The cell-surface straight and branched repeats of N-acetyllactosamine (LacNAc) units, called poly-LacNAc chains, characterize the histo-blood group i and I antigens, respectively. The transition of straight to branched poly-LacNAc chain (i to I) is determined by the I locus, which expresses 3 IGnT transcripts, IGnTA, IGnTB, and IGnTC. Our previous investigation demonstrated that the i-to-I transition in erythroid differentiation is regulated by the transcription factor CCAAT/enhancer binding protein α (C/EBPα). In the present investigation, the K-562 cell line was used as a model to show that the i-to-I transition is determined by the phosphorylation status of the C/EBPα Ser-21 residue, with dephosphorylated C/EBPα Ser-21 stimulating the transcription of the IGnTC gene, consequently resulting in I branching. Results from studies using adult erythropoietic and granulopoietic progenitor cells agreed with those derived using the K-562 cell model, with lentiviral expression of C/EBPα in CD34+ hematopoietic cells demonstrating that the dephosphorylated form of C/EBPα Ser-21 induced the expression of I antigen, granulocytic CD15, and also erythroid CD71 antigens. Taken together, these results demonstrate that the regulation of poly-LacNAc branching (I antigen) formation in erythropoiesis and granulopoiesis share a common mechanism, with dephosphorylation of the Ser-21 residue on C/EBPα playing the critical role.
Introduction
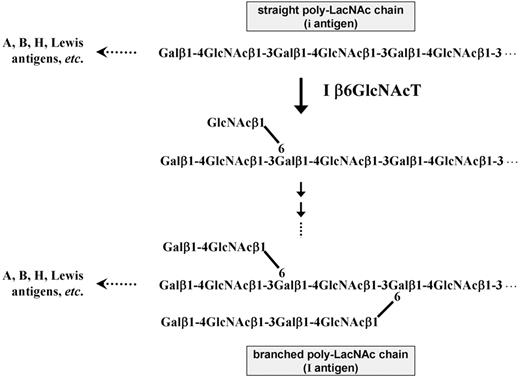
The glyco-chain consisting of repeats of N-acetyllactosamine (Gal-GlcNAc, LacNAc) units, called poly-LacNAc chains, is one of the major glyco-structures expressed on cell surfaces. Various glyco-epitopes, including histo-blood group A, B, and H antigens; the Lewis (Le) antigen series of Lea, Leb, Lex, Ley, sialyl Lea (sLea), and sialyl Lex (sLex); and others, are constructed on the terminals of poly-LacNAc chains through different enzymatic pathways (Figure 1).1,2 The straight LacNAc repeats are synthesized by the sequential actions of β-1,3-N-acetylglucosaminyltransferase and β-1,4-galactosyltransferase. Through the addition of the decisive GlcNAcβ1-6 branch by the activity of I β-1,6-N-acetylglucosaminyltransferase (I β6GlcNAcT) and the following actions of β-1,4-galactosyltransferase and β-1,3-N-acetylglucosaminyltransferase, the straight poly-LacNAc chains can be converted into branched structures (Figure 1).3,–5 Straight and branched poly-LacNAc chains characterize the structures for the human blood group i and I antigens, respectively.6,7 The i and I antigens, carried on glycolipids and glycoproteins,3,4 were first identified on human red cells,8,–10 and in later investigations,7,11 they were observed on the surfaces of most human cells and on soluble glycoproteins in various body fluids.
The expression of I antigen on red cells is developmentally regulated. Adult human red cells fully express I antigens, containing only a few i, which predominate in fetal and neonatal red cells. After birth, the quantity of I antigen gradually increases as the level of i antigen decreases, until the normal adult red cell Ii status is reached at approximately 18 months of life.10,12 However, it has been shown that the reverse conversion (I to i) is associated with several hematologic disorders, including thalassemia, hypoplastic anemia, and acute leukemia.13 These disorders demonstrate that the transition between straight and branched poly-LacNAc chains may play a significant role in erythrocyte differentiation.
Formation of the I antigenic structure on cell surfaces is determined by an uncommon molecular genetic architecture. The human I β6GlcNAcT gene, also designated IGnT14 (approved as GCNT2 by the HUGO Gene Nomenclature Committee), expresses 3 different transcripts, denoted IGnTA, IGnTB, and IGnTC, which have different exon 1, but identical exon 2 and exon 3, regions.15,16 The 3 IGnT cDNAs do not have any common 5′ region, and the 3 transcripts display differential expression patterns in different human tissues, indicating that transcription of the 3 IGnT forms is determined by different DNA regulatory regions and different regulatory mechanisms. The architecture of I β6GlcNAcT locus affords the complex regulation of the spatially and temporally specific formation of the poly-LacNAc branching structure.
In previous investigations15,17 we showed that, among the 3 IGnT forms, IGnTC is the gene responsible for the expression of blood group I antigen on red cells. In a recent study, we revealed that the i-to-I phenotypic transition during erythroid differentiation is regulated by the transcription factor CCAAT/enhancer binding protein α (C/EBPα), which enhances transcription of the IGnTC gene, consequently leading to the formation of the I antigen.18 In that study, our results further suggested that the activity of C/EBPα on IGnTC activation is modulated at the posttranslational level, and examination of the potential posttranslational modification demonstrated that dephosphorylation at Ser, but not Tyr and Thr, residues of C/EBPα occurs in erythroid differentiation of K-562 cells and is accompanied by the induction of IGnTC gene expression.
In the hematopoietic system, it is well known that transcription factor C/EBPα plays a crucial role in the development of granulocytes.19,20 In the present investigation, we show that the formation of poly-LacNAc branching (I antigen) in erythropoiesis and granulopoiesis is determined by a common mechanism, with dephosphorylation of the Ser-21 residue on C/EBPα playing the critical role in branch formation.
Methods
Culture and differentiation induction of K-562 cells
The human leukemia cell line K-562 (ATCC) was grown in 90% RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (50 U/mL), and streptomycin (50 μg/mL). To induce erythroid differentiation of K-562 cells, the cells (5 × 105/mL) were cultured in medium to which 2mM sodium butyrate (Sigma-Aldrich) was added for 2 days or 25μM hemin (Sigma-Aldrich) was added for 4 days.
Expression of wild-type and mutant C/EBPα in K-562 cells
Cellular total RNAs were prepared by use of the RNeasy Mini Kit (QIAGEN), and the first-strand cDNAs were primed with oligo-dT primer and synthesized by SuperScript III reverse transcriptase (Invitrogen). The full coding cDNAs for C/EBPα transcription factor was obtained by polymerase chain reaction (PCR) by use of the primer pair of CEBPAF9 and CEBPAR10His (the sequences and designs of PCR primers used in this study are described in the supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article) with the cDNA sample prepared from the mRNA of K-562 cells serving as the template. The mutant C/EBPα cDNAs were then generated through site-directed mutagenesis. By use of the wild-type C/EBPα cDNA as a template, the sense/antisense mutant primers S21AF/S21AR, S21DF/S21DR, S234AF/S234AR, and S234DF/S234DR were used, in combination with the forward and reverse primers CEBPAF9 and CEBPAR10His, during PCR amplification to generate C/EBPα cDNAs with nucleotide alterations of 61-62AG>GC, 61-62AG>GA, 700-701AG>GC, and 700-701AG>GA, respectively, which led to amino acid substitutions of S21A, S21D, S234A, and S234D, respectively. The amplified full coding cDNAs for wild-type and mutant C/EBPα were cloned into the NheI/KpnI sites of the mammalian expression vector pcDNA3.1− (Invitrogen).
K-562 cells, cultured in medium without addition of the inducing agent, were transfected with the constructed or mock vectors as described previously.21 At 72 hours after transfection, the transfected cells were enriched under the selection of antibiotic G418 (1 μg/mL) for 72 hours before harvesting. The expressions of the wild-type and mutant C/EBPα factors in the cells were confirmed by Western blotting by the use of Penta-His antibody (QIAGEN).
Flow cytometric analysis
To detect the cell-surface I antigen, cells were incubated with human monoclonal anti-I antibody (OSK14, a gift from Dr Yoshihiko Tani, Osaka Red Cross Blood Center) at 4°C for 2 hours after blocking with 5% bovine serum albumin. The bound monoclonal antibodies were detected by incubation with fluorescein isothiocyanate (FITC)–conjugated goat anti–human immunoglobulin M (IgM) antibody (AbD Serotec), and the cells were subjected to flow cytometry (EPICS XL; Beckman Coulter Inc). To detect the CD15 and CD71 antigens, the cells were incubated with anti-CD15 and anti-CD71 antibodies (BD Biosciences), respectively, at 4°C for 2 hours after blocking with 5% bovine serum albumin. The bound antibodies were detected by incubation with FITC-conjugated goat anti–mouse IgM (Santa Cruz Biotechnology Inc) or anti–mouse immunoglobulin G (IgG; Leinco Technologies) antibodies.
Quantification of IGnT and C/EBPα transcripts
The IGnTA, IGnTB, IGnTC, C/EBPα, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcripts in the cDNA samples were quantified by the use of TaqMan gene expression assay kits (Applied Biosystems) with ID codes Hs01941271_s1, Hs00377333_m1, Hs01106057_m1, Hs00269972_s1, and Hs99999905_m1, respectively. Real-time PCR detection was performed with the use of the GenAmp 7300 Sequence Detection System (Applied Biosystems) to quantify the transcripts. Serial dilutions of the pGEM-T vector (Promega) bearing IGnTA, IGnTB, IGnTC, C/EBPα, or GAPDH cDNAs were used to generate standard curves for each respective transcript.
Chromatin immunoprecipitation analysis
Chromatin immunoprecipitation (ChIP) analysis was performed to analyze the associations of wild-type and mutant C/EBPα with the IGnTC gene 5′ promoter region (−322 to +62 bp) with the ChIP assay kit (Upstate), as described previously.18
Nuclear protein extraction and Western blotting
For nuclear protein extractions, the described protocols22 were followed with minor modifications.18 A total of 20 μg of nuclear proteins was separated by use of 12% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes with the use of the iBlot Dry Blotting System (Invitrogen). After blocking with 5% bovine serum albumin, the blots were detected with Phospho-C/EBPα(Ser21) antibody (Cell Signaling Technology), which specifically detected C/EBPα transcription factor with phosphorylated Ser-21 residue. The bound antibody was then detected with horseradish peroxidase–conjugated goat anti–rabbit IgG antibody (Leinco Technologies), and the signals were developed by use of Western Lighting Chemiluminescence Reagent Plus (PerkinElmer). After stripping, the membrane was blotted successively with anti-C/EBPα (Santa Cruz Biotechnology Inc) and anti–histone H1 (Stressgen) antibodies.
Isolation of CD34+, CD71+, and CD15+ cells and E and G cultures
Human adult CD34+ hematopoietic cells were isolated from granulocyte colony-stimulating factor (CSF)–mobilized human peripheral blood stem cells, and adult CD71+ and CD15+ cells were isolated from general peripheral blood cells. Mononuclear cells were first isolated from the blood samples by Ficoll density gradient with the use of Ficoll-Plaque Plus (Amersham). CD34+, CD71+, and CD15+ cells were then purified by use of the CD34, CD71, and CD15 MicroBead kits (Miltenyi Biotec), respectively, following the manufacturer's protocols. For erythropoietic (E) culture, CD34+ cells were cultured in StemSPAN SFEM medium (StemCell Technologies) supplemented with stem cell factor (50 ng/mL; StemCell Technologies) and erythropoietin (3 U/mL; Janssen-Cilag) for 7 days. For granulopoietic (G) culture, CD34+ cells were cultured in the conditions identical with those for E culture except that granulocyte CSF and granulocyte/macrophage CSF (10 ng/mL each; StemCell Technologies) were substituted for erythropoietin in the cultures.
Consent to study human subjects was given by the Institutional Review Board of National Taiwan University, Taipei. Informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Lentiviral expression of wild-type and mutant C/EBPα
The full coding cDNAs of wild-type C/EBPα and mutant C/EBPα-S21A and C/EBPα-S21D were cloned into pLenti6/V5-D-TOPO vector (Invitrogen), constructing the gene transfer vectors. HEK 293T cells (ATCC), cultured in Dulbecco modified Eagle medium in 6-well plates (1 × 105 cells/well), were transfected with the mock pLenti6 vector or pLenti6 transfer construct (0.5 μg) together with the packaging vector pCMVdeltaR8.91 (0.5 μg) and the VSV-G envelope glycoprotein-expressing vector pMD.G (0.1 μg; provided by the National RNAi Core Facility, Academia Sinica), with Lipofectamine Transfection Reagent supplemented with Plus Reagent (Invitrogen). The medium was collected 48 hours after transfection, and the virion suspensions were concentrated through centrifugation. The CD34+ cells, cultured in StemSPAN SFEM medium supplemented with stem cell factor (50 ng/mL) in 24-well plates (3 × 105 cells/well), were transduced with the produced virions in the medium to which polybrene (8 μg/mL) was added. The cells were transferred to 6-well plates 16 hours after transduction and were cultured for 72 hours before harvest.
Results
Effect of C/EBPα-S21A and C/EBPα-S21D expression in K-562 cells
In the present investigation, the effect of the phosphorylation status of specific Ser residues in C/EBPα on the stimulation of I antigen expression was further examined. Phosphorylation can occur on several Ser residues in C/EBPα, including Ser-21 in human and mouse C/EBPα,23,24 Ser-193 and Ser-230 in mouse C/EBPα,25,26 and Ser-248 in rat C/EBPα,27 and phosphorylation of these Ser residues markedly affected the functions of C/EBPα in transcription activation and cell differentiation induction. However, the amino acid positions that are homologic with mouse C/EBPα Ser-193 and rat C/EBPα Ser-248 residues correspond to Pro and Ala, respectively, in human C/EBPα, and the position homologic with mouse C/EBPα Ser-230 residue is Ser-234 in human C/EBPα. Thus, the effects of the phosphorylation status of Ser-21 and Ser-234 residues in human C/EBPα on the expression of I antigen were explored.
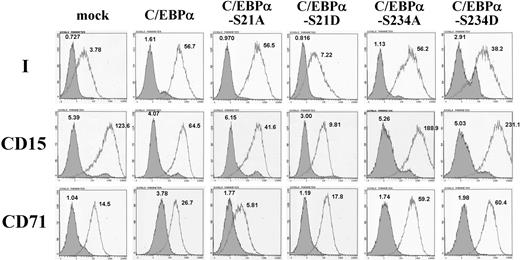
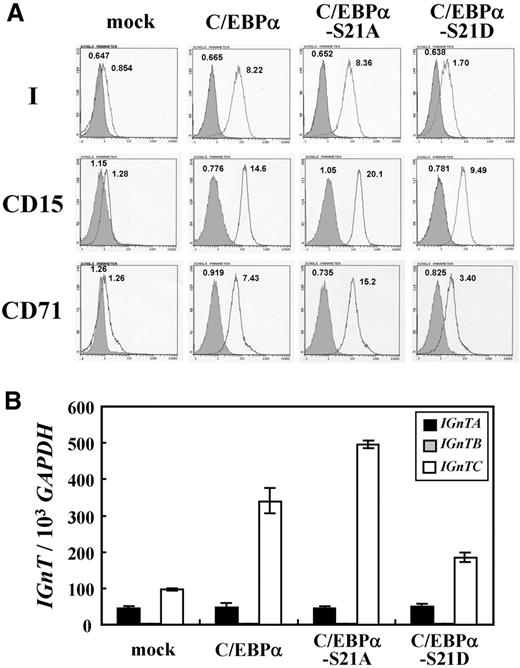
By use of site-directed mutagenesis, the Ser-21 and Ser-234 of human C/EBPα were substituted with Ala or Asp amino acids separately, which mimicked the dephosphorylated and phosphorylated states of the Ser residues, respectively. K-562 cells, which have been shown to exhibit i-to-I antigen conversion when induced to differentiate into erythrocyte lineage,28 were used. Overexpression of these C/EBPα constructs in K-562 cells was performed as described in “Expression of wild-type and mutant C/EBPα in K-562 cells” and Western blotting with Penta-His antibody demonstrated that the expressions of the wild-type and mutant C/EBPα factors were similar in each group of cells (supplemental Figure 1). Flow cytometric analysis demonstrated that expression of wild-type C/EBPα significantly elevated the expression of I antigen on cell surfaces (Figure 2). The C/EBPα mutants with S21A, S234A, or S234D changes exhibited the capability to induce I antigen expression similar to that of the wild-type C/EBPα; nevertheless, the S21D substitution abolished the I antigen–induction activity of C/EBPα in K-562 cells.
Structures of the straight and branched poly-LacNAc chains. The i antigen is characterized by the straight chain of repeating LacNAc units and is converted to the I antigen structure, characterized by the branched poly-LacNAc chain, through the activity of I β6GlcNAcT, which synthesizes the decisive GlcNAcβ1-6 branch. Various glyco-epitopes, including blood group A, B, and H antigens and the Lewis antigen series (Lea, Leb, Lex, Ley, sLea, and sLex), are constructed on the terminal ends of the straight and branched poly-LacNAc chains through different enzymatic pathways.
Structures of the straight and branched poly-LacNAc chains. The i antigen is characterized by the straight chain of repeating LacNAc units and is converted to the I antigen structure, characterized by the branched poly-LacNAc chain, through the activity of I β6GlcNAcT, which synthesizes the decisive GlcNAcβ1-6 branch. Various glyco-epitopes, including blood group A, B, and H antigens and the Lewis antigen series (Lea, Leb, Lex, Ley, sLea, and sLex), are constructed on the terminal ends of the straight and branched poly-LacNAc chains through different enzymatic pathways.
Expressions of I antigen and CD15 and CD71 markers in K-562 cells expressing wild-type and mutant C/EBPα. Wild-type C/EBPα and C/EBPα mutants with respective substitutions of S21A, S21D, S234A, and S234D were expressed in K-562 cells cultured in noninducing conditions. Expressions of cell-surface I antigen and CD15 and CD71 markers on the K-562 cells were analyzed by the use of flow cytometry and detected with monoclonal anti-I (1:15 dilution), anti-CD15 (1:200 dilution), and anti-CD71 (1:200 dilution) antibodies, respectively, with the bound antibodies on the cell surfaces revealed by 1:200 dilution of FITC-conjugated goat anti–human IgM, anti–mouse IgM, and anti–mouse IgG antibodies, respectively. The open and shaded areas represent results obtained from cells incubated with first antibody and FITC-conjugated secondary antibody, and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak.
Expressions of I antigen and CD15 and CD71 markers in K-562 cells expressing wild-type and mutant C/EBPα. Wild-type C/EBPα and C/EBPα mutants with respective substitutions of S21A, S21D, S234A, and S234D were expressed in K-562 cells cultured in noninducing conditions. Expressions of cell-surface I antigen and CD15 and CD71 markers on the K-562 cells were analyzed by the use of flow cytometry and detected with monoclonal anti-I (1:15 dilution), anti-CD15 (1:200 dilution), and anti-CD71 (1:200 dilution) antibodies, respectively, with the bound antibodies on the cell surfaces revealed by 1:200 dilution of FITC-conjugated goat anti–human IgM, anti–mouse IgM, and anti–mouse IgG antibodies, respectively. The open and shaded areas represent results obtained from cells incubated with first antibody and FITC-conjugated secondary antibody, and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak.
The effects of the wild-type and mutant C/EBPα on the expressions of granulocytic (CD15) and erythroid (CD71) markers in K-562 cells also were examined. The CD15 antigen was notably expressed in the original K-562 cells, and the expression appeared to retain high levels in the cells with overexpression of wild-type C/EBPα and mutant C/EBPα-S21A, although the geometric mean fluorescence intensities (MFIs) of CD15 expression in these 2 groups of cells were moderately lower than that of the control K-562 cells (Figure 2). However, overexpression of C/EBPα-S21D mutant significantly diminished the CD15 expression in K-562 cells. These results were consistent with a previous investigation23 in which the authors demonstrated that phosphorylation of Ser-21 in C/EBPα blocked its induction activity for granulopoietic differentiation. Overexpression of C/EBPα-S21A mutant in K-562 cells diminished the expression of CD71 marker, which expressed on the original K-562 cells notably, whereas CD71 expression was not diminished in the cells expressing C/EBPα-S21D, C/EBPα-S234A, and C/EBPα-S234D.
To discern which of the IGnT forms was responsible for the I antigen elevation in the different groups of K-562 cells, the expressions of the IGnTA, IGnTB, and IGnTC transcripts were quantitatively analyzed. As shown in Figure 3, expressions of wild-type C/EBPα and mutant C/EBPα with S21A, S234A, or S234D substitutions markedly increased the quantity of IGnTC, but not IGnTA and IGnTB, transcripts, compared with the IGnT profile in the mock control cells. This finding suggests that I antigen increase in the K-562 cells resulted from the activation of IGnTC gene expression. Overexpression of the C/EBPα-S21D mutant in K-562 cells did not stimulate any IGnT gene expression; this finding is consistent with the invalidation of C/EBPα-S21D on I antigen induction observed in the cells.
Expression profiles of IGnT genes in K-562 cells expressing wild-type and mutant C/EBPα. Wild-type C/EBPα and C/EBPα mutants with respective substitutions of S21A, S21D, S234A, and S234D were expressed in K-562 cells cultured in noninducing conditions. Real-time PCR was used to quantify the IGnTA, IGnTB, IGnTC, and GAPDH transcripts in the cDNA samples. The quantities of the IGnT transcripts were normalized to that of GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown.
Expression profiles of IGnT genes in K-562 cells expressing wild-type and mutant C/EBPα. Wild-type C/EBPα and C/EBPα mutants with respective substitutions of S21A, S21D, S234A, and S234D were expressed in K-562 cells cultured in noninducing conditions. Real-time PCR was used to quantify the IGnTA, IGnTB, IGnTC, and GAPDH transcripts in the cDNA samples. The quantities of the IGnT transcripts were normalized to that of GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown.
Association of C/EBPα-S21A and C/EBPα-S21D with the IGnTC gene promoter region
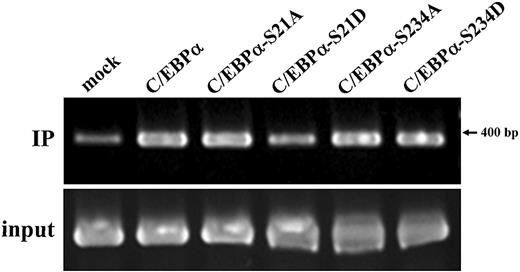
The associations of wild-type and mutant C/EBPα with the IGnTC gene 5′ promoter region (−322 to +62 bp) in K-562 cells were examined by the use of ChIP analysis (Figure 4),18 and the ChIP results were quantitatively analyzed by the use of densitometry (described in the legend). The results demonstrated the association of wild-type C/EBPα with the IGnTC promoter region, and the S21A, S234A, and S234D substitutions in C/EBPα did not alter the association noticeably; by contrast, the S21D change decreased the association of C/EBPα with the IGnTC promoter region. These association patterns are consistent with the induction profiles of IGnTC gene expression in K-562 cells (Figure 3), suggesting C/EBPα and C/EBPα-S21A, but not C/EBPα-S21D, play roles in the induction of IGnTC gene expression.
Association of wild-type and mutant C/EBPα with the IGnTC gene 5′ promoter region. Wild-type C/EBPα and C/EBPα mutants with respective substitutions of S21A, S21D, S234A, and S234D were expressed in K-562 cells cultured in noninducing conditions. The association of wild-type and mutant C/EBPα with the 5′ promoter region (−322 to +62 bp) of the IGnTC gene in K-562 cells was examined by the use of ChIP analysis. A total of 1 × 107 K-562 cells expressing wild-type C/EBPα, mutants C/EBPα-S21A, C/EBPα-S21D, C/EBPα-S234A, and C/EBPα-S234D or mock pcDNA3.1− vector were used. The chromatin DNAs were immunoprecipitated (IP) with a 1:500 dilution of anti-C/EBPα antibody and the input DNA controls were used in PCR amplification for the IGnTC −322 to +62-bp region.18 The products were analyzed by the use of 2.0% agarose gel electrophoresis. The K-562 cells expressing mock pcDNA3.1− vector were used for the no-antibody control in the PCR amplification, and only a trace of PCR product was observed (not shown). The results were analyzed by the use of densitometry, and the quantification analysis showed that the ratio of the band intensities (from left to right), after calibration with individual input controls, was 1.00:2.95:2.99:1.55:3.03:3.15.
Association of wild-type and mutant C/EBPα with the IGnTC gene 5′ promoter region. Wild-type C/EBPα and C/EBPα mutants with respective substitutions of S21A, S21D, S234A, and S234D were expressed in K-562 cells cultured in noninducing conditions. The association of wild-type and mutant C/EBPα with the 5′ promoter region (−322 to +62 bp) of the IGnTC gene in K-562 cells was examined by the use of ChIP analysis. A total of 1 × 107 K-562 cells expressing wild-type C/EBPα, mutants C/EBPα-S21A, C/EBPα-S21D, C/EBPα-S234A, and C/EBPα-S234D or mock pcDNA3.1− vector were used. The chromatin DNAs were immunoprecipitated (IP) with a 1:500 dilution of anti-C/EBPα antibody and the input DNA controls were used in PCR amplification for the IGnTC −322 to +62-bp region.18 The products were analyzed by the use of 2.0% agarose gel electrophoresis. The K-562 cells expressing mock pcDNA3.1− vector were used for the no-antibody control in the PCR amplification, and only a trace of PCR product was observed (not shown). The results were analyzed by the use of densitometry, and the quantification analysis showed that the ratio of the band intensities (from left to right), after calibration with individual input controls, was 1.00:2.95:2.99:1.55:3.03:3.15.
I antigen and IGnTC gene expression and dephosphorylation of C/EBPα Ser-21 in erythroid differentiation of K-562 cells
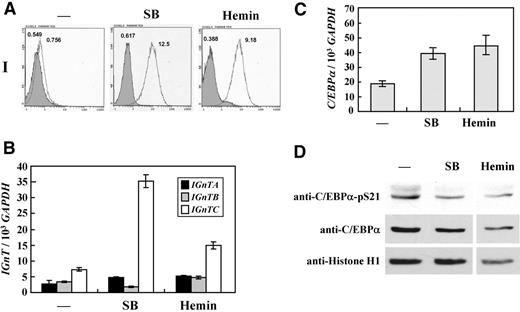
It has been shown that K-562 cells differentiate into erythroid lineage when treated with sodium butyrate and hemin.29,30 Benzidine staining was used to demonstrate the cells expressing hemoglobin. The original K-562 cells were virtually devoid of benzidine-positive particles; however, approximately 40% and 55% of the cells stained positively with benzidine after treatment with sodium butyrate and hemin, respectively (data not shown), indicating the induction of hemoglobin expression and erythroid differentiation of the cells. Flow cytometric analysis demonstrated marked elevation of I antigen expression in K-562 cells treated with sodium butyrate and hemin (Figure 5A). The expressions of the IGnT genes were quantitatively analyzed in K-562 cells with different treatments. The results showed that the expression of IGnTC, but not IGnTA and IGnTB, was notably stimulated in K-562 cells treated with sodium butyrate and hemin compared with the IGnT gene profile in the control cells (Figure 5B).
Expressions of I antigen and IGnT genes and phosphorylation status of C/EBPα Ser-21 in erythroid differentiation of K-562 cells. K-562 cells were cultured in medium supplemented with 2mM sodium butyrate (SB) for 2 days or 25μM hemin for 4 days to induce erythroid differentiation. (A) Flow cytometric analysis for I antigen expression detected with monoclonal anti-I antibody. Open and shaded areas represent cells detected with anti-I antibody and FITC-conjugated secondary antibody and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak. (B) Expression profiles for the IGnT transcripts. Real-time PCR was used to quantify the IGnTA, IGnTB, IGnTC, and GAPDH transcripts in the cDNA samples. The quantities of the IGnT transcripts were normalized to that of GAPDH transcript in each sample. Data were obtained from 3 detections; SD are shown. (C) Expression of C/EBPα transcript. Expressions of C/EBPα transcript in the cDNA samples were analyzed by the use of real-time PCR. The quantity of the C/EBPα transcript was normalized to that of the GAPDH transcript in each sample. Data were obtained from 3 detections; SD are shown. (D) Western blotting for C/EBPα and C/EBPα with phosphorylated Ser21. Nuclear fractions of the cells were analyzed using Western blotting with Phospho-C/EBPα(Ser21) antibody (1:1000 dilution), which specifically detected C/EBPα with phosphorylated Ser21 residue. After stripping, the membrane was detected successively with antibodies against C/EBPα (1:500 dilution) and histone H1 (1:500 dilution). The results were analyzed by densitometry, and quantification showed that the ratio of C/EBPα with phosphorylated Ser-21 in mock, SB-treated, and hemin-treated cells, after calibration with individual amounts of total C/EBPα and loading control histone, was 1.00:0.36:0.28.
Expressions of I antigen and IGnT genes and phosphorylation status of C/EBPα Ser-21 in erythroid differentiation of K-562 cells. K-562 cells were cultured in medium supplemented with 2mM sodium butyrate (SB) for 2 days or 25μM hemin for 4 days to induce erythroid differentiation. (A) Flow cytometric analysis for I antigen expression detected with monoclonal anti-I antibody. Open and shaded areas represent cells detected with anti-I antibody and FITC-conjugated secondary antibody and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak. (B) Expression profiles for the IGnT transcripts. Real-time PCR was used to quantify the IGnTA, IGnTB, IGnTC, and GAPDH transcripts in the cDNA samples. The quantities of the IGnT transcripts were normalized to that of GAPDH transcript in each sample. Data were obtained from 3 detections; SD are shown. (C) Expression of C/EBPα transcript. Expressions of C/EBPα transcript in the cDNA samples were analyzed by the use of real-time PCR. The quantity of the C/EBPα transcript was normalized to that of the GAPDH transcript in each sample. Data were obtained from 3 detections; SD are shown. (D) Western blotting for C/EBPα and C/EBPα with phosphorylated Ser21. Nuclear fractions of the cells were analyzed using Western blotting with Phospho-C/EBPα(Ser21) antibody (1:1000 dilution), which specifically detected C/EBPα with phosphorylated Ser21 residue. After stripping, the membrane was detected successively with antibodies against C/EBPα (1:500 dilution) and histone H1 (1:500 dilution). The results were analyzed by densitometry, and quantification showed that the ratio of C/EBPα with phosphorylated Ser-21 in mock, SB-treated, and hemin-treated cells, after calibration with individual amounts of total C/EBPα and loading control histone, was 1.00:0.36:0.28.
The expressions of the C/EBPα transcript and its protein product in K-562 cells were analyzed. compared with the control K-562 cells, the quantity of C/EBPα transcripts increased in the cells treated with sodium butyrate and hemin (Figure 5C); however, an increased protein level of 42-kDa C/EBPα was not observed in these cells (Figure 5D middle panel). Nevertheless, Western blot analysis with the use of antibody specifically detecting C/EBPα with phosphorylated Ser-21 revealed that the phosphorylation of Ser-21 in C/EBPα decreased in K-562 cells treated with sodium butyrate and hemin (Figure 5D upper panel). Densitometry was used to quantify the results of the Western blot analysis (shown in the legend). These results show that the I antigen formation in the erythroid differentiation of K-562 cells results from the activation of IGnTC gene expression and is accompanied by dephosphorylation of C/EBPα Ser-21.
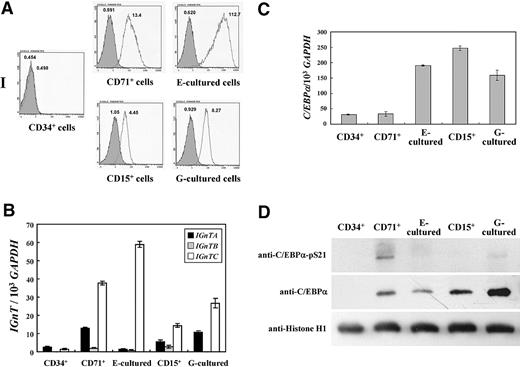
I antigen and IGnT gene profiles and phosphorylation status of C/EBPα Ser-21 in CD34+, CD71+, CD15+, E-cultured, and G-cultured cells
The expression profiles of I antigen and IGnT genes in in vivo erythropoietic and granulopoietic cells, CD34+, CD71+, and CD15+ blood progenitor cells, and E-cultured and G-cultured cells were investigated. CD34+ CD71+, and CD15+ cells were isolated as described in the section “Isolation of CD34+, CD71+, and CD15+ cells and E and G cultures”; flow cytometric analyses demonstrated that more than 95% of the collected cells possessed the respective CD marker, indicating considerable purities for the respective cells. CD34+ hematopoietic cells were induced to differentiate into erythroid and granulocytic lineages in E culture and G culture for 7 days, respectively. After the respective culture procedures, most of the cells (> 99%) became positive for CD71 or CD15 markers (data not shown), suggesting cellular differentiation into erythroid and granulocytic lineages, respectively.
As shown in Figure 6A, cell surface I antigen was not detected in CD34+ cells and was prominently expressed in CD71+ cells. The expression was even more prominent in the E-cultured cells. In the granulocytic lineage of CD15+ and G-cultured cells, the expression of I antigen was detected, although the expression amplitudes were not as prominent as those of CD71+ and E-cultured cells. Expression of the IGnTC gene was greatly elevated in CD71+ cells, and even more so in the E-cultured cells, compared with that in CD34+ cells (Figure 6B). In the CD15+ and G-cultured cells, IGnTC gene expression increased notably. Slight increases of IGnTA expression also were observed in CD71+, CD15+, and G-cultured cells but not in E-cultured cells. These profiles demonstrate good correlation between the expression of I antigen and the increase of IGnTC gene expression in the cells, illustrating that I antigen formation in both erythrocyte- and granulocyte-lineage cells is determined by the IGnTC gene.
Expression profiles of I antigen and IGnT genes and phosphorylation status of C/EBPα Ser-21 in CD34+, CD71+, CD15+, E-cultured, and G-cultured cells. (A) Flow cytometry analysis for I antigen expression. Adult CD34+, CD71+, and CD15+ cells were purified as described in “Isolation of CD34+, CD71+, and CD15+ cells and E and G cultures.” Flow cytometric analyses demonstrated that more than 95% of the collected cells possessed the respective CD marker. E-cultured and G-cultured cells were obtained through culture of CD34+ cells in erythropoietic and granulopoietic conditions, respectively, and more than 99% of the cells became positive for CD71 or CD15 markers after the respective culture procedures. Cell-surface I antigens of these cells were detected with the use of flow cytometry and with monoclonal anti-I antibody. Open and shaded areas represent cells detected with anti-I antibody and FITC-conjugated secondary antibody and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak. (B) Expressions of IGnT transcripts. The expressions of IGnT transcripts in the cDNA sample were analyzed with the use of real-time PCR. The quantities of IGnT transcripts were normalized to that of the GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown. (C) Expressions of C/EBPα transcripts. The C/EBPα transcript in the cDNA sample was quantitatively analyzed by the use of real-time PCR. The quantities of C/EBPα transcripts were normalized to that of the GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown. (D) Western blotting for C/EBPα and C/EBPα with phosphorylated Ser-21. Nuclear fractions of the cells were analyzed by the use of Western blotting with Phospho-C/EBPα(Ser21) antibody. After stripping, the membrane was detected successively with the antibodies against C/EBPα and histone H1.
Expression profiles of I antigen and IGnT genes and phosphorylation status of C/EBPα Ser-21 in CD34+, CD71+, CD15+, E-cultured, and G-cultured cells. (A) Flow cytometry analysis for I antigen expression. Adult CD34+, CD71+, and CD15+ cells were purified as described in “Isolation of CD34+, CD71+, and CD15+ cells and E and G cultures.” Flow cytometric analyses demonstrated that more than 95% of the collected cells possessed the respective CD marker. E-cultured and G-cultured cells were obtained through culture of CD34+ cells in erythropoietic and granulopoietic conditions, respectively, and more than 99% of the cells became positive for CD71 or CD15 markers after the respective culture procedures. Cell-surface I antigens of these cells were detected with the use of flow cytometry and with monoclonal anti-I antibody. Open and shaded areas represent cells detected with anti-I antibody and FITC-conjugated secondary antibody and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak. (B) Expressions of IGnT transcripts. The expressions of IGnT transcripts in the cDNA sample were analyzed with the use of real-time PCR. The quantities of IGnT transcripts were normalized to that of the GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown. (C) Expressions of C/EBPα transcripts. The C/EBPα transcript in the cDNA sample was quantitatively analyzed by the use of real-time PCR. The quantities of C/EBPα transcripts were normalized to that of the GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown. (D) Western blotting for C/EBPα and C/EBPα with phosphorylated Ser-21. Nuclear fractions of the cells were analyzed by the use of Western blotting with Phospho-C/EBPα(Ser21) antibody. After stripping, the membrane was detected successively with the antibodies against C/EBPα and histone H1.
The expressions of the C/EBPα transcript and its protein product, together with the phosphorylation status of C/EBPα Ser-21, in the cells were analyzed. Compared with CD34+ hematopoietic cells, elevations of the C/EBPα transcript level were observed in CD15+, E-cultured, and G-cultured cells, but not in CD71+ cells (Figure 6C). Expression of 42-kDa C/EBPα protein was demonstrated in all of the cells except CD34+ cells (Figure 6D middle panel), and the 42-kDa C/EBPα protein was prominently expressed in G-cultured cells. The absence of C/EBPα protein expression in adult CD34+ cells also was demonstrated in our previous investigation.18 The profiles of C/EBPα Ser-21 phosphorylation in these cells (Figure 6D top panel) showed that a very low proportion of the expressed 42-kDa C/EBPα possessed the phosphorylated Ser-21 residue in E-cultured, CD15+, and G-cultured cells compared with the portion of the phosphorylated C/EBPα Ser-21 to the total C/EBPα expressed in CD71+ cells. The extent of C/EBPα Ser-21 phosphorylation was especially low in the granulocytic lineage of CD15+ and G-cultured cells, consistent with the functional role of dephosphorylated C/EBPα Ser-21 in the development of granulocytes.
In the erythroid lineage of CD71+ and E-cultured cells, phosphorylation of C/EBPα Ser-21 in E-cultured cells clearly abated in comparison with that in CD71+ cells. Greater expression of dephosphorylated C/EBPα Ser-21 in granulocytic CD15+ and G-cultured cells than that in erythroid CD71+ and E-cultured cells was observed. This finding did not correspond with the IGnTC expression profiles in these 2 cell lineages, in which IGnTC expressions were greater in CD71+ and E-cultured cells than in CD15+ and G-cultured cells. However, when comparing C/EBPα Ser-21 phosphorylation and IGnTC gene expression in E-cultured cells to that in CD71+ cells, dephosphorylation of C/EBPα Ser-21 in erythropoietic processes was likely; the profile corresponded to the pattern of IGnTC gene induction in these 2 cell types.
C/EBPα, C/EBPα-S21A, and C/EBPα-S21D overexpression in CD34+ hematopoietic cells
The wild-type C/EBPα and mutant C/EBPα-S21A and C/EBPα-S21D were introduced into CD34+ hematopoietic cells by the use of lentiviral expression system. Western blotting with Penta-His antibody demonstrated that the expressions of the wild-type and mutant C/EBPα factors were similar in each group of the cells (supplemental Figure 2). The effects of these factors on the expressions of I antigen, CD15 and CD71 markers, and IGnT genes in CD34+ cells were analyzed. Overexpression of wild-type C/EBPα in CD34+ cells induced I antigen formation (Figure 7A) and stimulated the expression of IGnTC, but not IGnTA and IGnTB genes (Figure 7B). C/EBPα-S21A showed the capability of I antigen and IGnTC gene inductions in CD34+ cells, as did wild-type C/EBPα; nevertheless, the S21D substitution in C/EBPα diminished C/EBPα capacity to induce I antigen and IGnTC gene expression.
Lentiviral expression of C/EBPα, C/EBPα-S21A, and C/EBPα-S21D in CD34+ cells. (A) Flow cytometric analysis for I antigen expression. Expression of the I antigen on the cells transduced with the virions prepared from the pLenti6 gene transfer vectors harboring full coding cDNAs for C/EBPα, C/EBPα-S21A, or C/EBPα-S21D, and those transduced with the virions prepared from the mock pLenti6 vector were analyzed using flow cytometry and detected with monoclonal anti-I antibody. Open and shaded areas indicate cells detected with anti-I antibody and FITC-conjugated secondary antibody and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak. (B) Expression profiles of IGnT transcripts. Real-time PCR was used to quantify the IGnT and GAPDH transcripts in the cDNA sample. Quantities of the IGnTA, IGnTB, and IGnTC transcripts were normalized to that of GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown.
Lentiviral expression of C/EBPα, C/EBPα-S21A, and C/EBPα-S21D in CD34+ cells. (A) Flow cytometric analysis for I antigen expression. Expression of the I antigen on the cells transduced with the virions prepared from the pLenti6 gene transfer vectors harboring full coding cDNAs for C/EBPα, C/EBPα-S21A, or C/EBPα-S21D, and those transduced with the virions prepared from the mock pLenti6 vector were analyzed using flow cytometry and detected with monoclonal anti-I antibody. Open and shaded areas indicate cells detected with anti-I antibody and FITC-conjugated secondary antibody and with FITC-conjugated secondary antibody only, respectively. The geometric MFI detected is shown on the top of each peak. (B) Expression profiles of IGnT transcripts. Real-time PCR was used to quantify the IGnT and GAPDH transcripts in the cDNA sample. Quantities of the IGnTA, IGnTB, and IGnTC transcripts were normalized to that of GAPDH transcript in each sample. Data were obtained from 3 detections; SDs are shown.
The CD15 antigen profile resembled that of I antigen in these cells, with significant induction of CD15 expression by wild-type C/EBPα and mutant C/EBPα-S21A, and a much lesser extent by C/EBPα-S21D (Figure 7A). This profile of CD15 stimulation conforms to the known function of C/EBPα transcription factor, especially the form with dephosphorylated Ser-21, in the induction of granulopoiesis. It is noteworthy that the expression of CD71 antigen also paralleled that of antigens I and CD15 in these cells: marked induction of CD71 antigen expression was observed in the CD34+ cells with overexpression of wild-type C/EBPα and mutant C/EBPα-S21A, and the induction was greatly reduced in the cells with C/EBPα-S21D expression.
In combination with other results observed with the use of the K-562 cell model and in in vivo erythropoietic and granulopoietic cells, evidence obtained from wild-type and mutant C/EBPα expression in CD34+ hematopoietic cells demonstrated the functional role of C/EBPα with dephosphorylated Ser-21 residue in the stimulation of IGnTC gene expression, and consequently I antigen formation, in both erythroid and granulocytic differentiation.
Discussion
Transcription factor C/EBPα plays a crucial role in the development of granulocytes and controls granulopoietic differentiation in a stage-specific manner.19,20 The function of C/EBPα in granulopoiesis is critically affected by the phosphorylation status of its Ser-21 residue, with phosphorylation of Ser-21 blocking the ability of C/EBPα to induce granulopoiesis.23 With the exception of granulocytes, C/EBPα has not been implicated in the differentiation of other hematopoietic lineages.31 However, in a previous investigation, we found that C/EBPα transcription factor induces IGnTC gene expression, consequently leading to the formation of I antigen in erythropoietic processes.18 Our present investigation further demonstrates that phosphorylation of C/EBPα Ser-21 residue blocks C/EBPα activity on IGnTC gene stimulation and that dephosphorylation of C/EBPα Ser-21 occurs in both granulopoietic and erythropoietic processes, leading to the stimulation of IGnTC gene expression, and consequently, I antigen formation.
C/EBPα belongs to a family of leucine-zipper transcription factors.32,33 It conducts critical functions in regulating the balance between cell proliferation and differentiation in various cells, particularly in hepatocytes, adipocytes, and hematopoietic cells. In the hematopoietic system, C/EBPα is the key factor for granulopoiesis, and the amino terminal and E2F interaction domains on C/EBPα are critical for this function.34 Furthermore, C/EBPα also participates in regulating the repopulating activity of hematopoietic stem cells.35 Loss of C/EBPα function in myeloid cells leads to blocked myeloid differentiation, resulting in early blockage of granulocyte maturation. Furthermore, C/EBPα gene mutations have been observed frequently in patients with acute myeloid leukemia.20 Phosphorylation plays a critical role in the modulation of C/EBPα function. For example, protein kinase C can phosphorylate C/EBPα at Ser residues, leading to an attenuation of its DNA-binding activity.36 Cyclin D3-cdk4/cdk6 complex phosphorylates mouse C/EBPα at Ser-193 in the liver and supports the growth-inhibitory activity of C/EBPα.37 Signaling through the phosphatidylinositol 3-kinase-Akt pathway induces protein phosphatase 2A-mediated dephosphorylation of mouse C/EBPα Ser-193 in liver tumor cells, leading to blockage of C/EBPα-mediated growth inhibition.26 Furthermore, phosphorylation of rat C/EBPα Ser-248, through the Ras signaling pathway, enhances the activity of C/EBPα to induce granulocytic differentiation.27,38 Our present research shows cells of erythrocyte lineage use a mechanism crucial for the development of granulocyte lineage, dephosphorylation of C/EBPα Ser-21, to regulate the expression of cell-surface I antigen.
It is of interest to note that, in addition to the induction of I antigen and CD15 marker, overexpression of C/EBPα and C/EBPα-S21A in CD34+ hematopoietic cells also induced the expression of CD71 antigen (Figure 7). The S21D substitution greatly decreased the ability of C/EBPα to induce CD71 expression in CD34+ cells. Two other erythrocyte markers, glycophorin A and hemoglobin, also were examined in CD34+ cells with overexpression of C/EBPα; however, induction of these 2 markers was not observed (data not shown). In the K-562 cell model, the phenomenon of CD71 marker increase caused by C/EBPα transcription factor was indistinct (Figure 2); this is probably the result of the terminal-differentiated character of K-562 cells, which express considerable amounts of CD71 and glycophorin A antigens already. The CD71 antigen is a transferrin receptor, which is essential for iron transport into cells. Transferrin receptor is necessary for the development of erythrocytes.39 We are interested in elucidating the mechanism through which C/EBPα transcription factor induced the expression of transferrin receptor during erythropoiesis and whether C/EBPα exerted any influence on the development of erythrocytes.
It has been shown that the transition from straight to branched poly-LacNAc structure leads to drastic changes of antigenicity of the glyco-epitopes building on the terminals of poly-LacNAc chains. An evident example is the histo-blood group A and B antigens on fetal and neonatal red cells. Despite the presence of IgG anti-A and anti-B antibodies in the serum of group O mothers, severe hemolytic disease in the fetus and newborn (HDFN) caused by these antibodies is rare.40 Unlike adult red cells, which present strong reactivity with anti-A and anti-B antibodies, the fetal and neonatal red cells react very weakly with anti-A and anti-B antibodies. This weak reactivity of fetal and neonatal red cells is suggested to contribute to the prevention of severe HDFN because of an ABO-incompatible pregnancy. The fetal and neonatal red cells express straight poly-LacNAc chains (i antigen) predominantly; after birth the poly-LacNAcs on red cells are converted to the branched structure (I antigen) rapidly.10,12 An IgG antibody molecule is able to attach both of its combining sites to its binding antigen, a condition termed monogamous bivalency,41 and binding of antibody by 2 or more combining sites is favored over analogous single-site attachment by a factor greater than 103.42,43 The paucity and the spatial configuration of the A and B antigens carried by the straight poly-LacNAc chains restrain the monogamous bivalent binding of anti-A and anti-B antibodies,44 and thus the straight poly-LacNAc structures on the fetal and neonatal red cells lead to weak reactivity of the cells to anti-A and anti-B antibodies. Because the branching of poly-LacNAc structures on red cells would compromise ABO-incompatible pregnancies, it has been proposed that there is a strong biologic selection against the phenotypic transition from the straight-chain i to branched-chain I phenotype on red cells.44 However, formation of poly-LacNAc branching occurs on red cells, and to prevent severe ABO HDFN, is regulated to take place after birth, suggesting that the formation of the poly-LacNAc branching structure should have an elaborate biologic significance for postnatal red cells.
A study of embryonic stem cells expressing embryoglycan also illustrates the significance of poly-LacNAc branching structure.45 The study showed that the embryonic stem cells deficient in I β6GlcNAcT activity, although continuing to express stage-specific embryonic antigen-1, a Lex antigen, lost antigenicity of the 4C9 epitope, also a Lex antigen. The results suggest that 4C9 antigenicity requires either Lex epitope building on a structure with poly-LacNAc branching or clustering of Lex epitopes, accomplished by branching of poly-LacNAc chains. The study further indicates the significance of poly-LacNAc branching formation on the antigenicity of the glyco-epitopes on poly-LacNAc terminals.
In addition to developmentally regulated expression, altered expression patterns for I antigen often have been observed during oncogenetic processes.2,46 Because of the drastic effect of poly-LacNAc branching formation on the antigenicity of the terminal glyco-epitopes, it is rational that tight regulation of the transition between straight and branched poly-LacNAc chains has been observed during the developmental and cellular differentiation processes. The molecular genetic architecture of the I β6GlcNAcT gene locus, which expresses 3 different IGnT transcripts under the control of specific regulatory mechanisms, affords the complex regulation of the spatially and temporally specific expression of the poly-LacNAc branching in different cells. We demonstrated the mechanism for the regulation of poly-LacNAc branching formation in both erythropoietic and granulopoietic processes. Furthermore, we seek to reveal the possible effect or consequence on the expressions or antigenicities of terminal glyco-epitopes led by the i-to-I transition in granulocytes and to elucidate whether this transition in granulocytes occurs only postnatally, as observed in erythrocytes.
We believe the progress achieved in the present study encourages further investigation to elucidate the detailed cellular mechanisms responsible for modifying the C/EBPα Ser-21 phosphorylation status to stimulate IGnTC gene expression. It has been demonstrated that extracellular signal-regulated kinases 1 and 2 interacts with C/EBPα and phosphorylates the C/EBPα Ser-21 residue, leading to inhibition of C/EBPα to induce granulopoiesis.23,24 It should be especially noted that activation of p38 mitogen-activated protein kinase and the inhibition of the extracellular signal-regulated kinase signaling pathways for the induction of erythroid differentiation of K-562 cells upon treatment with histone deacetylase inhibitors (sodium butyrate, apicidin, or trichostatin) is well established.47,–49 However, activation of p38 mitogen-activated protein kinase stimulates phosphorylation of C/EBPα Ser-21 in hepatocytes and increases the transactivation activity of C/EBPα to stimulate the transcription of the gluconeogenic gene phosphoenolpyruvate carboxykinase.50 These studies illustrate the complexity and the involvement of different signaling pathways in different cells for regulation of C/EBPα Ser-21 phosphorylation. It is of particular interest to reveal whether erythrocyte and granulocyte lineages use the same cellular mechanisms to regulate the phosphorylation status of C/EBPα Ser-21, and elucidation of these detailed cellular mechanisms will provide information for further investigation to elucidate the molecular mechanism, leading to the postnatal transition of straight to branched poly-LacNAc chains.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was partially supported by grants NSC 96-2320-B-002-074-MY3 and NSC 98-3112-B-002-032 from the National Science Council, Taiwan.
Authorship
Contribution: Y.-C.T. designed and performed research; C.-Y.H. performed research; M.L. designed research and contributed vital reagents; C.-H.T. contributed vital reagents; C.-F.S. contributed vital reagents; and L.-C.Y. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lung-Chih Yu, Institute of Biochemical Sciences, National Taiwan University, P.O. Box 23-106, Taipei 106, Taiwan; e-mail: yulc@ntu.edu.tw.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal