Abstract
The description of hairy cell leukemia as a specific clinical entity was published 50 years ago. The clinical outcome for patients was hampered by ineffective chemotherapy, and splenectomy was the major therapeutic approach to improve peripheral blood counts. The median survival after diagnosis was 4 years. With the introduction of α-interferon in 1984, marked improvements in patient responses were observed. Shortly thereafter, the introduction of the purine nucleoside analogs transformed this disease into a highly treatable form of leukemia, and patients with the classic form of this rare leukemia now have a near-normal life expectancy. However, other clinical entities mimicking this disease do not respond; thus, accurate diagnosis is important. Immunophenotypic features in classic hairy cell leukemia show that the leukemic cells express CD11c, CD25, CD103, and CD123 and display bright CD20. Despite the high percentage of durable complete remissions with modern therapy, the long-term disease-free survival curves have not reached a plateau. Many patients who achieve a complete remission by morphologic criteria have minimal residual disease demonstrable by either flow cytometry or immunohistochemical staining, and this population may be at higher risk for earlier relapse. Continued clinical research is essential to optimize therapy for this disease.
Introduction
In 1958, Bouroncle et al described a series of patients with leukemic reticuloendotheliosis.1 Although this collection of cases established that the previously described isolated reports actually represented a distinct hematologic malignancy, the classic manuscript contained a very thorough presentation of the many clinical facets of this disease now known as hairy cell leukemia (HCL). Furthermore, it established that therapeutic intervention was limited either to careful titration of alkylating agents or to splenectomy. The ability to alter the clinical course of the patients with this rare form of leukemia did not substantially change until the introduction of α-interferon in 1984.2 Shortly thereafter, observations that a purine nucleoside analog (pentostatin) could induce a high degree of complete remissions (eg, 75%-89%) in this previously “untreatable” chronic leukemia changed the natural history of HCL in record time.3-8
Another purine nucleoside analog (cladribine) produced remarkably high remission rates (eg, 91%) with a single course of therapy.9,10 The outstanding results with this agent delivered as a single course of therapy led to cladribine being the initial therapy selected by most hematologists. Many of the initial studies with this agent excluded patients with active infection from enrollment, but studies from multiple institutions confirmed the high complete remission rate with this drug.11-14 Thus, patients who are treated with either purine nucleoside analog as front-line therapy will achieve a high rate of complete remission (75%-90%).7,10
The long-term studies reported at 5 to 10 years of follow-up with both pentostatin and cladribine show that the remissions are long-lived for the most part. Both agents have contributed to the improved overall survival in this disease. Despite this remarkable achievement with monotherapy, the disease-free survival curves for either agent have not plateaued, and both agents have a similar relapse rate of approximately 30% to 40% in longitudinal studies (Table 1).15-19 One of the most recent long-term follow-up studies from the Royal Marsden, reporting on 233 patients, found that pentostatin and cladribine are essentially the same with respect to outcome. With a median of 16 years of follow-up from diagnosis, in this study pentostatin and cladribine are considered interchangeable and equal in efficacy.18
Long-term follow-up therapy for HCL
| Therapy/reference (year) . | Median patient follow-up, y . | No. of patients . | CR, percentage . | Outcome . |
|---|---|---|---|---|
| Pentostatin | ||||
| Maloisel et al8 (2003) | 5.3 | 238 | 79 | Estimated DFS at 10 y 68.8% |
| Flinn et al17 (2000) | 9.3 | 241 | 76 | Estimated RFS at 10 y 67% |
| Else et al18 (2009) | 14 | 188 | 82 | Relapse at 15 y 47% |
| Cladribine | ||||
| Else et al18 (2009) | 9 | 45 | 76 | Relapse 48% at 15 y |
| Goodman et al19 (2003) | 9 | 207 | 95 | Relapse rate 37% |
| Chadha et al15 (2005) | 9.7 | 86 | 79 | Relapse rate 36% |
| Therapy/reference (year) . | Median patient follow-up, y . | No. of patients . | CR, percentage . | Outcome . |
|---|---|---|---|---|
| Pentostatin | ||||
| Maloisel et al8 (2003) | 5.3 | 238 | 79 | Estimated DFS at 10 y 68.8% |
| Flinn et al17 (2000) | 9.3 | 241 | 76 | Estimated RFS at 10 y 67% |
| Else et al18 (2009) | 14 | 188 | 82 | Relapse at 15 y 47% |
| Cladribine | ||||
| Else et al18 (2009) | 9 | 45 | 76 | Relapse 48% at 15 y |
| Goodman et al19 (2003) | 9 | 207 | 95 | Relapse rate 37% |
| Chadha et al15 (2005) | 9.7 | 86 | 79 | Relapse rate 36% |
DFS indicates disease-free survival (time from date of response until relapse, death, or last observation); and RFS, relapse-free survival (time from date of complete response until either first relapse or death from any cause).
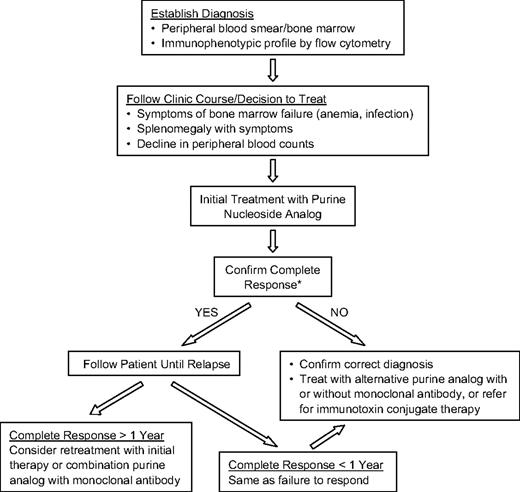
In this manuscript, I discuss the current approach to the diagnosis, management, and follow-up of patients with this rare form of chronic leukemia (Figure 1). For most reported studies, the definition of achieving a complete remission entails recovery of hemoglobin to more than 12 g/dL, absolute granulocyte count more than 1500/μL, and a platelet count more than 100 000/μL for at least 1 month. In addition, there should be no evidence of HCL cells by morphologic examination of the bone marrow biopsy or the peripheral blood. Patients should have had resolution of organomegaly by physical examination and be asymptomatic from their disease. However, on close inspection of the remission bone marrow with immunologic probes, minimal residual disease (MRD) is still demonstrable in a varying percentage of cases (ranging from 15% to 50% or more depending on the method of detection used).21,22 MRD is defined as identification of persistent HCL after treatment using immunophenotypic analysis, immunohistochemical staining, or DNA polymerase chain reaction in the absence of disease detectable by morphologic criteria.
Recommended treatment schema for HCL. *Confirmation of a complete response: If patient is participating in a clinical trial, consider using flow cytometry or immunohistochemical stains on bone marrow to document minimal residual disease. It is difficult to require these added studies for patients being treated off a clinical protocol.
Recommended treatment schema for HCL. *Confirmation of a complete response: If patient is participating in a clinical trial, consider using flow cytometry or immunohistochemical stains on bone marrow to document minimal residual disease. It is difficult to require these added studies for patients being treated off a clinical protocol.
Multicolor 4-channel flow cytometry (eg, CD11c, CD25, CD103, CD20) is highly sensitive and specific for detecting low levels of hairy cells in either the peripheral blood or the bone marrow aspirate (detection limit estimated at 0.003%-0.05%).23 Residual HCL can be difficult to identify with standard cytochemical staining. Immunohistochemical staining of the bone marrow (with either DBA.44 or anti-CD20) is a very useful measure for detecting MRD. Of the patients who relapse, many eventually require retreatment.17,19,24 Whether the patients with the greatest degree of residual disease are those likely to need retreatment forms the basis for ongoing investigation. Several authors have suggested a correlation between the extent of MRD and clinical relapse.23,25 However, larger studies will be necessary to identify the value and the optimal approach in eradicating MRD. In addition to relapse, there are patients who develop resistant disease or fail to respond to initial therapy.26 Failure to respond to initial therapy with a purine nucleoside analog should raise suspicion that the patient may not have classic HCL, and might instead have a variant of this disease.
HCL: establishing the diagnosis and considering the differential diagnosis
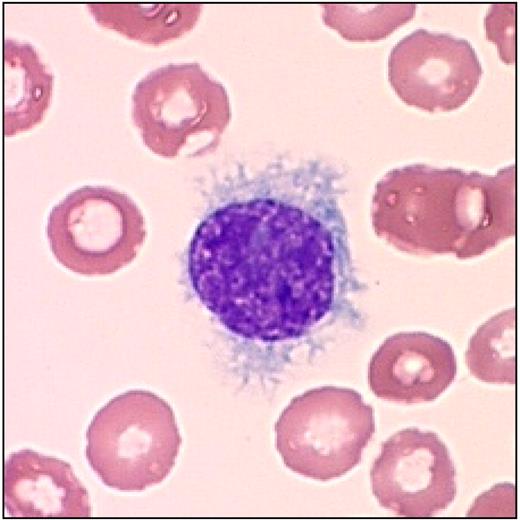
The original diagnosis of this disease was based on morphology of the leukemic cells in either the peripheral blood or the bone marrow biopsy.1,27 Many patients have a difficult marrow aspiration. Therefore, the cytologic diagnosis was made either on a supravital preparation or with a Wright stain of peripheral blood. Patients with HCL often have relatively low neutrophil counts, and monocytopenia is characteristically observed. The leukemic cells have a round to oval nucleus with a well-defined nuclear border. The cytoplasm has a serrated border with the characteristic projections that appear to be “hair-like” (Figure 2).
Typical leukemic cell in HCL. This figure (hairy cell) was obtained using an UPlanFL 100× Olympus objective in oil immersion. The image was collected using an MTI 3 CCD camera (DAGE-MTI Inc) with PAX-it 2.0 acquisition software (MIS).
Typical leukemic cell in HCL. This figure (hairy cell) was obtained using an UPlanFL 100× Olympus objective in oil immersion. The image was collected using an MTI 3 CCD camera (DAGE-MTI Inc) with PAX-it 2.0 acquisition software (MIS).
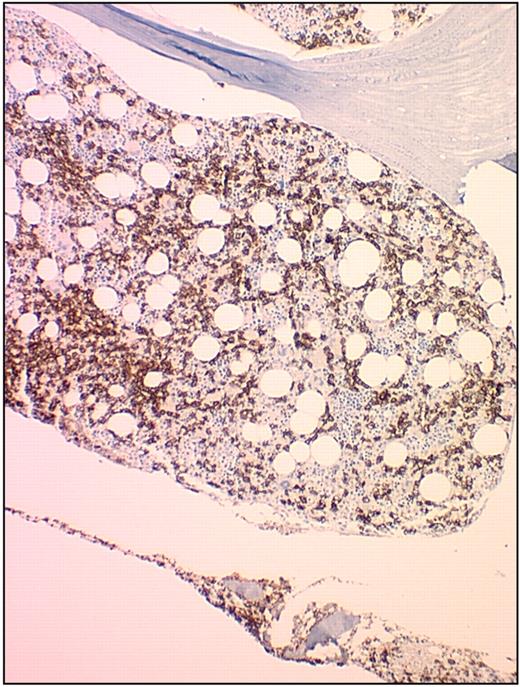
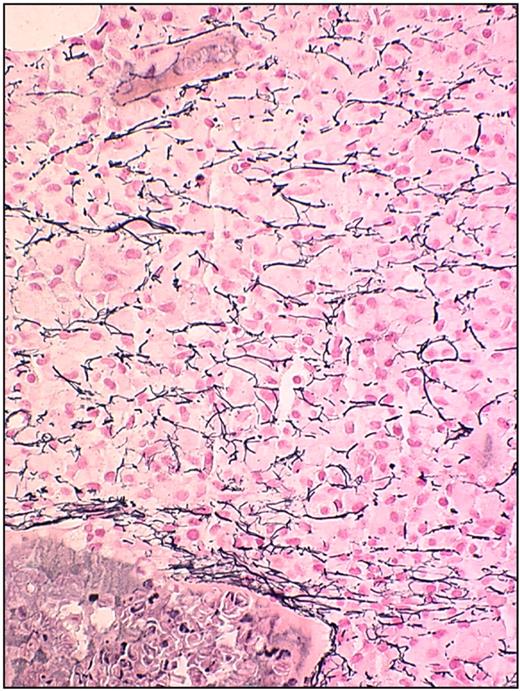
The bone marrow biopsy shows an infiltrating mononuclear cell population of characteristic leukemic cells that stain brightly positive for CD20 (Figure 3). The cells are usually well spaced with central nuclei. The bone marrow biopsy often demonstrates fibrosis, and there is a variable infiltration of the leukemic cells on scanning the specimen (Figure 4). In some patients, there is a striking hypocellular appearance to the bone marrow that may resemble hypoplastic or aplastic anemia. Recognizing this feature of diagnosis is critically important to avoid an error in diagnosis of aplastic anemia.28 Assessment of the cellularity is also important in deciding on a therapeutic regimen. Patients with a severely hypocellular marrow may require an initial dose reduction in purine nucleoside analog to avoid prolonged and profound therapy-induced myelosuppression. However, hypocellularity may be focal, and the need for specific dose reduction for these patients is still an unresolved issue. Certainly, hypocellularity after therapy has been reported.29
Bone marrow biopsy in HCL stained with anti-CD20 monoclonal antibody. This figure (HCL patient bone marrow biopsy) was stained with anti-CD20 monoclonal antibody (Dako North America) and detected using a horseradish peroxidase–conjugated mixed secondary detection system (LSAB; Dako North America). The image was obtained using an UPlanFL 20× Olympus objective. The image was collected using an MTI 3 CCD camera (DAGE-MTI Inc) with PAX-it 2.0 acquisition software (MIS).
Bone marrow biopsy in HCL stained with anti-CD20 monoclonal antibody. This figure (HCL patient bone marrow biopsy) was stained with anti-CD20 monoclonal antibody (Dako North America) and detected using a horseradish peroxidase–conjugated mixed secondary detection system (LSAB; Dako North America). The image was obtained using an UPlanFL 20× Olympus objective. The image was collected using an MTI 3 CCD camera (DAGE-MTI Inc) with PAX-it 2.0 acquisition software (MIS).
Bone marrow biopsy in HCL: reticulin stain. This figure (biopsy with reticulin stain) was stained using reagents according to the manufacturer's instructions (Ventana Medical Systems). The image was obtained using an UPlanFL 40× Olympus objective. The microscope used was an Olympus BX50 (Olympus America). The image was collected using an MTI 3 CCD camera (DAGE-MTI Inc) with PAX-it 2.0 acquisition software (MIS).
Bone marrow biopsy in HCL: reticulin stain. This figure (biopsy with reticulin stain) was stained using reagents according to the manufacturer's instructions (Ventana Medical Systems). The image was obtained using an UPlanFL 40× Olympus objective. The microscope used was an Olympus BX50 (Olympus America). The image was collected using an MTI 3 CCD camera (DAGE-MTI Inc) with PAX-it 2.0 acquisition software (MIS).
The use of peripheral blood immunophenotyping has made the initial diagnosis of HCL much easier than in the past.30 Furthermore, the characteristic antigen expression on the hairy cell can be documented with triple- or quadruple-color flow cytometry. HCL characteristically expresses CD11c, CD25, CD103, and CD123. In addition, the leukemic cells express CD20, CD22, CD52, and cyclin D1. In establishing the initial diagnosis, staining of the bone marrow with anti-CD20 monoclonal antibody may provide a more accurate appreciation for the extent of marrow involvement (Figure 3).
Several of these leukemic antigens have been used to target treatment with monoclonal antibodies and immunotoxin conjugates. Other antigens are used as biomarkers to accurately categorize the form of B-cell malignancy. Annexin 1, for example, is a relatively new marker and is under investigation as a promising tool in differentiating HCL from its variant form.31 Importantly, the hairy cell variant is usually CD25−CD123−. This distinction is important therapeutically, as patients with the variant do not respond as well to standard therapy.
Recognition of the variant of HCL and other subsets of patients with distinct pathologic entities is now possible with modern diagnostic studies. The clinical presentation may also be quite distinct from the typical form of this disease. Patients with the hairy cell variant may actually have an elevated lymphocyte count and lack the characteristic monocytopenia.21 Identification of specific therapy for those patients with the variant forms of the disease requires more study.
In Table 2, the presentation of other chronic lymphoid malignancies that must be differentiated by flow cytometry from HCL is summarized.21 It is important in planning the successful treatment course to establish the specific identity of the leukemic process and to assess the cellularity of the bone marrow to avoid therapeutic complications.
Differential diagnosis for HCL
| Disease . | Immunophenotype . | Other features . |
|---|---|---|
| HCL | CD11c, CD25, CD103, CD123, annexin A1+, CD20bright | Monocytopenia, frequent leukopenia |
| Hairy cell variant | CD11c, CD103, CD25− | No monocytopenia, high leukemic cell count |
| SMZL/SLVL | CD11c, CD25, CD24, CD79b | |
| Chronic lymphocytic leukemia | CD5, CD19, CD23 | |
| B-prolymphocytic leukemia | CD19, FMC7, CD79b, CD20, and CD22bright | High leukocyte count |
| Disease . | Immunophenotype . | Other features . |
|---|---|---|
| HCL | CD11c, CD25, CD103, CD123, annexin A1+, CD20bright | Monocytopenia, frequent leukopenia |
| Hairy cell variant | CD11c, CD103, CD25− | No monocytopenia, high leukemic cell count |
| SMZL/SLVL | CD11c, CD25, CD24, CD79b | |
| Chronic lymphocytic leukemia | CD5, CD19, CD23 | |
| B-prolymphocytic leukemia | CD19, FMC7, CD79b, CD20, and CD22bright | High leukocyte count |
SMZL/SLVL indicates splenic marginal zone lymphoma/splenic lymphoma with villous lymphocytes. This entity may occasionally be CD103+. In addition, CD11c has also been reported to be positive in a subset of these patients.
Clinical course and manifestations of HCL
In the original description of this disease, the most frequent symptoms at presentation were weakness and fatigue.1 The spleen was enlarged in 96% of the cases but was symptomatic in far fewer. Hepatomegaly was found in 58% of patients, and 35% had lymphadenopathy. Substantial peripheral adenopathy was rarely observed. The advent of noninvasive imaging revealed that more patients have intra-abdominal lymph node enlargement than was initially appreciated. The frequency and significance of intra-abdominal lymphadenopathy have not been extensively defined, although one report suggested that lymphadenopathy correlated with overall survival.8
Multiple investigators have found that age and hemoglobin level are important prognostic parameters. We previously reported that patient age, hemoglobin level, and massive splenomegaly are associated with a worse prognosis.7 The variable course of the disease has been well recognized, but the clinical parameters for predicting prognosis in the past were largely described before the era of effective chemo-immunotherapy.32 Opportunities now exist for clinical trials to incorporate an assessment of predictive biomarkers as well as clinical parameters for disease response and progression. Predictive biomarkers in the era of effective chemotherapy may identify patients who will do exceptionally well or those who will require different therapeutic approaches.33-38
Although the clinical course of the disease in the past was complicated by infection,27 this situation has improved with current therapy.39 It is also important to remember the impact of therapy on the immune system.40 Patients who have received a purine nucleoside analog have reduced cellular-based immunity for at least 9 to 12 months after completion of therapy.41-43 Addition of other immunosuppressive agents during this period of reduced T-cell numbers can further enhance the risk for infection.44 Therefore, future combination strategies must consider the impact of adding agents to the purine nucleoside analogs. Patients receiving multiple agents need consideration for prophylaxis for opportunistic infections. Prompt attention and therapy for viral exacerbations of either herpes or cytomegalovirus infection are definitely required. There is a paucity of data for recommending optimal infection prophylaxis and the duration of therapy for infection, although Ravandi and O'Brien suggest strategies for those who have received a purine analog or monoclonal antibody therapy.44 Consequently, the development of specific recommendations for dealing with infection in the “post–purine nucleoside” era represents another fertile area for clinical investigation.
In addition to complications associated with bone marrow failure, patients may develop disease-related autoimmune complications, including vasculitis and autoimmune hemolytic anemia.39,45-47 Lytic bone disease has also been observed, and extramedullary HCL has involved many tissues within the body.
Although the median age of diagnosis is 55 years of age, there is a wide range of age at diagnosis. For younger patients, there may be a higher overall response rate; however, there is also an increased chance that relapse of the disease may eventually require further therapy. One of the youngest reported cases involved a teenager; the patient has been successfully treated with splenectomy and interferon and followed for 30 years. Despite the major advances made with purine analogs, this case illustrates that interferon can have a benefit in certain patients with this disease.48 An unexplained curiosity of this disease is the male predominance. There is a 4:1 to 3:1 ratio of male to female patients.
There are conflicting reports regarding the association of this disease and an increased risk of developing a secondary malignancy, including a secondary lymphoid malignancy.49 Consequently, patients should be followed using appropriate evidence-based surveillance guidelines for specific age- and gender-related malignancies and infections.
A less frequent, but recognized, complication of HCL is spontaneous splenic rupture,50,51 which requires prompt intervention. Although splenectomy was once a standard therapy for HCL, the indications for removing the spleen now are quite limited. Certainly, splenic rupture represents one of the potential reasons. Patients with splenomegaly who have severe thrombocytopenia and active bleeding represent another cohort that should be considered for urgent removal of the spleen. Response to pharmacologic therapy for thrombocytopenia requires weeks, and a few patients have unfortunately died without splenectomy as an intervention. In those patients who have a splenectomy, efforts should be made to protect them from the consequences attendant to overwhelming infection after this procedure. Vaccination and prophylactic antibiotics should be mandated for all splenectomized patients as the infectious complications are often preventable.52
When to initiate therapy
Many patients do not require immediate therapy, and the indications for initiating treatment need to be firmly established. Patients who have symptomatic disease with fatigue that interferes with normal activities, or who have discomfort from an enlarged spleen, should be considered for therapy. Because bone marrow failure represents a major reason for initiating therapy, patients who are anemic (hemoglobin < 12 g/dL), thrombocytopenic (platelet count < 100 000/μL), or granulocytopenic (absolute granulocyte count < 1000) should have a bone marrow assessment in anticipation of starting therapy. Currently, patients are closely followed before actually starting therapy, and clinical judgment is important in making the decision to initiate treatment. If the patient is maintaining safe peripheral blood counts, the conservative approach is to “watch and wait” until counts begin to fall. The purine nucleoside analogs can cause worsening granulocytopenia before there is improvement. Consequently, they should be prescribed before the absolute granulocyte count has reached severely low levels.
In using an actual hemoglobin level for therapeutic intervention, the decision has largely been based on clinical symptoms. Repeated studies have observed that severely anemic patients and those with severe thrombocytopenia have a worse outcome. Therefore, I recommend that therapy be initiated when a declining trajectory predicts that the patient will reach a platelet count less than 100 000/μL or an absolute granulocyte count consistently less than 1000/μL. Anticipating that therapy will temporarily worsen the peripheral counts, I start therapy before the hematologic parameters have deteriorated to dangerously low levels. In following the patient before therapy, values can be obtained quarterly and the trend charted. For those patients who present with counts that are already severely depressed or if an infection has complicated the course, selection of the least myelosuppressive regimen may enable improvement to occur before full doses are delivered.
Patients require extensive explanation and counseling to accept a “watch and wait” approach. Most patients and their families are anxious to begin therapy, but they need to understand that current monotherapy involving a purine nucleoside analog is not “curative” but is appropriately begun when the counts show the inexorable trend to decline. Initiation of therapy before either symptoms or declining counts are observed carries risks that need to be explained to patients. Whereas most patients tolerate the purine nucleoside therapy well, some have had serious complications from prolonged and profound myelosuppression and immunosuppression.
How to treat: summary of standard therapeutic approaches
There is substantial heterogeneity in how patients are approached across the globe for this highly treatable disorder, and there is a lack of consensus for standard treatment of this disease.53-56 There is, however, general agreement that the goal should be to achieve a complete remission. The National Cancer Institute's PDQ published Treatment Summaries (www.cancer.gov/cancertopics/pdq/treatment/hairy-cell-leukemia/) provide some guidance, but there are no concise and specific recommendations regarding selection of dose or schedule of drug administration for patients with severely compromised bone marrow reserves. Furthermore, the summary admits that there have been limited to no studies addressing the impact of treating MRD, or the value of consolidation and maintenance therapy. There are no specific recommendations beyond monotherapy with a purine nucleoside analog.
The purine nucleoside analog used most often in clinical practice for induction therapy is cladribine.42,53 The experience from Scripps Clinic with a 7-day administration of this agent at 0.1 mg/kg per day by continuous intravenous infusion showed that 91% of patients achieved a complete remission. In a recent report summarizing the outcome of this experience with 349 patients, the relapse rate from this group was 37% with long-term follow-up.19
Other investigators both in the United States and Europe have administered cladribine with alternative schedules and routes of administration. If the agent is administered at 0.14 mg/kg per day by a 1- or 2-hour intravenous infusion for 5 doses, the results are reported to be similar.53 Subcutaneous administration for 5 or 7 days or weekly administration by intravenous route for 5 or 6 weeks produces comparable rates of complete remission.12,57,58 The weekly intravenous schedule reportedly had less myelosuppression.12,58 However, Robak et al54 recently reported a prospective randomized study with 132 patients who received either daily doses for 5 days or 6 weekly doses. They found no significant difference in the number of serious infections or septic deaths between these 2 arms, and concluded that the interrupted schedule was equally effective but no safer than the daily administration.54 A recent Swiss study reached the same conclusion that subcutaneously administered cladribine daily for 5 days versus weekly had similar outcomes and no difference in toxicity.59
Unfortunately, the long-term follow-up studies with cladribine administered by these alternative doses and schedules are not as uniformly mature as the extensive data reported by investigators at Scripps Clinic. A major advantage of using cladribine has been associated with the short course of initial therapy, but the major toxicity has been myelosuppression. Thus, long-term follow-up reports on response duration and patient outcomes continue to be very important for these other therapeutic strategies with alternative schedules using cladribine.
Pentostatin has routinely been administered by a short intravenous infusion followed by hydration as an outpatient every 2 weeks. Early reports showed very high complete remission rates with this agent exceeding 85%.4,6 In a large, multi-institutional study, Grever et al showed that pentostatin produced at least 76% complete remission in newly treated HCL.7 Patients with infection were not excluded from registration on this study, indicating that this agent can feasibly be used if necessary in this setting. Furthermore, long-term follow-up reports show that these remissions were very durable and equivalent to those produced with cladribine.17,18
Because pentostatin delivered on an interrupted schedule every 2 weeks may be less myelosuppressive than cladribine, the frequency of febrile neutropenia requiring systemic antibiotics appeared to be less with this agent (eg, 27% with pentostatin vs 37% to 58% after a 7-day course of cladribine).7,9,60,61 Some of the earlier trials used weekly pentostatin for the first 3 doses, but more recent recommendations suggest that every 2 weeks is equally effective. Indeed, the dose of pentostatin can be delayed to every 3 weeks if the absolute neutrophil count falls far below the baseline count as a consequence of treatment. This delay of a week may permit improvement of counts, and full dose administration can then be resumed. The disadvantage of the therapeutic approach with pentostatin requiring months of outpatient visits may be counterbalanced by fewer therapy-induced febrile episodes requiring hospitalization. The intermittent administration of pentostatin also permits titration of the dose depending on the neutropenia observed early in the course of therapy.
In administering either pentostatin or cladribine, careful attention should be directed to renal function, as these agents are excreted through a renal route. Consequently, our studies with pentostatin restricted eligibility to those with a serum creatinine less than 1.5 mg/dL. In following patients, determinations of serum creatinine are checked before each dose of pentostatin is administered. Patients who receive outpatient therapy with pentostatin are hydrated with 1.5 L of intravenous fluid with each dose of the drug. If an increase in the serum creatinine concentration is greater than 20% over the baseline, the dose is not administered until renal function returns to baseline. Using these precautions, the drug is very well tolerated in an outpatient setting.7
In the patients who have a hypocellular bone marrow, a reduced initial dose of pentostatin with prolongation of the usual 2-week interval between treatments has been used to avoid extensive myelosuppression. As the peripheral blood counts improve, the dose of pentostatin can be increased or titrated until the normal dose is tolerated. The usual pattern of response involves initial improvement in the platelet count with subsequent improvement in the red cells and white blood cells.
Studies involving use of growth factors to enhance white blood cell recovery have not yielded consistent results.55,62 Therefore, therapeutic decisions regarding which agent, dose, and schedule of administration are challenging at initiation of therapy. Although I personally prefer the use of pentostatin because it permits titration of dose and schedule, cladribine has generally been regarded as the treatment of choice, with pentostatin being recommended for those in relapse. The recent long-term data support the fact that these agents are indeed equivalent. A prospective randomized comparison of cladribine versus pentostatin as initial therapy is highly improbable considering the effectiveness of both of these agents and the rarity of the disease. Instead, attention for new therapeutic strategies will involve combined chemo-immunotherapy (eg, a purine analog and rituximab). Additional new agents are also under consideration, but the use of interferon under select considerations should not be forgotten. Benz et al recently updated the experience of low-dose interferon with maintenance.63 They suggest that there may be a role for this useful drug in specific patients, considering the lingering concern over secondary malignancies associated with the purine analogs; in addition, there may be an opportunity for this agent to help those too frail to receive a purine analog-based regimen.63
What should be done with MRD
Several reports indicate that MRD after induction therapy with a purine nucleoside analog can be eradicated by monoclonal therapy, such as rituximab.64-66 Although this appears to be a reasonable approach, there are few data on the effectiveness in preventing relapse. How much “residual disease” justifies continued therapy, and for how long? Furthermore, there are no data to discern whether simultaneous therapy would be better than sequential administration of the purine nucleoside analog and rituximab. Some persons have used 4 cycles of rituximab after the purine analog, whereas others have used 8 cycles of the monoclonal antibody. In a recent report of a randomized trial in treating patients with chronic lymphocytic leukemia, simultaneous administration of rituximab with another purine analog (fludarabine) was more effective than sequential therapy.67 There have been no carefully controlled randomized trials to define the optimal approach of using combined chemoimmunotherapy in HCL.
Before treating patients “off study,” the clinician should appreciate the importance of each patient in helping to better understand the optimal approach to treating this disease. If we are to be successful in predicting outcome based on MRD, more work needs to be done in the context of organized clinical trials. Immunophenotypic analysis of the peripheral blood and bone marrow after therapy often identifies the patient with residual disease. Those patients may have a higher chance of clinical relapse requiring further therapy. Whether the MRD will have an adverse impact on overall survival is not yet known. Drugs that are most effective in pursuing a “true” complete remission also carry an added risk for producing further suppression of the immune system. Our therapeutic advances must be applied cautiously to avoid causing harm. Rituximab may be useful in eliminating residual or resistant disease, and is relatively safe. In contrast, whereas alemtuzumab could theoretically be useful in this setting because of the common expression of CD52 on hairy cells, use of this agent comes at the cost of additional prolonged immunosuppression.68 A prospective randomized trial has recently been designed by Robert J. Kreitman at the National Institutes of Health to address the question of optimal scheduling of rituximab and cladribine (http://clinicaltrials.gov/ct2/show/NCT00781235?recr=Open&cond=Hairy+Cell+Leukemia&rank=3). Considering the durability of existing complete remissions after monotherapy and the cost of rituximab, it may be ultimately important to pursue the combination in those who have relapsed. Because of these current uncertainties, it is important to encourage patients to participate in organized clinical trials.
How to follow up on patients treated for HCL
Patients should be followed closely during treatment and for several months after completion of therapy, with special attention to appropriate surveillance and treatment for infection resulting from myelosuppression. The improvement in peripheral blood counts after purine nucleoside analog treatment may require weeks and sometimes months. Usually, the platelet count will improve earlier, showing that the other counts may also soon improve. It is wise to wait for 3 or even 4 months before doing the follow-up bone marrow biopsy to confirm a complete response. If the bone marrow biopsy does not show a complete response after several months, it is important to ensure that the original diagnosis has been correctly made. Patients with a variant of HCL are more likely to fail to achieve a complete response, and often relapse early after completion of initial therapy. It is important to ultimately confirm that a complete remission has been achieved because this information may be useful if the patient relapses. In the context of ongoing or future clinical trials, it will be important to quantitate MRD in patients achieving a complete remission to gain additional evidence as to the importance of this parameter in predicting relapse requiring therapy.
After the patient has achieved a complete remission, careful follow-up for the first year is important. Patients can be seen at monthly to quarterly intervals depending on the quality of peripheral count recovery. If patients are relapsing, there may be a fall in one of the cellular elements heralding the relapse. If relapse is suspected, the bone marrow examination should be repeated before restarting therapy. Immunohistochemical staining with DBA.44 or anti-CD20 (or other specific hairy cell–detecting monoclonal antibodies) frequently will show the extent of bone marrow infiltration.
Soluble interleukin-2 receptors can quantitatively parallel the course of the disease.37,38,69 For example, elevated levels at diagnosis will fall with effective therapy. Serial determinations have been useful in identifying those patients who will probably relapse. The decision to re-treat after relapse requires similar judgment compared with initiation of first-line therapy. Soluble CD22 is a recently described tumor marker that may be used to follow HCL patients. This may be particularly helpful in following the patient with CD22+CD25− disease.34
Treatment of HCL at relapse
Treatment of patients who have relapsed is typically effective, and the anticipated response to second-line therapy can often be judged by the duration and quality of the initial response. If there was an initial remission of short duration (eg, < 1 year), then repeat administration of the original therapy is less likely to result in a longer second remission. Although repeated therapy with a purine nucleoside often captures a second or more remission, the cumulative effects of repetitive courses of these agents may result in treatment-related bone marrow injury or immunodeficiency. Several patients have been successfully treated with combined chemo-immunotherapy using rituximab and a purine nucleoside analog at relapse.20,65
In patients who have had an initial, durable complete response to a purine analog lasting greater than one year, a reasonable course of action would be to re-treat the patient either with the same agent or the alternative purine analog, as there is evidence that these agents do not show cross-resistance. In the recent extensive review by Else et al, patients who achieved a complete remission with initial treatment and those with hemoglobin greater than 10 g/dL or platelet count more than 100 000/μL had the longest relapse-free survivals.18 Therefore, it is important to carefully evaluate the quality of response after therapy to know how best to re-treat the patient in case of a subsequent relapse. The overall complete response rate of patients in first relapse receiving a purine nucleoside analog approximates 69%.20 Achievement of a complete response with the second-line induction therapy also correlates with a longer second relapse-free survival. These investigators noted that third-line treatment was also successful in achieving high-quality remissions, but the percentage of those achieving a complete response declines with successive single-agent treatments.
Goodman et al noted that 75% of patients re-treated with cladribine achieved a second complete response.19 These responses were durable with a median of 35 months. The third-line therapy for patients with a second relapse was also successful in a limited number of patients, but in general the successive durations of the complete remissions were shorter.19 In our experience with pentostatin, patients can also achieve durable second and third remissions, but the ultimate outcome is similar to that described for cladribine. Successive remissions are progressively shorter. This requires consideration for combined, tolerable immunotherapy with chemotherapy.19
Resistant HCL
Innovative targeted therapy using immunotoxin conjugates has been successfully applied to patients with purine analog-resistant disease. Kreitman and Pastan have pioneered the use of these biologic approaches to therapy for refractory disease.70 LMB-2 is a recombinant immunotoxin directed against CD25. This agent shows promising results, despite the limited number of patients treated to date. Considering that patients with the variant of this disease may not express CD25, another very promising agent is BL22, a recombinant immunotoxin directed against CD22.71 Durable remissions with BL22 have been observed in heavily treated patients failing purine nucleoside analog therapy. Although a small percentage of patients have developed a hemolytic uremic syndrome after exposure to BL22, the high complete remission rate of 61% in previously treated patients is encouraging. The majority of patients achieved substantial remissions and did not exhibit further T-cell impairment that would have been observed with additional purine nucleoside analog therapy. Further clinical investigation is needed to optimize the incorporation of these novel therapies in the management of patients with HCL.
Defining the best therapy for induction and postinduction therapy requires additional clinical investigation. Optimization of therapy is now within our grasp, but achieving this outcome will probably involve combined chemo-immunotherapy with a purine nucleoside analog and a monoclonal antibody.72 Alternatively, combined therapy may take advantage of the incorporation of an immunotoxin conjugate after initial cytoreductive therapy with a purine nucleoside analog.70 Regardless of the route, there is a real need to discover new therapeutic strategies for those unfortunate patients not responding to initial therapy or those who have repeated relapse.71 Newer agents that have been highly effective in the treatment of refractory chronic lymphocytic leukemia may also be useful in treating patients with resistant HCL.
In conclusion, in celebrating the enormous advances achieved in the 50 years since Dr Bouroncle et al described HCL, we must remember that important clinical questions remain unanswered. Most patients can now be reassured that we have highly effective, but not curative, therapy for the disease. For those who ultimately relapse or fail to respond, we must continue the pursuit of laboratory-to-clinic translational research. Optimization of therapy for the majority is close at hand, and efforts to standardize approaches will be needed to ensure that all patients have a chance to benefit from the hard work contributed by many investigators. In attempting to improve therapy for this rare form of leukemia, scientific collaboration is crucial. In 2009, an international Hairy Cell Consortium was organized to link experts in this disease from across the globe (www.hairycell.org). Both patients and their physicians frequently are perplexed by their unusual clinical situation. It is our hope that all investigators interested in this rare disease will continue their efforts to address the remaining questions related to optimal therapy, the importance of eradicating MRD, and the investigation of novel directed therapies for those not responding to current therapy. Patients with this disease should be given realistic hope, but our work must continue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The author thanks Dr Gerard Lozanski for the excellent photomicrographs of hairy cell leukemia and Dr David Lucas for his excellent assistance with manuscript preparation, both of whom work with me at Ohio State University.
Authorship
Contribution: M.R.G. is the sole author of this manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Michael R. Grever, Department of Internal Medicine, Ohio State University, 395 W 12th Ave, Rm 392 North Doan Tower, Columbus, OH 43210; e-mail: michael.grever@osumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal