Abstract
Although their first application in clinical practice occurred in the 1940s, vitamin K antagonists remain the only form of oral anticoagulant medication approved for long-term use. Although the available vitamin K antagonists are highly effective for the prevention and/or treatment of most thrombotic disease, the significant interpatient and intrapatient variability in dose-response, the narrow therapeutic index, and the numerous drug and dietary interactions associated with these agents have led clinicians, patients, and investigators to search for alternative agents. Three new orally administered anticoagulants (apixaban, dabigatran, and rivaroxaban) are in the late stages of development and several others are just entering (or moving through) earlier phases of investigation. These novel anticoagulant medications are being studied for the prevention and treatment of venous thromboembolism, the treatment of acute coronary syndromes and the prevention of stroke in patients with atrial fibrillation. This review summarizes published clinical trial data pertinent to apixaban, dabigatran, and rivaroxaban.
Introduction
During the past 20 years, the approval of anticoagulants such as low-molecular-weight heparins (LMWHs), indirect factor Xa inhibitors (eg, fondaparinux), and direct thrombin inhibitors (eg, argatroban, bivalirudin, lepirudin, and desirudin) has signaled a growing interest in antithrombotic compounds that have relatively discrete targets within the coagulation pathway. Although these medications offer several potential advantages over unfractionated heparin, they all require parenteral administration and are substantially more expensive than oral vitamin K antagonists (VKAs). Thus VKAs, despite disadvantages such as variability in dose response, a narrow therapeutic index, and numerous drug and dietary interactions, are the only option for most patients requiring chronic anticoagulation.
An anticoagulant that shares some of the positive attributes (eg, wide therapeutic index, less complex pharamacodynamics) of the newer parenteral agents, but can be administered orally, may represent a significant improvement over warfarin and other currently available VKAs. Although several compounds are being considered, the 3 oral agents that are most advanced in clinical development programs achieve their anticoagulant effect by stoichiometrically inhibiting a single activated clotting factor, either thrombin (factor IIa) or factor Xa. This approach of directly antagonizing a single target in the clotting pathway is quite different from the mechanism of action for VKAs, whereby the hepatic synthesis of multiple clotting proteins is altered.
This review describes the pharmacology and clinical trial experience (both completed and planned) of apixaban, dabigatran etexilate, and rivaroxaban. A partial list of other compounds currently in various stages of development (along with their target/mechanism) can be found in Table 1.
Partial list of anticoagulants in development
| Agent . | Company . | Status, phase . |
|---|---|---|
| Direct thrombin inhibitors | ||
| Dabigatran etexilate | Boehringer Ingelheim | 3 |
| AZD0837 | Astra Zeneca | 2 |
| MCC 977 | Mitsubishi Pharma | 2 |
| Direct factor Xa inhibitors | ||
| Rivaroxaban | Bayer, Ortho-McNeill | 3 |
| Apixaban | Bristol-Myers Squibb, Pfizer | 3 |
| Betrixaban | Portola | 2 |
| YM150 | Astellas | 2 |
| Edoxaban (DU-176b) | Daichi Sankyo | 3 |
| TAK-442 | Takeda | 2 |
| Otamixaban* | Sanofi-Aventis | 2 |
| Indirect factor Xa inhibitor | ||
| Idraparinux* | Sanofi-Aventis | 3 |
| Idrabiotaparinux* | Sanofi-Aventis | 3 |
| Novel VKA | ||
| ATI-5923 | Aryx Therapeutics | 2b |
| Agent . | Company . | Status, phase . |
|---|---|---|
| Direct thrombin inhibitors | ||
| Dabigatran etexilate | Boehringer Ingelheim | 3 |
| AZD0837 | Astra Zeneca | 2 |
| MCC 977 | Mitsubishi Pharma | 2 |
| Direct factor Xa inhibitors | ||
| Rivaroxaban | Bayer, Ortho-McNeill | 3 |
| Apixaban | Bristol-Myers Squibb, Pfizer | 3 |
| Betrixaban | Portola | 2 |
| YM150 | Astellas | 2 |
| Edoxaban (DU-176b) | Daichi Sankyo | 3 |
| TAK-442 | Takeda | 2 |
| Otamixaban* | Sanofi-Aventis | 2 |
| Indirect factor Xa inhibitor | ||
| Idraparinux* | Sanofi-Aventis | 3 |
| Idrabiotaparinux* | Sanofi-Aventis | 3 |
| Novel VKA | ||
| ATI-5923 | Aryx Therapeutics | 2b |
Parenteral agent.
Pharmacology
Apixaban
Apixaban is a direct inhibitor of factor Xa (both within and outside the prothrombinase complex). When taken by mouth, apixaban has more than 50% bioavailability and reaches peak plasma concentration in 3 to 4 hours. The terminal half-life is 10 to 14 hours after repeated doses. Apixaban is metabolized in part by CYP3A4; it is partly eliminated by the kidneys (25%) and, to some extent, also processed via CYP-independent mechanisms in the liver.1,2 Apixaban does not induce or inhibit CYP enzymes and is expected to have a low likelihood of drug-drug interactions. It remains to be determined whether combined hepatic and renal elimination means that apixaban can be safely used in patients with mild (or moderate) hepatic or renal impairment.
Dabigatran etexilate
Dabigatran directly inhibits both free and clot-bound thrombin. Dabigatran etexilate (a pro-drug) is rapidly converted (after oral administration and hepatic processing) to dabigatran, with peak plasma dabigatran concentrations recorded approximately 1.5 hours after oral ingestion. Once at steady state, dabigatran has a half-life of 14 to 17 hours. With oral treatment, bioavailability is 7.2%, and dabigatran is predominantly excreted in the feces.3,4 Although part of the bioconversion from pro-drug to active metabolite occurs in the liver, the cytochrome p450 system is not involved. Potentially important drug interactions with quinine/quinidine and verapamil have been described. Elimination of dabigatran after hepatic activation occurs predominantly (up to 80%) in the kidneys; thus, patients with significant renal impairment have been excluded from most clinical trials involving dabigatran. Approved labels in Canada and elsewhere recommend an arbitrary dose reduction in the setting of moderate renal dysfunction, and recommend against use with severe renal dysfunction.
Rivaroxaban
Rivaroxaban, a direct factor Xa inhibitor, achieves maximum plasma levels approximately 3 hours after oral ingestion. Once at steady state, the terminal half-life is 4 to 9 hours (up to 12 hours in patients > 75 years old). Very few significant drug-drug interactions are anticipated, and food does not affect absorption from the gastrointestinal tract; the oral bioavailability is more than 80%.5 Like apixaban, rivaroxaban inhibits both the “free” and prothombinase-complex-bound forms of activated factor X. Sixty-six percent of orally ingested rivaroxaban is excreted by the kidneys6 ; and in jurisdictions where it is available, rivaroxaban is currently contraindicated in patients with a creatinine clearance less than 30 mL/min. Caution is advised if rivaroxaban is used in patients with less severe renal insufficiency. No specific dose reduction algorithm is available.
Clinical trial data
For each of the 3 agents, Tables 2,Table 3 through 4 provide an overview of the phase 3 clinical trials that are ongoing or completed.
Overview of completed/ongoing phase 3 clinical trials involving apixaban
| . | Comparator . | No. of patients (approximate) . | Results expected/published . | Acronym . |
|---|---|---|---|---|
| AF | VKA | 15 000 | 2011 | ARISTOTLE |
| VTE, primary prophylaxis | ||||
| Orthopedic | LMWH | 6 200 | 2009,7 2010 | ADVANCE-1,2 |
| Orthopedic | LMWH | 5 400 | 2010 | ADVANCE-3 |
| Medical | LMWH | 6 500 | Early 2010 | ADOPT |
| VTE acute treatment | LMWH + VKA | 2 900 | 2012 | AMPLIFY |
| VTE secondary prevention | Placebo (before randomization, all patients have received 6-12 months of anticoagulation) | 2 400 | 2012 | AMPLIFY extension study |
| Post–acute coronary syndrome | Placebo (all patients will receive standard treatment, such as aspirin, β-blocker) | 11 000 | 2012 | APPRAISE-2 |
| . | Comparator . | No. of patients (approximate) . | Results expected/published . | Acronym . |
|---|---|---|---|---|
| AF | VKA | 15 000 | 2011 | ARISTOTLE |
| VTE, primary prophylaxis | ||||
| Orthopedic | LMWH | 6 200 | 2009,7 2010 | ADVANCE-1,2 |
| Orthopedic | LMWH | 5 400 | 2010 | ADVANCE-3 |
| Medical | LMWH | 6 500 | Early 2010 | ADOPT |
| VTE acute treatment | LMWH + VKA | 2 900 | 2012 | AMPLIFY |
| VTE secondary prevention | Placebo (before randomization, all patients have received 6-12 months of anticoagulation) | 2 400 | 2012 | AMPLIFY extension study |
| Post–acute coronary syndrome | Placebo (all patients will receive standard treatment, such as aspirin, β-blocker) | 11 000 | 2012 | APPRAISE-2 |
Overview of completed/ongoing phase 3 clinical trials involving dabigatran
| . | Comparator . | No. (approximate) of patients . | Results expected/published . | Acronym . |
|---|---|---|---|---|
| AF | VKA | 18 000 | 200911 | RELY |
| VTE primary prophylaxis, orthopedic | LMWH | 7 500 | 2007-20098-10 | RE-NOVATE, RE-MOBILIZE, RE-MODEL |
| VTE acute treatment | VKA (patients will have LMWH therapy during the first week of therapy with dabigatran or VKA) | 2 600 | 2011, 2012 | RECOVER |
| VTE secondary prevention | Placebo (before randomization, all patients have received 6-18 months of anticoagulation) | 4 500 | 2011 | REMEDY, RECOVER II |
| . | Comparator . | No. (approximate) of patients . | Results expected/published . | Acronym . |
|---|---|---|---|---|
| AF | VKA | 18 000 | 200911 | RELY |
| VTE primary prophylaxis, orthopedic | LMWH | 7 500 | 2007-20098-10 | RE-NOVATE, RE-MOBILIZE, RE-MODEL |
| VTE acute treatment | VKA (patients will have LMWH therapy during the first week of therapy with dabigatran or VKA) | 2 600 | 2011, 2012 | RECOVER |
| VTE secondary prevention | Placebo (before randomization, all patients have received 6-18 months of anticoagulation) | 4 500 | 2011 | REMEDY, RECOVER II |
Overview of completed/ongoing phase 3 clinical trials involving rivaroxaban
| . | Comparator . | No. (approximate) of patients . | Results expected/published . | Acronym . |
|---|---|---|---|---|
| AF | VKA | 14 000 | 2010 | ROCKET-AF |
| VTE primary prophylaxis | ||||
| Orthopedic | LMWH | 8 500 | 2008, 200912-15 | RECORD 1-4 |
| Medical | LMWH | 8 000 | 2011 | MAGELLAN |
| VTE acute treatment | LMWH + VKA | 6 200 | 2010 | EINSTEIN DVT and EINSTEIN PE |
| VTE secondary prevention | Placebo (before randomization, all patients received 6-12 months of anticoagulation) | 1 300 | 2010 | EINSTEIN extension study |
| Post–acute coronary syndrome | Placebo (all patients will receive standard treatment, such as aspirin, β-blocker) | 16 000 | Unknown | ATLAS ACS TIMI 51 |
| . | Comparator . | No. (approximate) of patients . | Results expected/published . | Acronym . |
|---|---|---|---|---|
| AF | VKA | 14 000 | 2010 | ROCKET-AF |
| VTE primary prophylaxis | ||||
| Orthopedic | LMWH | 8 500 | 2008, 200912-15 | RECORD 1-4 |
| Medical | LMWH | 8 000 | 2011 | MAGELLAN |
| VTE acute treatment | LMWH + VKA | 6 200 | 2010 | EINSTEIN DVT and EINSTEIN PE |
| VTE secondary prevention | Placebo (before randomization, all patients received 6-12 months of anticoagulation) | 1 300 | 2010 | EINSTEIN extension study |
| Post–acute coronary syndrome | Placebo (all patients will receive standard treatment, such as aspirin, β-blocker) | 16 000 | Unknown | ATLAS ACS TIMI 51 |
Apixaban
Phase 2 data for apixaban in the prevention and treatment of venous thromboembolism (VTE) suggested that this compound may be a safe and effective anticoagulant over a wide range of doses.16,17 The first phase 3 orthopedic prophylaxis trial (ADVANCE-1) randomized 3195 patients, in double-blind fashion, to either apixaban (2.5 mg orally twice a day) or enoxaparin (30 mg subcutaneously every 12 hours). In both arms, treatments were begun 12 to 24 hours after surgery; all patients underwent venography approximately 2 weeks later. The proportion of patients who reached the primary endpoint (VTE, both symptomatic and venographically detected, or death from any cause) was similar for the apixaban (8.99%) and enoxaparin (8.85%) arms; however, the predetermined noninferiority endpoint was not met.7 It is unclear whether the failure to prove noninferiority can be explained by inaccurately low prestudy estimates of event rates, the dose/timing of apixaban, or some other cause. In any case, the comparable event rates (neither regimen was found superior to the other), along with less clinically relevant bleeding in the apixaban arm, strongly suggest that apixaban is an effective anticoagulant. Results from ADVANCE-2, a randomized double-blind multicenter trial comparing apixaban 2.5 mg orally twice a day with enoxaparin 40 mg subcutaneously once daily for preventing VTE after total knee replacement were presented at the congress of the International Society of Thrombosis and Hemostasis in July 2009. The primary efficacy outcome (all VTE) occurred in 147 of 976 evaluable patients (15.1%) in the apixaban group and 243 of 997 evaluable patients (24.4%) in the enoxaparin group (relative risk = 0.62; 95% confidence interval, 0.51-0.74, 1-sided P < .001). A nonsignificant trend toward less clinically relevant bleeding also favored apixaban (53 patients, 3.5% vs 72 patients, 4.8%; P = .09).
A phase 3 study (AMPLIFY) of patients with acute VTE (deep vein thrombosis [DVT] and/or pulmonary embolism [PE]) will compare apixaban (10 mg twice daily for 7 days, followed by apixaban 5 mg, twice daily for 6 months) to a standard strategy using enoxaparin followed by VKA. A phase 3 “extension” study will enroll patients for whom there is clinical uncertainty about whether to continue oral anticoagulation after 6 months of routine treatment with a VKA. Participants will be randomly assigned to receive 1 of 3 possible interventions for 12 months: placebo, apixaban 2.5 mg twice daily, or apixaban 5 mg twice daily. In both trials, the primary outcome measure will be a composite of symptomatic, objectively confirmed recurrent VTE or death during the treatment period.
The APPRAISE trial was a phase 2 dose-finding study in which 1700 patients were randomized to receive placebo or 1 of 4 apixaban doses for the 6 months after standard acute therapy for acute coronary syndrome.18 For patients receiving the 2 highest doses of apixaban, the trial was terminated prematurely because of excess bleeding. In this population of patients receiving one or more concomitant antiplatelet agents, the 2 lower-dose apixaban arms had higher rates of the primary endpoint (major bleeding plus clinically relevant nonmajor bleeding) than did the placebo group. The trial was not powered to detect a statistically significant difference in the rates in the composite efficacy endpoint of cardiovascular death, nonfatal heart attack, severe recurrent ischemia, and nonhemorrhagic stroke but showed trends favoring apixaban over placebo for these endpoints. A phase 3 trial (APPRAISE-2) comparing apixaban 5 mg twice daily versus placebo in this clinical setting is under way.
Finally, apixaban is being studied as a stroke prevention strategy for patients with atrial fibrillation (AF). When completed, the ARISTOTLE trial will randomize approximately 18 000 patients with chronic nonvalvular AF to apixaban 5 mg orally twice daily or warfarin, target international normalized ratio (INR) 2.0 to 3.0. This event-driven, double-blind, parallel arm study is designed to show that apixaban is noninferior to well-managed warfarin in the prevention of stroke or systemic embolism. In a different randomized, double-blind study (AVERROES), apixaban (5 mg orally twice daily) is being compared with aspirin (81-324 mg daily) among AF patients who have failed or are unsuitable for VKA treatment. This trial will include 5000 to 6000 patients; follow-up will be up to 36 months.
Dabigatran etexilate
After completing phase 2 dose-finding studies,19,20 the manufacturer of dabigatran designed 3 large phase 3 studies examining the utility of dabigatran for the prevention of VTE after orthopedic surgery. The RE-NOVATE trial randomized patients to either dabigatran 220 mg daily or 150 mg daily or enoxaparin 40 mg subcutaneously daily, with the first dose administered preoperatively.8 The primary endpoint was a composite of total VTE and death from all causes. Both doses of dabigatran were noninferior to enoxaparin; major bleeding was similar between dabigatran 220 mg, 2.0% (P = .44); dabigatran 150 mg, 1.3% (P = .6); and enoxaparin, 1.6%. Patients undergoing total knee replacement were studied in the RE-MODEL study.9 The primary outcome, the composite of total VTE and mortality, occurred in 36.4% of patients in the dabigatran 220 mg group and 40.5% of patients in the dabigatran 150 mg group and 37.7% of patients in the enoxaparin 40 mg group. Both trials demonstrated noninferiority for dabigatran compared with enoxaparin. The RE-MOBILIZE study compared dabigatran with enoxaparin administered at a dose of 30 mg twice daily, started postoperatively. The primary outcome of total VTE and death occurred in 31.1% of patients in the dabigatran 220 mg group, 33.7% of patients in the dabigatran 150 mg group, and 25.3% of those in the enoxaparin group. Dabigatran, as administered in the RE-MOBILIZE study, was thus inferior to enoxaparin administered at standard North American doses after knee replacement surgery.10 Large phase 3 studies of dabigatran versus warfarin for the secondary prevention of acute VTE are ongoing: patients in both arms will receive short-term “overlap” treatment with LMWH.
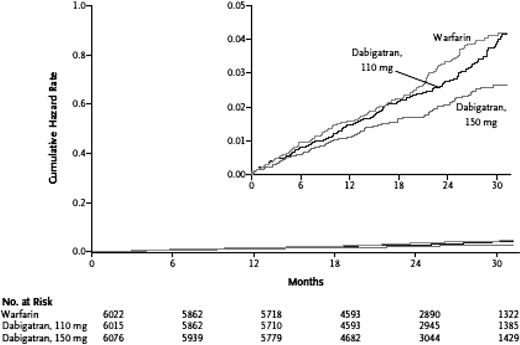
More than 18 000 patients with nonvalvular AF were enrolled in RELY, an open-label study of stroke prevention where 2 doses of dabigatran (110 mg or 150 mg twice daily) were compared with warfarin (target INR = 2-3); median follow-up was 2 years. Designed as a noninferiority trial with a primary outcome of stroke or systemic embolism, RELY demonstrated that dabigatran 110 mg twice daily not only provided antithrombotic protection similar to well-managed warfarin but also was associated with a lower annual rate of major bleeding (3.36% vs 2.71%, P = .003). The twice daily 150-mg dose of dabigatran resulted in a lower rate of stroke/systemic embolism, 1.11% vs 1.69%; (relative risk = 0.66; 95% confidence interval, 0.53-0.82; P < .001 for superiority), and was associated with a similar risk of major bleeding (Figure 1). Dyspepsia was reported by approximately 12% of patients taking both doses of dabigatran, compared with only 5.8% of patients taking warfarin. Because of concerns that arose with another direct thrombin inhibitor, ximelagatran, transaminase levels were monitored closely in this trial, and no evidence of hepatoxicity was reported.11
Dabigatran versus warfarin in patients with nonvalvular AF: cumulative hazard rates for the primary outcome of stroke or systemic embolism, according to treatment group. Reprinted from Connolly et al11 with permission.
Dabigatran versus warfarin in patients with nonvalvular AF: cumulative hazard rates for the primary outcome of stroke or systemic embolism, according to treatment group. Reprinted from Connolly et al11 with permission.
Rivaroxaban
Based on the results of 4 phase 3 clinical trials, rivaroxaban is now approved both in Canada and Europe for the prevention of VTE after major orthopedic surgery. In May 2009, the US Food and Drug Administration decided not to approve rivaroxaban for similar indications in the United States, citing a concern that rivaroxaban “could lead to bleeding events in significantly more patients” than enoxaparin on the Food and Drug Administration website. The agency has requested more safety data, and the possibility of approval at a later date remains open. In each of the 4 “REgulation of Coagulation in ORthopaedic surgery to prevent Deep-vein thrombosis and pulmonary embolism” (RECORD) studies, rivaroxaban 10 mg by mouth once daily proved superior to the comparator in the prevention of VTE.12-15 Taken together, the results from these clinical studies suggest that a 10-mg oral dose of rivaroxaban can, compared with standard doses of enoxaparin, reduce the risk of VTE after total hip or knee arthroplasty (Table 5). Overall rates of major hemorrhage were low, but the pooled results from more than 12 000 patients included in these 4 trials show a trend toward increased major bleeding (0.39% vs 0.21%, P = .08) with rivaroxaban. When the trial definition of major bleeding is combined with surgical site bleeding, the rates for rivaroxaban and enoxaparin are 1.80% and 1.37%, respectively (P = .06; Table 6).
Total VTE
| . | RECORD 1 (hip) . | RECORD 2 (hip) . | RECORD 3 (knee) . | RECORD 4 (knee) . |
|---|---|---|---|---|
| Rivaroxaban | ||||
| n | 1595 | 864 | 824 | 965 |
| Endpoint | 18 (1.1%) | 17 (2.0%) | 79 (9.6%) | 67 (6.9%) |
| Enoxaparin | ||||
| n | 1558 | 869 | 878 | 959 |
| Endpoint | 58 (3.7%) | 81 (9.3%) | 166 (18.9%) | 97 (10.1%) |
| . | RECORD 1 (hip) . | RECORD 2 (hip) . | RECORD 3 (knee) . | RECORD 4 (knee) . |
|---|---|---|---|---|
| Rivaroxaban | ||||
| n | 1595 | 864 | 824 | 965 |
| Endpoint | 18 (1.1%) | 17 (2.0%) | 79 (9.6%) | 67 (6.9%) |
| Enoxaparin | ||||
| n | 1558 | 869 | 878 | 959 |
| Endpoint | 58 (3.7%) | 81 (9.3%) | 166 (18.9%) | 97 (10.1%) |
Total VTE = occurrence of: any DVT (symptomatic or asymptomatic), nonfatal PE, or death of any cause in RECORD studies of rivaroxaban after major orthopedic surgery.12-15 All differences favor rivaroxaban, and all reach statistical significance (P < .05).
Pooled rates of bleeding from the 4 RECORD trials
| . | Rivaroxaban, no. per 1000 patients . | Enoxaparin, no. per 1000 patients . | P . |
|---|---|---|---|
| Major bleeding | 3.9 | 2.1 | .08 |
| Major bleeding + surgical site bleeding | 18.0 | 13.7 | .06 |
| . | Rivaroxaban, no. per 1000 patients . | Enoxaparin, no. per 1000 patients . | P . |
|---|---|---|---|
| Major bleeding | 3.9 | 2.1 | .08 |
| Major bleeding + surgical site bleeding | 18.0 | 13.7 | .06 |
Adapted from the FDA Advisory Committee Briefing Document (http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM181524.pdf; accessed October 5, 2009).
After a phase 2 trial of rivaroxaban for the treatment of acute VTE,21 2 phase 3 studies are currently underway: one for patients with DVT with or without PE and one for patients with PE with or without DVT. Both trials have a randomized, open-label design in which rivaroxaban 15 mg is given twice a day orally for the first 3 weeks; the rivaroxaban dose is then reduced to 20 mg once daily for the remainder of the study. The comparator in both trials is enoxaparin plus VKA. The average duration of therapy is expected to be 6 months. A secondary prophylaxis study is also underway; in this trial, patients who have received at least 6 to 12 months of anticoagulation (and for whom there is clinical equipoise about whether to continue anticoagulation) will be randomized to rivaroxaban 20 mg daily or placebo.
In patients with AF, rivaroxaban 10 mg once daily is being compared with warfarin therapy in a phase 3 trial where the composite of stroke and systemic embolism and the combination of major plus clinically relevant nonmajor bleeding are the primary endpoints. This large, randomized, double-blind multicenter study is now closed to recruitment, and results are expected in 2010 or early 2011. Participants are expected to be on study medication for an average of 2 years.
Rivaroxaban is also being studied in a phase 3 trial of patients with recent acute coronary syndrome. In this randomized, double-blind 16 000-patient trial, 2 doses of rivaroxaban (2.5 mg twice daily and 5 mg twice daily) will be compared with placebo as an adjunct to the current standard of care. The primary outcome measure is the reduction in the risk of the composite endpoint of cardiovascular death, myocardial infarction, or stroke. A dose-finding phase 2 study (ATLAS, TIMI 46) demonstrated benefit with respect to the primary (composite endpoint) but also showed increased bleeding with higher rivaroxaban doses.22 The decision to administer rivaroxaban twice daily in the phase 3 acute coronary syndrome study emerged from observations in ATLAS that, for this population (many of whom are also taking one or more antiplatelet agents), the risk-benefit ratio may be better when the total amount of drug is split into 2 doses rather than administered as a single tablet.
Practical considerations
All 3 drugs discussed in this review are eliminated, to some extent, by the kidneys; thus, whether (or at what dose) patients with moderate to severe renal insufficiency can use these agents may not be determined for some time. Although each of these agents affects conventional clotting assays to some degree (Table 7),23,24 further research will be needed to determine how clinicians can best assess plasma anticoagulant activity for each agent (when such an assessment is necessary). No evidence-based reversal strategy is available for a bleeding patient taking one of these novel anticoagulants. Although the need for such an intervention is expected to arise infrequently because all 3 drugs have relatively short half-lives (Table 8), the lack of an antidote or evidence-based bleeding management plan may be a disadvantage in the eyes of many clinicians. Principles for the management of anticoagulant-related bleeding (with a specific focus on new agents) have been outlined elsewhere32 ; at least one in vitro study has identified a possible strategy worthy of further investigation.33
Effect of novel anticoagulants on traditional laboratory assessments of coagulation in humans
| . | ECT . | Xa inhibition . | TCT . | PT . | aPTT . |
|---|---|---|---|---|---|
| Dabigatran 200 mg TID | 5.2* | No effect | 27* | Not reported | 2.3* |
| Rivaroxaban 30 mg BID | No effect | 68% | No effect | 2.6* | 1.8* |
| Apixaban 25 mg BID | No effect | Not reported | Not reported | Not reported | 1.2* |
| . | ECT . | Xa inhibition . | TCT . | PT . | aPTT . |
|---|---|---|---|---|---|
| Dabigatran 200 mg TID | 5.2* | No effect | 27* | Not reported | 2.3* |
| Rivaroxaban 30 mg BID | No effect | 68% | No effect | 2.6* | 1.8* |
| Apixaban 25 mg BID | No effect | Not reported | Not reported | Not reported | 1.2* |
Modified from Eriksson et al.24
ECT indicates ecarin clotting time; TCT, thrombin clotting time; PT, prothrombin time; aPTT, activated partial thromboplastin time; TID, three times daily; and BID, twice daily.
Estimated fold increase from baseline at peak levels.
Summary of the pharmacology and clinical status of apixaban, rivaroxaban, and dabigatran
| . | Apixaban . | Rivaroxaban . | Dabigatran . |
|---|---|---|---|
| Brand name | — | Xarelto | Pradaxa |
| Target | Factor Xa | Factor Xa | Factor IIa |
| tmax, h | 1-31 | 2-45 | 1.25-34 |
| Half-life, h | 8-151 | 9-135 | 12-144 |
| Renal excretion | ∼ 25%1 | 66%25 | 80%4 |
| Food effect | Not reported | Delays absorption26 | Delays absorption27 |
| Effect of age | Not reported | Variable28 | None29 |
| Effect of body weight | Not reported | None30 | None31 |
| Clinical status | — | Approved in Canada and Europe for VTE prevention after orthopedic surgery | Approved in Canada and Europe for VTE prevention after orthopedic surgery |
| . | Apixaban . | Rivaroxaban . | Dabigatran . |
|---|---|---|---|
| Brand name | — | Xarelto | Pradaxa |
| Target | Factor Xa | Factor Xa | Factor IIa |
| tmax, h | 1-31 | 2-45 | 1.25-34 |
| Half-life, h | 8-151 | 9-135 | 12-144 |
| Renal excretion | ∼ 25%1 | 66%25 | 80%4 |
| Food effect | Not reported | Delays absorption26 | Delays absorption27 |
| Effect of age | Not reported | Variable28 | None29 |
| Effect of body weight | Not reported | None30 | None31 |
| Clinical status | — | Approved in Canada and Europe for VTE prevention after orthopedic surgery | Approved in Canada and Europe for VTE prevention after orthopedic surgery |
— indicates not applicable; and tmax, time to maximum plasma concentration.
It is possible that one or more of these agents will have a wider therapeutic index than warfarin, thus reducing the risk of hemorrhage while preserving the beneficial antithrombotic effects. However, at least some of the large studies in orthopedic surgery suggest that there will continue to be a tradeoff (fewer thromboembolic events will be balanced by extra bleeding and vice versa). These novel anticoagulants will certainly be more expensive than warfarin (but will probably be less expensive than LMWH). Definitive cost-effectiveness analyses will have to account for the cost of regular INR measurement; the potential to eliminate such testing will certainly offset at least some of the additional acquisition cost of these novel agents. Whether their increased cost relative to VKAs can, in light of these concerns, be justified by increased patient convenience and a small reduction in the risk of stroke with dabigatran therapy in patients with AF remains to be seen. On the other hand, the greatest potential for net benefit from these novel agents may rest with patients who currently do not have access to the sort of frequent INR monitoring and dose adjustment that maximize the safety of VKAs. In addition, the pharmacologic characteristics of these drugs will probably eliminate the need for perioperative anticoagulation (“bridge therapy”) with parenteral agents. Finally, in some settings (such as for extended prophylaxis after major orthopedic surgery), these agents may replace LMWHs where their oral administration and price may provide a compelling case for their use.
In conclusion, clinical trial data published thus far suggest that oral agents may soon be widely available as alternatives to VKAs (and currently available parenteral therapies) for patients at risk of thromboembolism. It remains to be seen which of the 3 agents discussed here (and others in earlier phases of development) will be used for the various disease states in which they are being studied. The overall utility of any novel anticoagulant will depend on many factors, including efficacy compared with current agents, cost, risk-benefit profile, reversibility, and patient convenience/satisfaction.
Authorship
Contribution: D.G., E.L., and M.A.C. contributed to the literature search; D.G. created the first draft of the manuscript; and D.G., E.L., and M.A.C. participated in revising and preparing the final draft of the manuscript.
Conflict-of-interest disclosure: Within the past 2 years, D.G. has received research funding from and/or acted as a consultant for CSL Behring, Roche Diagnostics, Aryx Therapeutics, Boehringer Ingelheim, and Bristol-Myers Squibb. Within the past 2 years, M.A.C. has received honoraria from Sanofi-Aventis, Leo Laboratories, Pfizer, Bayer, Boehringer Ingelheim, and Artisan Pharma. E.L. declares no competing financial interests.
Correspondence: David Garcia, University of New Mexico, MSC08-4630, 900 Camino de Salud NE, Albuquerque, NM 87131-0001; e-mail: davgarcia@salud.unm.edu.