Abstract
Criteria for distinguishing among etiologies of thrombocytosis are limited in their capacity to delineate clonal (essential thrombocythemia [ET]) from nonclonal (reactive thrombocytosis [RT]) etiologies. We studied platelet transcript profiles of 126 subjects (48 controls, 38 RT, 40 ET [24 contained the JAK2V617F mutation]) to identify transcript subsets that segregated phenotypes. Cross-platform consistency was validated using quantitative real-time polymerase chain reaction (RT-PCR). Class prediction algorithms were developed to assign phenotypic class between the thrombocytosis cohorts, and by JAK2 genotype. Sex differences were rare in normal and ET cohorts (< 1% of genes) but were male-skewed for approximately 3% of RT genes. An 11-biomarker gene subset using the microarray data discriminated among the 3 cohorts with 86.3% accuracy, with 93.6% accuracy in 2-way class prediction (ET vs RT). Subsequent quantitative RT-PCR analysis established that these biomarkers were 87.1% accurate in prospective classification of a new cohort. A 4-biomarker gene subset predicted JAK2 wild-type ET in more than 85% patient samples using either microarray or RT-PCR profiling, with lower predictive capacity in JAK2V617F mutant ET patients. These results establish that distinct genetic biomarker subsets can predict thrombocytosis class using routine phlebotomy.
Introduction
Platelets mediate the initial first step in hemostasis while simultaneously providing the negatively charged phospholipid surface required for contact phase-mediated propagation of the coagulation cascade. Despite these key functions, molecular defects causally implicated in platelet-associated bleeding or thrombotic risk are largely unknown, best characterized by loss of glycoproteins (GP) IIb/IIIa (αIIbβ3; Glanzmann thrombasthenia) or the GPIb-IX-V complex (Bernard-Soulier syndrome).1 Similarly, protein overexpression may favor platelet activation and thrombus formation, providing conceptual support for the presence of biomarkers that may confer enhanced thrombosis susceptibility risk. Thus, polymorphisms within the ITGA2 gene encoding the α2 polypeptide of the heterodimeric α2β1 collagen receptor increase receptor surface density and are associated with an increased risk of ischemic heart disease in homozygotes (especially smokers),2,3 although confirmatory results using meta-analyses for a distinct subset of hemostatic proteins have been somewhat disappointing.4,5 Similarly, platelet membrane polymorphisms have been linked to stroke in small studies, but the evidence is not strong.6-9 Such studies highlight the relevance of identifying additional gene/protein biomarkers that may be causally linked to clinically relevant platelet phenotypes.
Platelets retain megakaryocyte-derived mRNA, although the platelet transcriptome is less complex than that of nucleated cells.10,11 Furthermore, platelets have evolved unique adaptive molecular signals for maintenance of genetic and protein diversity.12 Quiescent platelets generally display minimal translational activity, although maximally activated platelets retain the capacity for protein synthesis.13 Newly formed “reticulated” platelets retain larger quantities of mRNAs and have been associated with enhanced thrombotic risk in patients with thrombocytosis,14 suggesting that changes in mRNA levels may be associated with the prothrombotic phenotype. It remains unknown whether this risk is the result of globally altered expression profiles or specific changes in gene/protein subsets.
We have now applied gene-expression profiling to develop discriminatory class prediction models of a benign human disorder (thrombocytosis) as an initial paradigm for molecular classification of platelet disorders.15 The differentiation of clonal from reactive thrombocytosis (RT) has important diagnostic and therapeutic implications because thrombohemorrhagic complications arising in RT are unusual,16 in contrast to frequent events in patients with clonal disorders, such as essential thrombocythemia (ET).17,18 ET is a myeloproliferative disorder (MPD) subtype microscopically indistinguishable (unless accompanied by macrothrombocytosis) from the larger subset of nonclonal, thrombocytotic disorders associated with a wide array of human diseases. Recent data have identified an activating Janus kinase 2 mutation (JAK2V617F) in patients with polycythemia rubra vera (PV),19,20 although it appears with lower frequency in ET patients. Although an etiology for thrombocytosis is evident in many patients, its association with occult malignancies, coupled with the fact that ET remains a diagnosis of exclusion,21 support the need for well-defined algorithms for class prediction. Discriminatory class prediction models using gene-expression profiling have been developed for various human malignancies, with implications for diagnostics and clinical management.22-26 We now demonstrate that a comparable strategy can be developed for assigning class in patients with thrombocytosis, providing the foundation for a more generalizable strategy for platelet genetic classification.
Methods
Patient recruitment
Patients were randomly enrolled from the larger pool of patients referred to the Division of Hematology for evaluation of thrombocytosis. Any subject older than age 18 years was eligible for the study, and the primary ineligibility criteria were failure to provide consent; data are from the initial recruitment with no reentry to date. All subjects provided informed consent for an Institutional Review Board–approved protocol completed in conjunction with the National Institutes of Health–funded General Clinical Research Center at Stony Brook University in accordance with the Declaration of Helsinki; healthy controls were identified from the larger pool of General Clinical Research Center volunteers representing the ethnically diverse population of Long Island, NY; enrollment of healthy controls was not matched with that of thrombocytosis patients. Patient recruitment occurred over a 3.5-year period using preexisting, standard hematologic criteria (clinical and laboratory data) for the initial diagnosis of ET or RT.27,28 Patient recruitment followed 2 distinct phases; in the first phase, samples from all 3 phenotypes (ET, RT, healthy controls) were used for biomarker identification and confirmatory platform validation of biomarker subsets (cohort 1); the subsequent study phase applied these validated markers to a second population of thrombocytotic patients as a means of predicting class in newly recruited patient populations (cohort 2). Both sex and age distribution paralleled prevalence figures for ET with a male:female ratio of 1:1.4; age at diagnosis ranged from 23 to 78 years. Platelet counts at the time of blood isolation ranged from normal (reflecting treatment) to 1 724 000/μL; use of platelet-lowering drugs (ie, hydroxyurea, analgrelide, or untreated) was recorded for individual patients at the time of platelet isolation and purification (Table 1).
Patient characteristics
| Characteristic . | ET*† (n = 40) . | RT*‡ (n = 38) . | Healthy controls§ (n = 48) . |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 15 (38) | 16 (42) | 23 (48) |
| Female | 25 (52) | 22 (58) | 25 (52) |
| Age group, no. (%) | |||
| Younger than 35 y | 3 (8) | 9 (24) | 16 (33) |
| 36-60 y | 13 (32) | 24 (63) | 27 (57) |
| 60 y or older | 24 (60) | 5 (13) | 5 (10) |
| Platelet count, ×10−3/mm3 | |||
| Mean | 659 | 632 | 246 |
| Range | 203-1724 | 173-1044 | 160-415 |
| Hemoglobin, g/dL | |||
| Mean | 13.3 | 10.9 | 14.1 |
| Range | 9.5-20.8 | 8.1-15.3 | 12.3-15.9 |
| White cell count, × 10−3/mm3 | |||
| Mean | 10.2 | 14.9 | 6.3 |
| Range | 3.1-44.6 | 5.7-65.2 | 4.4-9.6 |
| JAK2 genotype, no. (%) | |||
| V617F heterozygous | 21 (52) | 0 (0) | 0 (0) |
| V617F homozygous | 3 (8) | 0 (0) | 0 (0) |
| Normal | 16 (40) | 38 (100) | 48 (100) |
| Characteristic . | ET*† (n = 40) . | RT*‡ (n = 38) . | Healthy controls§ (n = 48) . |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 15 (38) | 16 (42) | 23 (48) |
| Female | 25 (52) | 22 (58) | 25 (52) |
| Age group, no. (%) | |||
| Younger than 35 y | 3 (8) | 9 (24) | 16 (33) |
| 36-60 y | 13 (32) | 24 (63) | 27 (57) |
| 60 y or older | 24 (60) | 5 (13) | 5 (10) |
| Platelet count, ×10−3/mm3 | |||
| Mean | 659 | 632 | 246 |
| Range | 203-1724 | 173-1044 | 160-415 |
| Hemoglobin, g/dL | |||
| Mean | 13.3 | 10.9 | 14.1 |
| Range | 9.5-20.8 | 8.1-15.3 | 12.3-15.9 |
| White cell count, × 10−3/mm3 | |||
| Mean | 10.2 | 14.9 | 6.3 |
| Range | 3.1-44.6 | 5.7-65.2 | 4.4-9.6 |
| JAK2 genotype, no. (%) | |||
| V617F heterozygous | 21 (52) | 0 (0) | 0 (0) |
| V617F homozygous | 3 (8) | 0 (0) | 0 (0) |
| Normal | 16 (40) | 38 (100) | 48 (100) |
Splenomegaly was identified in 14 of 40 ET patients (35.0%) and in 2 of 38 RT patients (5.3%).
At the time of platelet isolation, 12 patients were being treated with hydroxyurea, 12 patients with anagrelide, 1 patient with both hydroxyurea and anagrelide, 1 patient with pegylated interferon alfa-2b, and 14 patients were untreated.
Underlying patient diagnoses associated with RT included the following: infection (19), malignancy (14), other (3), and inflammatory diseases (2).
Complete blood counts are from random subset of 10 males and 10 females.
Molecular studies
Leukocytes and gel-filtered platelets were isolated from peripheral blood (20 mL) as previously described10 ; the final platelet-enriched product contained no more than 3 to 5 leukocytes per 105 platelets. High-quality platelet RNA was isolated using Trizol,29 and platelet mRNA quantification and integrity were established using an Agilent 2100 Bioanalyzer. Mean platelet RNA concentrations among the 3 groups were comparable, ranging from approximately 0.3 to 1.0 fg/platelet. High-molecular-weight DNA was used as the source for genomic JAK2V617F (exon 12, 1849G→T transversion) genotyping,19,20 whereas platelet mRNA was used for transcript genotyping. Mutational screening was completed using both pyrosequence and dideoxy sequence analyses of polymerase chain reaction (PCR)–amplified fragments. Samples were defined as JAK2V617F-positive if the mutation was detected in more than 5% of the respective (DNA or RNA) nucleic acid pools.
Confirmatory studies of platelet gene expression were established using fluorescence-based real-time PCR (RT-PCR).10,30 Oligonucleotide primer pairs were generated using Primer3 software (www.genome.wi.mit.edu), designed to generate PCR products (200 ± 1 bp) at the same annealing temperature. mRNA levels were quantified using real-time fluorometric analysis of SYBR green I, and relative mRNA abundance was determined from triplicate assays performed in parallel for each primer pair using the comparative threshold cycle number (Δ-Ct method).31
Chip design and manufacture
Gene-expression profiles were determined using an oligonucleotide chip uniquely designed and fabricated for comparative analysis of platelet-expressed genes. The final 432-member gene list was established using microarray profiles from an initial cohort of normal (n = 5) and essential thrombocythemic (n = 6) platelet mRNAs hybridized to the Affymetrix HU133A GeneChip containing a 22 283 probe set representing approximately 13 000 genes10,30 ; leukocyte RNA from 3 normal controls was used to delineate leukocyte gene-expression profiles. The gene list included the following cohorts: (1) a group of platelet-restricted genes with no expression in leukocytes (n = 126); (2) a preliminary group of discriminatory genes distinguishing between thrombohemorrhagic ET phenotypes (n = 71; this gene subset was obtained from the original 2906-gene list30 and a P value cutoff of .05 that segregated a limited subset of ET patients [n = 6] into 3 phenotypes: thrombotic [n = 2], hemorrhagic [n = 2], and normal [n = 2]); (3) a list of genes with platelet expression greater than leukocyte expression by 10-fold (n = 285); and (4) the list of genes with leukocyte expression greater than platelet expression by 10-fold (n = 43; leukocyte contamination control). After removal of duplicates, the final list contained 432 genes, which cosegregated by cell type (platelet vs leukocyte) as determined by in silico analysis.32 The relative gene-expression patterns comprising the gene list were found to logically follow a Poisson distribution (not shown). Several Arabidopsis probe elements were included to serve as normalization controls and as quantitative measures of interslide and intraslide variability33 ; for all genes, 70-mer oligonucleotides were designed and synthesized based on the Ensembl (www.ensembl.org) Human 13.31 Database; all probe sets were spotted in quadruplicate to provide replicates and statistical robustness.
Gene-expression analysis
Platelet gene profiling was completed using a template-switching mechanism to optimize amplification from low-abundance mRNAs.34 Initially, 20 ng of purified platelet or human reference RNA was supplemented with a fixed amount of Arabidopsis mRNA to provide internal standards for hybridization and normalization. Human Universal Reference RNA was purchased from Stratagene (catalog no. 740000) and contains equal quantities of pooled DNase-treated total RNA from 10 human cell lines, thereby providing a diversified sampling suitable for cohybridization microarray experiments. Amplification and labeling were completed using the Ovation Aminoallyl system (NuGen Technologies), providing for 4 to 6 μg of cDNA/sample. After reaction termination (95°C for 5 minutes), the amplified aminoallyl dUTP cDNA products were extracted in RNAse-free water and concentrated to small volumes (< 5 μL) using Microcon filters (Millipore). cDNA solutions were vacuum-dried and coupled to Cy3 (human reference RNA) or Cy5 (patient RNA) dyes (GE Healthcare) for 1 hour at 25°C in light-protected tubes. After purification, the efficiency of dye coupling was measured by optical density (650 nm Cy5; 550 nm Cy3), and stoichiometrically equivalent mixes were then coincubated to achieve optimal Cy3/Cy5 ratios. After a concentration and drying step, labeled cDNA mixes were allowed to hybridize to individual platelet chips for 12 to 16 hours at 42°C, at which point they were washed and immediately scanned for quantification of fluorescence intensity using a Gene Pix 4000B scanner (Molecular Devices). The initial data processing (eg, gridding, technical spot analysis) was completed using GenePix Pro (Version 6.0) software. After rigorous inspection to exclude spotting irregularities, raw Cy3/Cy5 ratios were quantified for individual genes. Reproducibility of microarray profiles using biologic replicates from healthy donors was high (Spearman correlation coefficients of 0.93-0.95, n = 5). All microarray data were submitted to the GEO database in MIAME-compliant format, reported under NCBI tracking no. 15670131 (series GSE12295).
Bioinformatics and statistical analyses
Microarray data were analyzed and visualized using GeneSpring (Silicon Genetics) or in-house software. Expression data were sequentially normalized by spot, by gene, and by chip essentially as previously described,10,30 followed by a moderate filtering step to maximize our ability to identify differentially expressed genes. For each gene, the 4 ratios were averaged and log2-transformed before data analysis. Genes with fluorescence intensities less than 10 (the default background threshold in GeneSpring) in more than 70% of arrays were excluded from further analysis.
The Kruskal-Wallis, nonparametric one-way analysis of variance was performed to identify differentially expressed genes among the 3 cohorts (ie, ET, RT, and normal). The nonparametric Wilcoxon rank-sum test was used to examine median differences between 2 independent samples. This included sex effects, the comparison between ET and RT subjects, as well comparison within ET subjects by JAK2 genotype using either microarray or quantitative RT-PCR data. The significance level is set at .05 (2-sided) unless otherwise specified.
We used the genetic microarray profiles to develop a statistical classifier designed to categorize and predict clinical phenotypes (ie, normal, ET, RT class prediction). All statistical analyses and class prediction models were completed on deidentified samples blinded to phenotype. Stepwise discriminant analysis was used to identify an initial biomarker subset that separated class on the basis of microarray data. The fidelity of the genetic biomarker subsets as class prediction tools was established using nonparametric linear discriminant analysis (LDA) with a leave-one-out cross-validation analysis.35 Using cross-validation, the available datasets were divided into k disjoint sets; k models were then trained on a different combination of k-1 samples and tested on the remaining partition. The k-fold cross-validation estimate of a given performance statistic simply reflects the mean of that statistic evaluated for each of the k models over the corresponding test partitions of the data. An integral property of the leave-one-out cross-validation estimator is that it provides an almost unbiased estimate of the generalization ability of the genetic classifier.
Posterior classification probability for each subject was derived and the binary decision was made for group assignment based on subject highest probability. As part of the confirmatory studies, the same biomarker set using the microarray data was applied to the quantitative RT-PCR data; fidelity was established using LDA leave-one-out cross-validation in the initial patient cohort, and with direct prediction using the initial cohort (cohort 1) as the training set, and an entirely independent new cohort (cohort 2) as the testing set. This same biomarker identification and validation procedure was applied both for separation of ET versus RT, and for substratification of ET by JAK2 genotype (JAK2V617F vs wild type).
Results
Patient cohort analysis
A total of 126 subjects were recruited into the study. The cohort 1 patient mix included 95 subjects (ET [n = 24]; RT [n = 23]; healthy controls [n = 48]), whereas the cohort 2 population was restricted to those with thrombocytosis (ET [n = 16]; RT [n = 15]; Table 1). The mean platelet counts between ET and RT patient cohorts were nearly identical and not statistically different, although differences were identified in hemoglobin (P < .001) and leukocyte concentrations (P = .02). These latter differences are readily explained by concomitant illnesses in RT patients who typically cause chronic anemia or reactive leukocytosis. At the time of platelet collection, 7 of 40 ET and 1 of 38 RT patients had normal platelet counts, reflecting either medication (ET) or thrombocytotic resolution (RT). In patients with RT, infection and malignancy accounted for the majority of etiologies (87%). Compared with RT patients, a greater percentage of ET patients were 60 years of age or older; both advanced age and female preponderance reflected known demographics of ET.28 Of the ET patients, 52% were heterozygous for the JAK2V617F mutation (GT), whereas a smaller fraction (8%) were found to be homozygous for the mutation (TT); these characteristics are consistent with those previously reported.20,36 No RT or healthy controls harbored the JAK2V617F mutation.
Sex effect on gene-expression profiles
Given previous evidence demonstrating genetic differences between normal and ET platelets,30 a platelet-focused gene chip for screening cohort 1 subjects was fabricated. Initially, we sought to exclude any sex effect among the 3 groups, and calculated the Wilcoxon rank-sum test for each of the genes on the array. For both normal and ET cohorts, the preponderance of the genes was equally distributed within the 95% confidence interval, with only 4 of the genes in either group demonstrating any sex effect (Figure 1A). In normal controls, 2 genes displayed greater expression in males (CCDC88C and HIST1H2BF), whereas 2 other genes were differentially weighted toward females (LOC152719 and LOC390354). In ET platelets, a single gene (E2F1) was male-skewed, whereas 3 genes (GAS2L1, SASH3, and PPME1) were female-biased. In contrast, sex effects were more prominent in the RT cohort, with 11 genes falling outside the 95% confidence interval, all of which demonstrated male-skewed gene expression differences. Interestingly, 2 of these 12 genes (ITGA2B and ITGB3) encode the major polypeptide subunits of the platelet glycoprotein IIB/IIIA (αIIb/β3) integrin, suggesting that the molecular mechanisms that control gene expression of the heterodimeric receptor complex are concordantly regulated by sex during situations associated with reactive thrombocytosis.
Sex and discriminant analysis based on 3-group microarray expression profiles. (A) Scatter plots by patient cohort were generated by applying a nonparametric Wilcoxon rank-sum test to determine sex differences in gene expression for each of the 432 genes on the microarray chip. The z-score represents a weighted difference between the observed and the expected rank sums for the Wilcoxon test; thus, a z-score of 0 represents equivalent median expression (male gene expression = female gene expression), whereas the dashed lines represent 95% confidence intervals that are female (positive)- or male (negative)-skewed. The individual genes that are differentially expressed by sex are delineated in each phenotypic group. (B) List of the 11-biomarker gene subset identified by stepwise discriminant analysis. P values for 3-cohort discrimination are displayed (cut off at P < .001 for simplicity). (C) Plot of posterior classification probability demonstrates the segregation of the 3 phenotypes (ET = 24 [●], RT = 23 [○], normal = 48 [ ]) using the 11-biomarker gene subset via LDA with leave-one-out cross-validation (group means are depicted by the individual rhomboids with cross-hairs). Each symbol represents one patient microarray that incorporates aggregate expression data from the 11-gene subset. For each patient sample, the posterior classification probabilities that a given subject belongs to ET or RT cohorts are displayed by subject; the probability that a subject belongs to the normal cohort is equivalent to 1 − the sum of the corresponding ET and RT probabilities.
]) using the 11-biomarker gene subset via LDA with leave-one-out cross-validation (group means are depicted by the individual rhomboids with cross-hairs). Each symbol represents one patient microarray that incorporates aggregate expression data from the 11-gene subset. For each patient sample, the posterior classification probabilities that a given subject belongs to ET or RT cohorts are displayed by subject; the probability that a subject belongs to the normal cohort is equivalent to 1 − the sum of the corresponding ET and RT probabilities.
Sex and discriminant analysis based on 3-group microarray expression profiles. (A) Scatter plots by patient cohort were generated by applying a nonparametric Wilcoxon rank-sum test to determine sex differences in gene expression for each of the 432 genes on the microarray chip. The z-score represents a weighted difference between the observed and the expected rank sums for the Wilcoxon test; thus, a z-score of 0 represents equivalent median expression (male gene expression = female gene expression), whereas the dashed lines represent 95% confidence intervals that are female (positive)- or male (negative)-skewed. The individual genes that are differentially expressed by sex are delineated in each phenotypic group. (B) List of the 11-biomarker gene subset identified by stepwise discriminant analysis. P values for 3-cohort discrimination are displayed (cut off at P < .001 for simplicity). (C) Plot of posterior classification probability demonstrates the segregation of the 3 phenotypes (ET = 24 [●], RT = 23 [○], normal = 48 [ ]) using the 11-biomarker gene subset via LDA with leave-one-out cross-validation (group means are depicted by the individual rhomboids with cross-hairs). Each symbol represents one patient microarray that incorporates aggregate expression data from the 11-gene subset. For each patient sample, the posterior classification probabilities that a given subject belongs to ET or RT cohorts are displayed by subject; the probability that a subject belongs to the normal cohort is equivalent to 1 − the sum of the corresponding ET and RT probabilities.
]) using the 11-biomarker gene subset via LDA with leave-one-out cross-validation (group means are depicted by the individual rhomboids with cross-hairs). Each symbol represents one patient microarray that incorporates aggregate expression data from the 11-gene subset. For each patient sample, the posterior classification probabilities that a given subject belongs to ET or RT cohorts are displayed by subject; the probability that a subject belongs to the normal cohort is equivalent to 1 − the sum of the corresponding ET and RT probabilities.
Delineation of a genetic biomarker subset for discriminant analysis
Of the genes on the microarray chip, 267 displayed expression values that were significantly different among the 3 groups (P < .05), as established using the Kruskal-Wallis nonparametric one-way analysis of variance. This relatively large number is not unexpected because the chip was specifically fabricated to include the subset of differentially expressed genes from a previous study.30 Among this subset, 148 genes were found to be significantly different between RT and ET cohorts using the Wilcoxon rank-sum test. Stepwise LDA identified an 11-biomarker subset that segregated the 3 phenotypic cohorts (ET vs RT vs normal; Figure 1B). Gene Ontology annotations using GOstat37 demonstrated that the predominant functional annotations of this 11-biomarker subset were related to nucleosome and chromatin assembly (genes H3FA, HIST1H2AG, and WASF3), critically important components of gene transcription that may regulate megakaryocytopoiesis and/or proplatelet formation.38 The 11-biomarker gene subset did not include HSD17B3 or HSD17B12, which were previously shown to distinguish normal from ET platelets.30
The utility of the initial 11-biomarker subset to predict phenotypic class (ie, ET, RT, and normal) was initially confirmed using a leave-one-out cross-validation analysis. In this situation, each subject is classified by the profiles derived from all cases excluding that case.35 This approach confirmed the generalizability of the statistical classifier (ie, its performance on previously unseen data) using the available data as both training and test data. As shown, the 3 groups could be readily classified by phenotype (Figure 1C), thereby providing an additional, unbiased estimate of class prediction. The posterior classification probabilities applied in a binary decision model using this gene set for 3-cohort analysis confirmed that 82 of 95 (86.3%) of all subjects could be correctly classified, with the greatest sensitivity evident in RT patients (100%; Table 2). Of the patients with thrombocytosis, 1 of 47 (2.1%) was genetically classified as belonging to the normal cohort, although 6 of 48 (12.5%) normal subjects were incorrectly classified as belonging to ET (n = 1) or RT (n = 5) cohorts.
Three- or two-cohort classification using 11 biomarkers
| Phenotypic class . | Genotypic class (microarray) . | |||
|---|---|---|---|---|
| Normal . | RT . | ET . | Total . | |
| Three-way classification | ||||
| Normal | 42 (87.5) | 5 (10.4) | 1 (2.1) | 48 |
| RT | 0 | 23 (100) | 0 | 23 |
| ET | 1 (4.2) | 6 (25.0) | 17 (70.8) | 24 |
| Classification by group aggregate | 82/95 (86.3%) | |||
| Two-way classification | ||||
| RT | — | 23 (100) | 0 (0) | 23 |
| ET | — | 3 (12.5) | 21 (87.5) | 24 |
| Classification by group aggregate | 44/47 (93.6%) | |||
| Phenotypic class . | Genotypic class (microarray) . | |||
|---|---|---|---|---|
| Normal . | RT . | ET . | Total . | |
| Three-way classification | ||||
| Normal | 42 (87.5) | 5 (10.4) | 1 (2.1) | 48 |
| RT | 0 | 23 (100) | 0 | 23 |
| ET | 1 (4.2) | 6 (25.0) | 17 (70.8) | 24 |
| Classification by group aggregate | 82/95 (86.3%) | |||
| Two-way classification | ||||
| RT | — | 23 (100) | 0 (0) | 23 |
| ET | — | 3 (12.5) | 21 (87.5) | 24 |
| Classification by group aggregate | 44/47 (93.6%) | |||
Classification was completed using nonparametric LDA with a leave-one-out cross-validation (numbers in parentheses are percentage by group). A subject is classified to the group corresponding to his/her highest posterior classification probability (both 3-way and 2-way classifications are delineated).
— indicates not applicable.
Because a primary goal of classification is to predict phenotypic class among patients with thrombocytosis, we used the 11-biomarker subset as a discriminatory tool restricted to ET and RT. Two-cohort LDA confirmed that ET and RT profiles segregated by class, with only 3 of 47 (6.4%) outliers and an overall classification success rate of 93.6% (Table 2). Indeed, the ET versus RT discriminatory analyses were more accurate using a 2-cohort (as opposed to the 3-cohort) linear discriminatory classifier. Similar to the results evident in the 3-cohort analysis, RT was correctly classified in 100% of cases using microarray analysis, although 3 of 24 ET patients were classified as having RT. Of the 3 misclassified ET patients, 2 were JAK2V617F-negative and ET was diagnosed by exclusion.
Thrombocytosis classification and prediction models
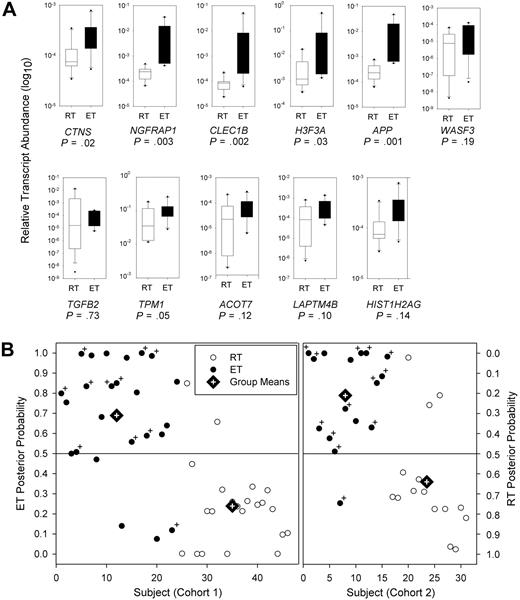
Two levels of validation were used to further establish the reliability of this biomarker subset to assign phenotypic class based on genetic profiles. Initially, the fidelity of the 11-member gene subset in discriminating between ET and RT cohorts was pursued by genetic profiling using a distinct, confirmatory platform. We generated oligonucleotide primers to the 11-biomarker gene set (supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article), and completed quantitative RT-PCR for a randomly selected subset of 10 subjects in each cohort. Six of the biomarkers were found to have significantly different median expression levels between ET and RT cohorts via quantitative RT-PCR at P < .05 (CTNS, NGFRAP1, CLEC1B, H3F3A, APP, and TMP1; Figure 2A). These confirmatory results provided strong presumptive evidence that ET and RT profiles were genetically distinct using 2 independent platforms (microarray and quantitative RT-PCR).
Thrombocytosis discriminant analysis using an 11-biomarker gene subset. (A) A randomly selected subset of ET (n = 10) and RT (n = 10) patient platelets (each group included 5 males and 5 females) was analyzed by quantitative RT-PCR using oligonucleotide primers specific to each of the 11 genetic biomarkers. Relative gene expression is displayed on a log10 scale standardized to β-actin mRNA, calculated from triplicate wells for each patient sample using the comparative threshold number (Δ-Ct). Boxes represent the interquartile range that encompasses 50% of the values, whereas the 95% confidence intervals and outliers are depicted; the horizontal bar within each box represents the group median; P values were calculated using nonparametric Wilcoxon rank-sum test. (B) LDA plot shows the posterior classification probability of each subject by cohort using the 11-biomarker gene subset based on quantitative RT-PCR profiles (individual group means are delineated by rhomboids with cross-hairs). Cohort 1 included 24 ET and 22 RT patient samples (1 RT sample was omitted because of lack of RNA), whereas cohort 2 included 16 ET and 15 RT patients. For binary decisions (ie, ET or RT), a subject is classified based on the highest posterior classification (ie, a group with probability > .5). Patient samples containing the JAK2V617F mutation (either homozygous or heterozygous) are indicated by +.
Thrombocytosis discriminant analysis using an 11-biomarker gene subset. (A) A randomly selected subset of ET (n = 10) and RT (n = 10) patient platelets (each group included 5 males and 5 females) was analyzed by quantitative RT-PCR using oligonucleotide primers specific to each of the 11 genetic biomarkers. Relative gene expression is displayed on a log10 scale standardized to β-actin mRNA, calculated from triplicate wells for each patient sample using the comparative threshold number (Δ-Ct). Boxes represent the interquartile range that encompasses 50% of the values, whereas the 95% confidence intervals and outliers are depicted; the horizontal bar within each box represents the group median; P values were calculated using nonparametric Wilcoxon rank-sum test. (B) LDA plot shows the posterior classification probability of each subject by cohort using the 11-biomarker gene subset based on quantitative RT-PCR profiles (individual group means are delineated by rhomboids with cross-hairs). Cohort 1 included 24 ET and 22 RT patient samples (1 RT sample was omitted because of lack of RNA), whereas cohort 2 included 16 ET and 15 RT patients. For binary decisions (ie, ET or RT), a subject is classified based on the highest posterior classification (ie, a group with probability > .5). Patient samples containing the JAK2V617F mutation (either homozygous or heterozygous) are indicated by +.
For the final validation phase, we analyzed patient profiles from this initial cohort (n = 46) but also studied a second, independent group composed of newly recruited cohort 2 patients with thrombocytosis (n = 31). We completed quantitative RT-PCR analyses using the 11 biomarkers and applied a binary class prediction model to predict (assign) phenotypic class to either ET or RT group. As shown in Figure 2B, these quantitative RT-PCR data alone gave similar and accurate phenotypic classification that paralleled those seen using the initial microarray data (compare with Table 2); indeed, 84.8% of cohort 1 and 87.1% of cohort 2 patients could be accurately classified into RT or ET phenotypes using quantitative RT-PCR, with aggregatory prediction rates of 85.7% for the combined groups.
Substratification by JAK2 genotype
We applied the identical discriminant and validation analyses for ET class prediction substratified by the absence (GG) or presence (GT/TT) of the JAK2V617F mutation. Stepwise discriminant analysis based on the microarray data alone resulted in a 4-member subset composed of genes HIST1H1A, SRP72, C20orf103, and CRYM. LDA with cross-validation based on the microarray data alone confirming that 20 of 23 (87%) patients were correctly classified, with the best classification evident in patients who were genotypically normally (GG) at the JAK2 locus (12 of 12 patients properly classified). Comparable results were seen using quantitative RT-PCR (as a validation platform) in which only 2 of 16 JAK2 wild-type (GG) patients were misclassified (Tables 3–4). In contrast, class prediction was less accurate in the subset of patients harboring the JAK2V6717F mutation. By microarray analyses, 3 of 11 JAK2V6717F patients were misclassified, whereas quantitative RT-PCR resulted in a higher misclassification rate (9 of 24; 37.5%). Thus, although the overall classification using confirmatory quantitative RT-PCR remained satisfactory (72.5% correct classification), the results were not as accurate as those using microarray. For both approaches, the specificity of the classification was best in the subset of patients who were genotypically normal at the JAK2 locus. One explanation may be the limited number of evaluable patients used for the discriminatory classifier. Alternatively, the transcript profiles of JAK2V617F platelets may be heterogeneous, with both phenotypic and genotypic differences affected by quantitative burden of mutant JAK2V617F.39
JAK2 class prediction using a 4-biomarker gene subset
| Genotype . | Class prediction . | |||||
|---|---|---|---|---|---|---|
| Microarray . | Quantitative RT-PCR* . | |||||
| JAK2 (GG) . | JAK2 (GT/TT) . | Total . | JAK2 (GG) . | JAK2 (GT/TT) . | Total . | |
| JAK2 (GG) | 12 (100.0) | 0 (0) | 12 | 14 (87.5) | 2 (12.5) | 16 |
| JAK2 (GT/TT) | 3 (27.3) | 8 (72.7) | 11 | 9 (37.5) | 15 (62.5) | 24 |
| Group aggregates | 20/23 (87.0) | 29/40 (72.5) | ||||
| Genotype . | Class prediction . | |||||
|---|---|---|---|---|---|---|
| Microarray . | Quantitative RT-PCR* . | |||||
| JAK2 (GG) . | JAK2 (GT/TT) . | Total . | JAK2 (GG) . | JAK2 (GT/TT) . | Total . | |
| JAK2 (GG) | 12 (100.0) | 0 (0) | 12 | 14 (87.5) | 2 (12.5) | 16 |
| JAK2 (GT/TT) | 3 (27.3) | 8 (72.7) | 11 | 9 (37.5) | 15 (62.5) | 24 |
| Group aggregates | 20/23 (87.0) | 29/40 (72.5) | ||||
GG indicates wild-type normal; GT, Jak2V617F heterozygote; and TT, Jak2V617F homozygote.
Classification includes aggregate data from the training set (cohort 1) using cross-validation, and test set (cohort 2) via prediction based on the training data.
Discriminant analysis function of 4-biomarker gene subset
| Gene discriminant power* . | ||||
|---|---|---|---|---|
| Gene† . | Microarray . | Quantitative RT-PCR . | ||
| JAK2 (GG) . | JAK2 (GT/TT) . | JAK2 (GG) . | JAK2 (GT/TT) . | |
| HIST1H1A | 91.6 | 36.4 | 93.8 | 37.5 |
| SRP72 | 91.6 | 36.4 | 87.5 | 0.0 |
| C20orf103 | 16.7 | 100 | 37.5 | 75.0 |
| CRYM | 75 | 54.6 | 87.5 | 25.0 |
| Gene discriminant power* . | ||||
|---|---|---|---|---|
| Gene† . | Microarray . | Quantitative RT-PCR . | ||
| JAK2 (GG) . | JAK2 (GT/TT) . | JAK2 (GG) . | JAK2 (GT/TT) . | |
| HIST1H1A | 91.6 | 36.4 | 93.8 | 37.5 |
| SRP72 | 91.6 | 36.4 | 87.5 | 0.0 |
| C20orf103 | 16.7 | 100 | 37.5 | 75.0 |
| CRYM | 75 | 54.6 | 87.5 | 25.0 |
Discriminant power of each individual gene was determined by entering only the given gene in the LDA. The gene discriminant power is the percentage of a given group correctly classified using one specific gene; thus, an entry of 100 means that all subjects in the given group were correctly classified. HIST1H1A indicates histone cluster 1, H1a; SRP72, signal recognition particle 72 kDa; C20orf, chromosome 20 open reading frame; and CRYM, crystallin, μ.
Classification includes aggregate data from the training set (cohort 1) using cross-validation, and test set (cohort 2) via prediction based on the training data.
Discussion
We now provide evidence that gene-expression profiling can be used to molecularly classify platelet phenotypes, using a common human disorder (thrombocytosis) as a paradigm for study. By applying a novel microarray platform to an extensive cohort of normal and diseased platelet phenotypes, we have identified an 11-member gene biomarker subset that effectively predicts phenotypic class in 86% of aggregate platelet samples, with more than 90% classification success when specifically applied to patients with thrombocytosis. We controlled for the false discovery rate using 4 distinct, iterative validation steps: (1) microarray profiling followed by a leave-one-out cross-validation algorithm for the original cohort; (2) application of a confirmatory platform (quantitative RT-PCR) for the original cohort, again confirmed by a leave-one-out cross-validation algorithm; (3) recruitment of a second independent cohort; and (4) application of the 11-member biomarker subset identified in the original cohort to classify newly recruited patients using quantitative RT-PCR. Thus, although the results await replication in larger patient cohorts, they nonetheless provide compelling evidence that transcriptomic approaches can be applied to clinically relevant platelet disorders.
Limitations of large-scale gene-expression profiling and reporting standards have been well described,40,41 with additive restrictions in platelets resulting from small mRNA quantities and potential leukocyte contamination.10,32 Nonetheless, comparable approaches have been successfully developed for patients with various human malignancies.42,43 Unlike human malignancies, which are typically diagnosed using standard histologic criteria, the majority of platelet disorders are morphologically indistinguishable under light microscopy, with electron microscopic abnormalities restricted to rare genetic variants.1 These observations suggest that clinically relevant molecular classification of platelet disorders may be achieved using comparable expression profiling and class prediction models. The feasibility of such an approach is further highlighted by recent evidence for efficient and reproducible platelet genetic profiling from small blood volumes (0.1 mL).44
Greater amounts of platelet RNA are identifiable in the subpopulation of newly generated “reticulated” platelets (RPs) that account for approximately 1% to 3% of the circulating platelet pool.14,45 RP quantification is achieved using an RNA-specific vital dye (thiazole orange) that does not distinguish between ribosomal and mRNA.10 We did not quantify RP percentage in this study, although such information has been previously reported,14,46 with evidence that thrombocytosis patients had RP percentages that were similar to those of normal controls and not different between RT and ET patients. Increased relative and absolute RP counts were primarily restricted to the subset of thrombocytotic patients with concomitant thromboses.14 Thus, although previous data have used RPs as surrogate markers for thrombotic risk, these studies fail to identify specific genetic differences that account for phenotypic differences. Our study, on the other hand, extends these observations by demonstrating clear delineation of platelet genetic profiles by phenotype. At this time, it remains unknown whether these alterations in genetic profiles are restricted to this smaller RP pool or reflect alterations broadly distributed in the circulating platelet pool.
The proper classification of thrombocytosis is of paramount importance given the frequency of thrombohemorrhagic complications known to occur in ET (to the exclusion of RT).15,16,28 Likewise, although platelet-lowering drugs are effective in controlling thrombocytosis, none are devoid of side effects, and persistent concerns remain about potential leukemogenic effects of hydroxyurea despite evidence to the contrary.47 Revised diagnostic criteria for the MPDs have been proposed,27 largely incorporating the presence of the JAK2V617F mutation as a major criterion in both PV and ET diagnosis. Whereas the presence of the JAK2V617F mutation is a highly predictive marker (major criterion) for PV diagnosis, the absence of the mutation in up to 60% of ET patients highlights its limited applicability in the larger cohort of thrombocytotic patients. With relevance to JAK2 wild-type ET, a 4-biomarker gene subset effectively subclassified ET using either microarray (100%) or quantitative RT-PCR (87.5%), although class prediction was less efficient in patients harboring the JAK2V617F mutation. Nevertheless, these data strongly suggest that algorithms can be developed for class prediction models of thrombocytosis, initially using combinations of JAK2V617F screens in conjunction with our 11-biomarker subset; confirmatory 4-biomarker profiles could be obtained for patients who are JAK2 wild-type. On a broader note, a unified molecular classification schema for MPDs could be envisioned, presumably integrating diagnostic and prognostic platelet biomarkers for formulation of tailored therapies by thrombohemorrhagic risk susceptibility.17,18
Of the 11-biomarker subset initially identified by microarray profiling, 6 genes demonstrated statistically significant gene-expression differences between ET and RT using quantitative RT-PCR. Of these genes, APP (encoding the amyloid β-precursor protein, AβPP) remains the best characterized in platelets, with limited platelet functional information on NGFRAP1, CLEC1B, CTNS, H3F3A, or TPM1. AβPP is the precursor to the amyloid β-peptide (Aβ) that accumulates in the brains of patients with Alzheimer disease. AβPP is an abundant platelet α-granule protein that is released on platelet activation and contains a Kunitz-type serine protease inhibitor domain analogous to protease nexin-2.48 Protease nexin-2/AβPP is a potent inhibitor of various serine proteases, notably coagulation factors IXa, XIa, Xa, and the tissue factor FVIIa complex. Megakaryocyte/platelet overexpression of human AβPP in transgenic mice modulates cerebral thrombotic risk, suggesting that changes in platelet AβPP expression may affect the thrombohemorrhagic balance in vivo.49 Although our studies were not focused on delineating genetic differences that could affect platelet-related thrombosis or hemorrhage known to occur in ET, these collective observations imply that enhanced AβPP expression may be causally implicated in the development of hemorrhagic complications known to occur in subsets of ET patients. Comparable gene-profiling studies focusing on platelet transcriptomic differences stratified by ET thrombohemorrhagic events may provide greater insight into its utility as a predictive biomarker for vascular (or other) thrombohemorrhagic complications.18
Limited information exists on sex-restricted genetic differences in platelets, despite evidence for dissimilarities in platelet function or platelet-targeted therapies.50,51 Our preliminary studies identified gene subsets that may be sex-skewed in normal and ET platelets, although these differences are more pronounced in RT patients. In this cohort, a male-weighted trend in higher gene expression was evident for 12 genes. Unlike the situation with ET or normal platelets, none of the RT gene expressions was weighted toward females. Although these observations are preliminary, they are noteworthy because 2 genes (ITGA2B and ITGB3) displayed congruent expression changes and encode both polypeptide chains of the GPIIb/IIIa fibrinogen receptor, the final common mediator of platelet aggregation. Interestingly, sex-related differences in GPIIb/IIIa function have been described52 ; furthermore, recent meta-analysis on efficacy of GPIIb/IIIa inhibitors in acute coronary syndromes confirmed reduced risk of death or myocardial infarction in men, although these differences disappeared when patients were substratified by troponin concentrations.53 Although there is no evidence that cell-surface GPIIb/IIIa expression is modulated by conditions known to cause RT (as suggested by our data), the sex-restricted increase is intriguing given the documented capacity for platelets to dynamically adjust their transcriptome when activated by potent agonists, such as thrombin,12,13 a critically important link between thrombotic and inflammatory pathways.54
Despite evidence that RT and ET (and normal) platelets are genetically distinct, analysis of larger patient cohorts will be required to determine whether the various RT subtypes can be further subclassified (eg, malignancy-associated, inflammatory, iron deficiency). In inflammation-associated thrombocytosis, interleukin-6 may function as a critical mediator by up-regulating thrombopoietin mRNA.55 Although a comparable interleukin-6 role may be evident in iron deficiency or malignancy-associated thrombocytosis,15 it remains likely that additional mechanisms may be operational. Because many of these ligands mediate their effects through receptors known to regulate transcription, we speculate that expression profiles may be further substratified by etiology, a hypothesis readily testable with more extensive cohort analysis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Xiao Wu for statistical analysis, Lesley Scudder and Jean Wainer for excellent technical support, and Dr Alexander Zuhoski for assistance with subject recruitment.
This work was supported by the National Institutes of Health (grants HL49141, HL53665, HL086376, and HL76457), Department of Defense (MPO48005), a Targeted Research Award from Stony Brook University, and National Institutes of Health Center grant MO1 10710-5 to the University Hospital General Clinical Research Center.
National Institutes of Health
Authorship
Contribution: D.V.G. designed experiments, performed research, analyzed data, and contributed to the manuscript writing and preparation; W.Z. provided analytical tools for data processing and analyzed data; X.X. performed statistical and bioinformatic data analysis; E.T.S. recruited patients for this study; M.M. performed research and analyzed data; M.H.Z. recruited patients; C.K. collected biologic samples; A.D. designed experiments and analyzed data; and W.F.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dmitri V. Gnatenko, Division of Hematology, Department of Medicine, T15-040 HSC, State University of New York at Stony Brook, Stony Brook, NY 11794-8151; e-mail: dgnatenko@notes.cc.sunysb.edu.

![Figure 1. Sex and discriminant analysis based on 3-group microarray expression profiles. (A) Scatter plots by patient cohort were generated by applying a nonparametric Wilcoxon rank-sum test to determine sex differences in gene expression for each of the 432 genes on the microarray chip. The z-score represents a weighted difference between the observed and the expected rank sums for the Wilcoxon test; thus, a z-score of 0 represents equivalent median expression (male gene expression = female gene expression), whereas the dashed lines represent 95% confidence intervals that are female (positive)- or male (negative)-skewed. The individual genes that are differentially expressed by sex are delineated in each phenotypic group. (B) List of the 11-biomarker gene subset identified by stepwise discriminant analysis. P values for 3-cohort discrimination are displayed (cut off at P < .001 for simplicity). (C) Plot of posterior classification probability demonstrates the segregation of the 3 phenotypes (ET = 24 [●], RT = 23 [○], normal = 48 []) using the 11-biomarker gene subset via LDA with leave-one-out cross-validation (group means are depicted by the individual rhomboids with cross-hairs). Each symbol represents one patient microarray that incorporates aggregate expression data from the 11-gene subset. For each patient sample, the posterior classification probabilities that a given subject belongs to ET or RT cohorts are displayed by subject; the probability that a subject belongs to the normal cohort is equivalent to 1 − the sum of the corresponding ET and RT probabilities.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/1/10.1182_blood-2009-05-224477/4/m_zh89990944770001.jpeg?Expires=1769096846&Signature=3wRsfWObNycsj45Xg6k93NOTp367BRECE6Mf3cMKfbk0IW-GIwjML8vkPbHLmanN-nKIwSKmzIu0oj9ht465z2lHTvawBaLQQ2zlbIDSJwyDe~Xb~ghICD94sua8pa2G5vYaB8oxH8OrVdW9T7INtdCgYGDSicU5gMmuYkwJSdjKhNmJ1CS4Z8wzhkG7etw2sRlOzCqncdBP3XqHaebBaJ1Y5B-0OaVraEb~2VIuZU~hT-vL5RANCFqgbmyWu1Qy2HCKWi4xxOEl73t1ZlWBnAzOSKTwNlBzzlbYRrx~4S2qkYXKs0fEuDQAxVHIlT2i5j9Jgq1njo1e3QcECH5ACQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal