34553; Invitrogen) for 15 minutes at 37°C. After this incubation period, the cells were mixed together in culture medium at a final concentration of 100 000/μL for 6 hours and subsequently prepared for microscopic analysis as described in “Platelet morphology.”
For studies described in Figure 4B, platelets were placed in suspension culture; and after 6 hours, the cells were incubated in 8-well borosilicate chamber slides that were coated with human fibrinogen (Calbiochem) to characterize adherence and spreading of long platelet processes by real-time microscopy.
Platelet morphology
Freshly isolated or aged platelets were fixed either immediately, to assess baseline morphology, or after 6 hours of suspension culture. In select studies, the platelets were coincubated with thrombin (Sigma-Aldrich), nocodazole (Sigma-Aldrich), or taxol (Invitrogen). At the end of the experimental period, paraformaldehyde (2% final) was added directly to the washed platelets as previously described2,5 to maintain the native morphology of the cells. The fixed platelets (10 000 total for each sample) were subsequently layered onto Vectabond-coated coverslips (Vector Laboratories) using a cytospin centrifuge (Shandon Cytospin; Thermo Fisher Scientific). Platelets were permeabilized, with the exception of the panels shown in Figure 4C and D. To determine the number of platelets with extensions and distinct cell bodies, fixed cells were counterstained with AlexaFluor 488 phalloidin (A12379; Invitrogen), a high-affinity probe for F-actin, and/or an AlexaFluor 555 (W32464; Invitrogen) conjugate of wheat germ agglutinin (WGA). Three random fields were captured from each independent experiment and at least 500 total cells, which encompassed individual platelets and platelets with 2 or more distinct cell bodies, were counted.
For the whole blood culture system displayed in Figure 2A, whole blood was passed through a small-bore needle to break apart any preexisting platelets that possessed 2 or more cell bodies. Then, the whole blood was directly resuspended in paraformaldehyde (1% final) or cultured for 6 hours followed by fixation. The fixed blood was subsequently placed on the bench for 2 hours, which resulted in a passive sedimentation of the red blood cells. The upper phase, which enriched in platelets, was gently layered onto Vectabond-treated slides as described in the previous paragraph.
For the ultrastructural analyses, cultured platelets were fixed in 2.5% glutaraldehyde/1% paraformaldehyde in cacodylate buffer for 20 minutes or in 2.5% glutaraldehyde in phosphate-buffered saline (PBS) buffer overnight. Platelets were washed with 0.1M phosphate buffer (pH 7.4), followed by distilled H2O by centrifugation at 800g (10 minutes). Platelets were then postfixed with 2% osmium tetroxide (60 minutes), washed twice with distilled H2O, dehydrated by a graded series of acetone concentrations (50%, 70%, 90%, and 100%; 2 × 10 minutes each) followed by embedding in Epon. Thin sections were examined with a JEOL JEM-1011 electron microscope after uranyl acetate and lead citrate staining. Digital images were captured with a side-mounted Advantage HR CCD camera (Advanced Microscopy Techniques), saved in TIFF format, and trimmed for publication using Adobe Photoshop CS3.
Protein expression
Fixed cells were stained with antibodies directed against P-selectin (sc-6941; Santa Cruz Biotechnology), β-tubulin (T-5293; Sigma-Aldrich), αIIbβ3 or annexin V (ab34407 and ab14196; Abcam). Specificity of the staining was confirmed for each antibody with isotype-matched nonimmune IgG. The cells were counterstained with either AlexaFluor 488 phalloidin or AlexaFluor 555 WGA conjugate as described in the previous paragraph. To assess mitochondrial function, we used MitoTracker Red CM-H2XRos (M7513; Invitrogen), a reduced probe that fluoresces when it enters actively respiring mitochondria. For these studies, MitoTracker (1μM) was incubated with the live platelets one hour before the end of the experiment.
For the studies in Figure 4A, freshly isolated (0 hours) or cultured (6 hours) platelets were activated with thrombin or its vehicle for 5 minutes. The cells were subsequently incubated with fluorescein isothiocyanate-conjugated antibodies against P-selectin (no. 555523; BD Biosciences PharMingen) or PAC-1 (no. 340535; BD Biosciences) and then fixed and analyzed on a 5-color FACScan analyzer (BD Biosciences) using the BD software CellQuest. Isotype-matched control samples were used to exclude nonspecific antibody binding.
Platelet morphologic measurements and counts
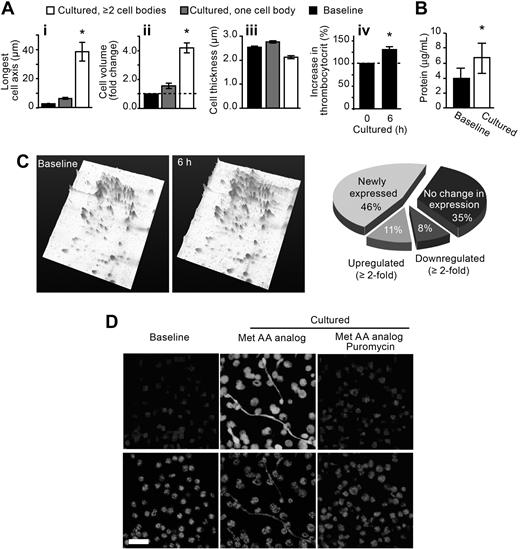
For the studies shown in Figure 5Ai through iii, CFDA-labeled platelets were fixed at baseline or after 6 hours of culture and then gently layered on a glass surface as described in “Platelet morphology.” Stepwise z-series (0.1-μm slices) were conducted to assess cell thickness. Minimum and maximum diameter as well as cell perimeter were determined using Volocity software, Version 4 (Improvision Inc, a PerkinElmer company). Platelet perimeter and thickness were used to calculate cell volume, and thrombocytocrits were performed to assess platelet biomass.
For the experiments shown in Figure 6D, platelet concentrates were rested for 2 hours to allow reconstitution of minor platelet activation. Then a separate bag was aligned to the platelet bag tube by sterile docking to obtain a baseline (day 0) sample of the platelet concentrate. Individual samples were diluted 1:4 in 2% PBS-ethylenediaminetetraacetic acid, and then platelet counts and mean platelet volume were determined using an automated particle counter (Sysmex). The same procedure was repeated after the platelet concentrates were stored under agitation for 5 days. Cell counts and volume were done as part of quality control experiments at the Institute of Transfusion Medicine at the University of Greifswald to assess the integrity of the platelet concentrates.
Microfluidic device for single-cell experiments
Microfluidic devices were fabricated by soft lithography.13 Negative photoresist SU-8 2025 (MicroChem) was spin-coated onto clean silicon wafers to a thickness of 25 μm, which defines the channel height, and patterned laterally by exposure to UV light through a transparency photomask (CAD/Art Services). Sylgard 184 polydimethysolixane (PDMS; Dow Corning) was mixed with cross-linker (ratio 10:1), degassed thoroughly, poured onto the photoresist patterns, and cured for at least 1 hour at 65°C. The PDMS replicas were peeled off the wafer and bonded to glass slides after oxygen-plasma activation of both surfaces. The microfluidic channels were coated with Aquapel (PPG Industries) by filling the channels with the solution as provided by the company and subsequently flushing them with air before the experiments to improve coating of the channel walls with fluorinated oil. Polyethylene tubing with an inner diameter of 0.38 mm and an outer diameter of 1.09 mm (BD Biosciences) was used to connect the channels to syringes. Glass and polycarbon syringes were used to load the fluids into the devices. Flow rates were set by computer-controlled syringe pumps.
For the time-resolved observation of platelet dynamics, we confined individual cells in drops that were approximately 33 pL in volume. Aqueous drops were fabricated in an inert carrier fluid (FC40 fluorocarbon oil; 3M) using the microfluidic devices. To stabilize the drops, a PFPE-PEG block-copolymer surfactant (RainDance Technologies), at a concentration of 1.8% (wt/wt), was added to the suspending oil. Before the experiments, the freshly isolated or aged platelets were resuspended in fresh media. The concentration was adjusted to 15 000/μL to achieve an average concentration of 1 cell per 2 drops according to the Poisson distribution.
Each microfluidic device combined a flow focusing geometry14 for drop production and a storage area where the drops were kept in place for real-time observation. During storage, the drops assumed the shape of an ellipsoid with a height of 25 μm, determined by the channel height, and a diameter of 50 μm or less that was determined by the volume. This setup provided adequate spatial confinement while simultaneously allowing the platelets to move freely within the drops, thereby minimizing inadvertent activation of the cells. Defined locations for the drops in the device allowed us to evaluate the same drop over time. Rigorous screens with the surfactant-rich oil were also performed to demonstrate that the oil did not activate the platelets (data not shown). For all of the studies, the platelets were cultured in the drops at 37°C in a humidified 5% CO2 atmosphere. The permeability of both the PDMS15 and fluorocarbon carrier oil16 to gas ensures sufficient exchange to maintain the cells at the CO2 level set by the controlled environment.
Two-dimensional electrophoresis and protein synthetic studies
Two-dimensional gel electrophoresis was performed as previously described.17,18 In brief, platelets were lysed in buffer containing 7M urea, 2M thiourea, 4% 3-(3-cholamidopropyl)dimethylamonio-1-propyl sulfonate, 40mM Tris (base), and 2 tablets of a protease inhibitor mix per 20 mL of buffer stock solution (complete mini; Roche). The final protein concentration of each sample was determined using the method of Popov et al.19 Isoelectric focusing was performed using the Protean IEF Cell (Bio-Rad) at a temperature of 20°C. Gel strips (pH 3-10 L; GE Healthcare) were rehydrated for 12 hours at 50 V using a buffer containing 7M urea, 2M thiourea, 2% 3-(3-cholamidopropyl)dimethylamonio-1-propyl sulfonate, 0.5% IPG buffer (pH 3-10 L), dithiothreitol (2.8 mg/mL), and traces of bromophenol blue. The samples were applied as part of the rehydration solution, and lysates were run on an 11-cm strip. For the second dimension (sodium dodecyl sulfate–polyacrylamide gel electrophoresis), IPG strips were equilibrated for 20 minutes in buffer (6M urea, 30% vol/vol glycerol [87% vol/vol], 2% wt/vol sodium dodecyl sulfate, 50mM Tris-Cl, pH 8.8, 100 mg dithiothreitol/10 mL, and traces of bromophenol blue). Gels were silver-stained according to Heukeshoven and Dernick20 using a silver staining kit (GE Healthcare). Gels were analyzed using the Proteomeweaver software package (Definiens).
For global protein synthesis, detection platelets were cultured in Dulbecco modified Eagle medium without L-methionine (no. 21013-024; Invitrogen,) for 2 hours to deplete any residual L-methionine. During these 2 hours, one part of the sample was also preincubated with puromycin (P-8833; Sigma-Aldrich) as previously described.10 The medium was then substituted with 250μM Click-iT AHA (no. MP10102; Invitrogen), an amino acid analog of L-methionine containing an azido moiety. After 6 hours, platelets were fixed in suspension and layered onto Vectabond-treated slides as described in “Platelet morphology.” The fixed platelets were washed, permeabilized (0.25% NP-40 in PBS), and the amino acid analog was detected using a custom-made AlexaFluor 488–conjugated alkyne (5μM). The chemoselective ligation between the azide and alkyne was performed using the Click-iT Protein Analysis Detection Kit (no. MP33370; Invitrogen), and the binding of fluorescent dye was analyzed by confocal microscopy.
Statistical analyses
The mean plus or minus SEM was determined for each experimental variable displayed in Figures 1C, 4A, 5A and B, and 6B through D. Analysis of variance was conducted to identify differences that existed among multiple experimental groups. If significant differences were found, a Student-Newman-Keuls post hoc procedure was used to determine the location of the difference. A paired t test was used for Figures 5B and 6D. P less than .05 was considered statistically significant.
Microscopy
Low-resolution wide-field microscopy used in Figure 1F was performed with a Nikon Eclipse E400 microscope (Nikon Instruments Inc) equipped with a 40×/0.65 NA objective and a PixeLINK PL-A662 camera (PixeLINK). Fluorescence microscopy and high-resolution confocal reflection microscopy was performed using an Olympus IX81, FV300 and a FV1000 (Olympus) confocal-scanning microscope equipped with a 60×/1.42 NA oil objective for viewing platelets. An Olympus FVS-PSU/IX2-UCB camera and scanning unit and Olympus Fluoview FV 300 and FV1000 image acquisition software Version 5.0 were used for recording. The images were further analyzed using Adobe Photoshop CS Version 8.0, Metamorph software (Molecular Devices), and ImageJ (National Institutes of Health). Real-time microscopy was performed using an Olympus IX81 microscope and images were assessed with Metamorph software. Single frames were further processed using Iprocess (Cell Imaging Core, University of Utah) as well as ImageJ.
Results
Discoid platelets form new cell bodies
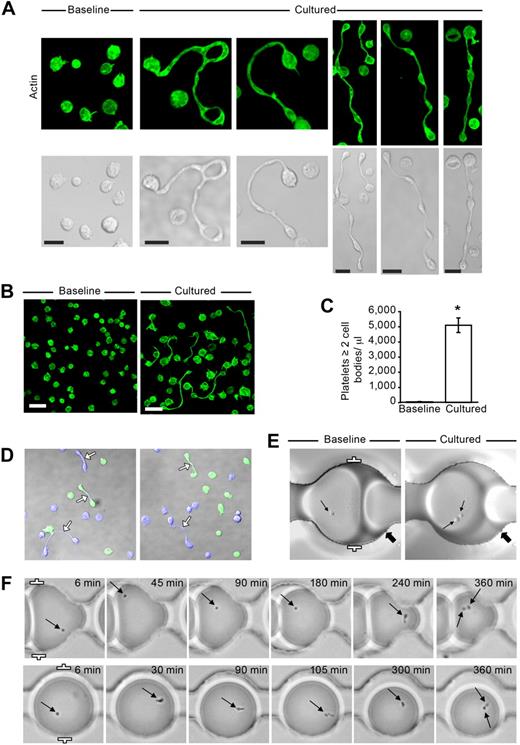
The ability of platelets to generate new cell bodies was examined by placing freshly isolated washed cells in suspension culture and “snap-fixing” samples at times ranging from 0 to 24 hours before analyzing morphology. As shown in Figure 1A and B, freshly isolated platelets (0 hours) were discoid and structural signs of activation were absent. In contrast, after 6 hours in culture, approximately 5% of the platelets extended projections with distinct cell bodies (Figure 1A-C). Extension formation was time-dependent; platelets with more than one cell body were first observed after 2 hours and continued to increase at successively later time points (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Morphologically, the extensions resembled proplatelets previously described in megakaryocytes21 ; however, new cell bodies did not develop exclusively from large platelets (ie, > 4 μm in diameter), which were infrequently observed in the cell preparations (Figures 1A-B; see also Figures 3Ai, 5Ai).
Freshly isolated platelets extend projections with distinct cell bodies. (A) Localization of actin (green, phalloidin) in human platelets that were fixed immediately after isolation (Baseline) or after 6 hours in suspension (Cultured). The bottom row displays corresponding transmission images. This figure is representative of more than 20 independent experiments. Scale bars represent 5 μm. (B) Lower magnification of freshly isolated platelets that were stained for actin at baseline or after they were cultured for 6 hours (scale bars, 10 μm). Panel is representative of more than 20 experiments. (C) The bar graph indicates the number of extended platelets with at least 2 cell bodies per microliter of culture media (mean ± SEM; n = 13). *P < .05, baseline versus cultured. (D) Two separate cultures of platelets were labeled (blue or green) and then incubated with one another for 6 hours. Left and right panels: 2 independent experiments, which are representative of 3. (E) Platelets were loaded into “parked” microdrops and examined at baseline or after 6 hours (Cultured). The thin arrows point to single platelets (Baseline) or the same platelet that formed 2 distinct cell bodies after 6 hours (Cultured). The thick arrows point to unique landmarks for each position in the microfluidic device. (F) Sequential images of platelets using low-resolution wide-field microscopy. The arrows highlight the location of the platelets within each drop during the course of the experiment and the formation of 2 distinct cell bodies after 6 hours (far right panels). Distance between the white brackets (E-F, far left panels) is 50 μm.
Freshly isolated platelets extend projections with distinct cell bodies. (A) Localization of actin (green, phalloidin) in human platelets that were fixed immediately after isolation (Baseline) or after 6 hours in suspension (Cultured). The bottom row displays corresponding transmission images. This figure is representative of more than 20 independent experiments. Scale bars represent 5 μm. (B) Lower magnification of freshly isolated platelets that were stained for actin at baseline or after they were cultured for 6 hours (scale bars, 10 μm). Panel is representative of more than 20 experiments. (C) The bar graph indicates the number of extended platelets with at least 2 cell bodies per microliter of culture media (mean ± SEM; n = 13). *P < .05, baseline versus cultured. (D) Two separate cultures of platelets were labeled (blue or green) and then incubated with one another for 6 hours. Left and right panels: 2 independent experiments, which are representative of 3. (E) Platelets were loaded into “parked” microdrops and examined at baseline or after 6 hours (Cultured). The thin arrows point to single platelets (Baseline) or the same platelet that formed 2 distinct cell bodies after 6 hours (Cultured). The thick arrows point to unique landmarks for each position in the microfluidic device. (F) Sequential images of platelets using low-resolution wide-field microscopy. The arrows highlight the location of the platelets within each drop during the course of the experiment and the formation of 2 distinct cell bodies after 6 hours (far right panels). Distance between the white brackets (E-F, far left panels) is 50 μm.
The extensions observed in cultured cells typically terminated in discoid cell bodies. These new cell bodies were close in size to platelets, and multiple bulges resembling smaller discoid cell bodies were also frequently observed (Figure 1A-B). Newly formed cell bodies were bounded by actin, and sialic acid–rich structures were concentrated at the core of each bulbar region. Newly formed cell bodies were usually separated from one another by thin elongated processes that often appeared segmented and bent and, in some cases, bifurcated. Many platelets with short tails (as distinct from membrane tethers) were also observed, indicating they may have been beginning the transition to extended cells or had recently separated from others. We also frequently observed ring-like cell bodies of various size (Figure 1A), reminiscent of previous observations of platelets in freshly isolated plasma.22
Coculturing of labeled platelet populations showed that the process responsible for cell body extension did not involve fusion of metabolically active platelets (Figure 1D). This was also evident in experiments where individual platelets were confined in drops of culture media suspended in oil and stabilized with inert surfactant. Figure 1E shows an example of an individual platelet that formed a second cell body after a 6-hour incubation period. Sequential cell tracking demonstrated that single platelets underwent morphologic changes at different times during incubation and, in numerous cases, developed 2 distinct cell bodies (Figure 1F).
Progeny formation occurs in whole blood
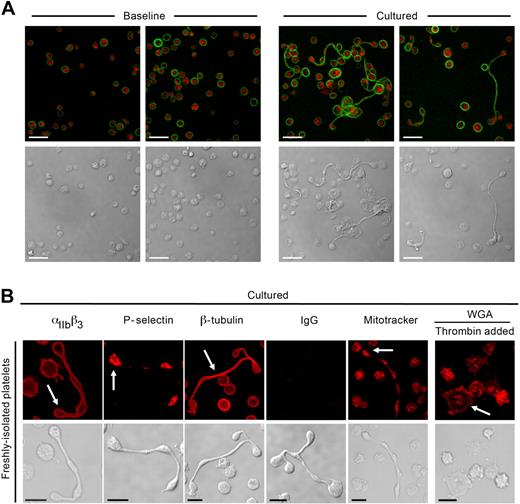
Platelets with multiple cell bodies have been observed in plasma immediately processed from whole blood,23-25 but their source (ie, megakaryocytes or platelets) is not known. We examined the ability of platelets to form progeny in whole blood by first eliminating preexisting platelet extensions by passing freshly drawn blood through a small-bore needle and then culturing the blood in a manner similar to the experiments described in “Platelet morphology.” Then, the whole blood was immediately processed or cultured for 6 hours. Single cells showing the classic β-tubulin peripheral ring staining pattern of resting platelets predominated in freshly isolated blood (Figure 2A left panels). After 6 hours, we observed increasing numbers of platelets that extended projections encompassing 2 or more cell bodies (Figure 2A right panels). β-tubulin was present in the shafts connecting the cell bodies and coiled around their rims, resembling β-tubulin expression in proplatelets that extend from megakaryocytes.26
Platelets develop new cell bodies in whole blood and express critical biomarkers. (A) Platelets were isolated from freshly collected (Baseline) or cultured (6 hours) whole blood as described in “Platelet isolation and culture.” The red and green stains identify sialic acids (WGA) and β-tubulin, respectively. (B) Freshly isolated platelets were cultured (6 hours) alone or in the presence (far right panels) of thrombin (0.01 U/mL). From left to right in the top row, the red stain identifies αIIbβ3, P-selectin, β-tubulin, control IgG, respiring mitochondria (Mitotracker), or sialic acids. Corresponding transmission images are shown in the bottom row. Scale bars represent 5 μm. Panels A and B are representative of 3 independent experiments.
Platelets develop new cell bodies in whole blood and express critical biomarkers. (A) Platelets were isolated from freshly collected (Baseline) or cultured (6 hours) whole blood as described in “Platelet isolation and culture.” The red and green stains identify sialic acids (WGA) and β-tubulin, respectively. (B) Freshly isolated platelets were cultured (6 hours) alone or in the presence (far right panels) of thrombin (0.01 U/mL). From left to right in the top row, the red stain identifies αIIbβ3, P-selectin, β-tubulin, control IgG, respiring mitochondria (Mitotracker), or sialic acids. Corresponding transmission images are shown in the bottom row. Scale bars represent 5 μm. Panels A and B are representative of 3 independent experiments.
Newly formed platelets express essential platelet proteins
αIIb, an integrin unique to megakaryocytes and platelets,27 was present on the surface of all platelets in suspension culture, including the newly formed cell bodies (Figure 2B). P-selectin coalesced to the center of the bulbar extensions (Figure 2B), indicating that α-granules were redistributed to the newly formed cell bodies. β-tubulin was present in the shafts of each extension and coiled around the rim of the newly formed cell bodies (Figure 2B). Respiring mitochondria were readily observed in cell bodies and occasionally in shafts, suggesting that functioning organelles traffic through these microtubular-rich thoroughfares to reach bulbar regions of the cell (Figure 2B).
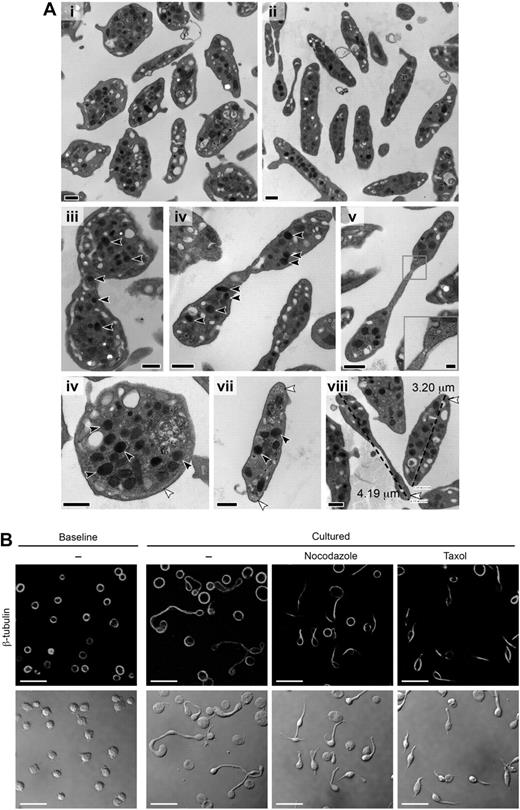
Transmission electron microscopic (TEM) analyses of the processes confirmed that the newly formed cell bodies were packed with granules and other organelles (Figure 3A). Nuclei were absent in platelets with 2 or more cell bodies, indicating that the extensions did not develop from megakaryocytes harvested from the bloodstream.23 The shafts lacked defined cellular membranes in some regions containing segmented constrictions (Figure 3Av), suggestive of cellular pinching and resembling constricted cleavage furrows (Figure 3Av inset) in dividing mitotic cells24 and proplatelet extensions.27
Newly formed platelets possess granules and an elaborate microtubule network. (A) Thin section TEMs of representative platelets at baseline (i,vi,vii) and after 6 hours in culture (ii-v,viii). The black scale bar represents 500 nm. Multiple α granules (black arrows) are observed in platelets with multiple cell bodies (iii-v) and occasionally in the connecting region (iii). A constricted region resembling a cleavage furrow is noted along the long shaft of a cultured platelet (v with inset, original magnification ×80 000; scale bar represents 100 nm). Original magnifications: iii, ×25 000; iv-v, ×30 000. Microtubules in cross section were also observed at ends of the cultured platelets (viii white arrows). (viii) How platelet diameters were measured by TEM (original magnification ×30 000), which confirmed that cell diameters significantly (P < .05) increased in cultured platelets compared with freshly isolated platelets (also see Figure 5Ai). (B) The panels display baseline platelets (far left) and cultured platelets (6 hours) that were left alone or treated with reagents that disrupt microtubular function (ie, nocodazole or taxol). Top row: Specific immunostaining for β-tubulin. Bottom row: Corresponding transmission images. This figure is representative of 3 independent experiments. Scale bars represent 10 μm.
Newly formed platelets possess granules and an elaborate microtubule network. (A) Thin section TEMs of representative platelets at baseline (i,vi,vii) and after 6 hours in culture (ii-v,viii). The black scale bar represents 500 nm. Multiple α granules (black arrows) are observed in platelets with multiple cell bodies (iii-v) and occasionally in the connecting region (iii). A constricted region resembling a cleavage furrow is noted along the long shaft of a cultured platelet (v with inset, original magnification ×80 000; scale bar represents 100 nm). Original magnifications: iii, ×25 000; iv-v, ×30 000. Microtubules in cross section were also observed at ends of the cultured platelets (viii white arrows). (viii) How platelet diameters were measured by TEM (original magnification ×30 000), which confirmed that cell diameters significantly (P < .05) increased in cultured platelets compared with freshly isolated platelets (also see Figure 5Ai). (B) The panels display baseline platelets (far left) and cultured platelets (6 hours) that were left alone or treated with reagents that disrupt microtubular function (ie, nocodazole or taxol). Top row: Specific immunostaining for β-tubulin. Bottom row: Corresponding transmission images. This figure is representative of 3 independent experiments. Scale bars represent 10 μm.
Microtubules and microfilaments were commonly observed in the shafts between the cell bodies, similar to the organization of microtubules in constricted areas of proplatelets extending from megakaryocytes.27 We found that platelets treated with taxol, which stabilizes microtubules, or nocodazole, which impairs polymerization of microtubules, do not form progeny (Figure 3B). Instead, platelets display a “teardrop-like” morphology, possessing short tails that are devoid of new cell bodies.
Newly formed platelets are functional
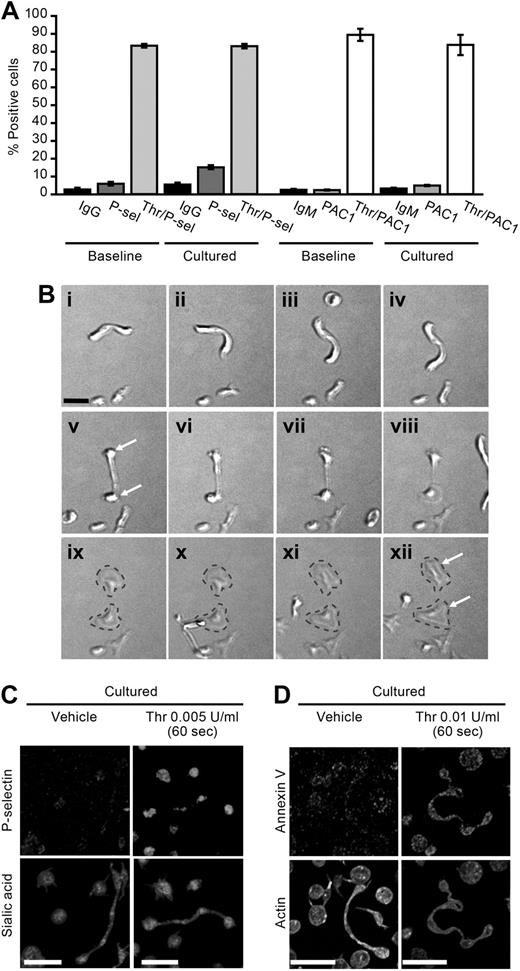
Figures 1D and 2B demonstrate that newly formed platelets are metabolically active and possess respiring mitochondria, respectively. Surface expression of PAC-1, a marker of αIIbβ3 activation, and P-selectin remained relatively stable during the culture period (Figure 4A). Similarly, the expression of annexin V did not significantly increase over 6 hours (1.5% ± 0.5% vs 1.7% ± 0.5%, baseline vs cultured). However, surface expression of all of these platelet activation markers increased in cultured platelets exposed to thrombin (Figure 4A,C-D). Similar responses were observed in ex vivo aged platelets (storage day 5) that were resuspended in culture medium (data not shown). Real-time microscopy also demonstrated that platelet processes, which previously developed in culture, adhered to and spread on immobilized fibrinogen (Figure 4B). In this example, which is representative of many others, the long process separated into 2 individual cells as the platelet spread on extracellular matrix (Figure 4B).
Newly formed cell bodies are functional. (A) The bar graph depicts P-selectin and PAC-1 surface expression (percentage of positive cells) as assessed by flow cytometry in freshly isolated (0 hours) or cultured (6 hours) platelets with or without thrombin (Thr) stimulation for 15 minutes. The data are compared with isotype-matched control antibodies (IgG, IgM). (B) Platelets were cultured in suspension for 6 hours and subsequently placed on immobilized fibrinogen. As shown in these sequential images (i-xii), a platelet process adheres, spreads, and forms 2 distinct cell bodies that eventually separate from one another (gray dashed lines). Scale bar represents 5 μm. Panel B is representative of 4 independent experiments. (C-D) Platelets were cultured for 6 hours and subsequently treated with vehicle or thrombin (0.005 U/mL). After 60 seconds, the platelets were fixed in solution, the permeabilization step was skipped, and then the cells were coimmunostained for (C) P-selectin and sialic acids (WGA) or (D) annexin V and actin. Panels C and D are representative of 3 independent experiments. Scale bars represent 10 μm.
Newly formed cell bodies are functional. (A) The bar graph depicts P-selectin and PAC-1 surface expression (percentage of positive cells) as assessed by flow cytometry in freshly isolated (0 hours) or cultured (6 hours) platelets with or without thrombin (Thr) stimulation for 15 minutes. The data are compared with isotype-matched control antibodies (IgG, IgM). (B) Platelets were cultured in suspension for 6 hours and subsequently placed on immobilized fibrinogen. As shown in these sequential images (i-xii), a platelet process adheres, spreads, and forms 2 distinct cell bodies that eventually separate from one another (gray dashed lines). Scale bar represents 5 μm. Panel B is representative of 4 independent experiments. (C-D) Platelets were cultured for 6 hours and subsequently treated with vehicle or thrombin (0.005 U/mL). After 60 seconds, the platelets were fixed in solution, the permeabilization step was skipped, and then the cells were coimmunostained for (C) P-selectin and sialic acids (WGA) or (D) annexin V and actin. Panels C and D are representative of 3 independent experiments. Scale bars represent 10 μm.
In addition, surface P-selectin expression was increased in platelets that developed new cell bodies when they were activated with low concentrations of thrombin (Figure 4C). Similarly, newly formed platelets displayed increased annexin V on their surface in response to cellular activation (Figure 4D).
Biosynthetic events indicative of cellular proliferation increase in cultured platelets
As nucleated cells prepare for cell division, they increase in size. To determine whether similar changes occur in progeny-forming platelets, we fixed the platelets in suspension and gently layered them on a flat surface to assess the entire cell in a single plane. We found that the maximal diameter (Figure 5Ai) and volume (Figure 5Aii) of platelets increased (P < .05) during the culture period whether the cells were actively forming new cell bodies or not. In contrast, the thickness of platelets decreased slightly, in large part, because the shafts that connect the cell bodies were very thin (Figure 5Aiii).
Cultured platelets increase in biomass and accumulate protein. (A) The bars represent the mean ± SEM for diameter (i), volume (ii), thickness (iii), and biomass (iv) of freshly isolated versus cultured platelets. *P < .05 versus baseline (i-iii) or 0 hours (iv). (B) The bars represent the mean ± SEM for total protein concentration of freshly isolated (Baseline) vs cultured platelets. *P < .05 versus baseline. (C) Left panels: Protein expression patterns for freshly isolated (Baseline) versus cultured (6 hours) platelets. These 2-dimensional gels, which are tilted in a third dimension to more effectively display the peak intensity and height of individual proteins, are representative of 5 independent experiments. (Right panel) The pie chart categorizes the protein expression patterns in freshly isolated versus cultured platelets. The categories are labeled as newly expressed (spots identified in cultured platelets that were not present at baseline), up-regulated (spots that were increased in cultured platelets compared with baseline), down-regulated (spots that were decreased in cultured platelets compared with baseline), or no change (spots that remained constant between cultured and baseline platelets). The percentages in the pie chart are the average of 5 independent experiments. (D) Platelets were coincubated with a fluorescent methionine analog (Met AA analog) in the presence or absence of puromycin. The top row identifies incorporation of methionine into newly synthesized protein. The bottom row panels (WGA) identify sialic acids. These panels are representative of 3 independent experiments.
Cultured platelets increase in biomass and accumulate protein. (A) The bars represent the mean ± SEM for diameter (i), volume (ii), thickness (iii), and biomass (iv) of freshly isolated versus cultured platelets. *P < .05 versus baseline (i-iii) or 0 hours (iv). (B) The bars represent the mean ± SEM for total protein concentration of freshly isolated (Baseline) vs cultured platelets. *P < .05 versus baseline. (C) Left panels: Protein expression patterns for freshly isolated (Baseline) versus cultured (6 hours) platelets. These 2-dimensional gels, which are tilted in a third dimension to more effectively display the peak intensity and height of individual proteins, are representative of 5 independent experiments. (Right panel) The pie chart categorizes the protein expression patterns in freshly isolated versus cultured platelets. The categories are labeled as newly expressed (spots identified in cultured platelets that were not present at baseline), up-regulated (spots that were increased in cultured platelets compared with baseline), down-regulated (spots that were decreased in cultured platelets compared with baseline), or no change (spots that remained constant between cultured and baseline platelets). The percentages in the pie chart are the average of 5 independent experiments. (D) Platelets were coincubated with a fluorescent methionine analog (Met AA analog) in the presence or absence of puromycin. The top row identifies incorporation of methionine into newly synthesized protein. The bottom row panels (WGA) identify sialic acids. These panels are representative of 3 independent experiments.
Dividing cells typically increase their biosynthetic activity in preparation for cytokinesis, which involves redistribution of cytoplasm, organelles, and cell membranes into daughter cells.25 Similarly, we found that the biomass (Figure 5Aiv) and intracellular protein content (Figure 5B) of platelets increased significantly (P < .05) as they produced new cells. Separation of the intracellular proteins by 2-dimensional gel electrophoresis also demonstrated an increase in total protein as well as a substantial shift in intracellular protein expression patterns (Figure 5C). Increased protein expression was in part the result of protein synthesis because an azido-modified methionine analog readily incorporated into platelets that develop new cell bodies (Figure 5D). Methionine incorporation was blocked by the translational inhibitor puromycin, which also reduced the development of new cell bodies (Figure 5D).
Ex vivo aged platelets develop new cell bodies and increase in number
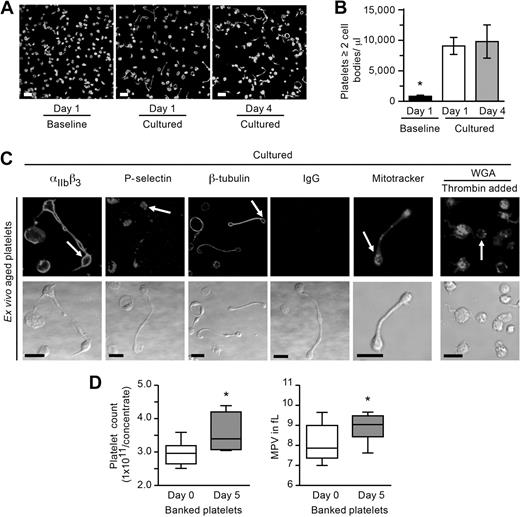
Long projections of megakaryocytes have been demonstrated in bone marrow sinusoids,28 and similar types of projections have been identified in freshly isolated plasma.22,28-30 To determine whether mature platelets have the capacity to generate new cell bodies and increase in number outside of the bone marrow milieu, we removed platelets from the circulation and aged them ex vivo. In the first set of studies, apheresed platelets were stored in plasma at 20°C to 24°C under constant agitation in a Food and Drug Administration–approved platelet bag. After 1 or 4 days of storage, the platelets were removed under sterile conditions, gently washed, and resuspended in culture medium. Baseline platelets (ie, time 0) were characteristically discoid; and similar to freshly isolated platelets, the ex vivo aged platelets readily formed new cell bodies (Figure 6A-B). The new cell bodies expressed integrin αIIb, P-selectin, β-tubulin, and contained respiring mitochondria (Figure 6C).
Stored platelets develop new cell bodies and increase in number. (A-B) Ex vivo aged (1 or 4 days) platelets were resuspended in M199 medium and immediately fixed (Baseline) or cultured in suspension for 6 hours. (A) The panels display a representative example of one study where the platelets were stained for actin. Scale bars represent 10 μm. (B) The bar graph indicates the number of ex vivo–aged platelets with at least 2 cell bodies per microliter (mean ± SEM; n = 4). *P < .05, cultured vs baseline. (C) Ex vivo–aged platelets (day 4) were resuspended in culture medium for 6 hours in the presence (far right panels) or absence of thrombin. From left to right in the top row, the top row panels identify αIIbβ3, P-selectin, β-tubulin, control IgG, respiring mitochondria (Mitotracker), or sialic acids (WGA). Corresponding transmission images are shown in the bottom row. Scale bars represent 5 μm. This figure is representative of 3 independent experiments. (D) Platelets were stored under standard blood bank conditions, and platelet counts as well as mean platelet volumes (MPV) were determined. The left graph shows the platelet count before (day 0) and after (day 5) storage (mean ± SEM; n = 10). The right panel displays the MPV obtained from platelets used for the counting studies. *P < .05, day 0 vs day 5, for both panels.
Stored platelets develop new cell bodies and increase in number. (A-B) Ex vivo aged (1 or 4 days) platelets were resuspended in M199 medium and immediately fixed (Baseline) or cultured in suspension for 6 hours. (A) The panels display a representative example of one study where the platelets were stained for actin. Scale bars represent 10 μm. (B) The bar graph indicates the number of ex vivo–aged platelets with at least 2 cell bodies per microliter (mean ± SEM; n = 4). *P < .05, cultured vs baseline. (C) Ex vivo–aged platelets (day 4) were resuspended in culture medium for 6 hours in the presence (far right panels) or absence of thrombin. From left to right in the top row, the top row panels identify αIIbβ3, P-selectin, β-tubulin, control IgG, respiring mitochondria (Mitotracker), or sialic acids (WGA). Corresponding transmission images are shown in the bottom row. Scale bars represent 5 μm. This figure is representative of 3 independent experiments. (D) Platelets were stored under standard blood bank conditions, and platelet counts as well as mean platelet volumes (MPV) were determined. The left graph shows the platelet count before (day 0) and after (day 5) storage (mean ± SEM; n = 10). The right panel displays the MPV obtained from platelets used for the counting studies. *P < .05, day 0 vs day 5, for both panels.
Next we determined whether platelets counts increased in cells that were stored under standard blood bank conditions. We found that platelets from 9 of 10 donors increased in number, which was accompanied by a significant increase in mean platelet volume (Figure 6D). The average increase was 21.1% plus or minus 5.1% (P < .01 vs day 0) with a maximal increase of 46.6% (ie, day 0 = 2.955 × 1011 platelets/concentrate vs day 5 = 4.369 × 1011 platelets/concentrate). The number of freshly isolated platelets also increased significantly (P < .05) after 6 hours in suspension culture (14.8% ± 2.6% increase over baseline).
Discussion
Our studies show that platelets, the products of one of the most intricate cellular differentiation pathways in the hematopoietic system, are capable of producing progeny. Unlike nucleated cells, platelets do not split into 2 uniform daughter cells; rather, they produce extended structures with 2 or more cell bodies that contain their own organelles, cytoskeletal system, and other cellular components. The newly formed platelets are capable of adhering to surfaces, spreading, and responding to agonist stimulation by expressing surface activation markers. The shafts connecting multiple platelet bodies are thin and often contain constricted regions that are difficult to preserve (requiring “snap-fixation” and gentle manipulation) and easily broken by centrifugation or pipetting. This indicates that multiple platelet bodies are primed to break down into individual cells, raising the possibility that circulatory shear stresses would separate such bodies if they form in the bloodstream in vivo.
The mechanisms that regulate progeny formation in platelets are incompletely understood, but our data demonstrate that an intact microtubular network is required for the formation of new cell bodies. Microtubules are essential for cell division, and dynamic microtubules accommodate shape changes in platelets31 and regulate the development of proplatelets that extend from the cytoplasm of megakaryocytes.32 This indicates that megakaryocytes and mature platelets use similar structural mechanisms to form bulbar extensions and that progeny formation may be an extension of proplatelet evolution in the circulation.21,28 Because of these similarities, one might expect progeny formation to occur more readily in young platelets that are recently shed from megakaryocytes. However, large platelets (> 4 μm), which are thought to be younger, were infrequently observed in our platelet preparations and ex vivo aged platelets readily developed new cell bodies. This suggests that the process may not be restricted to young platelets. If this is the case, it is intriguing to speculate that platelets may be expanded in storage bags under certain conditions. We did find that banked platelets increase in number and volume, but others have shown that platelet counts are stable during storage.33 The reasons for this disparity may be the result of differences in collection procedures, storage conditions, or counting methods used by the independent studies and need to be addressed more rigorously in the future. Platelets also have a tendency to become activated during the preparation process and prolonged storage,34,35 which may block or reduce progeny formation. We found that freshly isolated platelets fail to develop progeny when they are cocultured in the presence of low concentrations of thrombin (far right panels of Figures 2B, 6C) or Escherichia coli (data not shown). Inadvertent activation during the isolation procedure also prevents progeny from forming in freshly isolated platelets.
Similar to cell division in nucleated cells, progeny formation in platelets transpires over hours and is associated with increases in protein synthesis and mitochondrial DNA replication (data not shown). Presumably, this upsurge in synthetic activity accounts for the buildup in total intracellular protein content and cell biomass that occurs as platelets expand in number. The identity of synthesized products probably encompasses critical housekeeping proteins essential for normal platelet function. Booyse and Rafelson36 previously demonstrated that platelets incorporate amino acids into contractile protein. More recent studies also found that platelets constitutively synthesize integrin αIIbβ311,37 and that cultured platelets accumulate P-selectin.38 Whether these proteins and others are synthesized by platelets as they develop progeny is currently being investigated by our group. It will also be important to determine whether platelets synthesize membrane lipids as they form progeny. Majerus et al39,40 have shown that platelets possess requisite machinery for lipid metabolism and readily synthesize fatty acids.
A key question is this: does this process occur in vivo? Various forms of extended platelets have been observed in freshly prepared platelet-rich plasma from humans,22 and circulating “proplatelet-like” processes have also been identified in healthy and thrombocytopenic rats.29,30 Whether these processes are directly shed from the cytoplasm of megakaryocytes or morphed products of circulating platelets is not known. Using an ex vivo culture system, we found that platelets can develop new cell bodies in whole blood, and Behnke and Forer22 demonstrated that platelet processes continue to elongate in freshly prepared human plasma. However, future studies are required to determine whether platelet progeny formation occurs in flowing blood. If it does occur, this would mean that thrombopoiesis continues in the bloodstream, which may help explain how small populations of bone marrow megakaryocytes can maintain vast numbers of circulating platelets. Although we have shown that platelet progeny appear to be structurally and functionally normal, it has also yet to be determined whether platelets produced from other platelets represent a distinct population from those produced by megakaryocytes; for example, they may differ in thrombogenicity, life span, or susceptibility to apoptosis.
The biologic relevance of platelet progeny formation clearly requires further investigation. Nevertheless, our observations of this phenomenon in vitro are sufficient to challenge some long-held notions concerning how hematopoiesis works and what highly differentiated eukaryotic cells are capable of, even those that extrude their nuclei. Apart from the significance of these findings for platelet blood banking and transfusion, our observations could have important implications on thrombopoiesis during health and disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chris K. Rodesch and Keith R. Carney of the University of Utah Cell Imaging Core and Nancy Chandler of the University of Utah Electron Microscopy Core for technical assistance as well as Diana Lim for preparation of the figures, critical comments, and consultation regarding effective display of the images.
This work was supported by National Institutes of Health grants HL066277 (A.S.W.), HL044525 (G.A.Z.), and HL075507 (L.W.K.) and by the ARUP Institute for Clinical and Experimental Pathology. H.S. was supported by a postdoctoral fellowship (0625098Y) and a Beginning Grant-in-Aid (09BG1A 2250381) from the American Heart Association Western States Affiliate. W.H.A.K. was supported by an operating grant from the Canadian Institutes of Health Research (MOP-81208). S.K. was supported by a Deutsche Forschungsgemeinschaft grant (KO 3572/1). D.A.W. was supported by National Science Foundation grants DMR-0602684 and DBI-0649865 and by the Harvard Materials Research Science and Engineering Center (DMR-0213805). B.F.K. was supported by a fellowship from German Society of Cardiology (Pfizer Grant of the DGK, 2009).
National Institutes of Health
Authorship
Contribution: H.S., S.K., W.H.A.K., A.G., and A.S.W. conceived and designed the experiments; H.S., S.K., W.H.A.K., N.M., B.F.K., A.G., and A.S.W. performed the experiments; H.S., S.K., W.H.A.K., A.G., G.A.Z., and A.S.W. analyzed the data; H.S., S.K., W.H.A.K., N.M., D.A.W., R.C.B., A.G., and A.S.W. contributed reagents, materials, and analysis tools; and H.S., S.K., W.H.A.K., D.A.W., L.W.K., A.G., G.A.Z., and A.S.W. wrote and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew S. Weyrich, Department of Internal Medicine, Eccles Institute of Human Genetics, Bldg 533, Rm 4220, University of Utah, Salt Lake City, UT 84112; e-mail: andy.weyrich@hmbg.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal