Abstract
In patients with overt inflammatory diseases, up-regulated hepcidin impairs iron absorption and macrophage release, causing anemia. Whether the mild proinflammatory state of aging is associated with increased hepcidin is unknown. We characterized the relationships between urinary hepcidin, iron status, anemia, and inflammation in 582 patients 65 years or older participating in the InCHIANTI (Invecchiare in Chianti, “Aging in the Chianti Area”) study, a population-based study of aging in Tuscany, Italy. Compared with nonanemic persons, urinary hepcidin (nanograms/milligram of urinary creatinine) was significantly lower in iron deficiency and inflammation anemia compared with no anemia or other anemia types. Urinary hepcidin was positively correlated with log(ferritin) and negatively correlated with the soluble transferrin receptor/log(ferritin) ratio but not correlated with markers of inflammation: interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α, and C-reactive protein (CRP). Lower iron was significantly correlated with higher IL-6 and CRP. Adjusting for confounders, IL-6 and CRP remained significantly associated with serum iron, with no evidence that such a relationship was accounted for by variability in urinary hepcidin. In conclusion, elevated proinflammatory markers were associated with anemia and low iron status, but not with higher urinary hepcidin. Future studies should test whether hepcidin production becomes up-regulated only in situations of overt inflammation.
Introduction
The etiology of anemia in older persons is multifactorial, with iron deficiency and chronic inflammation found in approximately two-thirds of the cases.1,2
In iron deficiency anemia, reduced availability of iron causes a hypoproliferative disorder. In anemia of inflammation, proinflammatory cytokines affect iron metabolism, particularly iron turnover and ferritin synthesis causing low serum iron without evidence of iron deficiency.3,4 Research on the pathogenesis of anemia of inflammation has focused recently on hepcidin, a 25 amino-acid peptide, which is synthesized by hepatocytes as an 84 amino-acid precursor, subsequently processed into prohepcidin and hepcidin.5 Hepcidin binds to and induces the degradation of the unique iron transporter ferroportin, reduces intestinal absorption of iron6 and blocks mobilization of iron from hepatocytes and macrophages, thereby causing low serum iron and hypoproliferative anemia through decreased iron reutilization.7 Hepcidin levels rise in the iron-replete state and decline in response to anemia, active erythropoiesis, and hypoxia.8 Hepcidin synthesis is also stimulated by inflammatory mediators, a mechanism that may have evolved as a response to infection, aimed at depriving microorganisms of iron.8,9 Treatment of primary hepatocytes with interleukin-6 (IL-6),10 lipopolysaccharide (LPS), or LPS-stimulated macrophages increases hepcidin mRNA expression, and this induction is blocked by treatment with anti–IL-6 antibodies.11
Older persons are often affected by a chronic, mild proinflammatory state characterized by elevated levels of proinflammatory markers such as interleukin-6 (IL-6) and C-reactive protein (CRP).12 It may be hypothesized that this mild inflammatory state may cause a chronic elevation of circulating hepcidin and consequently promotes the development of anemia. Indeed, diseases in which hepcidin is inappropriately increased, because of mutations in its suppressor13 or autonomous production by hepatic adenomas,14 are characterized by iron-restricted anemia with resistance to iron supplementation. Increased levels of hepcidin have been detected in diseases characterized by overt inflammation, such as rheumatoid arthritis, abscesses, and sepsis.9,15 However, whether the age-associated mild proinflammatory state affects hepcidin and through this mechanism modulates iron metabolism and the risk of anemia in the general population is unknown. To address this question, we measured urinary hepcidin in subjects affected by different forms of anemia and nonanemic controls enrolled in a population-based study of aging. We expected to find high urinary hepcidin concentrations in participants with anemia of inflammation and in those with high serum concentrations of proinflammatory markers. We also hypothesized that participants with iron deficiency anemia would have low urinary hepcidin as an attempt to maximize iron absorption and bioavailability.
Methods
Study population
InCHIANTI (Invecchiare in Chianti, “Aging in the Chianti Area”) is a study conducted in the Tuscany Region of Italy.16 In 1998, 1270 adults 65 years or older, were randomly selected from the residents of 2 towns in the Chianti region of Tuscany, Greve in Chianti and Bagno a Ripoli. The rationale, design, and data collection methods have been described in greater detail elsewhere.16 Of 1256 eligible subjects, 1155 (90.1%) agreed to participate, of whom 1043 (90.3%) participated in the blood drawing. For the present study of anemia, 49 subjects were excluded because they had a positive medical history for cancer, gastrointestinal disease, or any medical condition potentially associated with bleeding, and 94 were excluded because the 24-hour urine collection had not been performed or was considered unreliable. None of the remaining participants had a thalassemia trait. Participants received an extensive description of the study and participated after providing written informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Institutional Review Board of the Italian National Institute of Research and Care of Aging.
At enrollment, there were 86 subjects with anemia. Anemia was defined according to the World Health Organization criteria as a hemoglobin level less than 130 g/L (13 g/dL) in men and less than 120 g/L (12 g/dL) in women. A random sample of 496 nonanemic control subjects was taken at a ratio of approximately 6:1 with the subjects who had anemia. The final study population included 582 participants (270 men, 312 women) who had complete data for the analysis presented and who were not taking iron, vitamin B12, or folate supplements.
Measurement of urinary hepcidin
Blood samples were obtained from participants after a 12-hour fast and a 15-minute rest. At the time of the home interview, participants were provided a plastic container and received detailed instructions for 24-hour urine collection. Aliquots of serum and 24-hour urine were stored at −80°C and were not thawed until analysis. Serum and urinary creatinine were measured using a modified Jaffe method and used to calculate creatinine clearance. Cationic peptides were extracted from urine using CM Macro-prep (Bio-Rad Laboratories Inc) eluted with 5% acetic acid, lyophilized, and resuspended in 0.01% acetic acid. Hepcidin urinary concentrations were determined by immunodot assay.9 Urine extracts equivalent to 0.1 to 0.5 mg of creatinine were dotted on Immobilon-P membrane (Millipore Corp), along with a range of synthetic hepcidin standards (0-40 ng). Hepcidin was detected on the blots using rabbit anti–human hepcidin antibodies with goat anti–rabbit horseradish peroxidase as a secondary antibody. Dot blots were developed by the chemiluminescent detection method (SuperSignal West Pico Chemiluminescent Substrate; Pierce Chemical Co) and quantified with the Chemidoc cooled camera running Quantity One software (Bio-Rad Laboratories Inc). Urinary hepcidin concentrations were normalized by urinary creatinine concentration and expressed as nanograms per milligram of creatinine.17 Repeated assays of hepcidin performed in the same urinary samples 1 year apart yielded values that were highly correlated (r = 0.988), with no evidence for systematic trends toward lower or higher levels with time.
To check for potential biases introduced by creatinine normalization of hepcidin levels and because the urine samples used in the assay were from a 24-hour urine collection, we repeated our analysis expressing hepcidin as total nanograms excreted in 24 hours.
Indicators of iron and nutritional status
Erythrocytes and hemoglobin were measured using an automatic hematology analyzer Coulter LH 750 (Beckman Coulter, Instrumentation Laboratory) within 6 hours from the time of phlebotomy. Folate and vitamin B12 were measured by a radioimmunoassay (ICN Pharmaceuticals). The minimum detectable concentrations were 1.3596nM (0.6 ng/mL) for folate and 18.445pM (25 pg/mL) for vitamin B12; the interassay coefficient of variations (CVs) were 7.1% and 12.3%, respectively. Vitamin B12 deficiency was defined as a concentration less than 147.56pM (200 pg/mL) and folate deficiency, as a concentration less than 4.9852nM (2.2 ng/mL).1 Serum ferritin and soluble transferrin receptor (sTfr) were measured in duplicate using chemiluminescent immunoassays (Abbott Diagnostics and Nichols Institute Diagnostics). The minimum detectable concentration in these assays was 5 ng/mL for ferritin and 0.1nM for sTfr, and the interassay CVs were less than 7%. Circulating iron was assessed by a colorimetric assay (Roche Diagnostics GmbH), having a sensitivity of .895μM (5 μg/dL) and an interassay CV less than 3%. A serum sTfr/log(ferritin) ratio higher than 1.5 was considered as indicating iron deficiency. In absence of direct bone marrow examination, the sTfr/log(ferritin) ratio, also known as the transferrin receptor–ferritin index, is considered the most reliable indicator of iron stores.18
Inflammatory markers
Serum levels of interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α (TNF-α) were measured in duplicate by high-sensitivity enzyme-linked immunosorbent assays using commercial kits (Biosource International). The lowest detectable concentration was 0.1 pg/mL for IL-6, 0.09 pg/mL for TNF-α, and 0.01 pg/mL for IL-1β. The interassay CVs were less than 7% for all 3 cytokines. C-reactive protein (CRP) was measured in duplicate using an enzyme-linked immunosorbent assay and colorimetric competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies. The minimum detectable threshold was 0.28 572nM (0.03 mg/L) and the interassay coefficient of variation was 5%.
Chronic diseases
Diseases were ascertained by an experienced clinician according to pre-established criteria that combined information from self-reported physician diagnoses, current pharmacologic treatment, medical records, clinical examinations, and blood tests. Diseases included in the current analysis were coronary heart disease (7.7%), congestive heart failure (4.5%), hypertension (62%), stroke (4.3%), and diabetes (13.0%). In addition, we considered participants who were affected by recent or current infection, malignant cancer, rheumatoid arthritis, inflammatory bowel diseases, connective tissue disorders, and skin ulcer as having an “inflammatory condition.” Diagnostic algorithms for diseases were modified versions of those created for the Women's Health and Aging Study.19
Causes of anemia
Anemia and its pathophysiologic forms were classified by applying sequentially the following criteria: (1) women with hemoglobin level less than 120 g/L (12 g/dL) and men with hemoglobin level less than 130 g/L (13 g/dL) were considered as anemic; (2) anemic participants with creatinine clearance less than 30 mL/min were classified as having anemia of chronic kidney disease; (3) those with Tfr/log(ferritin) ratio higher than 1.5 and/or ferritin less than 12 ng/mL were classified as having iron deficiency anemia; (4) those with low circulating iron (< 10.74μM [< 60 μg/dL]) and no evidence of iron deficiency (Tfr/log(ferritin) < 1.5) were classified as having anemia of inflammation; and (5) anemic participants with serum folate less than 2.2 ng/mL or vitamin B12 less than 147.56pM (200 pg/mL) were classified as having nutritional (“B12/folate deficiency”) anemia. In accordance with Guralnik et al, participants with normal renal function, normal circulating iron, and no iron, folate, or vitamin B12 deficiency (namely, lack of criteria usually associated with anemia of kidney disease, anemia of inflammation, or nutrient deficiency anemia) were classified as having “unexplained anemia of aging.”2
Statistical analysis
Variables are reported as mean values plus or minus standard deviations (SDs), medians, and interquartile ranges (Q1-Q3) or percentages, and compared between participants with and without anemia. Variables with skewed distribution were log-transformed for subsequent analyses. Distributions of hepcidin were explored in participants without anemia and in those with different pathophysiologic forms of anemia. Differences between groups were formally tested by analysis of variance, and analysis of covariance was adjusted for age, sex, and hemoglobin.
The relationship of hepcidin with markers of iron status (serum iron, ferritin, and sTfr/log(ferritin)) and markers of inflammation (IL-6, IL-1β, TNF-α, and CRP) was explored with scatter plots and formally tested with linear regression models, which were subsequently adjusted for covariates. To verify whether inflammation was associated with low circulating iron, we examined mean iron levels according to quartiles of the major inflammatory markers and the relationship was tested both by analysis of variance and test for trend, after adjustment for age, sex, calorie intake, iron intake, sTfr/log(ferritin) ratio, major chronic disease, and hemoglobin. Finally, to explore the possible mediating effect of hepcidin on the relationship between inflammation and low iron, the analysis was also adjusted for log(hepcidin). The analysis was repeated after excluding participants with iron deficiency. All analyses were performed using the SAS statistical package, Version 9.1 (SAS Institute Inc).
Results
Participants' characteristics according to presence and type of anemia are reported in Table 1. In the nonanemic group, the levels of inflammatory markers and urinary hepcidin were slightly higher than those reported for the same age range. With the exception of iron deficiency anemia, participants with anemia were significantly older than those without anemia. Adjusting for age and sex, participants with CKD anemia and anemia of inflammation were significantly more likely to have a body mass index lower than 25 kg/m2, to be affected by one or more proinflammatory conditions, and to have higher levels of proinflammatory markers (IL-6, CRP, and TNF-α) than those without anemia. Compared with nonanemic controls, participants with CKD, iron deficiency, and inflammation anemia but not those with B12/folate deficiency or unexplained anemia had higher erythropoietin levels. Of note, compared with anemic controls, urinary hepcidin was significantly lower among participants with inflammation anemia and especially those with iron deficiency, but not in those with other anemia types. In alternative analyses in which mean urinary hepcidin concentrations were expressed as nanograms excreted in 24 hours rather than normalized by creatinine concentrations, iron deficiency anemia was the only form of anemia in which absolute urinary hepcidin concentrations differed from nonanemic controls and other forms of anemia (P < .001).
Characteristics of the study population according to anemia and anemia type
| Characteristic . | No anemia . | CKD anemia . | Iron-deficiency anemia . | Anemia of inflammation . | Anemia with B12/folate deficiency . | Anemia unexplained . |
|---|---|---|---|---|---|---|
| Total no. | 496 | 9 | 15 | 21 | 9 | 32 |
| Age, y | 73.3 (69.9-77.9) | 89.0‡ (84.0-90.8) | 72.2 (68.8-90.7) | 78.5‡ (74.7-83.1) | 85.2† (73.6-87.9) | 78.8† (73.8-85.5) |
| Male, % | 46.5 | 44.4 | 73.3 | 61.9 | 33.3 | 27.3 |
| Female, % | 53.5 | 55.6 | 26.7 | 38.1 | 66.7 | 72.7 |
| Years of education | 5.0 (4.0-6.0) | 4.0† (4.0-5.0) | 5.0 (3.0-5.0) | 5.0 (4.0-5.0) | 5.0 (3.0-5.0) | 5.0 (4.0-5.0) |
| Body mass index, % | ||||||
| Less than 25 kg/m2 | 29.6 | 55.6‡ | 26.7 | 57.1‡ | 33.3 | 30.2 |
| More than 25 kg/m2 | 70.4 | 44.4 | 73.3 | 42.9 | 66.7 | 69.7 |
| Hepcidin, ng/mg creatinuria | 60.7 (34.5-114.2) | 64.0 (57.5-81.0) | 15.0‡ (0.0-21.6) | 50.9‡ (17.7-105.5) | 49.4 (38.7-91.1) | 80.0 (42.0-103.4) |
| Hemoglobin, g/dL | 14.0 (13.3-14.8) | 10.4‡ (8.8-11.3) | 11.1‡ (9.9-12.2) | 11.8‡ (11.0-11.9) | 11.9‡ (11.4-12.5) | 11.7‡ (11.4-12.0) |
| Ferritin, mg/L | 118 (61-206) | 108 (62-134) | 8‡ (6-11) | 67† (18-109) | 106 (67-147) | 103 (71-179) |
| Soluble transferrin receptor, nM/L | 16.0 (13.6-19.2) | 19.2 (16.8-28.8) | 28.8‡ (19.2-38.4) | 20.8‡ (17.4-27.2) | 14.4 (13.6-16.0) | 16.0 (14.4-18.4) |
| Iron, μg/dL | 82 (67-99) | 61 (38-68) | 32 (18-41) | 49 (38-55) | 84 (73-95) | 82 (73-97.0) |
| Erythropoietin, mU/mL | 9.7 (7.4-12.5) | 25‡ (19-34) | 27‡ (19-58) | 11.5‡ (9.5-23.4) | 9.7 (8.7-11.0) | 11.5 (9.7-14.2) |
| Vitamin B12, pg/mL | 374 (266-508.0) | 340† (274-1108) | 313 (194-404) | 214‡ (175-376.0) | 309 (157-362) | 453 (318-567) |
| Folate, ng/mL | 3.1 (2.4-4.2) | 3.7 (2.8-6.0) | 3.1 (2.1-4.8) | 2.5 (2.2-4.1) | 1.8‡ (1.7-2.1) | 4.1‡ (3.0-5.3) |
| Creatinine clearance, mL/min | 75.6 (58.6-93.8) | 19.6‡ (13.6-24.1) | 69.1 (42.2-102.7) | 57.8 (45.2-83.6) | 62.9 (48.9-69.5) | 68.6 (47.9-87.4) |
| Presence of an inflammatory condition, %* | 2.4 | 55.5‡ | 13.3 | 42.9‡ | 0.0 | 9.1 |
| Interleukin-6, pg/mL | 0.73 (0.37-1.43) | 3.4‡ (3.4-5.4) | 1.5‡ (0.8-2.6) | 1.8‡ (1.1-2.1) | 1.5† (1.2-2.7) | 1.3† (0.8-2.0) |
| C-reactive protein, mg/L | 1.3 (0.6-2.8) | 9.2‡ (3.2-13.7) | 3.5‡ (1.4-5.2) | 3.6‡ (2.0-9.0) | 2.3 (1.3-2.6) | 1.5 (0.8-3.4) |
| Tumor necrosis factor-α, pg/mL | 1.9 (1.4-3.2) | 5.5‡ (3.3-7.2) | 2.0 (1.7-3.4) | 2.9‡ (1.8-5.2) | 2.5 (1.9-3.0) | 2.5 (1.9-3.6) |
| Interleukin-1β, pg/mL | 0.01 (0.00-0.67) | 0.08 (0.00-7.9) | 0.00 (0.00-6.6) | 0.00 (0.00-0.93) | 0.00 (0.00-0.00) | 0.00 (0.00-0.71) |
| Interleukin-1 receptor antagonist, pg/mL | 130.0 (95.8-185.3) | 193† (158-306) | 136 (89.0-173.0) | 124.7 (87.5-187.2) | 154.1 (86.7-178.3) | 128.0 (89.9-179.3) |
| Interleukin-18, μg/mL | 382 (303-479) | 545 (463-574) | 410 (345-482) | 376.6 (286.4-555) | 343 (255-390.3) | 337 (268-518) |
| Characteristic . | No anemia . | CKD anemia . | Iron-deficiency anemia . | Anemia of inflammation . | Anemia with B12/folate deficiency . | Anemia unexplained . |
|---|---|---|---|---|---|---|
| Total no. | 496 | 9 | 15 | 21 | 9 | 32 |
| Age, y | 73.3 (69.9-77.9) | 89.0‡ (84.0-90.8) | 72.2 (68.8-90.7) | 78.5‡ (74.7-83.1) | 85.2† (73.6-87.9) | 78.8† (73.8-85.5) |
| Male, % | 46.5 | 44.4 | 73.3 | 61.9 | 33.3 | 27.3 |
| Female, % | 53.5 | 55.6 | 26.7 | 38.1 | 66.7 | 72.7 |
| Years of education | 5.0 (4.0-6.0) | 4.0† (4.0-5.0) | 5.0 (3.0-5.0) | 5.0 (4.0-5.0) | 5.0 (3.0-5.0) | 5.0 (4.0-5.0) |
| Body mass index, % | ||||||
| Less than 25 kg/m2 | 29.6 | 55.6‡ | 26.7 | 57.1‡ | 33.3 | 30.2 |
| More than 25 kg/m2 | 70.4 | 44.4 | 73.3 | 42.9 | 66.7 | 69.7 |
| Hepcidin, ng/mg creatinuria | 60.7 (34.5-114.2) | 64.0 (57.5-81.0) | 15.0‡ (0.0-21.6) | 50.9‡ (17.7-105.5) | 49.4 (38.7-91.1) | 80.0 (42.0-103.4) |
| Hemoglobin, g/dL | 14.0 (13.3-14.8) | 10.4‡ (8.8-11.3) | 11.1‡ (9.9-12.2) | 11.8‡ (11.0-11.9) | 11.9‡ (11.4-12.5) | 11.7‡ (11.4-12.0) |
| Ferritin, mg/L | 118 (61-206) | 108 (62-134) | 8‡ (6-11) | 67† (18-109) | 106 (67-147) | 103 (71-179) |
| Soluble transferrin receptor, nM/L | 16.0 (13.6-19.2) | 19.2 (16.8-28.8) | 28.8‡ (19.2-38.4) | 20.8‡ (17.4-27.2) | 14.4 (13.6-16.0) | 16.0 (14.4-18.4) |
| Iron, μg/dL | 82 (67-99) | 61 (38-68) | 32 (18-41) | 49 (38-55) | 84 (73-95) | 82 (73-97.0) |
| Erythropoietin, mU/mL | 9.7 (7.4-12.5) | 25‡ (19-34) | 27‡ (19-58) | 11.5‡ (9.5-23.4) | 9.7 (8.7-11.0) | 11.5 (9.7-14.2) |
| Vitamin B12, pg/mL | 374 (266-508.0) | 340† (274-1108) | 313 (194-404) | 214‡ (175-376.0) | 309 (157-362) | 453 (318-567) |
| Folate, ng/mL | 3.1 (2.4-4.2) | 3.7 (2.8-6.0) | 3.1 (2.1-4.8) | 2.5 (2.2-4.1) | 1.8‡ (1.7-2.1) | 4.1‡ (3.0-5.3) |
| Creatinine clearance, mL/min | 75.6 (58.6-93.8) | 19.6‡ (13.6-24.1) | 69.1 (42.2-102.7) | 57.8 (45.2-83.6) | 62.9 (48.9-69.5) | 68.6 (47.9-87.4) |
| Presence of an inflammatory condition, %* | 2.4 | 55.5‡ | 13.3 | 42.9‡ | 0.0 | 9.1 |
| Interleukin-6, pg/mL | 0.73 (0.37-1.43) | 3.4‡ (3.4-5.4) | 1.5‡ (0.8-2.6) | 1.8‡ (1.1-2.1) | 1.5† (1.2-2.7) | 1.3† (0.8-2.0) |
| C-reactive protein, mg/L | 1.3 (0.6-2.8) | 9.2‡ (3.2-13.7) | 3.5‡ (1.4-5.2) | 3.6‡ (2.0-9.0) | 2.3 (1.3-2.6) | 1.5 (0.8-3.4) |
| Tumor necrosis factor-α, pg/mL | 1.9 (1.4-3.2) | 5.5‡ (3.3-7.2) | 2.0 (1.7-3.4) | 2.9‡ (1.8-5.2) | 2.5 (1.9-3.0) | 2.5 (1.9-3.6) |
| Interleukin-1β, pg/mL | 0.01 (0.00-0.67) | 0.08 (0.00-7.9) | 0.00 (0.00-6.6) | 0.00 (0.00-0.93) | 0.00 (0.00-0.00) | 0.00 (0.00-0.71) |
| Interleukin-1 receptor antagonist, pg/mL | 130.0 (95.8-185.3) | 193† (158-306) | 136 (89.0-173.0) | 124.7 (87.5-187.2) | 154.1 (86.7-178.3) | 128.0 (89.9-179.3) |
| Interleukin-18, μg/mL | 382 (303-479) | 545 (463-574) | 410 (345-482) | 376.6 (286.4-555) | 343 (255-390.3) | 337 (268-518) |
Data are reported as median values and interquartile ranges or percentages.
Anemia was defined as hemoglobin < 12 g/dL in women and < 13 g/dL in men.
CKD indicates chronic kidney disease.
Any of the following: recent or current infection, malignant cancer, rheumatoid arthritis, inflammatory bowel diseases, connective tissue disorders, or skin ulcer.
P < .05 compared with the “no anemia” group, adjusted for age and sex.
P < .01 compared with the “no anemia” group, adjusted for age and sex.
After adjusting for age and sex, IL-6 was significantly associated with higher likelihood of having anemia (odds ratio [OR] for 1 unit higher log (IL-6): 1.94; 95% confidence interval [CI]: 1.45-2.59). The association was even higher with anemia of inflammation (OR: 2.20; 95% CI: 1.33-3.63). After further adjusting these analyses for hepcidin, results did not change substantially (anemia vs no anemia: OR: 1.98; 95% CI: 1.49-2.66; anemia of chronic disease vs no anemia: OR: 2.29; 95% CI: 1.38-3.79). Using CRP or TNF-α instead of IL-6 in analogous analyses provided substantially similar results.
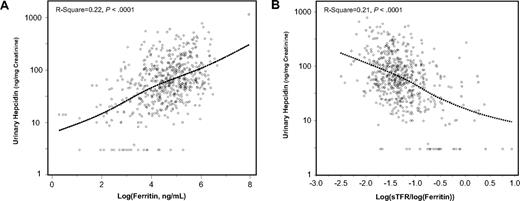
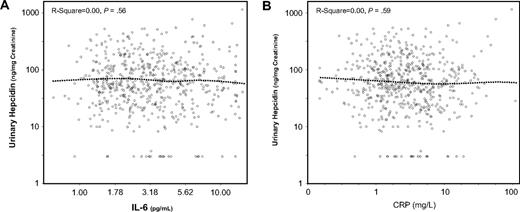
As expected, log(ferritin) was positively correlated, whereas the sTfr/log(ferritin) ratio was negatively correlated with urinary hepcidin (Figure 1). Contrary to our expectations, we found no correlation of IL-6 (Figure 2A), CRP (Figure 2B), IL-1β (r = −0.03, P = .52), and TNF-α (r = −0.07, P = .11) with urinary hepcidin concentrations. Adjusting for age, sex, and other covariates did not change these findings. In alternative analyses in which absolute urinary hepcidin concentrations were expressed as nanograms excreted in 24 hours, the correlations between urinary hepcidin and IL-6, CRP, IL-1β, and TNF-α were r = −0.04, P = .34, r = −0.03, P = .42, r = −0.07, P = .07, and r = −0.05, P = .20, respectively. We found no significant relationship between hepcidin and an “inflammatory score” constructed as the number of inflammatory markers (IL-6, CRP, IL-1β, and TNF-α) in the upper quintile (P = .87). Analogously, we found no significant correlation between urinary hepcidin and IL-6, CRP, IL-1β, and TNF-α when the analysis was restricted to participants with anemia and those with anemia of inflammation only (data not shown).
Urinary hepcidin and log(ferritin). Scatter plots of urinary hepcidin versus log(ferritin) (A) and sTfr/log(ferritin) ratio (B). Relationships are summarized as locally weighted regression curves. R-squares and P values are unadjusted.
Urinary hepcidin and log(ferritin). Scatter plots of urinary hepcidin versus log(ferritin) (A) and sTfr/log(ferritin) ratio (B). Relationships are summarized as locally weighted regression curves. R-squares and P values are unadjusted.
Urinary hepcidin. Scatter plots of urinary hepcidin vs IL-6 (A) and C-reactive protein (B). Relationships are summarized as locally weighted regression curves. R-squares and P values are unadjusted.
Urinary hepcidin. Scatter plots of urinary hepcidin vs IL-6 (A) and C-reactive protein (B). Relationships are summarized as locally weighted regression curves. R-squares and P values are unadjusted.
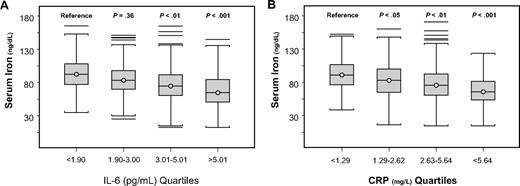
Serum iron was progressively lower with increasing quartiles of IL-6 (Figure 3A) and CRP (Figure 3B). In a linear regression model, IL-6 and CRP were significant, negative predictors of serum iron, and the effect these inflammatory markers had on serum iron did not change after adjusting for age, sex, hemoglobin, iron intake, energy intake, sTfr/log(ferritin) ratio, and major chronic disease, even after further adjustment for urinary hepcidin (Table 2). In an alternative analysis that used urinary hepcidin expressed as nanograms excreted in 24 hours, results were not substantially changed. In additional analyses, exclusion of participants with iron deficiency anemia did not substantially change the results.
Serum iron. Serum iron by quartiles of IL-6 (A; test for trend, P < .001) and C-reactive protein (B; test for trend, P < .001). P values shown for mean iron level are for comparison with the lowest quartile and are adjusted for age, sex, and body mass index.
Serum iron. Serum iron by quartiles of IL-6 (A; test for trend, P < .001) and C-reactive protein (B; test for trend, P < .001). P values shown for mean iron level are for comparison with the lowest quartile and are adjusted for age, sex, and body mass index.
Linear regression models assessing the relationship of IL-6 and CRP with serum iron, after adjusting for confounders
| Variable . | Model 1 . | Model 2 . | Model 3* . | |||
|---|---|---|---|---|---|---|
| Beta (SE) . | P . | Beta (SE) . | P . | Beta (SE) . | P . | |
| Age, y | 0.09 (0.15) | .52 | 0.10 (0.15) | .49 | 0.11 (0.15) | .47 |
| Sex | −6.48 (2.16) | .003 | −5.63 (2.18) | .01 | −4.37 (2.30) | .06 |
| Body mass index, kg/m2 | −0.04 (0.25) | .87 | −0.02 (0.25) | .95 | 0.15 (0.25) | .55 |
| Hemoglobin, g/dL | 7.8 (0.8) | < .001 | 7.5 (0.8) | < .001 | 6.3 (1) | < .001 |
| Loge interleukin-6, pg/mL | −2.99 (1.84) | .10 | −2.98 (1.83) | .10 | −1.91 (1.80) | .29 |
| Loge C-reactive protein, mg/L | −6.45 (1.07) | < .001 | −6.39 (1.07) | < .001 | −6.96 (1.03) | < .001 |
| Loge hepcidin, ng/mg creatinine | 1.00 (0.43) | .02 | 0.04 (0.45) | .92 | ||
| Variable . | Model 1 . | Model 2 . | Model 3* . | |||
|---|---|---|---|---|---|---|
| Beta (SE) . | P . | Beta (SE) . | P . | Beta (SE) . | P . | |
| Age, y | 0.09 (0.15) | .52 | 0.10 (0.15) | .49 | 0.11 (0.15) | .47 |
| Sex | −6.48 (2.16) | .003 | −5.63 (2.18) | .01 | −4.37 (2.30) | .06 |
| Body mass index, kg/m2 | −0.04 (0.25) | .87 | −0.02 (0.25) | .95 | 0.15 (0.25) | .55 |
| Hemoglobin, g/dL | 7.8 (0.8) | < .001 | 7.5 (0.8) | < .001 | 6.3 (1) | < .001 |
| Loge interleukin-6, pg/mL | −2.99 (1.84) | .10 | −2.98 (1.83) | .10 | −1.91 (1.80) | .29 |
| Loge C-reactive protein, mg/L | −6.45 (1.07) | < .001 | −6.39 (1.07) | < .001 | −6.96 (1.03) | < .001 |
| Loge hepcidin, ng/mg creatinine | 1.00 (0.43) | .02 | 0.04 (0.45) | .92 | ||
Model 3 adjusted for iron intake, energy intake, sTFR/log(ferritin) ratio, and major chronic diseases.
Discussion
In this study performed on a representative sample of the general population 65 years or older, we found that subjects with anemia of inflammation, characterized by low serum iron without evidence of iron deficiency, did not have higher urinary hepcidin compared with nonanemic controls. In addition, urinary hepcidin concentrations were not associated with markers of inflammation. These findings were contrary to our original hypothesis that high urinary hepcidin concentrations would be found in participants with anemia of inflammation and in those with high serum concentrations of proinflammatory markers. The findings were consistent whether using urinary hepcidin normalized by creatinine or absolute urinary hepcidin excreted in 24 hours. Our findings are consistent with those reported by Lee et al, who studied 8 subjects with anemia of aging. However, our findings are more robust because of larger and representative samples and detailed measures of cytokine levels.20
IL-6 has been directly implicated in the up-regulation of hepcidin, and in preclinical studies the evidence for such regulation is overwhelming.3 However, the relationship between IL-6 and hepcidin in humans has been demonstrated mostly in subjects with overt, severe inflammation such as subjects with acute sepsis,21 patients with multiple myeloma,22 and healthy volunteers challenged with IL-69 or LPS.15 The present study suggests that the mild, proinflammatory state found in older, community-dwelling adults is not associated with high urinary hepcidin. Interestingly, in our study a proinflammatory state characterized by elevated IL-6 and CRP was strongly associated with lower serum iron concentrations, with little evidence for a mediating effect of hepcidin on the relationship between inflammation and low iron. Thus, abnormally elevated hepcidin may not be necessary to sustain a mild hypoferremia and anemia of inflammation, either because alternative mechanisms have a similar effect (eg, direct regulation of ferroportin by inflammatory mediators) or because even normal hepcidin restricts the iron supply sufficiently to prevent the correction of the anemia, once it is established.
As originally hypothesized, subjects with iron deficiency anemia had lower urinary hepcidin concentrations compared with those who were not anemic. Urinary hepcidin was closely correlated with both serum ferritin and with the transferrin receptor–ferritin index, as consistent with previous studies in humans.10,23 Thus, it appears that hepcidin is a key regulator of iron metabolism for compensation of iron deficiency and the down-regulation of hepcidin production is easily detected by measuring urinary excretion. The failure to detect urinary hepcidin overproduction is likely not due to technical limitations of the assay, as the assay previously detected more than 100 times higher urinary hepcidin concentrations than those found in this study. Another possible reason for the inability to detect a moderate overproduction of hepcidin in urine may be because the hepcidin molecule is degraded locally in cells after binding to ferroportin. This hypothesis should be tested in preclinical studies.
The major strength of this study is the measure of hepcidin in a large population-based sample of persons who were well characterized for iron status, anemia, and inflammation. In contrast to most clinical series, this population did not include many persons with overt inflammatory conditions, such as a sepsis or connective tissue disorders. Thus, our findings should not be generalized to patients affected by these conditions. Aliquots of 24-hour urine used in this study were collected between November 1998 and March 2000, stored at −80°C, and never thawed before 2006 when assays were performed. In a pilot study, measures obtained 1 year apart in the same urinary samples were highly correlated and showed no evidence of degradation. However, the stability of hepcidin over longer periods is unknown. Another limitation of this study is that hepcidin was measured in the urine and expressed as nanograms per milligram of creatinine. At the time this analysis was performed, measuring hepcidin in the urine was the only reliable option. However, at least theoretically, hepcidin urinary excretion may be potentially affected by kidney function, muscle mass, and diurnal variation in glomerular filtration rate and tubular reabsorption. However, when we reanalyzed our data expressing hepcidin as nanograms/24 hours, results were substantially similar. In addition, our method was sensitive enough to detect reduction in hepcidin production that occurred in participants with iron deficiency anemia. Because of the epidemiologic nature of this study, the diagnoses of iron deficiency, anemia secondary to vitamin B12 deficiency, and anemia of chronic inflammation/anemia of chronic disease could not be definitively confirmed by comprehensive clinical and laboratory assessments and clinical follow-up that cannot be performed in the context of a population-based survey. Thus, a limitation of this study is that the etiologic classification of anemia was not based on clinical gold standards. It would have been useful to know the transferrin saturation to distinguish iron deficiency anemia from anemia of inflammation but, unfortunately, this test was not available. The “unexplained anemia” may have been unexplained because of a lack of a comprehensive medical assessment. Consistent with this view, the group of unexplained anemia had IL-6 levels that were slightly higher than the nonanemic group. Although we screened for β-thalassemia, we cannot exclude that some participants had α-thalassemia trait. Finally, although older people tend to have anemia from multiple causes, by using a sequential approach we defined mutually exclusive diagnostic groups. Indeed, participants with CKD in this cohort had a more powerful inflammatory signature than those with anemia of inflammation. However, when the analysis was repeated using an alternative definition for anemia of inflammation (sTfr/log(ferritin) < 1 ng/mL and ferritin > 100 ng/mL)24 and also allowing for multiple diagnoses and overlapping between diagnostic groups, results did not change. In addition, the lack of any correlation between proinflammatory markers and urinary hepcidin, the main result of this study, stands independent of possible etiologic misclassification of anemia. Finally, because of the observational and cross-sectional nature of this study, our findings should be interpreted with caution, and possibly should be replicated in a different population and using a prospective design.
In conclusion, urinary hepcidin concentrations were closely associated with markers of iron status but not with markers of inflammation in older, community-dwelling adults. Urinary hepcidin concentrations were not elevated among participants with the anemia of inflammation compared with nonanemic controls. This finding raises the possibility that hepcidin-independent mechanisms can cause hypoferremia and anemia of inflammation, and/or that even normal hepcidin levels are sufficient to sustain the anemia once it is initiated. An improved assay that measures hepcidin in serum25 is now available and should be used to confirm these results. The mechanisms by which mild inflammation affects iron status need to be further elucidated.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institute on Aging and the Intramural Research Program, National Institute on Aging, National Institutes of Health. The InCHIANTI study was supported as a targeted project (ICS-110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (contracts N01-AG-919413 and N01-AG-821336; grants R01 AG027012 and R01 AG029148). Measures of hepcidin were supported by an unrestricted grant from Orthobiotech.
None of the sponsoring institutions had any role in the analysis, interpretation, and reporting of the results of this study.
National Institutes of Health
Authorship
Contribution: L.F. collected data, designed the study, and wrote the paper; R.D.S. designed the study, analyzed data, and critically reviewed the paper; J.M.G., W.B.E., K.V.P., R.C.W., N.C.A., R.J.C., T.G., and E.N. designed the study and critically reviewed the paper; S.B. collected data, designed the study, and critically reviewed the paper; K.S. analyzed data; and D.L.L. designed the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigi Ferrucci, Clinical Research Branch, National Institute on Aging, Harbor Hospital Center, 5th Fl, 3001 S. Hanover St, Baltimore, MD 21225; e-mail: ferruccilu@grc.nia.nih.gov.
References
Author notes
L.F. and R.D.S. contributed equally to this work.