Abstract
The activating mutation JAK2 V617F plays a central role in the pathogenesis of polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Inhibition of JAK2 activity leads to growth inhibition and apoptosis in cells with mutated JAK2. However, the proapoptotic proteins involved in JAK2 inhibition-induced apoptosis remain unclear. In this study, we show that JAK2 inhibition-induced apoptosis correlated with up-regulation of the nonphosphorylated form of the BH3-only protein Bim in hematopoietic cell lines bearing JAK2 mutations. Knockdown of Bim dramatically inhibited apoptosis induced by JAK2 inhibition, which was reversed by the BH3 mimetic agent ABT-737. In addition, ABT-737 enhanced the apoptosis induced by JAK2 inhibition in JAK2 V617F+ HEL and SET-2 cells. The combination of JAK inhibitor I and ABT-737 reduced the number of erythroid colonies derived from CD34+ cells isolated from JAK2 V617F+ polycythemia vera patients more efficiently than either drug alone. These data suggest that Bim is a key effector molecule in JAK2 inhibition-induced apoptosis and that targeting this apoptotic pathway could be a novel therapeutic strategy for patients with activating JAK2 mutations.
Introduction
Myeloproliferative disorders (MPDs) are clonal hematopoietic diseases characterized by the excess production of 1 or more lineages of mature blood cells leading to complications of organomegaly, thrombosis, and hemorrhage.1 Recently, a somatic activating mutation in Janus kinase 2 (JAK2), a nonreceptor tyrosine kinase, was identified in patients with polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).2-6 A valine to phenylalanine substitution at position 617 (V617F) of JAK2 in the pseudokinase domain is the most common mutation, occurring in more than 95% of PV cases and in approximately 50% of patients with ET and PMF.7 Other mutations, such as K539L and T875N, have been identified in a small subset of PV patients and in a megakaryoblastic leukemia cell line, CHRF-288-11 (CHRF) cells, respectively.7
Conventional therapy for PV, ET, and PMF with cytoreductive chemotherapy or phlebotomy is not curative and does not reduce the risk of clonal evolution into myelodysplastic syndrome and acute leukemia. Thus, inhibition of mutant JAK2 may be a novel approach in the treatment of PV and other MPDs harboring JAK2 mutations. Various JAK inhibitors are currently under development and/or investigation in phase 1 and 2 clinical trials. However, initial reports from a clinical trial with one such JAK inhibitor, INCB018424, indicated that one-fourth of patients developed serious, although reversible, hematologic toxicities with initial dosing regimens.8 Furthermore, only a modest decrease in JAK2 V617F allele burden was seen in bone marrow and peripheral blood from advanced myelofibrosis patients.9 A phase 1 study of XL019, another JAK2 inhibitor, has shown that reversible peripheral neuropathy can occur at high doses (> 100 mg).10 Therefore, a better understanding of JAK2 inhibition-induced cell death may lead to the development of more efficient and less toxic therapeutic strategies for treating patients with MPDs.
Recently, our group and others have shown that BH3-only proteins, especially Bim, mediate apoptosis induced by tyrosine kinase inhibitors (TKIs), including imatinib,11 gefitinib,12-15 and mitogen-activated extracellular kinase (MEK) inhibitors.16 In addition, several lines of evidence suggest that there may be a shared common mechanism by which tumor cells driven by most, if not all, oncogenic kinases undergo apoptosis.17 These “oncogene addicted” tumor cells may use Bim as a common mediator during apoptosis induced by multiple TKIs. Therefore, we hypothesized that activation of Bim is necessary for apoptosis induced by JAK2 inhibition in cells carrying JAK2 mutations.
In the present study, we investigated the involvement of Bcl-2 family proteins in JAK2 inhibitor-induced apoptosis. We showed that Bim is a key effector of apoptosis induced by JAK2 inhibition. Moreover, a synthetic BH3 mimetic, ABT-737, potentiated apoptosis induced by JAK inhibitor I in JAK2 mutant cells. Importantly, the combination of JAK inhibitor I and ABT-737 reduced the number of primary JAK2 V617F+ erythropoietin-dependent and independent erythroid colonies derived from CD34+ cells isolated from PV patients. These results show that the combination of ABT-737 and JAK2 inhibitors could be a novel therapeutic strategy in treating patients with activating JAK2 mutations.
Methods
Patients
Informed consent was obtained through an Institutional Review Board–approved protocol by the Beth Israel Deaconess Medical Center in accordance with the Declaration of Helsinki. All patients in this study were followed at Beth Israel Deaconess Medical Center, met the World Health Organization diagnostic criteria for PV, and carried the JAK2 V617F mutation.
Reagents
JAK inhibitor I was purchased from Calbiochem. ABT-73718 was provided by Abbott Laboratories. CEP-701 was purchased from LC Laboratories. All reagents were dissolved in dimethyl sulfoxide (DMSO) and stored at −80°C.
Cell culture
HEL, CHRF-288-11 (CHRF), SET-2, and K562 cells were maintained in RPMI supplemented with 10% fetal bovine serum. Ba/F3 cells expressing murine erythropoietin (Epo) receptor (EpoR; Ba/F3-EpoR), Ba/F3-EpoR cells expressing wild-type (WT) JAK2 (Ba/F3-EpoR-wtJAK2), and Ba/F3-EpoR cells expressing JAK2 V617F (Ba/F3-EpoR-V617F) were maintained in RPMI supplemented with 10% fetal bovine serum and 1 unit/mL Epo. For cytokine starvation, cells were washed 3 times and resuspended in RPMI supplemented with 10% fetal bovine serum in the absence of Epo. Then the cells were collected as indicated.
shRNA constructs and transfection
Bim-specific (pSuper-bim73) and scrambled control (pSuper-bim75) short hairpin RNA (shRNA) constructs19 were transfected, and individual clones were selected by limiting dilution in the presence of 1 mg/mL G418 (Sigma-Aldrich).
In addition, 3 additional Bim shRNA constructs cloned in pLKO.1 vector (Open Biosystems) were used. shRNA sequences are provided in supplemental Figure 4A (available on the Blood website; see the Supplemental Materials link at the top of the online article). Lentiviral production and infection were performed as previously described.20 Cells resistant to 1 μg/mL puromycin were established and maintained.
Western blotting and antibodies
Whole-cell lysates were prepared as previously described.21 Bcl-xL and total STAT5 antibodies were purchased from Santa Cruz Biotechnology. Total extracellular signal–related kinase (ERK) antibody was purchased from BD Transduction Laboratories. Phospho-STAT5 (pTyr694), phospho-Akt (pS473), phospho-ERK 1/2 (pT202/pY204), Bcl-2, phospho-Bad (pS136), Bad, poly(ADP-ribose) polymerase (PARP), cleaved-PARP, Puma, and Akt antibodies were purchased from Cell Signaling Technology. Bim antibody was purchased from Stressgen. Phospho-Bim antibody (pS69) was purchased from Invitrogen. Actin antibody was purchased from Sigma-Aldrich.
Cell proliferation assay
Growth inhibition was assessed in triplicate using 10 000 cells/well by CellTiter 96 AQueous One solution proliferation kit (Promega) as previously described.12 Absorbance of formazan products was measured at 490 nm using the Wallac VICTOR3 (PerkinElmer) spectrophotometer; 50% inhibitory concentration (IC50) was calculated using Kaleidagraph 4.0 software (Synergy Software).
Flow cytometric analysis
Cell-surface exposure of phosphatidylserine after induction of apoptosis was assessed using an annexin V-FLUOS staining kit (Roche Diagnostics) as previously described.12 DNA fragmentation was assessed as previously described22 with minor modifications. Briefly, 1 million cells were permeabilized by fixation with 70% ethanol at −20°C, washed once with phosphate-buffered saline (PBS), and incubated with propidium iodide staining solution (BD Biosciences) for 20 minutes at 25°C. Mitochondrial membrane potential was assessed using 3,3-dihexyloxacarbocyanine iodide (DiOC6(3); Invitrogen) as previously described.12 Briefly, treated cells were washed and incubated with 40nM DiOC6(3) in PBS for 15 minutes at room temperature and analyzed. Bax activation was detected by flow cytometry as previously described.13,23 Briefly, cells were washed in PBS and fixed in 2% formaldehyde for 10 minutes at room temperature. Cells were washed in PBS and incubated in the presence of 1 mg/mL of anti-Bax clone 3 monoclonal antibody (BD Transduction Laboratories) diluted in permeabilization buffer (0.5% bovine serum albumin, 0.1% saponin in PBS) for 45 minutes on ice. Cells were washed in permeabilization buffer and incubated with species-specific Alexa 488–conjugated secondary antibody (Invitrogen), diluted 1: 100 in permeabilization buffer for 30 minutes on ice. Cells were washed in permeabilization buffer, resuspended in PBS, and analyzed using a Cytomics FC500 flow cytometer (Beckman Coulter).
Real-time PCR analysis
The mRNA levels of genes were measured by SYBR Green real-time polymerase chain reaction (PCR) using a Corbett Rotor Gene 6000 sequence detection system (Corbett Life Science). RNA was reverse transcribed, and the resulting cDNA was used in amplification reactions with SYBR Green PCR master mix (Applied Biosystems). The reactions consisted of 1 cycle of 95°C (10 minutes) followed by 40 cycles of 95°C (20 seconds) and 60°C (1 minute). Primers for human Bim; forward primer, 5′-AGTGGGTATTTCTCTTTTGACACAG-3′; reverse primer, 5′-GTCTCCAATACGCCGCAACT-3′. Human Bcl-xL; forward primer, 5′-GTAAACTGGGGTCGCATTGT-3′; reverse primer, 5′-TGCTGCATTGTTCCCATAGA-3′. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH); forward primer, 5′-CCACATCGCTCAGACACCAT-3′; reverse primer, 5′-CCAGGCGCCCAATACG-3′.
To monitor the presence of the JAK2 V617F mutation, erythropoietin-dependent and -independent colonies were individually picked from cultures in semisolid medium at day 14. Genomic DNA was isolated using QIAmp DNA isolation Micro Kit according to the manufacturer's recommendation (QIAGEN). To detect the JAK2 V617F mutation, quantitative real-time PCR was performed as described previously.24 Reactions were performed in technical duplicates. Results were corrected for differences in reaction efficiencies by standard curves using genomic DNA from a mix of HEL and HL-60 cells.
Colony assay
Parental and stably transfected HEL cells were pretreated with JAK inhibitor I as indicated for 24 hours. The cells were washed 3 times, and then 1000 cells/mL of human MethoCult H4230 was plated in duplicate according to the manufacturer's recommendations (StemCell Technologies). Colonies were counted on an inverted microscope after 14 days of incubation.
Peripheral blood samples were obtained from PV patients and healthy volunteers. CD34+ cells were isolated using immunomagnetic beads according to the manufacturer's recommendation (Miltenyi Biotec). For all colony assays conducted, 1000 CD34+ cells/mL of human MethoCult GF H4434 or H4534 were plated in duplicate according to the manufacturer's recommendations. Cultures contained either the JAK inhibitor I alone, ABT-737 alone, or both inhibitors together. Colonies were counted on an inverted microscope after 14 days of incubation. Benzidine staining was carried out to identify endogenous erythroid colonies (EECs) as previously described.25
Statistical analysis
Statistical analysis was done using SPSS 13.0 (SPSS Inc). Differences between the experimental groups were tested with independent-samples t test after normal distribution was confirmed using Kolmogorov-Smirnov testing. P values of less than .05 were considered statistically significant.
Results
Inhibition of JAK2 induces growth inhibition and apoptosis in cells with constitutively activated JAK2
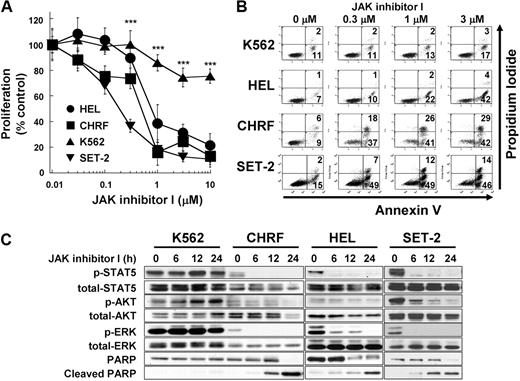
Previous reports have shown that inhibition of JAK2 induces growth inhibition and apoptosis in JAK2 mutant cells in vitro.5,26 To verify these findings, we treated 4 myeloid leukemia cell lines with JAK inhibitor I, which predominantly inhibits JAK2 tyrosine kinase activity. HEL and SET-2 cells harbor JAK2 V617F, and CHRF cells contain the JAK2 T875N mutation. All 3 cell lines show constitutive activation of JAK2.5,26,27 JAK inhibitor I inhibited growth of HEL, CHRF, and SET-2 cells with IC50 of 0.53, 0.36, and 0.14μM, respectively, whereas proliferation of K562 cells, harboring the BCR-ABL fusion protein, was significantly less affected by the treatment with JAK inhibitor I (P < .001, IC50 > 10μM, Figure 1A). The inhibition of proliferation in HEL, CHRF, and SET-2 cells can be explained by JAK2 inhibition-induced apoptosis, detected by cell-surface exposure of phosphatidylserine (Figure 1B) and cleavage of PARP (Figure 1C). JAK inhibitor I treatment led to rapid and sustained inactivation of STAT5, AKT, and ERK in all 3 JAK2 mutant cells, whereas these proteins remained phosphorylated in K562 cells (Figure 1C).
JAK2 inhibition induces growth inhibition and apoptosis in cells with mutated JAK2. (A) Dose-dependent growth inhibition detected by 3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium assay. HEL cells (HEL), SET-2 cells (SET-2), CHRF 288-11 cells (CHRF), and K562 cells (K562) were plated in 96-well plates and treated with increasing doses of JAK inhibitor I (range, 0-10μM) for 48 hours. Data are mean ± SD of at least 4 independent experiments comparing the JAK2 mutated cell lines with the bcr-abl+ cell line K562. JAK2 mutated cell lines versus K562 cells: ***P < .001. (B) Annexin V apoptosis assay. The cells were treated with increasing JAK inhibitor I concentrations (0, 0.3, 1, and 3μM) for 24 hours. Then, cells were harvested, stained with for annexin V and propidium iodide, and analyzed. Data are results from a representative experiment repeated 3 times with similar results. (C) Modulation of signaling after JAK inhibitor I treatment in HEL cells. The cells were treated with 3μM JAK inhibitor I for 0, 6, 12, and 24 hours. Cells were harvested, and extracts were analyzed by Western blotting.
JAK2 inhibition induces growth inhibition and apoptosis in cells with mutated JAK2. (A) Dose-dependent growth inhibition detected by 3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium assay. HEL cells (HEL), SET-2 cells (SET-2), CHRF 288-11 cells (CHRF), and K562 cells (K562) were plated in 96-well plates and treated with increasing doses of JAK inhibitor I (range, 0-10μM) for 48 hours. Data are mean ± SD of at least 4 independent experiments comparing the JAK2 mutated cell lines with the bcr-abl+ cell line K562. JAK2 mutated cell lines versus K562 cells: ***P < .001. (B) Annexin V apoptosis assay. The cells were treated with increasing JAK inhibitor I concentrations (0, 0.3, 1, and 3μM) for 24 hours. Then, cells were harvested, stained with for annexin V and propidium iodide, and analyzed. Data are results from a representative experiment repeated 3 times with similar results. (C) Modulation of signaling after JAK inhibitor I treatment in HEL cells. The cells were treated with 3μM JAK inhibitor I for 0, 6, 12, and 24 hours. Cells were harvested, and extracts were analyzed by Western blotting.
The BH3-only protein Bim is up-regulated during apoptosis induced by inhibition of JAK2 activity
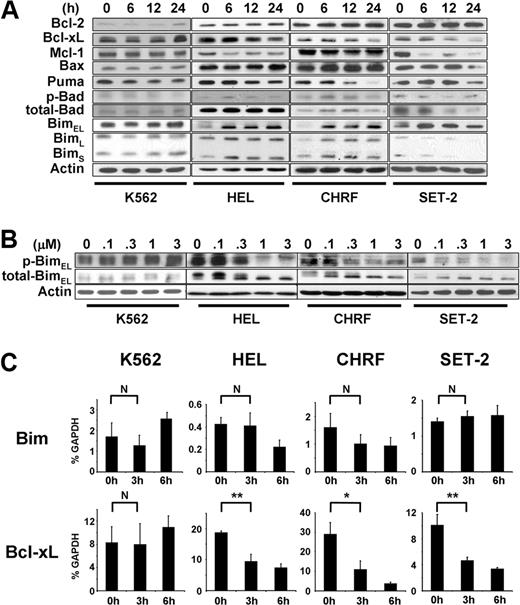
It has been shown that an increase in Bim activity by inhibition of ERK1/2 is critical for apoptosis induced by imatinib,11 gefitinib,12-14 and MEK inhibitors.16 Thus, our observation of rapid inactivation of ERK1/2 after JAK2 inhibition in JAK2 mutant cells but not in K562 cells (Figure 1C) prompted us to test the hypothesis that up-regulation of Bim may be involved in JAK2 inhibition-induced apoptosis as well. The Bim gene encodes 3 major isoforms: Bim short (BimS), Bim long (BimL), and Bim extra long (BimEL).28 Our results show that treatment of JAK2 mutant cells with JAK inhibitor I induced sustained induction of nonphosphorylated, active BimEL (Figure 2A). This induction of Bim was accompanied by a decrease in phosphorylation of ERK1/2 (Figure 1C). Furthermore, it appeared that inactivation of ERK1/2 resulted in up-regulation of active Bim, as we found that BimEL showed a faster migration and nonphosphorylation at serine 69 (Figure 2B). As previously shown, Bim can be phosphorylated by ERK1/2 at serine 69, rendering the protein inactive and susceptible to degradation, which is mediated by RSK1/2 and βTrCP.29-32 Therefore, the nonphosphorylated form of Bim seems to be resistant to proteasomal degradation. Indeed, Bim was more stable when Ba/F3-EpoR cells expressing JAK2 V617F were treated with JAK inhibitor I or 2 MEK/ERK inhibitors, PD98059 or U0126, compared with control cells (supplemental Figure 1). Sustained up-regulation of nonphosphorylated Bim specifically occurred in cell lines carrying activating JAK2 mutations, whereas Bim remained phosphorylated in K562 cells at concentrations as high as 3μM (Figure 2B), without obvious induction of apoptosis (Figure 1B). We found no significant down-regulation of phosphorylated Bad, another member of the BH3-only family, in any of the cell lines with JAK inhibitor I (Figure 2A).
Bim is up-regulated during JAK2 inhibition-induced apoptosis in cells harboring activating JAK2 mutations. (A) Western blot analysis of bcl-2 family proteins. The cells were treated with 3μM JAK inhibitor I for 0, 6, 12, and 24 hours. (B) Dose-response of the JAK inhibitor I on phosphorylation of Bim. The cells were treated with JAK inhibitor I as indicated for 6 hours. (C) Real-time PCR analysis. The cells were treated with 3μM JAK inhibitor I for 0, 3, and 6 hours. Then, cells were harvested, total RNA was extracted, and mRNA levels were assessed by real-time PCR. The mRNA levels were normalized to those of GAPDH using the comparative threshold cycle method. Data are mean ± SD of GAPDH-normalized mRNA expression of 3 independent experiments. Error bars represent SD. *P < .05. **P < .01. N indicates not significant.
Bim is up-regulated during JAK2 inhibition-induced apoptosis in cells harboring activating JAK2 mutations. (A) Western blot analysis of bcl-2 family proteins. The cells were treated with 3μM JAK inhibitor I for 0, 6, 12, and 24 hours. (B) Dose-response of the JAK inhibitor I on phosphorylation of Bim. The cells were treated with JAK inhibitor I as indicated for 6 hours. (C) Real-time PCR analysis. The cells were treated with 3μM JAK inhibitor I for 0, 3, and 6 hours. Then, cells were harvested, total RNA was extracted, and mRNA levels were assessed by real-time PCR. The mRNA levels were normalized to those of GAPDH using the comparative threshold cycle method. Data are mean ± SD of GAPDH-normalized mRNA expression of 3 independent experiments. Error bars represent SD. *P < .05. **P < .01. N indicates not significant.
In addition to the ERK1/2 pathway, Bim can also be induced through the PI3K-AKT pathway. Inhibition of PI3K-AKT leads to dephosphorylation and nuclear entry of the forkhead transcription factor FOXO-3A, which induces Bim mRNA expression.33 To test whether this pathway is also involved in JAK2 inhibition-induced Bim up-regulation, we performed quantitative real-time PCR analysis (Figure 2C). We found that mRNA expression of Bim was not increased in HEL, CHRF, SET-2, or K562 cells treated with JAK inhibitor I for 3 hours. In CHRF cells, Bim mRNA was rather down-regulated by JAK inhibitor I treatment, although this was not statistically significant (Figure 2C). Therefore, it is probable that inactivation of ERK1/2 is the predominant mediator of up-regulation of nonphosphorylated Bim by inhibiting protein degradation.
We also monitored expression of Bcl-2 family members after JAK2 inhibition in K562, HEL, and CHRF cells (Figure 2C). Consistent with a previous report,34 Bcl-xL was significantly down-regulated after JAK2 inhibition both at mRNA (Figure 2C) and protein (Figure 2A) levels only in JAK2 mutant cells. This could be the result of inactivation of STAT3/5 by JAK2 inhibition,34-36 as we detected a significant decrease in phospho-STAT5 levels in HEL, SET-2, and CHRF, but not in K562 cells after JAK2 inhibition (Figure 1C). Puma appeared to be down-regulated after JAK2 inhibitor I treatment in HEL, SET-2, and CHRF cell lines (Figure 2A). Mcl-1 was down-regulated in SET-2 cells (Figure 2A), which may contribute to JAK2 inhibition-induced apoptosis. Bcl-2 and Bax remained unchanged after JAK inhibitor I treatment. Similar results were obtained in HEL cells with another JAK2 inhibitor, CEP-701 (supplemental Figure 2).
These data show that JAK2 inhibition induces down-regulation of the antiapoptotic protein Bcl-xL and up-regulation of the proapoptotic BH3-only protein, Bim, suggesting a key role of these Bcl-2 proteins in JAK2 inhibition-induced apoptosis.
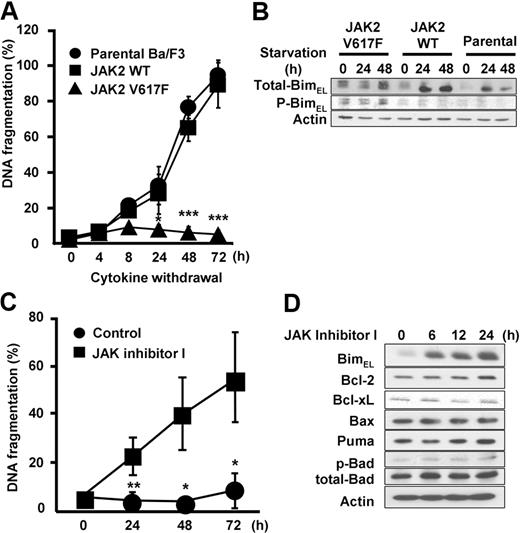
Next, to investigate the regulation of Bim by WT or mutant JAK2, we used Epo-dependent cells expressing either WT or JAK2 V617F (Figure 3).5 Ba/F3-EpoR cells show erythropoietin-dependent growth,37 and expression of JAK2 V617F in Ba/F3-EpoR cells confers erythropoietin-independent survival. Consistent with this observation, we observed that withdrawal of Epo led to significant induction of apoptosis in parental Ba/F3-EpoR and Ba/F3-EpoR-wtJAK2, whereas Ba/F3-EpoR-V617F cells did not show increased numbers of apoptotic cells in culture over the monitored period of 72 hours (Figure 3A). Western blots demonstrated that nonphosphorylated Bim was up-regulated in Ba/F3-EpoR and Ba/F3-EpoR-wtJAK2 cells, but Bim remained phosphorylated and not induced in Ba/F3-EpoR-V617F cells (Figure 3B). Next, we asked whether suppression of JAK2 V617F would induce Bim expression and apoptosis in this system as well. As shown in Figure 3C, treatment of Ba/F3-EpoR-V617F cells with JAK inhibitor I resulted in a significant increase of apoptosis after 24 to 72 hours. BimEL was up-regulated as early as 6 hours after treatment (Figure 3D). Whereas Bcl-xL expression slightly decreased, we did not observe changes in the expression of Bcl-2, Bax, Puma, or Bad in Ba/F3-EpoR-V617F cells (Figure 3D). These results suggest that constitutively activated JAK2 V617F is responsible for preventing Bim induction and apoptosis.
Induction of Bim is regulated by JAK2 kinase activity. (A) Apoptosis induced by Epo withdrawal. Ba/F3-EpoR cells (parental), Ba/F3-EpoR-wtJAK2, and Ba/F3-EpoR-V617F cells were washed 3 times with RPMI only and resuspended in RPMI supplemented with 10% fetal calf serum in the absence of Epo for the indicated hours. Cells were harvested, and apoptosis was measured for incorporation of propidium iodide by flow cytometry. Data are mean ± SD of 3 independent experiments. JAK2 WT versus JAK2 V617F: *P < .05, ***P < .001. (B) Western blot analysis of total-Bim and phospho-Bim. Ba/F3 stable cells was cultured in the presence of Epo for 0, 12, and 24 hours. (C) Apoptosis induced by JAK2 V617F inhibition. Ba/F3-EpoR-V617F cells were treated with either 3μM JAK inhibitor I or 0.1% DMSO (control) for 0, 24, 48, and 72 hours in RPMI supplemented with 10% fetal calf serum. Cells were harvested and apoptosis was assessed by flow cytometry. Data are mean ± SD of 3 independent experiments. Control versus JAK inhibitor I: *P < .05, **P < .01. (D) Induction of Bim by JAK2 V617F inhibition. Ba/F3-EpoR-V617F cells were treated with either 3μM JAK inhibitor I or 0.1% DMSO (control) for 0, 6, 12, and 24 hours. Cells were harvested and analyzed by Western blot.
Induction of Bim is regulated by JAK2 kinase activity. (A) Apoptosis induced by Epo withdrawal. Ba/F3-EpoR cells (parental), Ba/F3-EpoR-wtJAK2, and Ba/F3-EpoR-V617F cells were washed 3 times with RPMI only and resuspended in RPMI supplemented with 10% fetal calf serum in the absence of Epo for the indicated hours. Cells were harvested, and apoptosis was measured for incorporation of propidium iodide by flow cytometry. Data are mean ± SD of 3 independent experiments. JAK2 WT versus JAK2 V617F: *P < .05, ***P < .001. (B) Western blot analysis of total-Bim and phospho-Bim. Ba/F3 stable cells was cultured in the presence of Epo for 0, 12, and 24 hours. (C) Apoptosis induced by JAK2 V617F inhibition. Ba/F3-EpoR-V617F cells were treated with either 3μM JAK inhibitor I or 0.1% DMSO (control) for 0, 24, 48, and 72 hours in RPMI supplemented with 10% fetal calf serum. Cells were harvested and apoptosis was assessed by flow cytometry. Data are mean ± SD of 3 independent experiments. Control versus JAK inhibitor I: *P < .05, **P < .01. (D) Induction of Bim by JAK2 V617F inhibition. Ba/F3-EpoR-V617F cells were treated with either 3μM JAK inhibitor I or 0.1% DMSO (control) for 0, 6, 12, and 24 hours. Cells were harvested and analyzed by Western blot.
Knockdown of Bim inhibits apoptosis induced by JAK2 inhibition in HEL cells
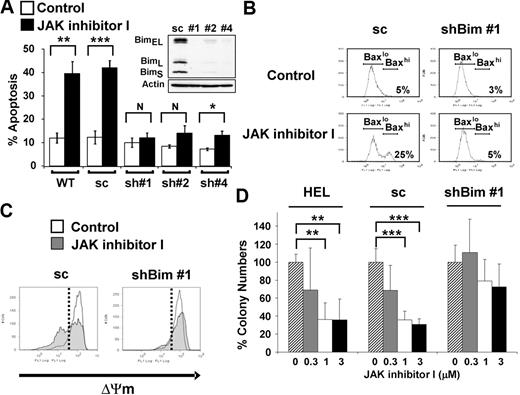
Next, we tested whether Bim activity is necessary for apoptosis induced by JAK2 inhibition by assessing the effects of Bim knockdown in HEL cells (Figure 4). We transfected HEL cells with an shRNA construct against Bim (pSuper-bim73),19 and individual clones were selected by limiting dilution. Three individual clones of stably transfected HEL cells (sh#1, sh#2, and sh#4) showed significant lower Bim expression at the protein level compared with HEL cells stably transfected with a construct (pSuper-bim75)19 expressing the scrambled shRNA (sc) sequence (Figure 4A inset). As shown in Figure 4A, apoptosis induced by JAK inhibitor I was significantly attenuated in all 3 knockdown clones (P < .01, comparing each clone either with WT or sc). In particular, shBim #1 cells, which represented the maximal stable knockdown of Bim, showed no significant difference in cell death between DMSO-treated and JAK inhibitor I-treated cells. The resistance to JAK inhibitor I in shBim #1 cells was observed for up to 72 hours (supplemental Figure 3). To exclude the possibility of off-target effects, we used 3 additional shRNA constructs targeting Bim mRNA (#1051, #1053, and #1054) to verify the effect of Bim knockdown on JAK2 inhibition-induced apoptosis. As shown in supplemental Figure 4, 2 of the 3 knockdown cells (#1051 and #1054) showed decreased apoptosis induced by JAK inhibitor I.
Knockdown of Bim expression leads to attenuation of apoptosis induced by JAK2 inhibition. (A) Annexin V apoptosis assay. WT HEL cells and HEL cells stably transfected with vector constitutively expressing either scrambled (pSuper-bim75, 1 stably transfected clone analyzed: sc) or shRNA sequences targeting Bim (pSuper-bim73; 3 stably transfected clones analyzed: sh#1, sh#2, sh#4) were treated with either DMSO or 3μM of JAK inhibitor I for 24 hours. Data are mean ± SD of annexin V+ cells from 5 independent experiments. Error bars represent SD. *P < .05. **P < .01. ***P < .001. N indicates not significant. (Inset) Expression analysis of knockdown of all isoforms of Bim (BimEL, BimL, BimS) in shBim #1, shBim #2, and shBim #4 cells compared with scrambled shRNA (sc) transfected cells by Western blot. (B) Flow cytometric analysis for Bax activation. The HEL cells stably transfected with shRNA targeting Bim (clone: shBim #1) or scrambled shRNA (sc) were treated with or without 3μM JAK inhibitor I for 18 hours. Data are results from a representative experiment repeated 3 times with similar results. (C) Flow cytometric analysis of the inner mitochondrial membrane potential (ΔΨm) breakdown. The HEL cells stably transfected with vectors constitutively expressing either shRNA targeting Bim (clone: shBim #1) or scrambled shRNA (sc) were treated with or without 3μM JAK inhibitor I for 24 hours. Data are results from a representative experiment repeated 3 times with similar results. (D) Colony-forming assay. The parental HEL cells (HEL), the HEL cells stably transfected with shRNA targeting Bim (clone: shBim #1; shBim), and scrambled shRNA (sc) were pretreated with JAK inhibitor I and plated in human MethoCult H4230. Data are mean plus or minus SD of colony numbers expressed as percentage of DMSO-treated cultures. Error bars represent SD (n = 3). **P < .01. ***P < .001.
Knockdown of Bim expression leads to attenuation of apoptosis induced by JAK2 inhibition. (A) Annexin V apoptosis assay. WT HEL cells and HEL cells stably transfected with vector constitutively expressing either scrambled (pSuper-bim75, 1 stably transfected clone analyzed: sc) or shRNA sequences targeting Bim (pSuper-bim73; 3 stably transfected clones analyzed: sh#1, sh#2, sh#4) were treated with either DMSO or 3μM of JAK inhibitor I for 24 hours. Data are mean ± SD of annexin V+ cells from 5 independent experiments. Error bars represent SD. *P < .05. **P < .01. ***P < .001. N indicates not significant. (Inset) Expression analysis of knockdown of all isoforms of Bim (BimEL, BimL, BimS) in shBim #1, shBim #2, and shBim #4 cells compared with scrambled shRNA (sc) transfected cells by Western blot. (B) Flow cytometric analysis for Bax activation. The HEL cells stably transfected with shRNA targeting Bim (clone: shBim #1) or scrambled shRNA (sc) were treated with or without 3μM JAK inhibitor I for 18 hours. Data are results from a representative experiment repeated 3 times with similar results. (C) Flow cytometric analysis of the inner mitochondrial membrane potential (ΔΨm) breakdown. The HEL cells stably transfected with vectors constitutively expressing either shRNA targeting Bim (clone: shBim #1) or scrambled shRNA (sc) were treated with or without 3μM JAK inhibitor I for 24 hours. Data are results from a representative experiment repeated 3 times with similar results. (D) Colony-forming assay. The parental HEL cells (HEL), the HEL cells stably transfected with shRNA targeting Bim (clone: shBim #1; shBim), and scrambled shRNA (sc) were pretreated with JAK inhibitor I and plated in human MethoCult H4230. Data are mean plus or minus SD of colony numbers expressed as percentage of DMSO-treated cultures. Error bars represent SD (n = 3). **P < .01. ***P < .001.
BH3-only proteins, including Bim, bind to and inactivate Bcl-2 or Bcl-xL proteins, keeping them from restraining Bax or Bak, which can permeabilize the mitochondrial outer membrane and initiate caspase activation.38 To explore the consequences of inhibition of Bim up-regulation on the mitochondrial pathway, we examined whether Bax is activated on JAK inhibitor I treatment. Knockdown of Bim prevented Bax activation on JAK inhibitor I treatment (Figure 4B). In addition, JAK inhibitor I failed to cause breakdown of the inner mitochondrial membrane potential (ΔΨm), which is caused by a sudden increase in permeability of the mitochondrial membrane, in shBim #1 cells (Figure 4C). To assess whether Bim is required for clonogenic survival, we characterized the colony-forming capacity of HEL shBim #1, HEL sc and parental HEL cells in semisolid medium (Figure 4D). Our results show that Bim knockdown led to an increased colony formation when cells were pretreated with JAK inhibitor I. Compared with the DMSO-treated control cells, parental HEL (mean ± SD, 36% ± 23%, P < .01) and HEL sc cells (mean ± SD, 31% ± 6%, P < .01) showed a significant reduction of colony numbers at 3μM. The colony number from Bim knockdown shBim #1 cells was not significantly reduced even on treatment with 3μM JAK inhibitor I (mean ± SD, 73% ± 26%, P = .07). Taken together, our data show that Bim plays an important role in the mitochondrial apoptotic pathway induced by inhibition of JAK2 in JAK2 V617F+ cells.
Resistance to apoptosis caused by Bim knockdown is reversed by the BH3 mimetic ABT-737
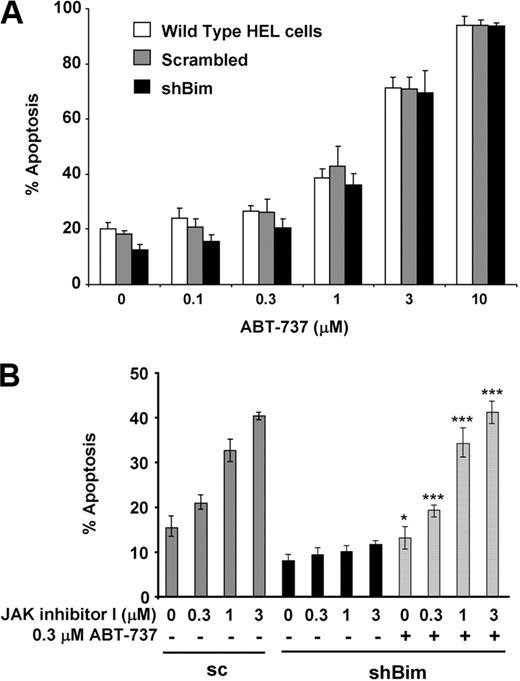
ABT-737 is a synthetic small-molecule inhibitor produced by structure-based drug design, which strongly binds to Bcl-2, Bcl-xL, and Bcl-w.18 In principle, BH3 mimetics, such as ABT-737, behave like BH3-only proteins. Therefore, we tested whether ABT-737 reverses resistance to apoptosis in Bim knockdown HEL cells. As shown in Figure 5A, ABT-737 treatment induced apoptosis in a dose-dependent manner in cells expressing Bim at normal levels as well as in Bim knockdown cells.
ABT-737 reverses resistance to apoptosis caused by Bim knockdown. (A) WT HEL cells (open bars) and HEL cells stably transfected with vectors constitutively expressing either scrambled (pSuper-bim75; 1 stably transfected clone analyzed: Scrambled; gray bars) or shRNA sequences targeting Bim (pSuper-bim73, clone #1: shBim; black bars) were treated with increasing doses of ABT-737 for 24 hours. Apoptosis was assessed by an annexin V assay. Data are mean ± SD of annexin V+ cells. Error bars represent SD (n = 3). (B) HEL cells stably transfected with pSuper-bim73 constitutively expressing scrambled shRNA (sc) were treated with increasing doses of JAK inhibitor I in the absence of ABT-737 (gray bars). HEL cells stably transfected with pSuper-bim75 (clone #1), constitutively expressing shRNA targeting Bim (shBim), were treated with increasing doses of JAK inhibitor I in the absence (black bars) or presence (hatched bars) of 0.3μM ABT-737 for 24 hours. Apoptosis was assessed by an annexin V assay. Data are mean ± SD of annexin V+ cells. Error bars represent SD (n = 3). JAK inhibitor alone versus JAK inhibitor I and ABT-737: *P < .05, ***P < .001.
ABT-737 reverses resistance to apoptosis caused by Bim knockdown. (A) WT HEL cells (open bars) and HEL cells stably transfected with vectors constitutively expressing either scrambled (pSuper-bim75; 1 stably transfected clone analyzed: Scrambled; gray bars) or shRNA sequences targeting Bim (pSuper-bim73, clone #1: shBim; black bars) were treated with increasing doses of ABT-737 for 24 hours. Apoptosis was assessed by an annexin V assay. Data are mean ± SD of annexin V+ cells. Error bars represent SD (n = 3). (B) HEL cells stably transfected with pSuper-bim73 constitutively expressing scrambled shRNA (sc) were treated with increasing doses of JAK inhibitor I in the absence of ABT-737 (gray bars). HEL cells stably transfected with pSuper-bim75 (clone #1), constitutively expressing shRNA targeting Bim (shBim), were treated with increasing doses of JAK inhibitor I in the absence (black bars) or presence (hatched bars) of 0.3μM ABT-737 for 24 hours. Apoptosis was assessed by an annexin V assay. Data are mean ± SD of annexin V+ cells. Error bars represent SD (n = 3). JAK inhibitor alone versus JAK inhibitor I and ABT-737: *P < .05, ***P < .001.
Previously, we and others have shown that activation of Bim is reduced in gefitinib-resistant non–small cell lung cancer cells12-15 or imatinib-resistant CML cells.11 Interestingly, resistance to apoptosis caused by loss of Bim is overcome by a combination of ABT-737 and imatinib in CML cells.11 In addition, it has been shown that ABT-737 can sensitize cells to various chemotherapeutic agents.38 Thus, we hypothesized that low doses of ABT-737 could resensitize Bim knockdown cells to JAK inhibitor I. The JAK inhibitor I-exposed cells were cotreated with 0.3μM ABT-737, which did not induce apoptosis on its own at this dose level (Figure 5A). As shown in Figure 5B, addition of ABT-737 fully restored induction of apoptosis by JAK inhibitor I treatment, whereas JAK inhibitor I alone had minimal effect on inducing apoptosis in Bim knockdown cells. These results suggest that ABT-737 binds to and antagonizes antiapoptotic Bcl-2 family proteins and thereby renders cells more susceptible to apoptotic signals. These results also suggest that Bim activity is specifically required for induction of JAK2 inhibition-induced apoptosis in JAK2 mutant cells.
ABT-737 enhances the effect of JAK2 inhibition both in JAK2 V617F+ cell lines and primary CD34+ hematopoietic progenitor cells from PV patients
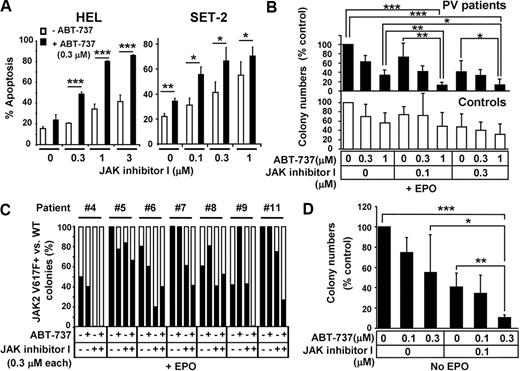
Based on observations that ABT-737 enhances the effect of TKIs,13-16 we investigated whether addition of ABT-737 could enhance JAK2 inhibition-induced apoptosis in cells with JAK2 mutations. As shown in Figure 6A, ABT-737 significantly enhanced apoptosis induced by JAK inhibitor I in HEL and SET-2 cells. Next, we investigated the effect of ABT-737 and JAK inhibitor I treatment on primary cells carrying mutant JAK2. CD34+ hematopoietic stem/progenitor cells isolated from normal or PV patients were treated with various combinations of JAK inhibitor I and ABT-737, and colony growth in the presence or absence of Epo was assessed (Figure 6B-D; supplemental Tables 1-2). Epo-dependent colony formation from PV patients (Figure 6B top panel) showed that 0.1μM JAK inhibitor I did not significantly reduce colony formation compared with DMSO (P = .08). However, the combination of 0.1μM JAK inhibitor I and 1μM ABT-737 significantly inhibited colony formation compared with either DMSO (P < .001), 0.1μM JAK inhibitor I (P < .01), or 1μM ABT-737 (P < .01) alone. Similarly, the combination of 0.3μM JAK inhibitor I and 1μM ABT-737 was significantly more potent than DMSO (P < .001), 0.3μM JAK inhibitor I (P < .05), or 1μM ABT-737 (P < .05) alone. In contrast, treatment with 1μM ABT-737 failed to enhance reduction of erythroid colonies induced by 0.1 or 0.3μM JAK inhibitor I in normal CD34+ cells (P = .14 and P = .34, respectively; Figure 6B lower panel). We also monitored the frequency of the JAK2 V617F mutation by allelic real-time PCR in individually isolated colonies grown in presence of Epo to determine whether addition of ABT-737 enhanced JAK inhibitor I-induced reduction of JAK2 V617F+ erythroid colony numbers. Combination treatment of 0.3μM ABT-737 and 0.3μM JAK inhibitor I reduced the frequency of JAK2 V617F+ colonies more efficiently than treatment with JAK inhibitor I alone in 4 of 7 PV patients tested (PV #5, #7, #9, and #11; Figure 6C). However, it is difficult to conclude that the combination was more efficient with mutated than normal colonies from these experiments. The colonies treated with 1μM ABT-737 in combination with either 0.1 or 0.3μM JAK inhibitor I were small and dysmorphic, and we failed to obtain sufficient amounts of genomic DNA from these colonies for allele-specific PCR (data not shown).
ABT-737 enhances the effect of JAK inhibitor I on HEL cells and primary CD34+ cells isolated from PV patients. (A) Annexin V assay. HEL and SET-2 cells were treated with increased doses of JAK inhibitor I in the absence (open bars) or presence (black bars) of 0.3μM of ABT-737 for 24 hours. Then, cells were harvested and apoptosis was measured by flow cytometry. Data are mean ± SD of annexin V+ cells. Error bars represent SD (n = 3). *P < .05. **P < .01. ***P < .001. (B) Colony formation assay of primary CD34+ cells in the presence of Epo. CD34+ cells were isolated from JAK2 V617F+ PV patients (top panel, black bars) and healthy volunteers (bottom panel, open bars). Cells were seeded in methylcellulose medium containing Epo (H4434) and various concentrations of ABT-737 (0, 0.3, and 1μM) and JAK inhibitor I (0, 0.1, and 0.3μM), where indicated. Erythroid colonies were scored after 14 days. Data are the mean ± SD of erythroid colony numbers expressed as a percentage of DMSO-treated cultures. Error bars represent SD of PV patients (n = 5) and healthy controls (n = 5). *P < .05. **P < .01. ***P < .001. (C) Assessment of JAK2 V617F mutation frequency in colony-forming cells. CD34+ cells were isolated from 7 JAK2 V617F+ PV patients. Cells were seeded in methylcellulose medium in the presence of Epo (H4434) and/or 0.3μM of ABT-737 and 0.3μM of JAK inhibitor I, where indicated. After 14 days of culture, individual erythroid colonies were isolated from each plate and genomic DNA was extracted. Quantitative real-time PCR was used to detect the presence of JAK2 V617F. Black bars represent the percentage of JAK2 V617F+ colonies; open bars, percentage of JAK2 WT colonies in cultured cells as detected in 4 to 8 genomic DNA samples/condition from informative PCRs (samples showing signals detectable in the JAK2 V617F and/or the JAK2 WT reaction were considered informative). (D) Colony formation assay in the absence of Epo. CD34+ cells were isolated from JAK2 V617F+ PV patients. Cells were seeded in methylcellulose medium lacking Epo (H4534) in the presence or absence of indicated concentrations of ABT-737 and/or JAK inhibitor I. Independent EECs were counted based on benzidine staining (supplemental Figure 5). Data are mean ± SD of EEC colony numbers expressed as percentage of DMSO-treated cultures. Error bars represent SD (n = 5). *P < .05. **P < .01. ***P < .001.
ABT-737 enhances the effect of JAK inhibitor I on HEL cells and primary CD34+ cells isolated from PV patients. (A) Annexin V assay. HEL and SET-2 cells were treated with increased doses of JAK inhibitor I in the absence (open bars) or presence (black bars) of 0.3μM of ABT-737 for 24 hours. Then, cells were harvested and apoptosis was measured by flow cytometry. Data are mean ± SD of annexin V+ cells. Error bars represent SD (n = 3). *P < .05. **P < .01. ***P < .001. (B) Colony formation assay of primary CD34+ cells in the presence of Epo. CD34+ cells were isolated from JAK2 V617F+ PV patients (top panel, black bars) and healthy volunteers (bottom panel, open bars). Cells were seeded in methylcellulose medium containing Epo (H4434) and various concentrations of ABT-737 (0, 0.3, and 1μM) and JAK inhibitor I (0, 0.1, and 0.3μM), where indicated. Erythroid colonies were scored after 14 days. Data are the mean ± SD of erythroid colony numbers expressed as a percentage of DMSO-treated cultures. Error bars represent SD of PV patients (n = 5) and healthy controls (n = 5). *P < .05. **P < .01. ***P < .001. (C) Assessment of JAK2 V617F mutation frequency in colony-forming cells. CD34+ cells were isolated from 7 JAK2 V617F+ PV patients. Cells were seeded in methylcellulose medium in the presence of Epo (H4434) and/or 0.3μM of ABT-737 and 0.3μM of JAK inhibitor I, where indicated. After 14 days of culture, individual erythroid colonies were isolated from each plate and genomic DNA was extracted. Quantitative real-time PCR was used to detect the presence of JAK2 V617F. Black bars represent the percentage of JAK2 V617F+ colonies; open bars, percentage of JAK2 WT colonies in cultured cells as detected in 4 to 8 genomic DNA samples/condition from informative PCRs (samples showing signals detectable in the JAK2 V617F and/or the JAK2 WT reaction were considered informative). (D) Colony formation assay in the absence of Epo. CD34+ cells were isolated from JAK2 V617F+ PV patients. Cells were seeded in methylcellulose medium lacking Epo (H4534) in the presence or absence of indicated concentrations of ABT-737 and/or JAK inhibitor I. Independent EECs were counted based on benzidine staining (supplemental Figure 5). Data are mean ± SD of EEC colony numbers expressed as percentage of DMSO-treated cultures. Error bars represent SD (n = 5). *P < .05. **P < .01. ***P < .001.
Because constitutive activation of JAK2 drives cell growth in the absence of Epo,39,40 we also assessed the effect of JAK inhibitor I alone and in combination with ABT-737 on cytokine-independent colony growth of CD34+ cells isolated from JAK2 V617F-carrying patients. Patient-derived CD34+ cells were subjected to combinations of JAK inhibitor I (0 and 0.1μM) and ABT-737 (0, 0.1, and 0.3μM) and EEC growth in the absence of Epo was monitored (Figure 6D). EECs were confirmed by benzidine staining (supplemental Figure 5)25 and by allelic real-time PCR detecting the JAK2 V617F mutation in all individually isolated EECs monitored (data not shown). EEC formation in PV patients (supplemental Table 2) demonstrated a 59% (mean ± SD, 40.8% ± 13.9%, P < .001) reduction when cells were treated with 0.1μM JAK inhibitor I and a 45% (mean ± SD, 55% ± 37.4%, P < .01) reduction when treated with 0.3μM ABT-737 alone, compared with DMSO-treated cells (Figure 6D). Notably, the most efficient reduction in EEC numbers was achieved by treating cells with 0.1μM JAK inhibitor I and 0.3μM ABT-737 simultaneously, resulting in a 89% (mean ± SD, 10.8% ± 2.2%, P < .001) reduction of cytokine-independent growth of mutant cells, compared with DMSO-treated cells (Figure 6D). This combination treatment was significantly more effective than JAK inhibitor I or ABT-737 alone in suppressing growth of mutant cells (P < .01 and P < .05, respectively, Figure 6D). Taken together, these results show that ABT-737 is able to enhance the effects of JAK2 inhibition in JAK2 V617F mutant cells.
Discussion
PV and other MPDs have been associated with gain-of-function mutations in JAK2, most commonly V617F.2-6 Sustained activation of JAK2 and its downstream pathways leads to uncontrolled growth and a block of apoptosis, supporting a rationale for targeting JAK2 mutant cells as a therapeutic strategy in MPD. Currently, there are various selective JAK inhibitors under development and investigation in phase 1 and 2 clinical trials. However, the complete block of JAK2 may lead to suppression of normal hematopoiesis as well.8,41 Therefore, better understanding of JAK2 inhibition-induced cell death may lead to the development of more efficient and less toxic therapeutic strategies for treating patients with MPD carrying activating JAK2 mutations.
In this study, we confirmed previous results5,26 that JAK inhibitor I impairs proliferation and induces apoptosis in JAK2 mutant cell lines. Moreover, we were able to demonstrate that JAK2 inhibition initiated the intrinsic mitochondrial pathway of apoptosis in JAK2 mutant cell lines, accompanied by up-regulation of the active, nonphosphorylated form of Bim. Importantly, knockdown of Bim abrogated apoptosis induced by JAK inhibitor I treatment, which was reversed by the BH3 mimetic ABT-737. Furthermore, we have shown that ABT-737 was able to augment apoptosis induced by JAK inhibitor I in JAK2 mutant cells. Finally, ABT-737 enhanced the suppression of Epo-dependent and Epo-independent colony growth and also reduced the frequency of JAK2 V617F+ colony-forming progenitors by JAK inhibitor I treatment of primary, patient-derived hematopoietic progenitor cells.
The Bcl-2 family proteins regulate the intrinsic mitochondrial apoptosis pathway and compose 3 subgroups based on structure and function: the proapoptotic Bax- and Bak-like proteins, the antiapoptotic Bcl-2 proteins, and the BH3-only proteins. The BH3-only proteins, especially Bim, initiate apoptosis signaling by binding and antagonizing the prosurvival Bcl-2 proteins, thereby releasing inhibition of the proapoptotic Bax and Bak proteins, which then cause apoptosis.42,43
Bim is regulated by multiple stimuli, including the PI3K-AKT-FOXO-3A and the ERK 1/2 MAP kinase pathways, both of which can be activated by JAK signaling.44 We failed to see up-regulation of Bim mRNA after JAK inhibitor I treatment of mutant JAK2 cells, suggesting that the AKT-FOXO-3A pathway may not play an important role in Bim up-regulation in these cells. However, JAK inhibitor I strongly inhibited ERK 1/2 phosphorylation in HEL cells and dephosphorylated Bim at an ERK phosphorylation site. In addition, Bim in its nonphosphorylated form promotes its rapid dissociation from Bcl-xL or Mcl-1.45 Therefore, inhibition of the ERK signal after JAK2 inhibition not only prevents Bim degradation but also increases its activity. Thus, it appears that ERK inactivation is the dominant contributor to the activation of Bim by JAK2 inhibition. A key role of Bim in JAK2 inhibition-induced apoptosis is supported by our Bim knockdown experiments, which demonstrated that Bim knockdown caused complete resistance to apoptosis after JAK inhibitor I treatment in a cell line carrying the activating mutation JAK2 V617F.
ABT-737 acts like or “mimics” BH3-only proteins,38 and our data showed that this BH3 mimetic was able to reverse the resistance to JAK inhibitor I in Bim knockdown cells. It could be speculated that more ABT-737 is required to achieve endogenous Bim concentrations that antagonize antiapoptotic Bcl-2 proteins, and ultimately initiate the apoptotic process in Bim knockdown cells.
The antiapoptotic Bcl-2 protein Bcl-xL is transcriptionally regulated by STAT3/5 and overexpressed in erythroid cells from patients with PV.46 In addition, STAT5 and Bcl-xL can induce erythroid colony formation from erythroid precursors in the absence of erythropoietin.39 In addition, a novel JAK2 inhibitor, AZ960, inhibits phosphorylation of STAT5, down-regulates Bcl-xL, and induces apoptosis.34 Consistent with these studies, we observed that JAK inhibitor I dephosphorylated STAT5 and down-regulated the expression of Bcl-xL. Because knockdown of Bcl-xL also leads to apoptosis in JAK2 mutant cells,34 it is possible that the apoptotic process may be initiated when Bim exceeds the point where it neutralizes all prosurvival Bcl-2 family members, including Bcl-xL. Indeed, in our Bim knockdown cells, ABT-737 at a nonapoptotic dose “primed” these cells to the apoptotic effects of JAK inhibitor I treatment that had been lost with the absence of a functional Bim signal. In this setting, ABT-737 may bind to and antagonize prosurvival Bcl-2 family proteins, such as Bcl-xL, with subsequent inactivation of JAK2 leading to further decreases in Bcl-xL that eventually trigger the apoptotic machinery. Therefore, our results suggest that the balance of Bim/Bcl-xL may be critical for induction of apoptosis caused by JAK2 inhibition.
ABT-737 has recently been reported to induce cell death in PV,47 albeit at high doses that may not be achievable in vivo. However, lower doses of BH3 mimetics, such as ABT-737, could increase the ratio of BH3-only proteins to antiapoptotic Bcl-2 family members sufficiently to enhance apoptosis induced by JAK2 tyrosine kinase inhibitors in PV. Analogous hypotheses have been verified in cases of the epidermal growth factor receptor inhibitor gefitinib, the BCR-ABL inhibitor imatinib, and MEK inhibitors in other oncogene-driven cancers.11-14,16 In the present study, we demonstrated the enhanced efficacy of ABT-737 in combination with JAK2 inhibition in cell lines and primary CD34+ hematopoietic progenitor cells from PV patients carrying mutant JAK2. Our data suggest that modulating Bcl-2 family members could be a potential therapeutic target in JAK2 mutant cells. This could be especially useful in MPD patients with mutated JAK2, as the combination treatment with a JAK inhibitor and a BH3 mimetic could decrease the doses required for efficacy of each individual compound, and thereby limit adverse side effects, such as significant cytopenias. Studies with larger numbers of patients will be necessary to further confirm this hypothesis.
In conclusion, the data presented here indicate that Bim is a key mediator of apoptosis caused by JAK2 inhibition in cells carrying constitutively activated forms of JAK2. Modulating Bcl-2 family members, with the use of BH3 mimetics, such as ABT-737, in combination with JAK2 inhibitors may be a promising novel strategy for the treatment of MPD patients with mutant JAK2.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Philippe Bouillet (Walter and Eliza Hall Institute) for providing Bim shRNA constructs, Dr Kimiharu Uozumi (Kagoshima University) for providing SET-2 cells, Drs Priya Koppikar and Ross Levine (Memorial Sloan-Kettering Cancer Center) for providing Ba/F3 stable cells, Dr Ann Mullally (Brigham and Women's Hospital) for helpful discussion, and Natasha Sng and Daniel Schanne for technical assistance. ABT-737 was kindly provided by Abbott Laboratories.
This work was supported by National Institutes of Health (grant R00CA126026, S.K.; grant R00CA131503, U.S.; and grants CA66996 and CA118316, D.G.T.), American Association for Cancer Research–AstraZeneca-Cancer Research (D.B.C), Dana-Farber Cancer Institute Claudia Adams Barr Program in Innovative Basic Cancer Research (T. Shimamura), and a Predoctoral Fellowship from German José Carreras Leukemia Foundation (B.W.).
National Institutes of Health
Authorship
Contribution: B.W., T. Siddiqi, D.B.C., U.S., D.G.T., and S.K. designed and conducted experiments and analyzed data; M.A.J., T. Shimamura, K.L., and P.B.S. conducted experiments and analyzed data; and B.W., T. Siddiqi, D.B.C., and S.K. wrote the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susumu Kobayashi, Division of Hematology/Oncology, Beth Israel Deaconess Medical Center, E/CLS-0430, 330 Brookline Ave, Boston, MA 02215; e-mail: skobayas@bidmc.harvard.edu.
References
Author notes
B.W. and T. Siddiqi contributed equally to this study.