Abstract
Autologous stem cell transplantation (ASCT) is recommended for younger patients with newly diagnosed multiple myeloma. Achieving complete response (CR) or at least very good partial response (VGPR) is a major prognostic factor for survival with 20% to 30% of patients achieving CR after ASCT. Bortezomib has shown synergistic effects with melphalan and no prolonged hematologic toxicity. In this Intergroupe Francophone du Myélome (IFM) phase 2 study, 54 untreated patients were enrolled between July and December 2007 to receive bortezomib (1 mg/m2 × 4) and melphalan (200 mg/m2) as conditioning regimen (Bor-HDM). Overall, 70% of patients achieved at least VGPR, including 17 patients with CR (32%) after ASCT. No toxic deaths were observed. Bortezomib did not increase hematologic toxicity. Only 1 grade 3 to 4 peripheral neuropathy was reported. A matched control analysis was conducted comparing our cohort with patients from the IFM 2005-01 trial (HDM alone). Patients were matched for response to induction therapy and type of induction: CR was higher in the Bor-HDM group (35% vs 11%; P = .001), regardless of induction therapy. These results suggest that Bor-HDM is a safe and promising conditioning regimen. Randomized studies are needed to assess whether this conditioning regimen is superior to HDM alone. This trial was registered at www.clinicaltrials.gov as NCT00642395.
Introduction
High-dose therapy (HDT) with autologous stem cell transplantation (ASCT) is recommended as part of the initial treatment strategy or at time of relapse in patients with newly diagnosed multiple myeloma (MM) eligible to undergo the procedure. Several trials have shown the superiority of intensive therapy over conventional chemotherapy in terms of response rate (RR), progression-free survival (PFS)1-4 and/or overall survival (OS).5-7 The outcome after ASCT is related to the quality of response. Achieving a complete response (CR) or at least a very good partial response (VGPR) is a major prognostic factor for long-term survival.8-12 Emerging data show that novel agents, such as immunomodulatory derivatives of thalidomide (IMiDs) and proteasome inhibitors, in combination with conventional agents in the setting of high-dose strategies, have considerably improved RRs. Currently, in intent-to-treat analyses, 20% to 30% of patients achieve CR with new induction regimen followed by HDT.13-18 High-dose melphalan (HDM; 200 mg/m2) is the recommended conditioning regimen before ASCT. Combination of HDM with total body irradiation, busulfan and/or other drugs have generally failed to increase survival because they frequently induced additional hematologic and nonhematologic toxicities.19-23 Bortezomib is a potent, selective, and reversible proteasome inhibitor.24 Synergistic effects have been reported both in vitro25,26 and in vivo27,28 between bortezomib and melphalan. The combination of bortezomib and HDM was consequently a logical and attractive approach to improve the efficacy of the conditioning regimen. Furthermore, this association was expected to be safe because bortezomib and melphalan do not share common toxicities (mainly neurologic toxicity for bortezomib and hematologic toxicity for HDM).
We therefore conducted a phase 2 trial of this combination. The aim of the study was to evaluate CR and VGPR rates after intensive therapy and to assess the toxicity of this new conditioning regimen.
Methods
Patient eligibility criteria
Patients younger than 65 years with symptomatic MM could be included in this trial, provided that they were eligible for HDT and they had nonprogressive disease after induction therapy. The exclusion criteria were a serum creatinine level of 2.5 mg/dL or more at time of HDT; liver insufficiency, for example, a serum total bilirubin level of 2.0 mg/dL or more, serum aspartate/alanine aminotransferase levels or alkaline phosphatases levels more than 2.5 times the upper limit of normal; a left ventricular ejection fraction of no more than 50% and a pulmonary diffusing capacity of less than 50% of predicted; a grade 3 or worse peripheral neuropathy, a significant comorbid disease that would preclude ASCT; and a history of any other malignant disease with the exception of basal cell carcinoma and stage I cervical cancer. Between July and December 2007, 54 patients with de novo MM were enrolled in this study at the time of HDT in 16 Intergroupe Francophone du Myélome (IFM) transplantation centers. Enrollment started at the end of the IFM 2005-01 trial's accrual. The choice of induction therapy was not specified in the protocol. All patients were evaluated after induction therapy and at 3 months after ASCT. There was no consolidation or maintenance therapy planned in the trial. The institutional ethics committees of all participating centers approved the study, and all patients gave written informed consent before entering the study in accordance with the Declaration of Helsinki. The original clinical trial was registered at www.clinicaltrials.gov as NCT00642395.
Study design
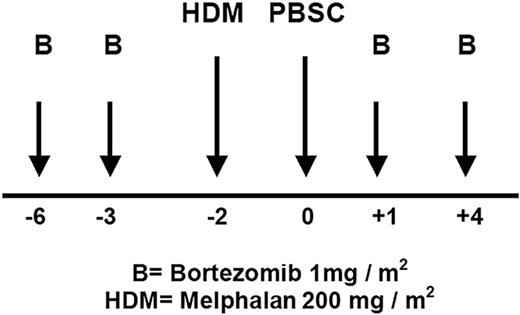
This multicenter, single-arm, open-label, phase 2 study evaluated the efficacy and safety of bortezomib in combination with HDM 200 mg/m2 (Bor-HDM) as transplantation conditioning regimen in patients with MM. Bortezomib was administered intravenously at 1 mg/m2 on days −6, −3, 1, and 4. Melphalan was administered intravenously at 200 mg/m2 on day −2. Peripheral blood stem cells (≥ 2 × 106 CD34+ cells/kg) were infused on day 0. The treatment schema is shown in Figure 1. All patients received standard supportive care measures, including growth factor support, blood transfusions, and prophylactic or therapeutic antibiotics according to local departmental guidelines at the time. Patients were discharged home after neutrophil (absolute neutrophil count [ANC] ≥ 0.5 × 109/L for 3 consecutive days) and platelet recovery (≥ 20 × 109/L without transfusion).
Criteria for evaluation
The primary objective of the trial was achievement of CR and VGPR at 3 months after ASCT. The secondary end points were safety profile of the combination regimen (eg, frequency of grade 3 or worse toxicities), PFS, and OS. Initial diagnostic and staging evaluations, initial therapies, and disease status at enrollment were documented for all patients. Myeloma testing included screening for chromosomal abnormalities by fluorescence in situ hybridization analysis, β2-microglobulin and albumin levels, serum and urine electrophoresis plus immunofixation, and radiographic studies (skeletal survey, total spine magnetic resonance imaging). Patients were staged by the Durie–Salmon staging system and by the International Staging System (ISS) at diagnosis.29,30 Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria of Adverse Events, version 3.0. Myeloma response and relapse definitions were based on the International Myeloma Working Group uniform response criteria,31 including bone marrow (BM) evaluation. Responses were assessed at the time of enrollment (after induction therapy) and at 3 months after ASCT. All patients were followed until death or reference date (March 31, 2009).
Matched control study
In an attempt to determine whether this Bor-HDM conditioning regimen was superior to HDM alone, we secondarily compared our cohort with patients from the IFM 2005-01 trial. This later study evaluated induction therapy with bortezomib and dexamethasone (Bor/Dex; arm B1) versus classic vincristine, Adriamycin, and dexamethasone (VAD; arm A1), followed by ASCT prepared with HDM 200 mg/m2 alone.17 To be eligible for the matched study, patients from the IFM 2005-01 trial should have nonprogressive disease after induction therapy and should have actually received HDM. Overall, 192 patients from the IFM 2005-01 trial were preselected for the comparison. According to predictive factors of CR after ASCT in this later group, 115 patients were subsequently matched for response to induction therapy (CR, VGPR, partial response, or stable disease) and type of induction therapy (VAD or Bor/Dex) in a 5:2 ratio. Therefore, 161 patients were selected and analyzed for response after ASCT, including 46 patients from the Bor-HDM study and 115 patients from the IFM 2005-01 trial (control). Seven patients in the Bor-HDM study, who needed more than 2 lines of induction therapy to achieve response before ASCT, were disqualified for this matched study because they had no suitable control in the IFM 2005-01 cohort.
Statistical considerations
Fisher exact tests and χ2 tests were used to compare differences in categorical variables and RRs. Comparisons were 2-sided unless otherwise specified, using P value less than .05 as significance level. To evaluate the effect of prognostic factors on CR after Bor-HDM, univariate analysis was conducted using age, sex, chromosomal abnormalities [del(13); del(17p); t(4;14)], ISS, induction therapy (VAD, Bor/Dex, other), and response to induction therapy (VGPR or better: yes, no). To determine the matching criteria for the comparison between our patients and patients from the IFM 2005-01 trial, similar univariate and multivariate analyses were conducted using age, sex, chromosomal abnormalities, ISS, induction therapy (VAD or Bor/Dex), and response to induction therapy (CR, VGPR, partial response or less). The duration of PFS was calculated for all patients from the start of induction therapy to the time of progression, relapse, death from any cause, or reference date. The duration of OS was calculated for all patients from the start of induction therapy to the time of death or reference date. Kaplan-Meier method was used to estimate survival functions with comparisons made by log-rank test. All statistical analyses were performed with SPSS software (SPSS Inc).
Results
Baseline characteristics
Fifty-four untreated patients with MM with nonprogressive disease after induction therapy were enrolled in this trial at time of HDT and underwent a first ASCT with Bor-HDM as conditioning regimen. Patients' demographics and baseline characteristics are shown in Table 1. Fluorescence in situ hybridization analysis was informative in 49 patients, including the presence of a 13q deletion in 23 patients, a 17p deletion in 3 patients, and a t(4;14) translocation in 6 patients. The choice of induction therapy was not specified in the protocol and was made on an individual patient basis by the treating clinician. Patients either received VAD (n = 29; 54%) or a Bor/Dex-based regimen (n = 18; 33%). Seven patients (13%) needed more than 2 lines of therapy to achieve response before ASCT (VAD, Bor/Dex, lenalidomide/Dex, and/or DCEP [dexamethasone-cyclophosphamide-etoposide-cisplatin]).
Baseline demographics and disease-related characteristics of patients
| Characteristic . | Bor-HDM (n = 54) . |
|---|---|
| Sex, M/F, n | 31/23 |
| Median age, y (range) | 58 (40-65) |
| Isotype, n (%) | |
| IgG | 37 (69) |
| IgA | 10 (18) |
| LC | 5 (9) |
| Others | 2 (4) |
| ISS stage, n (%) | |
| I | 29 (54) |
| II | 13 (24) |
| III | 12 (22) |
| Durie-Salmon stage, n | |
| I | 8 |
| II | 8 |
| III | 43 |
| Median creatinine level, mg/dL (range) | 1.02 (0.55-7.43) |
| Median calcium level, mmol/L (range) | 2.3 (2.0-3.5) |
| Median β2-microglobulin level, mg/L (range) | 3.0 (1.7-15.7) |
| Median albumin level, g/L (range) | 38 (18-52) |
| Median hemoglobin level, g/dL (range) | 10.0 (5.0-11.2) |
| Median bone marrow plasma cells, % (range) | 30 (4-90) |
| FISH analysis (n = 49), n (%) | |
| del(13q) | 23 (47) |
| del(17p) | 3 (6) |
| t(4;14) | 6 (12) |
| Frontline treatments, n (%) | |
| VAD | 29 (54) |
| Bor/Dex | 18 (33) |
| More than 2 lines of therapy | 7 (13) |
| Characteristic . | Bor-HDM (n = 54) . |
|---|---|
| Sex, M/F, n | 31/23 |
| Median age, y (range) | 58 (40-65) |
| Isotype, n (%) | |
| IgG | 37 (69) |
| IgA | 10 (18) |
| LC | 5 (9) |
| Others | 2 (4) |
| ISS stage, n (%) | |
| I | 29 (54) |
| II | 13 (24) |
| III | 12 (22) |
| Durie-Salmon stage, n | |
| I | 8 |
| II | 8 |
| III | 43 |
| Median creatinine level, mg/dL (range) | 1.02 (0.55-7.43) |
| Median calcium level, mmol/L (range) | 2.3 (2.0-3.5) |
| Median β2-microglobulin level, mg/L (range) | 3.0 (1.7-15.7) |
| Median albumin level, g/L (range) | 38 (18-52) |
| Median hemoglobin level, g/dL (range) | 10.0 (5.0-11.2) |
| Median bone marrow plasma cells, % (range) | 30 (4-90) |
| FISH analysis (n = 49), n (%) | |
| del(13q) | 23 (47) |
| del(17p) | 3 (6) |
| t(4;14) | 6 (12) |
| Frontline treatments, n (%) | |
| VAD | 29 (54) |
| Bor/Dex | 18 (33) |
| More than 2 lines of therapy | 7 (13) |
Bor-HDM indicates bortezomib and high-dose melphalan; Ig, immunoglobulin; LC, light chain; ISS, International Staging System; FISH, fluorescence in situ hybridization; VAD, vincristine, Adriamycin, and dexamethasone; Bor/Dex, bortezomib and dexamethasone.
Response evaluation
One patient did not receive the planned ASC transplant because of pulmonary aspergillosis at time of HDT. The RRs at enrollment and after ASCT are shown in Table 2. Within 3 months after preparative regimen with Bor-HDM, 70% of patients achieved at least VGPR, including 17 patients with CR (32%). Three patients had negative immunofixation but no BM evaluation and were therefore assessed as VGPR following the International Myeloma Working Group response criteria. In the VAD subgroup (n = 28), 68% of patients achieved VGPR or more, including 9 patients with CR (32%). In the Bor/Dex subgroup (n = 18), 72% of patients attained at least VGPR, including 7 patients with CR (39%). In univariate analysis, response to induction therapy (VGPR or better) was the only prognostic factor identified as a predictor of CR after HDT (P = .01). Adverse cytogenetics had no effect on RR. All 3 patients with a 17p deletion achieved VGPR or better, including 1 patient (33%) with CR after HDT. Five (83%) of 6 patients with a t(4;14) translocation achieved at least VGPR, including 2 patients with CR (33%). Among patients with a 13q deletion, 18 (78%) of 23 patients achieved VGPR or better, including 10 patients with CR (44%).
Summary of clinical responses at enrollment and after Bor-HDM
| Response . | After induction therapy . | After ASCT with Bor-HDM . | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients (n = 54) . | VAD (n = 29) . | Bor/Dex (n = 1)8 . | 2 or more lines (n = 7) . | All patients (n = 53)* . | VAD (n = 28)* . | Bor/Dex (n = 18) . | 2 or more lines (n = 7) . | |
| CR | 2 (4) | 0 | 2 (11) | 0 | 17 (32) | 9 (32) | 7 (39) | 1 (14) |
| VGPR | 15 (28) | 6 (21) | 8 (44) | 1 (14) | 20 (38)† | 10 (36) | 6 (33) | 4 (57) |
| PR | 31 (57) | 20 (69) | 5 (28) | 6 (86) | 13 (24) | 7 (25) | 5 (28) | 1 (14) |
| SD | 6 (11) | 3 (10) | 3 (17) | 0 | 2 (4) | 2 (7) | 0 | 0 |
| PD | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 | 1 (14) |
| CR + VGPR | 17 (32) | 6 (21) | 10 (55) | 1 (14) | 37 (70) | 19 (68) | 13 (72) | 5 (71) |
| Response . | After induction therapy . | After ASCT with Bor-HDM . | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients (n = 54) . | VAD (n = 29) . | Bor/Dex (n = 1)8 . | 2 or more lines (n = 7) . | All patients (n = 53)* . | VAD (n = 28)* . | Bor/Dex (n = 18) . | 2 or more lines (n = 7) . | |
| CR | 2 (4) | 0 | 2 (11) | 0 | 17 (32) | 9 (32) | 7 (39) | 1 (14) |
| VGPR | 15 (28) | 6 (21) | 8 (44) | 1 (14) | 20 (38)† | 10 (36) | 6 (33) | 4 (57) |
| PR | 31 (57) | 20 (69) | 5 (28) | 6 (86) | 13 (24) | 7 (25) | 5 (28) | 1 (14) |
| SD | 6 (11) | 3 (10) | 3 (17) | 0 | 2 (4) | 2 (7) | 0 | 0 |
| PD | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 | 1 (14) |
| CR + VGPR | 17 (32) | 6 (21) | 10 (55) | 1 (14) | 37 (70) | 19 (68) | 13 (72) | 5 (71) |
Values are number (%) of patients.
Bor-HDM indicates bortezomib and high-dose melphalan, ASCT, autologous stem cell transplantation; VAD, vincristine, Adriamycin, and dexamethasone; Bor/Dex, bortezomib and dexamethasone; CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; and PD, progressive disease.
One patient, who was in PR after VAD induction therapy, did not receive the planned ASCT because of pulmonary aspergillosis.
Three patients with negative immunofixation but without bone marrow evaluation were assessed as VGPR.
Engraftment and treatment-related toxicities
All patients, who actually underwent the procedure, completed the full conditioning regimen with Bor-HDM. Patients received at transplantation a median of 4 × 106 CD34+ cells/kg (range, 2-12 × 106 CD34+ cells/kg). The CD34+ cell dose for each patient was selected by the treating physician and represented a fraction of the total CD34+ cells collected. Toxicities are summarized in Table 3. All patients except 4 received granulocyte growth factors. There was no engraftment failure. Neutrophils (ANC ≥ 0.5 × 109/L) and platelets (≥ 20 × 109/L without transfusion) recovered in median times of 7 days (range, 4-15 days) and 3 days (range, 0-11 days), respectively. The median time to platelet level of 50 × 109/L or greater was 9 days (range, 3-31 days). Patients were discharged from the transplantation unit in median times of 19 days (range, 14-29 days). There was no treatment-related death. Only 5 serious adverse events were reported: 1 pulmonary embolism, 1 seizure, 1 acute lithiasic cholecystitis, and 2 pneumonias. Fourteen bacteremias were documented: 7 Staphylococcus epidermidis, 3 Streptococcus viridans, 2 Enterococcus faecalis, 1 Pseudomonas aeruginosa, 1 Escherichia coli, and 1 Branhamella catarrhalis. No patients died of sepsis. Nine patients developed pneumonia, and 2 of them were suspected of aspergillosis pneumonia. Three transient and moderate acute renal failures were observed. The most frequently reported grade 3 or 4 nonhematologic toxicities were mucositis of upper and lower digestive tract sites (47%) and peripheral neuropathy (PN; 1 case). Other grade 1 or 2 adverse events were digestive (diarrhea, 72%), dermatologic reactions such as rash and erythrodermia (34%), headache (28%), and de novo PN (2 cases). It should be noted that PN was present at the time of ASCT in 9 patients and did not get worse after Bor-HDM treatment.
Engraftment and transplantation-related toxicities (n = 53*)
| . | Value . |
|---|---|
| Median duration of neutropenia (ANC < 0.5 × 109/L), d (range) | 7 (4-15) |
| Median duration of thrombocytopenia, d (range) | |
| Less than 20 × 109/L | 3 (0-11) |
| Less than 50 × 109/L | 9 (3-31) |
| Median no. of platelet transfusions (range) | 2 (0-7) |
| Median no. of packed red blood cell transfusions (range) | 1 (0-4) |
| Median duration of fever, d (range) | 3 (0-10) |
| Mucositis | |
| All grades, n (%) | 37 (70) |
| Grade 3 or 4, n (%) | 25 (47) |
| Median duration of mucositis, d (range) | 9 (2-13) |
| GI tract/diarrhea grade 1 or 2, n (%) | 38 (72) |
| Dermatologic/allergic reactions grade 1 or 2, n (%) | 18 (34) |
| Peripheral neuropathy (de novo), n (%) | 3 (6) |
| Headache, n (%) | 15 (28) |
| Toxic death, n | 0 |
| . | Value . |
|---|---|
| Median duration of neutropenia (ANC < 0.5 × 109/L), d (range) | 7 (4-15) |
| Median duration of thrombocytopenia, d (range) | |
| Less than 20 × 109/L | 3 (0-11) |
| Less than 50 × 109/L | 9 (3-31) |
| Median no. of platelet transfusions (range) | 2 (0-7) |
| Median no. of packed red blood cell transfusions (range) | 1 (0-4) |
| Median duration of fever, d (range) | 3 (0-10) |
| Mucositis | |
| All grades, n (%) | 37 (70) |
| Grade 3 or 4, n (%) | 25 (47) |
| Median duration of mucositis, d (range) | 9 (2-13) |
| GI tract/diarrhea grade 1 or 2, n (%) | 38 (72) |
| Dermatologic/allergic reactions grade 1 or 2, n (%) | 18 (34) |
| Peripheral neuropathy (de novo), n (%) | 3 (6) |
| Headache, n (%) | 15 (28) |
| Toxic death, n | 0 |
ANC indicates absolute neutrophil count; and GI, gastrointestinal.
One patient did not receive the planned autologous stem cell transplantation because of pulmonary aspergillosis.
PFS and OS
At time of reporting, median follow-up time from induction therapy was 22 months (range, 12-28 months). Thirteen patients relapsed with an estimated 2-year PFS of 71% (SE, 8%). Among patients who achieved CR, estimated 2-year PFS is 88% (SE, 8%). For patients who failed to achieve CR, estimated 2-year PFS is 63% (SE, 11%; P = .134). Two patients died of progressive disease at 5 and 15 months after ASCT with an estimated 2-year OS of 96% (SE, 3%).
Matched control study for CR rates
To determine whether this Bor-HDM conditioning regimen was better than HDM alone, 46 patients of the present study were matched with 115 patients from the IFM 2005-01 trial, according to response to induction therapy and induction treatment. Baseline characteristics of patients and RRs are shown in Tables 4 and 5, respectively. Overall, 35% of patients (n = 16) in the Bor-HDM study attained CR compared with 11% (n = 13) of the selected cohort in the IFM 2005-01 trial (P = .001; odds ratio [OR], 4.2; 95% CI, 1.8-9.7). Ten patients had negative immunofixation but no BM evaluation and were actually assessed as VGPR, including 3 patients (6.5%) in the Bor-HDM study and 7 patients (6%) in the control study. In the VAD induction subgroup (n = 98), 32% of patients (9 of 28) in the Bor-HDM study attained CR compared with 10% (7 of 70) in the IFM 2005-01 trial (P = .013; OR, 4.3; 95% CI, 1.4-13.0). In the Bor/Dex induction subgroup (n = 63), 39% of patients (7 of 18) in the Bor-HDM study attained CR compared with 13% (6 of 45) in the IFM 2005-01 trial (P = .038; OR, 4.1; 95% CI, 1.2-14.9). The advantage of Bor-HDM compared with HDM on CR rates was confirmed to be independent of induction therapy (P = .972).
Characteristics of patients in the matched control study
| Characteristic . | IFM 2005-01 (n = 115) . | IFM Bor-HDM (n = 46) . |
|---|---|---|
| Sex, M/F, n | 60/55 | 24/22 |
| Median age, y (range) | 58 (32-65) | 58 (40-65) |
| Isotype, % | ||
| IgG | 69 | 67 |
| IgA | 20 | 20 |
| LC | 7 | 9 |
| Others | 4 | 4 |
| ISS stage, % | ||
| I | 48 | 52 |
| II | 36 | 22 |
| III | 16 | 26 |
| Median β2-microglobulin level, mg/L (range) | 3.3 (1.3-41.9) | 3.3 (1.7-15.7) |
| Median albumin level, g/L (range) | 39 (22-54) | 39 (18-52) |
| FISH analysis, n/evaluable patients (%) | ||
| del(13q) | 59/113 (52) | 21/42 (50) |
| del(17p) and/or t(4;14) | 14/113 (12) | 8/37 (21) |
| Frontline treatments, n (%)* | ||
| VAD | 70 (61) | 28 (61) |
| Bor/Dex | 45 (39) | 18 (39) |
| Response to induction therapy, n (%)* | ||
| SD | 15 (13) | 6 (13) |
| PR | 60 (52) | 24 (52) |
| VGPR | 35 (30) | 14 (30) |
| CR | 5 (5) | 2 (5) |
| Characteristic . | IFM 2005-01 (n = 115) . | IFM Bor-HDM (n = 46) . |
|---|---|---|
| Sex, M/F, n | 60/55 | 24/22 |
| Median age, y (range) | 58 (32-65) | 58 (40-65) |
| Isotype, % | ||
| IgG | 69 | 67 |
| IgA | 20 | 20 |
| LC | 7 | 9 |
| Others | 4 | 4 |
| ISS stage, % | ||
| I | 48 | 52 |
| II | 36 | 22 |
| III | 16 | 26 |
| Median β2-microglobulin level, mg/L (range) | 3.3 (1.3-41.9) | 3.3 (1.7-15.7) |
| Median albumin level, g/L (range) | 39 (22-54) | 39 (18-52) |
| FISH analysis, n/evaluable patients (%) | ||
| del(13q) | 59/113 (52) | 21/42 (50) |
| del(17p) and/or t(4;14) | 14/113 (12) | 8/37 (21) |
| Frontline treatments, n (%)* | ||
| VAD | 70 (61) | 28 (61) |
| Bor/Dex | 45 (39) | 18 (39) |
| Response to induction therapy, n (%)* | ||
| SD | 15 (13) | 6 (13) |
| PR | 60 (52) | 24 (52) |
| VGPR | 35 (30) | 14 (30) |
| CR | 5 (5) | 2 (5) |
Ig indicates immunoglobulin; LC, light chain; ISS, International Staging System; FISH, fluorescence in situ hybridization; VAD, vincristine, Adriamycin, and dexamethasone; Bor/Dex, bortezomib and dexamethasone; SD, stable disease; PR, partial response; VGPR, very good partial response; and CR, complete response.
Distributions are identical due to matching.
Response rates after transplantation in the matched control study
| Response, n (%) . | All patients . | VAD induction . | Bor/Dex induction . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IFM 2005-01 (n = 115) . | Bor-HDM (n = 46) . | P . | IFM 2005-01 (n = 70) . | Bor-HDM (n = 28) . | P . | IFM 2005-01 (n = 45) . | Bor-HDM (n = 18) . | P . | |
| CR | 13 (11) | 16 (35) | .001 | 7 (10) | 9 (32) | .013 | 6 (13) | 7 (39) | .038 |
| VGPR* | 49 (43) | 16 (35) | 23 (33) | 10 (36) | 26 (58) | 6 (33) | |||
| PR | 50 (43) | 12 (26) | 38 (54) | 7 (25) | 12 (27) | 5 (28) | |||
| SD | 3 (3) | 2 (4) | 2 (3) | 2 (7) | 1 (2) | 0 | |||
| CR + VGPR | 62 (54) | 32 (70) | .078 | 30 (43) | 19 (68) | .043 | 32 (71) | 13 (72) | |
| Response, n (%) . | All patients . | VAD induction . | Bor/Dex induction . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IFM 2005-01 (n = 115) . | Bor-HDM (n = 46) . | P . | IFM 2005-01 (n = 70) . | Bor-HDM (n = 28) . | P . | IFM 2005-01 (n = 45) . | Bor-HDM (n = 18) . | P . | |
| CR | 13 (11) | 16 (35) | .001 | 7 (10) | 9 (32) | .013 | 6 (13) | 7 (39) | .038 |
| VGPR* | 49 (43) | 16 (35) | 23 (33) | 10 (36) | 26 (58) | 6 (33) | |||
| PR | 50 (43) | 12 (26) | 38 (54) | 7 (25) | 12 (27) | 5 (28) | |||
| SD | 3 (3) | 2 (4) | 2 (3) | 2 (7) | 1 (2) | 0 | |||
| CR + VGPR | 62 (54) | 32 (70) | .078 | 30 (43) | 19 (68) | .043 | 32 (71) | 13 (72) | |
VAD indicates vincristine, Adriamycin, and dexamethasone; Bor/Dex, bortezomib and dexamethasone; IFM, Intergroupe Francophone du Myélome; Bor-HDM, bortezomib and high-dose melphalan; CR, complete response; VGPR, very good partial response; PR, partial response; and SD, stable disease.
Ten patients with negative immunofixation but without bone marrow evaluation were assessed as VGPR, including 3 patients (6.5%) in the Bor-HDM study and 7 patients (6%) in the IFM 2005-01 trial.
Discussion
HDM (200 mg/m2) with ASCT is the standard of care for younger patients with MM. However, HDT is not curative, and most patients relapse in a median time of 3 years. Several approaches have been tried to enhance the efficacy of transplantation. These approaches have included the use of a higher dose of melphalan,32 the use of total body irradiation,19,20 as well as the incorporation of others drugs into the conditioning regimen.21-23 These approaches usually resulted in higher morbidity and mortality, and HDM is the recommended preparative regimen. Recently, promising results have been reported with intravenous busulfan33 and arsenic trioxide.34 In the past few years, several novel and highly effective agents, such as immunomodulatory derivatives of thalidomide and proteasome inhibitors, have improved RRs and patient outcome, both in the setting of relapse and frontline therapies. These novel agents may also increase the efficacy of HDM and transplantation outcomes with deeper and long-lasting responses. The main aim of this IFM phase 2 clinical trial was to determine whether combining bortezomib and HDM (Bor-HDM) would provide high CR and VGPR rates after transplantation without the burden of increased toxicity.
Our study shows that bortezomib can safely be combined with HDM as a preparative regimen followed by ASCT. This regimen was well tolerated with no treatment-related mortality or increased toxicity. All patients except one received the full Bor-HDM conditioning regimen. Engraftment was not affected by the addition of bortezomib. Median durations of neutropenia (ANC < 0.5 × 109/L) and thrombocytopenia (platelet counts < 50 × 109/L) were 7 days (range, 4-15 days) and 9 days (range, 3-31 days), respectively. These results are comparable with those reported in the IFM 9502 trial20 in which patients were treated with HDM alone (arm B). In the IFM 9502 trial, median durations of neutropenia and thrombocytopenia were 8 days (range, 4-34 days) and 7 days (range, 0-30 days), respectively. PN, which is the main adverse effect of bortezomib, was not, in this setting, a matter of concern. Actually, Bor-HDM conditioning regimen did not enhance severity of preexisting PN, and only 3 patients presented de novo PN (6%). Other nonhematologic toxicities with this regimen were mild and manageable (grade 1 or 2 skin reactions and headache).
The primary end point of this trial was to evaluate the efficacy of HDT and especially to assess CR and VGPR rates in this MM population. Although still controversial, CR is a surrogate marker for outcome and survival, especially in poor-risk patients.12,35 In this trial, 70% of patients attained VGPR or better with at least 32% of patients in CR after a single course of HDT prepared by the Bor-HDM conditioning regimen, regardless of the type of induction therapy. According to current strategies with induction therapies containing new drugs, 20% to 30% of patients attain CR after ASCT prepared by HDM alone (intent-to-treat results of Gruppo Italiano Malattie Ematologiche Maligne dell' Adult [GIMEMA],16 IFM 2005-01,17 and Hemato-oncologic volwassen Nederland [HOVON]18 trials). Our promising CR rate might therefore be related to the addition of bortezomib into the conditioning regimen. This hypothesis was confirmed by the matched control study between our cohort and patients of the IFM 2005-01 trial (who did actually undergo ASCT). The addition of bortezomib in the conditioning regimen dramatically improved CR from 11% (HDM alone) to 35% (Bor-HDM) (P = .001). Comparison between our patients and matched patients from the IFM 2005-01 trial could be biased because we used historical controls. Nevertheless, it should be noted that initial evaluations and response criteria were identical in both trials. Furthermore, enrollment in this trial started just at the end of the 2005-01 trial's accrual. Because these trials were conducted at the same time period, patients' management was unlikely to be different during and after HDT. Therefore, these results suggest that this combination of bortezomib and HDM could be more effective to achieve CR than HDM alone. Several mechanisms could support these high RRs after the Bor-HDM conditioning regimen. The proteasome inhibitor bortezomib is able to sensitize MM cells in vitro to DNA-damaging drugs (such as melphalan)25 and to overcome chemoresistance.36 Mitsiades et al25 demonstrated also that the sequence of administration (bortezomib after chemotherapy) might be critical to maximize the synergistic effects. These findings provided the rationale for the 2 doses of bortezomib after HDM in our schedule of administration, which might be, in part, responsible for the increased CR rates we observed. Furthermore, Lonial et al37 and Kaufman et al38 confirmed, in a phase 1 trial, that bortezomib, when infused after HDM, dramatically enhanced the apoptotic pattern of MM cells, resulting in high RRs. Bortezomib is known to target the BM microenvironment.36 The interaction between the myeloma cell and the BM microenvironment is central to the growth and survival of myeloma cells. Adherence of MM cells to BM stromal cells triggers nuclear factor-κB–dependent transcription and secretion of interleukin-6 (IL-6),39 a MM cell growth and survival factor.40 IL-6 overproduction occurring after HDM might favor reparation and survival of the residual plasma cells.41 By virtue of its inhibition of nuclear factor-κB activation,42 bortezomib can inhibit this synthesis of IL-6 and enhance apoptosis of MM cells. Consequently, this Bor-HDM conditioning regimen could concurrently target both the tumor cells and their BM microenvironment to improve RRs and treatment outcomes for patients with MM.
Combination of bortezomib and HDM is a safe and promising conditioning regimen. In our matched comparison, the CR rate after a single course of HDT in untreated patients with MM appears to be higher than those attained after HDM alone. These data give support for developing this Bor-HDM combination followed by ASCT. Prospective randomized trials are needed to assess whether this combining regimen is effectively better than HDM alone in the setting of induction therapies containing new drugs. Subsequent consolidation and/or maintenance therapies could further enhance the depth and the duration of response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the work performed by individual research teams at all participating study sites; we are also indebted to Pr T. Facon for his kind support, Pr M. Mohty for his good advice, and Dr P. Campbell for his critical reading of the manuscript.
This work was supported by research funding from Janssen-Cilag.
Authorship
Contribution: M.A. designed the study; M.R., P.M., A.H., C.D., D.C., C.H., C.F., G.M., B.P., P.L., C.A., B.K., E.R., B.R., A.-M.S., M.D., V.D., L.G., and J.-L.H. recruited subjects for the study; M.R. collected the data; P.M., H.A.-L., C.M., and J.-L.H. provided the IFM 2005-01 data for the matched control study; J.-Y.M. and M.R. analyzed the data and performed the statistical analyses; and M.R. and M.A. wrote the paper. All authors were involved in analyzing and interpreting the data and checked the final version of the manuscript; the authors were fully responsible for content and editorial decisions for this manuscript.
Conflict-of-interest disclosure: P.M., J.-L.H., H.A.-L., and M.A. have participated in advisory boards for Janssen-Cilag and Celgene Corporation. The remaining authors declare no competing financial interests.
A complete list of the members of the Intergroupe Francophone du Myélome appears as a supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Murielle Roussel, Service d'Hématologie, Hôpital Purpan, Place du Dr Baylac, TSA 40031, 31059 Toulouse cedex 9, France; e-mail: roussel.m@chu-toulouse.fr.