The vascular pathobiology of sickle cell anemia involves inflammation, coagulation, vascular stasis, reperfusion injury, iron-based oxidative biochemistry, deficient nitric oxide (NO) bioavailability, and red cell sickling. These disparate pathobiologies intersect and overlap, so it is probable that multimodality therapy will be necessary for this disease. We have, therefore, tested a histone deacetylase (HDAC) inhibitor, trichostatin A (TSA), for efficacy in reducing endothelial activation. We found that pulmonary vascular endothelial VCAM-1 and tissue factor (TF) expression (both are indicators of endothelial activation) are powerfully and significantly inhibited by TSA. This is seen both with pretreatment before the inducing stress of hypoxia/reoxygenation (NY1DD sickle transgenic mouse), and upon longer-term therapy after endothelial activation has already occurred (hBERK1 sickle mouse at ambient air). In addition, TSA prevented vascular stasis in sickle mice, it exhibited activity as an iron chelator, and it induced expression of the antisickling hemoglobin, hemoglobin F. Notably, the TSA analog SAHA (suberoylanilide hydroxaminc acid) that is already approved for human clinical use exhibits the same spectrum of biologic effects as TSA. We suggest that SAHA possibly could provide true, multimodality, salubrious effects for prevention and treatment of the chronic vasculopathy of sickle cell anemia.
Introduction
The vascular pathobiology of sickle cell anemia is strikingly complex and involves multiple pathobiologies that intersect and often overlap. These include not only red cell sickling, but also reperfusion injury physiology, endothelial activation and dysfunction, a systemic inflammatory state with oxidative stress and abnormal endothelial adhesion biology, defective vasoregulation with biodeficient nitric oxide (NO), and marked activation of the coagulation system.1 It is notable that available data indicate that this situation is true for both sickle human and sickle transgenic mouse.1 In an attempt to organize these disparate pathobiologies into a logical sequence, we have argued that the proximate stimulus for all of this is very likely to be the near-constant occurrence of reperfusion injury physiology. There is not only a strong conceptual argument to support this,1 but also experimental evidence.2,3 It is likely initiated by proximate abnormal endothelial adhesion of white and red blood cells as well as the sickling phenomenon, with consequent reversible stasis and occlusion.1
This complexity of sickle disease pathobiology has led to the concept that only a chemotherapy-like model of multimodality therapy is likely to be of maximal clinical benefit in this disease.4 In this regard, it would be most cost-effective, safe, and tolerable if the needed multimodality therapy could be provided by a single agent. To that end, we have performed the present study of the experimental compound, trichostatin A (TSA), in sickle transgenic mice.
TSA is a selective and reversible hydroxamate inhibitor of the class I and II HDACs (histone deacetylases).5 By inhibiting these enzymes, it causes increased histone acetylation, which opens promoter loci to facilitate interaction with transcription factors. In addition, it alters acetylation of some transcription factors. These effects cause altered expression of multiple genes. Overall, the HDAC inhibitors include short-chain fatty acids, hydroxamates, cyclic tetrapeptides, benzamides, and some miscellaneous types.5 However, only class I and II HDAC inhibitors, of which TSA is the paradigmatic representative, specifically inhibit the HDAC enzymes. Butyrate, for example, inhibits HDAC only nonspecifically. TSA is observed to be nontoxic to adult mice, and importantly, does not disturb postnatal or embryonic development of mice.5 In cell culture systems, TSA inhibits a limited set of genes, but particularly affects cell-cycle, growth, and apoptosis genes, hence the current clinical interest in such drugs for cancer therapy. It also inhibits expression of proinflammatory cytokines and mediators in some inflammation models.
Our interest in TSA was specifically prompted by previous observations that TSA (along with other HDAC inhibitors) is an inhibitor of vascular cell adhesion molecule-1 (VCAM-1)6 and tissue factor (TF)7 expression by stimulated cultured HUVECs (human umbilical vein endothelial cells). Both VCAM-1 and TF expression are of high relevance to sickle cell disease; the former mediates abnormal adhesion of sickle red cells8 and white cells9 to endothelium, and TF is the trigger of coagulation activation. Expression of both VCAM-1 and TF are indicators of endothelial cell activation, with TF expression being an indicator of especially strong endothelial perturbation. Given these activities, we hypothesized that HDAC inhibitors might confer benefits in sickle disease.
Our present results demonstrate remarkable multimodality beneficial effects of TSA in vivo, specifically inhibition of adhesive inflammatory endothelial phenotype, endothelial TF expression, and vascular stasis in sickle mice. In addition, we report that TSA is an iron chelator, and that TSA promotes expression of antisickling fetal hemoglobin (HbF).10 We further report that the clinically approved related compound SAHA (suberoylanilide hydroxamic acid) exhibits all the same benefits as the experimental compound, TSA. Thus, TSA and SAHA each are demonstrated to have 5 separate modalities of action that are predicted to be of clinical benefit in sickle cell anemia.
Methods
Reagents
TSA was prepared for administration by dissolving in 0.5% dimethylsulfoxide (DMSO) in mouse saline and was usually administered at 1 mg/kg per dose intraperitoneally. SAHA also was dissolved in DMSO and mouse saline and was administered at 100 mg/kg intraperitoneally. Hemin and butyrate were purchased from Sigma-Aldrich; and SAHA for HbF induction was from Aton Pharma.
Mice
All of the genetically modified animals, along with C57BL6 wild-type control animals, were raised and housed in the same specific pathogen–free room at the University of Minnesota under Institutional Animal Care and Use Committee (IACUC) monitoring and approval. Our colonies of transgenic animals had previously been established after we performed sterile rederivization. All mice were fed standard mouse chow and allowed water ad libitum.
The sickle transgenic mouse models used were the mild-phenotype NY1DD mouse (homozygous for murine β-thalassemia, one copy of the human sickle transgene [with linked αH and βS], C57BL6 background); the more severe-phenotype S+SAntilles mouse (homozygous for murine β-thalassemia, one copy each of human transgenes for both βS-globin [linked to αH] and for βS-Antilles-globin [also linked to αH]); and the hBERK1 mouse (homozygous for knockout of murine α-globin, heterozygous for knockout of murine β-globin, one copy of the human sickle transgene, mixed genetic background). Their features and the methods used to characterize them have been previously published.11 Notably, in terms of endothelial activation (at least as evidenced by immunofluorescent detection of pulmonary vein TF and VCAM), we earlier showed that the hBERK1 mouse is identical to the most severe BERK mouse, and that the S+SAntilles mouse has a degree of activation intermediate between that of NY1DD and BERK.11
H/R model
Animals were studied at ambient air. In addition, some NY1DD animals were studied after hypoxia/reoxygenation (H/R), which was imposed by placing mice into an environmental chamber with 8% O2 for 3 hours (the H period), followed by a return to ambient air for 18 hours (the R period).11
It is important to note that “H/R” refers to the model (in which the stress is hypoxia, followed by reoxygenation). And indeed, at the level of mouse physiology, H/R is what the normal control animal experiences. However, the sickle mouse actually experiences I/R (ischemia/reperfusion), because the induction of sickling by the short-term exposure to hypoxia2 statistically increases the chance for vascular occlusion to occur. Thus, the H/R stress model is used here because the phenotype switch induced by it, consequent to occurrence of ischemia/reoxygenation, is a robust model of the physiology that patients with sickles are most likely undergoing all of the time.1 It does not comprise an attempt to simulate any specific clinical event.
TF expression
As an assessment of extreme endothelial activation, we measured expression of TF by the pulmonary vein endothelium (because these are the only vessels that consistently express it in these models) exactly as we previously described,11 using immunofluorescence microscopic review of tissue sections triple-stained for nuclei and endothelial marker murine CD31 (B2) and murine TF, with fluorescein isothiocyanate (FITC)– and tetramethylrhodamine-5 (and 6)-isothiocyanate (TRITC)–labeled secondary antibodies. For this, we used the partially purified polyclonal antibody preparation to murine TF12 that we previously validated and described.11 Quality control measures and negative controls were as previously described.11 TF was expressed as a percentage of pulmonary veins with positive endothelium.
To verify that increased TF expression by immunofluorescence is accompanied by increased endothelial TF mRNA, we examined NY1DD mice before or after H/R (here using 2.5 hours of reoxygenation). Lungs were harvested, diced, and digested with 300 U/mL of collagenase for 1 hour at 37°C. Lung endothelial cells were isolated using P1H12 antibody-coated Dynabeads. Endothelial cell enrichment was confirmed by immunostaining: cells were more than 88% CD31+, less than 1% smooth muscle, and less than 1% fibroblast. Lung endothelial cells from 3 mice were pooled and subjected to RNA isolation using Trizol Reagent. A total of 1.2 μg of total RNA from each pooled sample was used for cDNA synthesis using the SuperScript III 1st Strand Synthesis Supermix for qRT-PCR Kit. The cDNA was then subjected to real-time quantitative polymerase chain reaction (qPCR) using the TaqMan assay method in an I-Cycler q-PCR machine. The primer and TaqMan probe specific to murine sequence (mTF, mPECAM, and mVCAM-1) were purchased from Applied Biosystems. GAPDH was used as an internal control (Rodent GAPDH Control Reagent). The normalized expression level of each gene of each pooled sample was calculated. We compared 4 pools each of animals at air and after H/R.
These assays demonstrated that in parallel to TF protein expression increasing and VCAM protein expression remaining stable (Figure 1; NY1DD animals), mRNA signal for TF increased significantly, whereas that for VCAM-1 did not; as expected, the expression of the control molecule, PECAM, did not change (Figure 1). This validates our immunofluorescence observations at the mRNA level. For the larger-scale conduct of the present experiments, we used the immunofluorescence method because it detects protein rather than mRNA, is less cumbersome, and requires one-third as many experimental animals.
qPCR quantitation of (whole-lung) pulmonary endothelial antigens. qPCr quantitation in NY1DD mice shows that the stress of H/R, compared with controls at ambient air, does increase level of murine TF mRNA (left panel; P = .023), and does not significantly increase the level of murine VCAM1 mRNA (middle panel; P = .119). In the right panel, level of a control mRNA (murine PECAM) does not change, as expected (P = .383). These measurements are consistent with the protein data shown in Figure 2. To obtain each data point (n = 4 for each antigen), endothelial cells from 3 mice had to be pooled for analysis. Error bars show ±SD.
qPCR quantitation of (whole-lung) pulmonary endothelial antigens. qPCr quantitation in NY1DD mice shows that the stress of H/R, compared with controls at ambient air, does increase level of murine TF mRNA (left panel; P = .023), and does not significantly increase the level of murine VCAM1 mRNA (middle panel; P = .119). In the right panel, level of a control mRNA (murine PECAM) does not change, as expected (P = .383). These measurements are consistent with the protein data shown in Figure 2. To obtain each data point (n = 4 for each antigen), endothelial cells from 3 mice had to be pooled for analysis. Error bars show ±SD.
VCAM-1 and ICAM-1 expression
Adjacent sections were stained for VCAM-1 using rat anti–murine CD106 and intercellular cell adhesion molecule-1 (ICAM-1) using hamster anti–murine CD54, respectively, both biotinylated. Visualization was accomplished using avidin-biotin complex (ABC kit) and Nova Red substrate. ICAM-1 and VCAM-1 were expressed as percentages of reviewed pulmonary veins plus arteries that were positive, because both vessel types express this in these models.
TSA and SAHA treatments
TSA and SAHA were always given intraperitoneally, at doses indicated in “Reagents” or in “Results” if different from above. For pretreatment experiments, TSA and SAHA were given in 3 consecutive daily doses (except for the described single-dose studies), with the last dose give an hour before exposure to hypoxia. For non-pretreatment studies, it was given once daily for the durations indicated in “TSA in the hBERK1 sickle transgenic mouse”. In all studies, control animals that were given vehicle control were studied in parallel.
mPVEC cultures
Briefly, dissectible pulmonary vein was cut into pieces and treated with collagenase II. To grow murine pulmonary vein endothelial cells (mPVECs), we used the method we had previously developed for microvascular endothelial cells.13 The resulting cells were identified as endothelial by showing cobblestone morphology; positive staining for CD31, von Willebrand factor, VE-cadherin, VCAM-1, and flk-1; and negative staining for vimentin and smooth-muscle actin. From the same lungs, fibroblasts and smooth muscle cells were cultured for use as staining controls.
To verify in vitro that mPVECs actually do respond with TF expression, mPVECs that had reached approximately 80% confluence were kept overnight in serum-free conditions. Fresh medium was then added without (control) or with 100 ng/mL TSA. After 1 hour of incubation, cells were stimulated with 40 ng/mL murine tissue necrosis factor (TNFα) or 50 ng/mL phorbol myristate acetate (PMA), or by addition of 0.5% fetal bovine serum (FBS) for another 4 hours. TF expression was then determined using the anti–murine-TF preparation described and an anti–rabbit alkaline phosphatase–labeled secondary antibody. The reaction was monitored at 405 nm in a microplate reader after the reaction mixture was transferred to a 96-well plate. Because TSA did not affect TF on unstimulated mVPECs, we used those cells as a baseline control.
Stasis of blood flow
H/R-induced stasis of subcutaneous venular blood flow was measured in hBERK1 mice or in S+SAntilles mice with an implanted dorsal skin-flow chamber (DSFC) using intravital microscopy as previously described.14,15 All measurements of blood flow parameters were made 3 days after DSFC implantation. At baseline, with the mice in ambient air, flowing venules were selected at random and their relative locations were noted on a map of the microscopic field. After this, the mice were subjected to 1 hour of hypoxia (7% O2/93% N2), followed by reoxygenation in room air. (The degree of hypoxia used for these experiments is slightly different that that used for TF/VCAM-1 studies, because each type of experiment used the exact same conditions that had previously been standardized and used by the performing laboratory.) After 1 hour and 4 hours of reoxygenation, the same, previously flowing venules were re-examined for blood flow. Venules with no observable blood flow were counted as static. The percentage of static vessels was calculated. Number of animals and venules studied are indicated in the relevant figure legends. For these stasis experiments, TSA and SAHA were administered as stated, and the third and last injection was given 1 hour before exposure to hypoxia.
Iron chelation studies
TSA and SAHA were evaluated for their hypothesized ability to chelate bioavailable iron. We dissolved ferric ammonium citrate in Tris buffer containing 0.5% DMSO for TSA or 2.5% DMSO for SAHA. Absorption spectra of solutions of iron/DMSO without chelator were compared with those obtained in the presence of 10:1 molar ratio of chelator to iron (3.3mM SAHA or TSA, and 0.33mM iron) to promote full chelation, with spectra read 2 hours after reagent mixing. These data were compared with the effects of desferrioxamine at 1mM.
γ-Globin and HbF induction analysis
K562 cells were cultured in Iscove modified Dulbecco medium containing 10% FBS. Drug inductions were completed with hemin (50μM), butyrate (2mM), TSA (0.5μM), or SAHA (5μM) for 24, 48, and 72 hours. Total RNA was isolated from K562 cells using RNA Stat-60. Reverse transcription–qPCR (RT-qPCR) analysis was performed as previously described10 using SyberGreen iQ Supermix and 100pM γ-globin and glyceraldehyde-3-phosphate dehydrogenase (GAPD) primers.
For ELISA (enzyme-linked immunosorbent assay), K562 cells were lysed with water, and protein extracts were mixed with Drabkin reagent; cyanmethemoglobin levels were measured at 540 nm on a spectrophotometer. HbF was measured using the Hemoglobin F ELISA Quantitation Kit. The 96-well plates were coated with sheep anti-HbF human antibody, washed, and then incubated with sheep anti-HbF horseradish peroxidase–conjugated secondary antibody. Tetramethyl benzidine substrate was read at 450 nm; HbF was normalized by total hemoglobin and protein levels and compared with values obtained in untreated K562 cells.
Statistics
Testing for significance generally used Student t testing or analysis of variance (ANOVA) if multiple comparisons were made. The number of independent observations is shown for all data in the figure and/or table legends. Statistical analyses on the stasis data were performed using the Fisher exact test, comparing the proportions of venules exhibiting stasis at each time point.
Results
For reasons explained in “VCAM and ICAM expression” and “TF expression,” data on VCAM and ICAM are expressed as percentages of pulmonary veins plus arteries that were VCAM+, whereas data on TF are expressed as percentages of pulmonary veins that were TF+.
Effect of H/R on endothelial activation in NY1DD sickle mice
Normal control C57BL6 animals have a moderate, constitutive level of VCAM-1 expression (approximately 40%) and very low TF expression (approximately 7%) at ambient air (Figure 2). In response to H/R, the endothelial activation of these normal control mice is enough to show a significant increase in VCAM-1 expression to approximately 70% (P = .006), but it is not strong enough for TF to show any increase whatsoever.
VCAM and TF in the control and sickle mouse. VCAM (A) and TF (B) in the C57BL6 control and NY1DD sickle mice, which were pretreated with TSA for 3 days; hBERK1 mice were treated for 3, 7, or 21 days only at ambient air. NY1DD mice were studied at air and after H/R, as indicated, whereas hBERK1 animals were studied only at air. (A) *P < .001; **P < .001; #P = .027; &P = .019. (B) *P < .001; #P = .029; and &P < .001. Number of experimental animals, for bars left to right: top panel, 8, 4, 8, 4, 4, 6, 4, 6, 6, 6, 6, and 6; bottom panel, 4, 4, 4, 4, 4, 6, 4, 6, 6, 6, 6, and 6. These data illustrate that TSA decreases expression of VCAM and TF when given as pretreatment before the H/R-induced boost in endothelial stimulation (NY1DD model). In the hBERK1 model, with already-boosted VCAM and TF at ambient air, the TSA reduced VCAM but not TF unless it was given for a prolonged period. Error bars show ±SD.
VCAM and TF in the control and sickle mouse. VCAM (A) and TF (B) in the C57BL6 control and NY1DD sickle mice, which were pretreated with TSA for 3 days; hBERK1 mice were treated for 3, 7, or 21 days only at ambient air. NY1DD mice were studied at air and after H/R, as indicated, whereas hBERK1 animals were studied only at air. (A) *P < .001; **P < .001; #P = .027; &P = .019. (B) *P < .001; #P = .029; and &P < .001. Number of experimental animals, for bars left to right: top panel, 8, 4, 8, 4, 4, 6, 4, 6, 6, 6, 6, and 6; bottom panel, 4, 4, 4, 4, 4, 6, 4, 6, 6, 6, 6, and 6. These data illustrate that TSA decreases expression of VCAM and TF when given as pretreatment before the H/R-induced boost in endothelial stimulation (NY1DD model). In the hBERK1 model, with already-boosted VCAM and TF at ambient air, the TSA reduced VCAM but not TF unless it was given for a prolonged period. Error bars show ±SD.
In contrast, the mild-phenotype NY1DD sickle mouse, even at ambient air, shows very elevated VCAM-1 expression of approximately 70% (P = .006), but it still has the low-TF phenotype exhibited by the normal animals at air. After H/R, the NY1DD sickle mouse shows no further increase in VCAM-1 expression. (Earlier experiments had shown that VCAM, measured under the conditions of these experiments, never exceeds approximately 70%, so that level seems to represent the maximal extent of its expression.) But, H/R caused a phenotype conversion with a significant increase in TF expression to 20% (P < .001).
Thus, the sickle mouse at air has some endothelial activation, but the level of that is increased further after H/R. ICAM-1 was expressed at greater than 90% in all animals, of all types, under all conditions, so those data are not presented.
TSA pretreatment in NY1DD mice
A 3-day treatment of normal mice at air with 1 mg/kg per TSA dose (Figure 2) did not suppress their lower constitutive VCAM-1 level, but significantly diminished the high VCAM-1 expression in the NY1DD mice at air (P < .001). In parallel, this treatment had no effect on the low TF level in NY1DD animals at ambient air. In contrast, in NY1DD animals after H/R, this 3-day pretreatment significantly lowered VCAM expression (P < .001). It also inhibited the high-TF state these animals had assumed after H/R (P < .001). Thus, TSA given before H/R exposure effectively decreased the endothelial activation consequent to this stress.
TSA dose-response in NY1DD mice
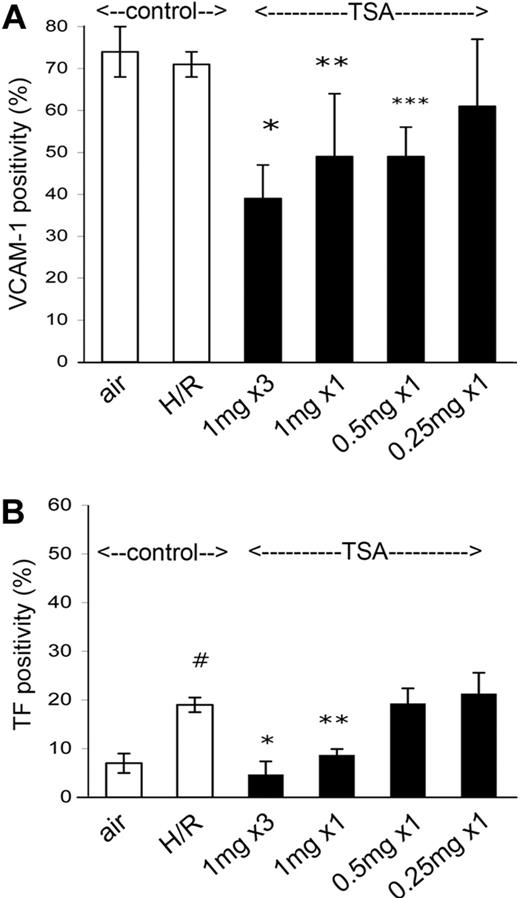
To evaluate lower TSA doses, we compared the standard 3-dose pretreatment (at 1 mg/kg/dose) to giving only a single pretreatment dose of TSA (1 mg/kg). Compared with the strong inhibitory effect of 3 doses on post-H/R NY1DD animals, a single dose (1 mg/kg) still reduced VCAM-1 (P = .04) and TF (P = .003) expression significantly, but clearly not as strongly (Figure 3). Single doses of TSA at the further reduced levels of 0.5 mg/kg and 0.25 mg/kg had little or no effect at all on the VCAM-1 and TF expression induced by H/R (Figure 3), so it appears that the dose of 1 mg/kg is the likely minimal effective dose of TSA.
Dose response to TSA in the NY1DD mouse. VCAM (A) and TF (B) in the NY1DD mouse illustrate dose-response to TSA pretreatment. In both panels, the 2 leftmost open bars illustrate the already described (in Figure 1) status in NY1DD animals at air and after H/R. Black bars show effect of TSA pretreatment of NY1DD animals then exposed to H/R. (A) *P < .001; **P = .04; ***P = .002. (B) #P = .009; *P < .001; **P = .003. Number of animals for bars left to right: top panel, 4, 4, 6, 3, 3, and 3; bottom panel, 4, 4, 6, 3, 3, and 3.These data illustrate that a pretreatment TSA dose of 1 mg/kg is the minimal efficacious dose. Error bars show ±SD.
Dose response to TSA in the NY1DD mouse. VCAM (A) and TF (B) in the NY1DD mouse illustrate dose-response to TSA pretreatment. In both panels, the 2 leftmost open bars illustrate the already described (in Figure 1) status in NY1DD animals at air and after H/R. Black bars show effect of TSA pretreatment of NY1DD animals then exposed to H/R. (A) *P < .001; **P = .04; ***P = .002. (B) #P = .009; *P < .001; **P = .003. Number of animals for bars left to right: top panel, 4, 4, 6, 3, 3, and 3; bottom panel, 4, 4, 6, 3, 3, and 3.These data illustrate that a pretreatment TSA dose of 1 mg/kg is the minimal efficacious dose. Error bars show ±SD.
TSA given after H/R in NY1DD mice
We also compared the efficacy of a single dose of TSA (1 mg/kg) given after exposure to the hypoxia period. In this situation, TSA was totally ineffective in reducing endothelial activation level (data not shown). Thus, the agent has to be present during the TF-inducing event to be effective (see “Discussion” regarding drug mechanism).
TSA in the hBERK1 sickle transgenic mouse
To test this therapy in a murine model of sickle disease that is on a different genetic strain background, we also studied hBERK1 animals.11 Even at ambient air, hBERK1 animals exhibit abnormal endothelial activation, with VCAM-1 expression at approximately 55% and TF at approximately 40% (Figure 2). These are the levels of activation we earlier documented not only for hBERK1 but also its more severe related mouse, BERK.11 We also previously reported that the best same-strain control for hBERK1 animals (the HbA-BERK mouse that produces entirely normal human hemoglobin A) does not exhibit increased endothelial activation, identical to the control C57BL6 mouse;11 therefore, the elevated activation manifested by these sickle animals is not simply due to a strain difference. Because hBERK1 has marked endothelial activation already even at ambient air, administration of TSA would necessarily be taking place after the activation-inducing stress has already taken place.
Figure 2 shows that 3 days of TSA treatment significantly lowered expression of VCAM (P = .001) but not expression of their already established TF in the hBERK1 mouse. Continuation of TSA for longer periods did result in significant depression of both VCAM (P = .002 at 7 days; P = .019 at 21 days) and TF (P = .002 at 7 days; P < .001 at 21 days). Thus, the longer treatment of hBERK1 completely lowered VCAM expression to the constitutive level seen in normal mice, and reduced TF expression significantly. TF and VCAM did not decrease in normal control mice treated for these longer time periods (data not shown).
This observation of development of TSA effect with longer-term therapy is consistent with the notion that TSA prevents induction of TF expression but does not actually reverse it once it has already occurred. Rather, the increasing efficacy seen with repeated dosing suggests that, over time, new TF expression is being prevented as the endothelium naturally remodels its surface.
Effect of TSA on mPVECs in vitro
To document in vitro that the mPVECs assayed for TF expression in the mouse lungs in vivo do respond with TF expression, we stimulated cultured mPVECs in each of 3 ways in 3 experiments: addition of 40 ng/mL TNFα, addition of 50 ng/mL PMA, or addition of 0.5% FBS after a period of serum deprivation. These treatments stimulated TF expression by 11% plus or minus 2.5%, 24.3% plus or minus 3.0%, and 53.7% plus or minus 11.9%, respectively. In parallel, TSA was added at 100nM for 1 hour before addition of the stimulants. In these 3 systems, TSA significantly prevented increased TF expression (P = .039, P = .049, and P = .017, respectively). This assures us that the TF changes observed in the mouse in vivo are actually part of the biology of mPVECs.
Action of TSA on vascular stasis
To test if TSA could prevent stasis in subcutaneous venules, hBERK1 mice with an implanted DSFC were pretreated for 3 days with TSA or vehicle control and then subjected to H/R (Figure 4). TSA-treated sickle mice had significantly less stasis than vehicle-treated mice after 1 hour of hypoxia and either 1 hour or 4 hours of reoxygenation (P = .006 for both by Fisher exact test).
Vascular stasis as monitored in a DSFC model using hBERK1 mice subjected to H/R. Mice were treated with or without TSA for 3 days. After the third TSA dose, flowing venules were selected and studied. Animals were then subjected to H/R. After 1 hour and 4 hours of reoxygenation, the same venules were re-examined for blood flow. There were 5 mice and 251 venules in the TSA-treated group, and 4 mice and 85 venules in the vehicle group. At least 18 venules per mouse were evaluable. Expressed on a per-total venule basis, asterisk (*) shows P = .006 for both at 1 hour and 4 hours of reoxygenation. This demonstrates an antistasis effect of TSA, consistent with that of other hydroxamic acids we previously studied using a different intravital microscopy model.15 Error bars show SE.
Vascular stasis as monitored in a DSFC model using hBERK1 mice subjected to H/R. Mice were treated with or without TSA for 3 days. After the third TSA dose, flowing venules were selected and studied. Animals were then subjected to H/R. After 1 hour and 4 hours of reoxygenation, the same venules were re-examined for blood flow. There were 5 mice and 251 venules in the TSA-treated group, and 4 mice and 85 venules in the vehicle group. At least 18 venules per mouse were evaluable. Expressed on a per-total venule basis, asterisk (*) shows P = .006 for both at 1 hour and 4 hours of reoxygenation. This demonstrates an antistasis effect of TSA, consistent with that of other hydroxamic acids we previously studied using a different intravital microscopy model.15 Error bars show SE.
TSA as an iron chelator
We studied this because TSA and SAHA are hydroxamic acids,5 2 examples of which (didox and trimadox) are iron chelators that were previously reported by us to be of vascular benefit (improved microvascular flow, diminished leukocyte/endothelial interaction) in a murine sickle model16 (“Discussion”). As shown in Figure 5, in the presence of iron, the paradigmatic hydroxamic acid chelator desferrioxamine shows color development in the visible region of the absorption spectrum. TSA and SAHA showed a similar effect. Thus, these agents are iron chelators (“Discussion”), though one cannot infer anything about relative affinity or degree of chelation from these comparative absorption spectra.
Iron chelation. Ferric ammonium citrate in buffer plus DMSO exhibits an absence of absorbance in the visible region (curve 1). Solutions of 3 chelators in DMSO were identical to curve 1. In contrast, iron chelation by 3 hydroxamic acids is illustrated by color development: curve 2, SAHA; curve 3, TSA; and curve 4, desferrioxamine. These data confirm iron chelation by all 3 hydroxamic acids, DFO, TSA, and SAHA.
Iron chelation. Ferric ammonium citrate in buffer plus DMSO exhibits an absence of absorbance in the visible region (curve 1). Solutions of 3 chelators in DMSO were identical to curve 1. In contrast, iron chelation by 3 hydroxamic acids is illustrated by color development: curve 2, SAHA; curve 3, TSA; and curve 4, desferrioxamine. These data confirm iron chelation by all 3 hydroxamic acids, DFO, TSA, and SAHA.
Induction of HbF
Because we previously had shown that TSA induces γ-globin gene transcription in K562 cells and hematopoietic cells in culture,10 we compared the ability of SAHA to stimulate HbF synthesis in K562 cells. Time course studies were completed at 24, 48, and 72 hours with the known HbF inducers hemin and butyrate and with the experimental HDAC inhibitor TSA. In a time-dependent manner, γ-globin mRNA levels were increased by hemin and butyrate to 5.89-fold and 3.46-fold inductions, respectively, at 48 hours (Table 1). TSA induced γ-globin transcription significantly at 2.76-fold (P = .002) and 1.83-fold (P = .02) at 48 and 72 hours. To complement the γ-globin gene expression data, we performed ELISA at 48 hours to quantify HbF protein synthesis. The data summarized in Table 2 demonstrate significant HbF induction by TSA (3.6-fold) and SAHA (2.13-fold), levels that are comparable with the known inducers hemin and butyrate. These data demonstrate that the ability of SAHA to induce γ-globin transcription and HbF synthesis is comparable with that of TSA in K562 cells.
Time-course studies of γ-globin gene activation by drug treatments in K562 cells
| Compound . | Drugs . | 24 h, g/GAPD mRNA . | 48 h, GAPD mRNA . | 72 h, GAPD mRNA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Avg ± SD . | P . | Fold increase . | Avg ± SD . | P . | Fold increase . | Avg ± SD . | P . | Fold increase . | ||
| Untreated | None | 1.17 ± 0.62 | – | 1.00 | 0.80 ± 0.20 | – | 1.00 | 6.36 ± 1.81 | – | 1.00 |
| Hemin | 50μM | 3.15 ± 1.13 | .016 | 2.69 | 4.68 ± 0.28 | 0.0001 | 5.89 | 17.23 ± 3.26 | .007 | 2.71 |
| Butyrate | 2mM | 1.45 ± 0.37 | .441 | 1.24 | 2.77 ± 0.76 | 0.0008 | 3.46 | 17.63 ± 2.16 | .002 | 2.77 |
| Trichostatin A | 0.5μM | 1.70 ± 0.16 | .200 | 1.46 | 2.21 ± 0.59 | 0.0023 | 2.76 | 11.64 ± 1.51 | .018 | 1.83 |
| SAHA | 5μM | 1.53 ± 0.18 | .368 | 1.31 | 1.37 ± 0.23 | 0.0052 | 1.71 | 12.34 ± 0.83 | .007 | 1.94 |
| Compound . | Drugs . | 24 h, g/GAPD mRNA . | 48 h, GAPD mRNA . | 72 h, GAPD mRNA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Avg ± SD . | P . | Fold increase . | Avg ± SD . | P . | Fold increase . | Avg ± SD . | P . | Fold increase . | ||
| Untreated | None | 1.17 ± 0.62 | – | 1.00 | 0.80 ± 0.20 | – | 1.00 | 6.36 ± 1.81 | – | 1.00 |
| Hemin | 50μM | 3.15 ± 1.13 | .016 | 2.69 | 4.68 ± 0.28 | 0.0001 | 5.89 | 17.23 ± 3.26 | .007 | 2.71 |
| Butyrate | 2mM | 1.45 ± 0.37 | .441 | 1.24 | 2.77 ± 0.76 | 0.0008 | 3.46 | 17.63 ± 2.16 | .002 | 2.77 |
| Trichostatin A | 0.5μM | 1.70 ± 0.16 | .200 | 1.46 | 2.21 ± 0.59 | 0.0023 | 2.76 | 11.64 ± 1.51 | .018 | 1.83 |
| SAHA | 5μM | 1.53 ± 0.18 | .368 | 1.31 | 1.37 ± 0.23 | 0.0052 | 1.71 | 12.34 ± 0.83 | .007 | 1.94 |
Data for each time point, shown as mean ± SD, are represented by 3 to 5 independent drug inductions.
Fetal hemoglobin induction in K562 cells by drug inducers
| Compound . | Drugs . | HbF/TH/TP* . | P . | Fold increase . |
|---|---|---|---|---|
| Untreated cells | None | 0.29 ± 0.04 | – | 1.00 |
| Hemin | 50μM | 0.92 ± 0.09 | < .001 | 3.17 |
| Butyrate | 2mM | 0.58 ± 0.05 | .002 | 2.52 |
| Trichostatin A | 0.5μM | 1.02 ± 0.16 | .002 | 3.76 |
| SAHA | 5μM | 0.62 ± 0.06 | .002 | 2.13 |
| Compound . | Drugs . | HbF/TH/TP* . | P . | Fold increase . |
|---|---|---|---|---|
| Untreated cells | None | 0.29 ± 0.04 | – | 1.00 |
| Hemin | 50μM | 0.92 ± 0.09 | < .001 | 3.17 |
| Butyrate | 2mM | 0.58 ± 0.05 | .002 | 2.52 |
| Trichostatin A | 0.5μM | 1.02 ± 0.16 | .002 | 3.76 |
| SAHA | 5μM | 0.62 ± 0.06 | .002 | 2.13 |
Fetal hemoglobin (HbF) levels were quantified by ELISA after 48 hours of drug treatment. Readings were normalized by total hemoglobin (TH) and total protein (TP) levels measured by spectrophotometry and expressed as mean ± SD.
SAHA exhibits the same benefits as TSA
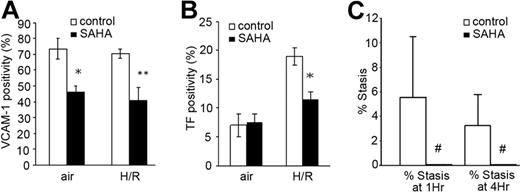
Because of the apparent beneficial effects of the experimental compound TSA, we tested the effect of SAHA, an analog that is already approved for human clinical use at 100 mg/kg per dose (see “Discussion” for dose level). Given for 3 days to NY1DD animals at air, it diminished VCAM-1 (P < .001; (Figure 6A) but not TF (Figure 6B) expression. However, given as a 3-day pretreatment, it significantly diminished H/R-induced expression of both VCAM (P < .001) and TF (P = .017).
Effect of SAHA on sickle transgenic mice in vivo. Three-day pretreatment with SAHA diminished both VCAM (A) and TF (B) in the NY1DD mouse at air and after H/R. (A) *P < .001; **P < .001. (B) *P = .017. For panels A and B, the number of animals for bars left to right was 4, 5, 4, and 5, respectively. (C) In post-H/R S+SAntilles sickle animals, SAHA inhibits stasis measured at 1 hour and 4 hours of reoxygenation. with P = .006 for both (#). Number of observations was 4 animals and 236 venules in the vehicle-treated group, and 4 animals and 245 venules in the SAHA-treated group. Overall, these data demonstrate that the clinically approved analog, SAHA, exhibits the same spectrum of effects as TSA. Error bars show SD (A-B) or SE (C).
Effect of SAHA on sickle transgenic mice in vivo. Three-day pretreatment with SAHA diminished both VCAM (A) and TF (B) in the NY1DD mouse at air and after H/R. (A) *P < .001; **P < .001. (B) *P = .017. For panels A and B, the number of animals for bars left to right was 4, 5, 4, and 5, respectively. (C) In post-H/R S+SAntilles sickle animals, SAHA inhibits stasis measured at 1 hour and 4 hours of reoxygenation. with P = .006 for both (#). Number of observations was 4 animals and 236 venules in the vehicle-treated group, and 4 animals and 245 venules in the SAHA-treated group. Overall, these data demonstrate that the clinically approved analog, SAHA, exhibits the same spectrum of effects as TSA. Error bars show SD (A-B) or SE (C).
In studies of the more severe S+SAntilles mouse stressed with H/R, we observed that SAHA significantly inhibited vascular stasis in the DSFC model (Figure 6C; P = .006 both at 1 and 4 hours, respectively, by Fisher exact test).
Discussion
Earlier studies showed that TSA (and other HDAC inhibitors) are strong inhibitors of VCAM-1 and of TF protein and mRNA induction in cultured HUVECs stimulated with various agonists (TNFα, IL1β, lipopolysaccharide, HOSCN).6,7 TSA does not alter TF translational efficiency or ubiquitinization-dependent TF degradation. Rather, TSA inhibits the binding of NFκB components cRel/p65 and p65/p507 to the unique NFκB sites that are separately specific for VCAM17 and to TF.18 It does not affect the other major transcriptional regulators of TF (AP-1, SP1, Egr-1).7
We note that this TF-inhibiting effect was also observed to be exerted in human peripheral blood mononuclear cells and murine peritoneal macrophages.7 Moreover, a related compound, SAHA, has been shown to down-regulate VCAM-1's counter-adhesion partner on monocytes, VLA4.19 Thus, although the present study focuses upon endothelial biology, the agents discussed here also are highly relevant to the adhesive and coagulation-promoting biology of monocytes. In addition, if this VLA4 inhibition extends to the erythron, it is relevant because enhanced VLA4 expression on sickle reticulocytes, and its binding to endothelial VCAM-1, is one of the significant mechanisms underlying the abnormal adhesion of sickle red cells to endothelium, a possible proximate event in vaso-occlusion.8,20
The present studies used 3 types of sickle transgenic mice. For one of these, the mild-phenotype NY1DD mouse, vigorous endothelial activation (as evidenced by TF expression) only occurs after the animal has been subjected to H/R, which provides a robust and tractable model of endothelial activation.11 As noted in “H/R model,” this stress actually causes the inflammatory changes of ischemia/reperfusion.21 Separate studies presented elsewhere22,23 have demonstrated the mechanism underlying the consequent endothelial activation consists of inflammation, specifically involving NFκB component p50 in peripheral blood monocytes (but not the p50 in vessel walls), and that TF expression is down-modulated by NO coming from endothelial nitric oxide synthase (eNOS), the generator of endothelial NO. Therefore, the present study did not repeat those mechanistic assessments but rather focused on the possible beneficial effects of TSA and SAHA.
Our present results demonstrate efficacy of TSA for administration both before endothelial-activating stress (NY1DD mouse) and for administration after activation has already been induced (hBERK1 mouse). The latter, of course, requires repeated dosing over a longer period of time. In these studies, we gave it daily; it is not yet known if it can be given less often and still exhibit efficacy. These results are consistent with the known mechanism of TSA inhibition of VCAM-1 and TF expression noted here, an effect on transcriptional regulation.7 In turn, this is consistent with our understanding that the triggering mechanism for marked endothelial activation occurs during the hypoxia period plus the first hour or so upon reoxygenation in the NY1DD mouse.2,3,11
Our previous studies14,15 demonstrated that vascular stasis, as visualized in the DFSC in this model, is accompanied by increased adhesion of red cells and white cells to endothelium and by sickling of red cells.14,15 Hence, we did not repeat such mechanistic studies in the present context, so we cannot yet be certain as to the mechanism of the protective effect conferred by the HDAC inhibitors in this regard. We previously did observe that subcutaneous stasis in this model is inhibited by anti–VCAM-1 antibody.14,15 However, it is quite possible that it involves other factors related to stasis, in addition to those studied herein. This will have to be examined by dedicated studies.
Interestingly, the hydroxamic acid category of agents are iron chelators, such as desferrioxamine, didox, and trimadox. Indeed, we previously demonstrated that didox and trimadox inhibit white blood cell–endothelial interaction, TF expression, and microvascular stasis in the sickle mouse, in addition to exerting an antioxidant effect by chelating bioavailable iron.16 Thus, there was reason to expect that the hydroxamate HDAC inhibitors TSA and SAHA might also beneficially chelate iron, which could impede iron-dependent redox chemistry, depending upon stoichiometry achieved. This was examined because iron is important in sickle disease pathobiology for multiple reasons.24 In fact, we found that TSA does chelate iron. Based on TSA and SAHA structure, we would predict that fully active chelation by these agents would require a 3:1 chelator-iron molar ratio. However, it will require dedicated, additional studies to identify affinity and Ksol (which predicts ability to strip iron from the membrane/cytosol interface), ability to permeate membranes, oral absorbability and pharmacokinetics, ability to inhibit oxidative reactions, and stoichiometry required for inactivating iron redox cycling. Performance of such studies will be complicated by the fact that TSA and SAHA are insoluble in aqueous solution.
Because the results with TSA were so promising, we tested a related HDAC inhibitor, SAHA (vorinostat), which is already approved for clinical human use (in cutaneous T-cell lymphoma). We observed that in the crucial experiments (inhibition of VCAM and TF, improvement of stasis, induction of γ-globin synthesis, and chelation of iron), SAHA exhibited the same spectrum of activity as the experimental compound, TSA. The dose required of SAHA is higher than that of TSA because it is a weaker HDAC inhibitor, and we used dosing that was not atypical for preclinical cancer studies in mice. To our knowledge, the only previous use of any HDAC inhibitor in the sickle context (butyrate, a nonspecific inhibitor) was limited to its ability to induce HbF expression.25
Interestingly, it is not inconceivable that TSA and SAHA would improve bioavailability of NO. We note that histone acetylation is an important regulator of eNOS expression, and TSA has been reported to actually increase eNOS transcription in nonendothelial cells.26 Whether this has any physiologic relevance to vasoregulation is not known at this time, but it should be studied. In addition, hydroxyurea, another hydroxamic acid, converts to NO upon oxidation by peroxidase or catalase,27 so TSA and SAHA might well do the same.
The present studies are not without caveats. First, these mouse models are not adequate for monitoring in vivo effect on HbF level, and that will need to be tested in a human pilot study. We are aware of no pre-existing data on HbF induction in the published Phase 1/2 trials of SAHA. Whether the multiple benefits observed here could actually be achieved in humans, all with the same dosing and with tolerable levels of side effects, is unknown and must be tested. We made no attempt here to identify the optimum dose for each individual biologic effect. There certainly are many questions that need to be addressed to evaluate the implications of the present data. In addition to the issues noted here, a longer-term, preclinical study of these drugs will need to be done in the sickle mouse model to test for benefits on development of organ pathology, and to find whether the effectiveness of these drugs is generalizable (ie, to other organs), given the role of endothelial heterogeneity.28
Nonetheless, the present data offer a promising beginning to potential development of a new agent for application in sickle disease. Considering the clinical implications of pulmonary vein VCAM and TF expression, this should be pursued (VCAM may play a central role in the acute chest syndrome,28 and TF would cause formation of thrombin immediately upstream of the most common area for sickle thrombosis, the Circle of Willis). Of course, SAHA should not be used in the clinic unless pilot testing has been accomplished in a controlled setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof:
Since the acceptance of this paper, an additional relevant publication has appeared.29 It provides a much more detailed argument regarding the probable hierarchy of sub-biologies that underlie sickle vascular pathobiology, as referred to in the present “Introduction”. Since this argument comprises the rationale for multi-modality therapy, we add this note.
Acknowledgments
This work was supported by the National Institutes of Health (PO1 HL55552 and R01 AI079219).
National Institutes of Health
Authorship
Contribution: R.P.H. designed the VCAM and TF studies, supervised their conduct, evaluated results, and wrote this paper; G.M.V. designed and supervised the stasis studies, which were conducted by C.F.A., J.V., and J.B., and helped edit the paper; B.S.P. performed the γ-globin and HbF induction study; J.N. developed and performed the culture of mPVECs, fibroblasts, and smooth muscle cells; R.K. and A.N.S. performed the TF and CAM detection studies; F.A. bred and genetically characterized the transgenic mice; S.O. assisted in performing these studies; R.J.K. created and provided the anti–murine-TF antibody; J.W.E. performed the iron chelation studies and helped edit the paper; and A.S. helped edit the paper and assisted in experimental design and interpretation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert P. Hebbel, 480 MMC, Division of Hematology-Oncology-Transplantation, University of Minnesota, Minneapolis, MN 55455; e-mail: hebbe001@umn.edu.