Abstract
Treatment options for patients with chronic refractory immune thrombocytopenic purpura (ITP) are limited. Because combination immunosuppressant therapy appeared to be effective in ITP and other disorders, we used this approach in patients with particularly severe and refractory ITP. In this retrospective, observational study, we determined the response (platelet count above 30 × 109/L and doubling of baseline) among 19 refractory ITP patients. Treatment consisted of azathioprine, mycophenolate mofetil, and cyclosporine. The patients had failed a median of 6 prior treatments, including splenectomy (in all except 1). Of 19 patients, 14 (73.7%) achieved a response lasting a median of 24 months, after which time 8 (57.1%) relapsed. Of the 8 relapsing patients, 6 responded to additional treatments. Of the 14 patients who achieved an initial response, 2 (14.3%) remained in remission after eventually stopping all medications. Severe adverse events did not occur. Combination immunosuppressant therapy can produce a rise in the platelet count that is sometimes sustained in refractory ITP patients.
Introduction
Immune thrombocytopenic purpura (ITP) is an acquired bleeding disorder characterized by autoantibody-mediated platelet destruction and impaired platelet production. Patients with chronic refractory ITP have the highest risk of death and disease-related or therapy-related complications.1,2 Treatment options include aggressive immunosuppressant therapy, and most recently thrombopoietin (TPO) receptor agonists.3,4 Single-agent immunosuppressant drugs such as azathioprine and cyclosporine have been used to treat refractory patients with moderate success5 ; however dose escalation can cause morbidity, and other options are needed.
Over the past several decades, physicians have noted that greater efficacy can be achieved using a combination of unrelated but synergistic medications.6,7 In this report, we describe our experience using a combination of azathioprine, mycophenolate mofetil, and cyclosporine to treat patients with particularly severe and refractory ITP.
Methods
Patients in this report had a platelet count less than 20 × 109/L that persisted for at least 12 months with an inadequate or transient response to multiple therapies. The senior author (J.G.K.) offered the option of a combination of immunosuppressant therapy. Patients with comorbidities such as liver failure or uncontrolled hypertension were not offered this treatment. Institutional Review Board approval from McMaster University was obtained to retrospectively review the medical charts of all patients with ITP treated in our clinic; this report describes only those patients treated with combination immunosuppressant therapy. Institutional Review Board approval was not required for the administration of the combination of immunosuppressant agents (each on its own an accepted therapy for ITP8 ), which was given per clinical need.
Medical records of each patient were reviewed by 3 independent assessors and data were abstracted in triplicate and verified for consistency. Platelet count measurements and follow-up visits were done as per routine care and mean monthly platelet counts were calculated. Target doses of immunosuppressant medications were azathioprine 2 mg/kg per day; mycophenolate mofetil 1 to 2 g/d; and cyclosporine 2 mg/kg per day. Low-dose cyclosporine was chosen to minimize toxicity and avoid the need for drug level monitoring.
We defined overall response as a platelet count level of 30 × 109/L or higher and doubling of baseline maintained for at least 4 weeks9 to reflect the goals of treatment for this group of refractory patients.10 Other outcomes were bleeding and toxicity. Relapse was defined as a drop in platelet count to below 30 × 109/L and/or the need for ITP rescue treatments. Proportions of patients achieving a platelet count response were calculated with 95% confidence intervals.
Results and discussion
Nineteen adults with chronic refractory ITP were treated with the combination of azathioprine, mycophenolate mofetil, and cyclosporine, representing 2% of ITP patients encountered during that time. The majority of nontreated patients were either not refractory or did not have platelet counts low enough to merit aggressive treatment. Median age of treated patients was 51 years, 74% were female, and the median baseline platelet count was 7 × 109/L (interquartile range [IQR], 4-19 × 109/L). The median duration of the ITP was of 8 years (IQR, 3.7-12.3) and the median number of prior treatments was 6 (IQR, 5-7), which included splenectomy (all except 1 who refused), prednisone, intravenous immune globulin, danazol, cyclophosphamide, vincristine, azathioprine, and cyclosporine. Most patients (17; 89.5%) had previous bleeding episodes, the most severe of which were intracerebral hemorrhage (n = 3), vaginal bleeding (n = 2), epistaxis (n = 3), or mucocutaneous bleeding (n = 9). Combination immunosuppressant therapy was administered for a median of 36 months (IQR, 23.0-47.5) and duration of follow-up was 47 months (IQR, 30.0-53.0).
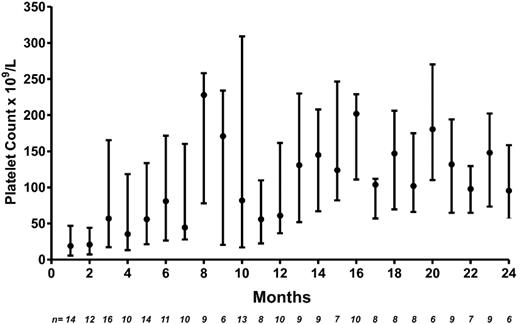
Of 19 patients treated, 14 (73.7%) achieved an overall response (platelet count above 30 × 109/L and doubling of baseline) that lasted for a median of 24 months (IQR, 11.5-46.8). Typically, there was a lag in the response of 2 months (IQR, 1.3-4.5). Thirteen patients (68.4%) achieved a platelet count higher than 50 × 109/L and 11 (57.9%) achieved a platelet count higher than 100 × 109/L. Among the 14 responders, the median platelet count on treatment was 72 × 109/L (IQR, 22-166 × 109/L; Figure 1). Nine patients had previously failed to respond to either 1 or 2 drugs (taken together) of the 3-drug combination.
Platelet count response after combination immunosuppressant therapy in patients with refractory ITP. Shown are median (●) platelet counts (×109/L) and first and third quartiles (upper and lower bars). N = number of patients with platelet count available at each time point.
Platelet count response after combination immunosuppressant therapy in patients with refractory ITP. Shown are median (●) platelet counts (×109/L) and first and third quartiles (upper and lower bars). N = number of patients with platelet count available at each time point.
Eight patients (57.1%) relapsed, of whom 6 responded to the addition of different ITP treatments. Two patients successfully stopped all medications and remain in remission after 4 and 20 months of follow-up. Major bleeding did not occur. Adverse events were reported in 11 patients (57.9%), and included transient and mild leukopenia (n = 4; lowest total leukocyte count, 2.4 × 109/L); mild infection (n = 6; deemed unrelated to treatment); and 1 infection requiring hospitalization due to worsening of thrombocytopenia. None of the infectious episodes were associated with leukopenia. Three patients experienced cyclosporine-related toxicities including gum hypertrophy and reversible tremors.
Patients with chronic refractory ITP represent less than 10% of ITP patients,10 yet they have an associated mortality of 10% to 30% from bleeding or, perhaps more frequently, toxicities of therapy.1,2 Currently, the options for the management of these patients are limited, although the recent introduction of TPO mimetics offers considerable promise.3,4 In this report, we describe the successful use of combination immunosuppressants.
The rationale for combination immunosuppressants is to target multiple pathways to inhibit the pathologic platelet autoantibody with minimal overlapping toxicities. In that way, lower doses can be used. Observations implicating T-cell regulation in the pathogenesis of ITP support this strategy because each of these agents has anti–T-cell activity. Azathioprine is a purine analog that inhibits DNA and RNA synthesis and inhibits T- and B-lymphocyte proliferation. Mycophenolate mofetil inhibits inosine monophosphate dehydrogenase resulting in the inhibition of T and B lymphocytes.11 Cyclosporine inhibits T lymphocytes by inhibiting calcineurin and the transcription of interleukin-2.12
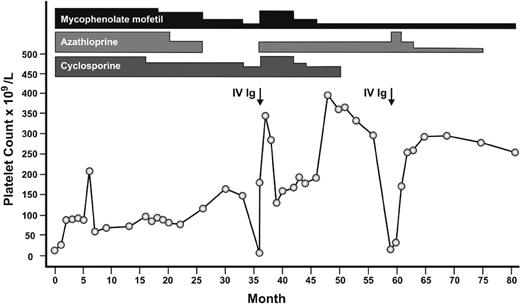
Single-agent immunosuppressant therapy has been used for patients with chronic ITP with moderate success, although, in general, patients were less refractory than those reported here. In a systematic review, azathioprine resulted in at least a partial response in 40 of 58 (66%) patients.5 Mycophenolate has also been shown to improve platelet counts above 50 × 109/L in 7 of 18 (38.9%) patients with refractory ITP13 and in 13 of 21 (62%) patients with severe ITP.14 Cyclosporine has been associated with a platelet count response in 44% to 75% of patients.15,16 Of the 19 patients in our study, 9 (47.4%) had previously failed treatment with either 1 or 2 of the 3-drug combination, suggesting that all 3 drugs together have an additive or synergistic effect. Figure 2 depicts the platelet count response in 1 such representative patient.
Representative patient with refractory ITP who responded to the combination of azathioprine, mycophenolate mofetil, and cyclosporine. Attempts at gradual dose reductions and discontinuation of azathioprine and subsequently cyclosporine each resulted in relapses. The height of the bar for each medication represents relative dosages. IVIg indicates intravenous immunoglobulin.
Representative patient with refractory ITP who responded to the combination of azathioprine, mycophenolate mofetil, and cyclosporine. Attempts at gradual dose reductions and discontinuation of azathioprine and subsequently cyclosporine each resulted in relapses. The height of the bar for each medication represents relative dosages. IVIg indicates intravenous immunoglobulin.
Others have used combination therapy as a treatment for refractory ITP. For example, combinations of cyclophosphamide, procarbazine, vincristine, etoposide, and prednisone were able to achieve a remission in 6 of 12 patients with severe refractory ITP6 ; and in 4 patients, remission was maintained for 60 to 150 months.17 Our strategy was to build on this approach using medications with more focused immune suppressive activity and potentially fewer toxicities. Another study describes the success of combinations of IVIg, steroids, vincristine, and anti-D for remission induction (25/35 [71%]) and combination azathioprine and danazol for maintenance (13/17 [76.5%]); however, only half of the patients in that study had failed splenectomy.7
Our study provides one approach for the severely refractory ITP patient who has limited treatment options. The use of lower doses of cyclosporine helps reduce the need for frequent monitoring and parenteral administration can be avoided for all drugs; 2 issues that help simplify treatment. Strengths of this study are the long duration of follow-up and the use of methodologic approaches (triplicate chart review, standardized outcome criteria) to minimize bias in a retrospective study. Limitations inherent to the retrospective design included nonregular follow-up visits, selection bias, and potential underreporting of minor toxicities and bleeding.18 In addition, the lack of a control group limits inferences about treatment effect.
The combination of azathioprine, mycophenolate and cyclosporine resulted in a platelet count response in 73.7% of patients with severe, refractory ITP. Treatment was well tolerated. The success of combination therapy suggests that a safe platelet count may be achievable with sufficient immunosuppression, even in severely affected patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Rumi Clare and Diana Moffat for independent data abstraction and Genie Leblanc for her help with paper preparation.
D.M.A. is supported by a New Investigator Award from the Canadian Institutes of Health Research in partnership with Hoffman-LaRoche.
Authorship
Contribution: D.M.A. designed and performed the research, analyzed the data, and wrote the paper; I.N., H.C., and N.M.H. designed the research and edited and approved the paper; A.S. and T.E.W. analyzed the data and edited and approved the paper; and J.G.K. conceived the research and edited and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald M. Arnold, FRCP(C), HSC 3V-48, Rm 3N-43, 1200 Main St West, Hamilton, ON, Canada L8N3Z5; e-mail: arnold@mcmaster.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal