Abstract
The processing of pro-interleukin-1β depends on activation of caspase-1. Controversy has arisen whether Toll-like receptor (TLR) ligands alone can activate caspase-1 for release of interleukin-1β (IL-1β). Here we demonstrate that human blood monocytes release processed IL-1β after a one-time stimulation with either TLR2 or TLR4 ligands, resulting from constitutively activated caspase-1 and release of endogenous adenosine triphosphate. The constitutive activation of caspase-1 depends on the inflammasome components, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and NALP3, but in monocytes caspase-1 activation is uncoupled from pathogen-associated molecular pattern recognition. In contrast, macrophages are unable to process and release IL-1β solely by TLR ligands and require a second adenosine triphosphate stimulation. We conclude that IL-1β production is differentially regulated in monocytes and macrophages, and this reflects their separate functions in host defense and inflammation.
Introduction
Much interest has been generated regarding processing and release of bioactive interleukin-1β (IL-1β) since the discovery that autoinflammatory disorders specifically respond to blockade of IL-1 receptor with the IL-1 receptor antagonist (IL-1Ra), or neutralization of IL-1β by the monoclonal anti–IL-1β antibodies. These syndromes include familial Mediterranean fever,1 familial cold autoinflammatory syndrome,2 Muckle-Wells syndrome,3 neonatal onset multisystem inflammatory disease (NOMID),4 hyperimmunoglobulin D syndrome,5 and adult-onset Still disease.6 Blood monocytes from patients with some of these disorders, especially cryopyrinopathies, readily release more IL-1β than monocytes from unaffected controls, revealing a loss of the tight control regulating processing and release of active IL-1β.
Several mechanisms control the production and activity of IL-1β, including the processing of the 31-kDa inactive IL-1β precursor form into the bioactive 17-kDa IL-1β,7 and the release from secretory lysosomes through K+-dependent mechanisms.8,9 In addition, control over IL-1 activity is exerted by the IL-1Ra or the type II decoy receptors.10 Processing of bioactive IL-1β depends on activation of caspase-1 by the protein complex termed the inflammasome.11 Several protein platforms/inflammasomes have been described for the activation of caspase-1, each of them include members of the NOD-like receptor (NLR) family of proteins.12 The most intensely studied have been the inflammasomes formed by the NLR family members NALP3 and NALP1, which also include the adapter protein ASC (apoptosis-associated speck-like protein containing a CARD) for the activation of caspase-1. Mutations in NALP3 exits in familial cold auto-inflammatory syndrome,2 Muckle-Wells syndrome, and NOMID, whereas specific NALP-1 polymorphisms have been associated with vitiligo and autoimmune diseases.13 In addition to the NALPs, another NLR member, IPAF, forms an inflammasome that activates caspase-1 in response to intracellular flagellin in an ASC-independent manner.14,15
However, controversy surrounds the capacity of toll-like receptor (TLR) ligands, such as lipopolysacchardie (LPS) to activate caspase-1, resulting in the release of active IL-1β. Using transfected cell lines and/or NALP3 knockout mice, a broad panel of stimuli have been proposed to activate the NALP3 inflammasome, including bacterial products such as peptidoglycans, muramyl dipeptide (MDP),16 and bacterial toxins,17 but also endogenous products such as uric acid18 or adenosine triphosphate (ATP).17 Based on responses in the leukemic cell line THP-1, a concept has arisen that IL-1β production induced by the often-used monocyte stimulant LPS is the result of contamination with non-LPS ligands, such as peptidoglycans,16 whereas LPS by itself is ineffective as a stimulator of IL-1β release. A second signal, such as muramyldipeptide or ATP, is required, and this would induce activation of caspase-1 followed by IL-1β processing and release.19 This model is derived from data in THP-1 cells16 and in primary mouse macrophages20 ; however, it is inconsistent with many studies showing abundant production and release of IL-1β from blood monocytes by purified LPS, lipopeptides, lipoteichoic acid, as well as cytokines such as tumor necrosis factor-α (TNF-α) and IL-1 itself.21,22 In addition, several studies report that synthetic products, which exclude contamination with NALP1 or NALP3 ligands, stimulate IL-1β release.23,24
In the present study, we demonstrate a remarkable difference in the level of constitutive caspase-1 activation and subsequent IL-1β release between freshly obtained blood monocytes and macrophages. We conclude that there is no basis for the concept that LPS is unable to induce processing and release of IL-1β from blood monocytes. We also conclude that, unlike the blood monocytes, the macrophages require not only LPS but also a second signal for the activation of the inflammasome and release of processed IL-1β.
Methods
Reagents
LPS (Escherichia coli serotype 055:B5) and staphylococcal peptidoglycan (PepG) were purchased from Sigma-Aldrich (St Louis, MO). LPS was repurified as previously described.25 Synthetic Pam3Cys was purchased from EMC Microcollections (Tubingen, Germany). Synthetic MDP was obtained from Sigma-Aldrich. The reversible caspase-1 inhibitor (termed ICE-i) Ac-Tyr-Val-Ala-Asp-2,6-dimethylbezoyloxymethylketone (YVAD) was purchased from Axxora Life Sciences (San Diego, CA) and solubilized in dimethyl sulfoxide at 10 mg/mL. The ICE-i was diluted to the desired concentration in RPMI. Antihuman caspase-1 p10 (sc515), anticaspase-1 p45 (sc-622), and anti–human ASC (sc30153) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–human IL-1β antibodies were purchased from Cell Signaling Technology (Danvers, MA). Recombinant granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-4 were purchased from R&D Systems (Minneapolis, MN). The TLR4 antagonist Bartonella quintana LPS was prepared as previously described.26 oxATP was purchased from Sigma-Aldrich.
Chinese hamster ovary cells
The Chinese hamster ovary (CHO)/CD14 cell line (clone 3E10) was transfected with plasmids expressing either human TLR4 or human TLR2 surface proteins, kindly provided by Douglas Golenbock (University of Massachusetts, Worcester, MA). On engagement of TLR4 or TLR2, a nuclear factor-κB–dependent reporter plasmid drives the expression of surface CD25, as a result of nuclear factor-κB translocation. Surface CD25 was assessed by flow cytometry after stimulation of CHO/TLR2 or CHO/TLR4 cells with 1 μg/mL LPS. In addition, peritoneal macrophages of TLR2 and TLR4 knockout mice were stimulated with LPS (1 μg/mL) for 24 hours at 37°C, and TNF was measured by specific enzyme-linked immunosorbent assay (ELISA).
Isolation of mononuclear cells and stimulation of cytokine production
These studies were approved by the Colorado Multiple Institutional Review Board. After informed consent was obtained in accordance with the Declaration of Helsinki, the peripheral blood mononuclear cell (PBMC) fraction was obtained by differential centrifugation over Ficoll-Paque (Sigma-Aldrich). Cells adjusted to 5 × 106 cells/mL were suspended in culture medium (RPMI 1640) supplemented with gentamicin 10 μg/mL, l-glutamine 10 mM, and pyruvate 10 mM; 100-μL cells were incubated with either 100 μL of culture medium (negative control) or purified LPS (various concentrations as described in the figure legends), Pam3Cys (10 μg/mL), heat-killed Staphylococcus epidermidis (106 microorganisms/mL), MDP (10 μg/mL), or combinations of MDP and LPS. In separate experiments, inhibitors (YVAD caspase-1 inhibitor, 20 μM) or IL-1Ra (R&D Systems) were added. After 24 hours, the supernatants were collected and stored at −70°C until assayed. Intracellular were assessed after adding 200 μL RPMI to the adherent cells and cell lysis by 2 cycles of freeze-thaw.
To investigate the role of monocytes for the production of IL-1β, the monocytes were purified using a separation assay with magnetic beads coated with anti-CD14 antibodies, as described by the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany).
To investigate the effect of LPS followed by the second stimulus of ATP on cytokine production, PBMCs or purified monocytes were initially stimulated for 4 hours with LPS. After 4 hours, the supernatants were collected and RPMI containing 1 mM ATP was added to the cells for another 15 minutes. The LPS-dependent IL-1β production during the first 4 hours and the ATP-dependent IL-1β secretion after the additional 15 minutes were assessed in the supernatant. The role of the endogenous ATP release for the stimulation of IL-1β was investigated by blocking P2X7 receptors with oxATP (300 μM) during the stimulation of cells for 4 hours with LPS.27
Cytokine and ATP determinations
Cytokine concentrations were determined by electrochemiluminescence assays. The IL-1β precursor and the mature IL-1β were measured by specific ELISA from R&D Systems. ATP concentrations in the supernatants were assessed using a firefly luciferase assay (ATP determination kit, Invitrogen, Carlsbad, CA).
Macrophage and dendritic cell differentiation
The adherent monocytes were obtained after incubation of PBMCs for 2 hours at 37°C, after which the nonadherent lymphocytes were discarded. The monocytes were incubated for 5 days with either 10% pooled human plasma (macrophage differentiation), 50 ng/mL G-CSF (macrophage differentiation), or a combination of GM-CSF (50 ng/mL) and IL-4 (20 ng/mL) (dendritic cell differentiation). On day 3, medium was refreshed. After 5 days, the supernatant was removed and cells were stimulated with the various stimuli. In addition, alveolar macrophages collected from healthy volunteers by bronchoalveaolar lavage were suspended to a concentration of 5 × 106 cells/mL and stimulated for cytokine production.
Immunoblotting
For immunoblotting, 10 × 106 cells were lysed in 100 μL lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 2 mM ethyleneglycoltetraacetic acid, 10% glycerol, 1% Triton X-100, 40 mM α-glycerophosphate, 50 mM sodium fluoride, 200 μM sodium vanadate, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 μM pepstatin A, and 1 mM phenylmethylsulfonyl fluoride). The homogenate was frozen, thawed, then centrifuged at 4 for 10 minutes at 14 000g, and the supernatant was taken for Western blotting. Equal amounts of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using 10% and 15% polyacrylamide gels at a constant voltage of 100 V. After SDS-PAGE, proteins were transferred to nitrocellulose membrane (0.2 μm). The membrane was blocked with 3% (wt/vol) milk powder in phosphate-buffered saline for 1 hour at room temperature followed by incubation overnight at 4°C with the primary antibody in 5% bovine serum albumin/Tris-buffered saline/Tween 20 (5% bovine serum albumin/TBST). After overnight incubation, the blots were washed 3 times with TBST and incubated with horseradish peroxidase-conjugated goat antirabbit antibody at a dilution of 1:10 000 in 3% (wt/vol) milk powder in phosphate-buffered saline for 1 hour at room temperature. After washing the blots 3 times with TBST, the blots were developed with electro chemiluminescence according to the manufacturer's instructions. The quantification of protein expression was performed by densitometry (GS-670, Bio-Rad, Hercules, CA) and signal analysis using Molecular Analyst software (Bio-Rad). The ratio between the intensity of the protein of interest and β-actin was calculated. The activation of caspase-1 was assessed by calculating the ratio between the p10 and p45 fragments.
siRNA experiments
For siRNA experiments, specific cell line and primary monocyte protocols for electroporation in the Amaxa chamber (Amaxa Biosystems, Gaithersburg, MD) were used, according to the instructions of the manufacturer. Specific sets of siRNA for ASC and NALP3 as well as control, nonsilencing siRNA were obtained from Dharmacon RNA Technologies (Lafayette, CO) or Ambion (Huntingdon, United Kingdom). After counting with trypan blue, PBMCs were centrifuged at 200g for 10 minutes and resuspended in 100 μL prewarmed Human Monocyte Nucleofector solution (Amaxa Biosystems) per transfer condition. Without delay, siRNA was added and electroporation was performed using program Y-001. After electroporation, the primary monocytes were allowed to recover overnight in polypropylene tubes, to avoid adhesion; 2 μg siRNA was used (control-sense 5′-UUCUCCGAACGUGUCACGUtt-3′; control-antisense 5′-ACGUGACACGUUCGGAGAAtt-3′; ASC-sense 5′-GAUGCGGAAGCUCUUCAGUtt-3′; ASC-antisense 5′-ACUGAAGAGCUUCCGCAUCtt-3′; Nalp3-sense 5′-GGUGUUGGAAUUAGACAACtt-3′; Nalp3-antisense GUUGUCUAAUUCCAACACCtg-3′) per 106 cells. On the next day, in case of THP1 cells, they were treated with phorbol-12-myristate-13-acetate (PMA) for 24 hours, washed, and incubated for additional 24 hours in culture medium. The next day, the cells were stimulated with LPS or LPS/ATP as described in “Isolation of mononuclear cells and stimulation of cytokine production.” Control experiments to check the inhibition of ASC or NALP3 expression were performed by reverse-transcribed polymerase chain reaction (RT-PCR) and immunoblotting.
RT-PCR
A total of 10 million freshly isolated PBMCs were incubated with the various stimuli. After 4 hours of incubation at 37°C, total RNA was extracted in 1 mL of TRIzol reagent. Isolated RNA was treated with DNase before being reverse-transcribed into complementary DNA using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase. PCR was performed using a Peltier Thermal Cycler-200 (Watertown, MA). Primer sequences for human IL-1β are sense, 5′-GGA-TAT-GGA-GCA-ACA-AGT-GG-3′ and antisense, 5′-ATG-TAC-CAG-TTG-GGG-AAC-TG-3′ and, for TNF-α, sense, 5′-ACA-AGC-CTG-TAG-CCC-ATG-TT-3′ and antisense, 5′-AAA-GTA-GAC-CTG-CCC-AGA-CT-3′. GAPDH was used as a reference gene, for which the primers were 5-GGC-AAA-TTC-AAC-GGC-ACA-3 (forward) and 5-GTT-AGT-GGG-GTC-TCG-CTC-TG-3 (reverse). PCR conditions were as follows: 2 minutes at 50°C and 10 minutes at 95°C, followed by 30 cycles of PCR reaction at 94°C for 45 seconds, 70°C for 2 minutes, and 59°C for 1 minute. The PCR products were run on 1% agarose gels stained with ethidium bromide.
Statistical analysis
The cytokine induction experiments were performed in triplicate wells, and the data are presented as cumulative results. The differences were analyzed by paired t test. The data are given as mean plus or minus SEM.
Results
Purified LPS stimulates IL-1β synthesis and release by freshly obtained blood monocytes
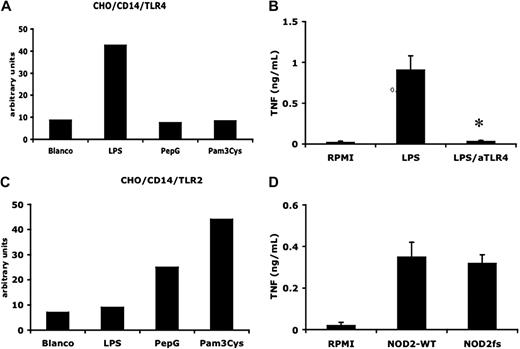
It has been suggested that LPS induces the release of active IL-1β only because of contamination with bacterial cell wall products, such as peptidoglycans and MDP.16 To exclude the presence of non-LPS microbial products, we performed a double purification of the standard commercial preparation of E coli O55:B5 LPS.25 We tested the properties of the preparation in several ways: purified LPS stimulated CD14/TLR4 but not CD14/TLR2 transfected CHO cells (Figure 1A,C); TNF-α production by the purified preparation was completely reversed in the presence of the specific TLR4 antagonist B quintana LPS26 (Figure 1B) or in TLR4−/− mice (not shown); moreover, there was no reduction in LPS-induced TNF-α production from PBMCs isolated from persons carrying the frame shift mutation in the MDP-receptor NOD2 (homozygous for the 3020insC mutation; Figure 1D). In addition, we did not observe a reduction in production induced by the purified LPS in TLR2-deficient mice (data not shown).
Double-purified LPS is a specific TLR4 agonist. (A) Effect of LPS (1 μg/mL), PepG (10 μg/mL), and Pam3Cys (10 μg/mL) on surface expression of CD25 in CD14/TLR4-transfected CHO cells (relative flow cytometry units are shown). The results of 1 representative experiment of 3 are presented. (B) Mean ( ± SEM) TNF-α levels in supernatants from LPS (10 ng/mL)–stimulated PBMCs in the absence or absence of the TLR4 antagonist B quintana (100 ng/mL) indicated by aTLR4 (N = 5, P < .01, compared with LPS stimulation). (C) Effect of LPS (1 μg/mL), PepG (10 μg/mL), and Pam3Cys (10 μg/mL) on surface expression of CD25 in CD14/TLR2-transfected CHO cells (relative flow cytometry units are shown). The results of 1 representative experiment of 3 are presented. (D) Mean (± SEM) TNF-α levels in 24-hour supernatants of LPS-stimulated PBMCs from 5 volunteers bearing wild-type NOD2 allele (NOD2 WT) and 4 persons with a frame-shift mutation in NOD2 (NOD2fs).
Double-purified LPS is a specific TLR4 agonist. (A) Effect of LPS (1 μg/mL), PepG (10 μg/mL), and Pam3Cys (10 μg/mL) on surface expression of CD25 in CD14/TLR4-transfected CHO cells (relative flow cytometry units are shown). The results of 1 representative experiment of 3 are presented. (B) Mean ( ± SEM) TNF-α levels in supernatants from LPS (10 ng/mL)–stimulated PBMCs in the absence or absence of the TLR4 antagonist B quintana (100 ng/mL) indicated by aTLR4 (N = 5, P < .01, compared with LPS stimulation). (C) Effect of LPS (1 μg/mL), PepG (10 μg/mL), and Pam3Cys (10 μg/mL) on surface expression of CD25 in CD14/TLR2-transfected CHO cells (relative flow cytometry units are shown). The results of 1 representative experiment of 3 are presented. (D) Mean (± SEM) TNF-α levels in 24-hour supernatants of LPS-stimulated PBMCs from 5 volunteers bearing wild-type NOD2 allele (NOD2 WT) and 4 persons with a frame-shift mutation in NOD2 (NOD2fs).
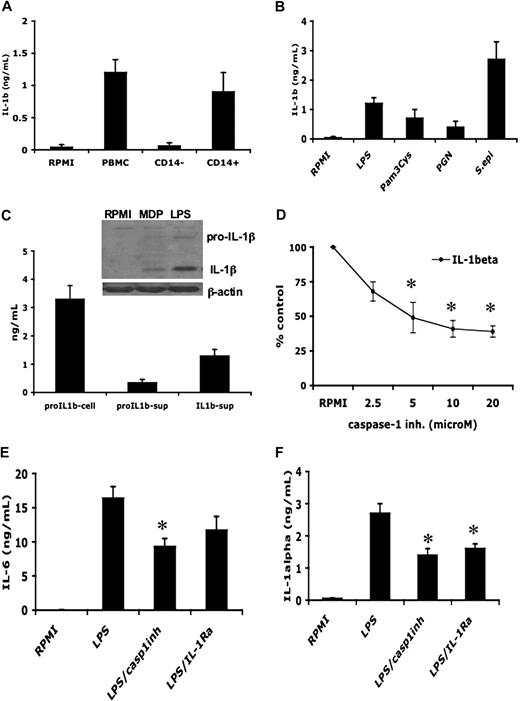
When human PBMCs were stimulated with this purified LPS, the levels of both proIL-1β precursor and the mature IL-1β were significantly increased (Figure 2C). To demonstrate the presence of the mature processed form of the IL-1β, Western blots of supernatants of cells stimulated with control medium, MDP, or LPS were performed. As shown in the inset to Figure 2C, LPS stimulates the release of the active 17-kDa form of IL-1β.
LPS induces bioactive IL-1β in primary monocytes. (A) Human PBMCs, purified CD14+ monocytes, or the CD14− lymphocytes were incubated with 10 ng/mL LPS, and the IL-1β concentrations were measured in the supernatant by specific ELISA after 24-hour incubation. (B) CD14+ monocytes were stimulated with various stimuli for the induction of IL-1β. (C) Human PBMCs were incubated with 10 ng/mL LPS and the levels of mature and precursor IL-1β or were measured in the supernatant by specific ELISA after 24-hour incubation. (Inset) Western blot of IL-1β and pro-IL-1β the supernatant derived from PBMCs stimulated with LPS (100 ng/mL) or MDP (10 μg/mL). (D) Dose-response of caspase-1 inhibitor ZVAD-fmk on the 24-hour levels of LPS-induced IL-1β from PBMCs. (E,F) The inhibitory effects of the caspase-1 inhibitor (20 μM) and of IL-1Ra (10 μg/mL) on the induction of IL-6 (E) and intracellular IL-1α measured in cell lysates (F) by 10 ng/mL LPS. Data from all 4 panels are presented as mean plus or minus SEM of cells harvested from 6 volunteers. *P < .05 compared with LPS stimulation alone.
LPS induces bioactive IL-1β in primary monocytes. (A) Human PBMCs, purified CD14+ monocytes, or the CD14− lymphocytes were incubated with 10 ng/mL LPS, and the IL-1β concentrations were measured in the supernatant by specific ELISA after 24-hour incubation. (B) CD14+ monocytes were stimulated with various stimuli for the induction of IL-1β. (C) Human PBMCs were incubated with 10 ng/mL LPS and the levels of mature and precursor IL-1β or were measured in the supernatant by specific ELISA after 24-hour incubation. (Inset) Western blot of IL-1β and pro-IL-1β the supernatant derived from PBMCs stimulated with LPS (100 ng/mL) or MDP (10 μg/mL). (D) Dose-response of caspase-1 inhibitor ZVAD-fmk on the 24-hour levels of LPS-induced IL-1β from PBMCs. (E,F) The inhibitory effects of the caspase-1 inhibitor (20 μM) and of IL-1Ra (10 μg/mL) on the induction of IL-6 (E) and intracellular IL-1α measured in cell lysates (F) by 10 ng/mL LPS. Data from all 4 panels are presented as mean plus or minus SEM of cells harvested from 6 volunteers. *P < .05 compared with LPS stimulation alone.
The secretion of IL-1β by LPS from monocytes was significantly inhibited by a caspase-1 inhibitor (Figure 2D). We used 2 strategies to assess the bioactivity of endogenous IL-1β stimulated by LPS. On the one hand, the release of the mature form of IL-1β was inhibited in the presence of a caspase-1 inhibitor, and that in turn reduced significantly the production of IL-1α and IL-6, as shown in Figure 2E,F. Moreover, the activity of IL-1β can be blocked at the level of the receptor by the competitive binding of IL-1Ra. Blockade of IL-1 receptors by adding IL-1Ra to the medium reduced the production and release of IL-6 and IL-1α (Figure 2E,F).
Different capacity of monocytes, macrophages, and dendritic cells to produce and release IL-1β
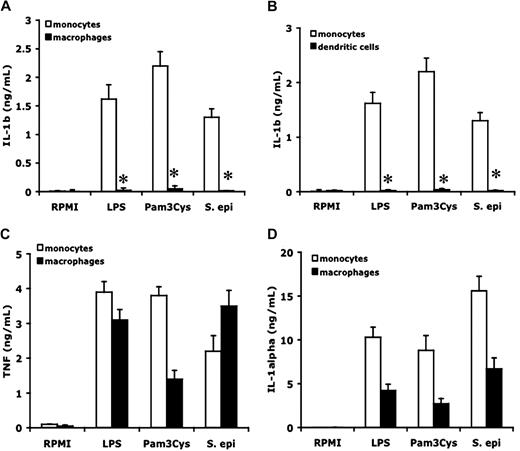
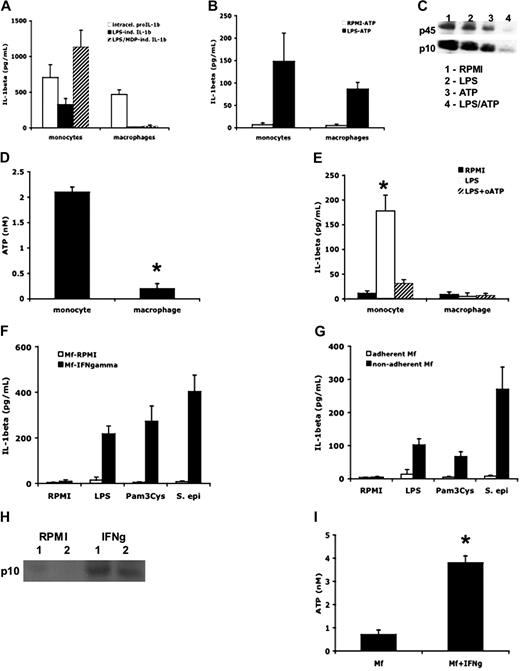
To compare the capacity of monocytes and macrophages to stimulate IL-1β synthesis and release, freshly isolated monocytes and monocyte-derived macrophages were stimulated with either purified TLR4 (LPS) or TLR2 (Pam3Cys) ligands, or with whole heat-killed S epidermidis, an inducer of IL-1β.28 As shown in Figure 3A, monocytes released large amounts of IL-1β in the supernatant after 24 hours of stimulation, whereas no IL-1β was present in the supernatant of stimulated macrophages. Macrophages that were differentiated in the presence of 10% pooled human plasma did not release IL-1β with each of the 3 stimulants. Similarly, there was no production of IL-1β by macrophages differentiated for 5 days with 50 ng/mL G-CSF (data not shown).
Differential production of cytokines by monocytes, macrophages, and dendritic cells. Freshly isolated human PBMCs, macrophages differentiated after 5-day incubation with 10% human plasma, or dendritic cells differentiated after 5 days GM-CSF (50 ng/mL) + IL-4 (20 ng/mL), were stimulated with culture medium, LPS (100 ng/mL), Pam3Cys (10 μg/mL), or S epidermidis (106 organisms/mL). IL-1β (A,B), TNF (C), or IL-1α (D) was measured in the supernantant after 24-hour incubation. Data are presented as mean plus or minus SEM of cells harvested from 6 volunteers. *P < .01 compared with the stimulation in monocytes.
Differential production of cytokines by monocytes, macrophages, and dendritic cells. Freshly isolated human PBMCs, macrophages differentiated after 5-day incubation with 10% human plasma, or dendritic cells differentiated after 5 days GM-CSF (50 ng/mL) + IL-4 (20 ng/mL), were stimulated with culture medium, LPS (100 ng/mL), Pam3Cys (10 μg/mL), or S epidermidis (106 organisms/mL). IL-1β (A,B), TNF (C), or IL-1α (D) was measured in the supernantant after 24-hour incubation. Data are presented as mean plus or minus SEM of cells harvested from 6 volunteers. *P < .01 compared with the stimulation in monocytes.
In addition to monocyte-derived macrophages, differentiation of monocytes into dendritic cells also resulted in a loss in the capacity to release mature IL-1β (Figure 3B). In contrast, both monocytes and monocyte-derived macrophages released comparable amounts of TNF-α (Figure 3C) or IL-1α (Figure 3D) in the same cultures. IL-1α concentrations were lower in macrophages than in monocytes, most probably because of the absent autocrine stimulatory loop induced by endogenous IL-1β.
Differential activation of caspase-1 and IL-1β excretion in monocytes and macrophages
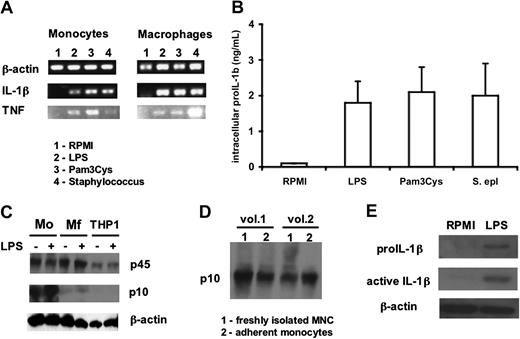
Despite the absence of IL-1β in the supernatants of macrophages stimulated with microbial stimuli, LPS induced abundant amounts of precursor IL-1β mRNA in both the monocytes and macrophages (Figure 4A). Moreover, significant amounts of the IL-1β precursor were present intracellularly in the macrophages (Figure 4B), demonstrating that a posttranslational defect is the mechanism of defective release of IL-1β in macrophages.
mRNA levels and processing of IL-1β in monocytes versus macrophages. (A) Steady-state levels of IL-1β and TNF-α in freshly isolated PBMCs or monocyte-derived macrophages after 4 hours of incubation with, LPS (100 ng/mL), Pam3Cys (10 μg/mL), or S epidermidis (106 organisms/mL). (B) Intracellular levels of precursor IL-1β in monocyte-derived macrophages stimulated for 24 hours with the various stimuli as in panel A. (C) Western blot of caspase-1 peptides in monocytes (Mo), macrophages (Mf), or THP-1 cells after 2 hours with and without LPS (100 ng/mL). (D) Western blot of the active p10 fragment of caspase-1 (sc515 antibody from Santa Cruz Biotechnology) in freshly isolated monocytes (column 1) and in monocytes that have adhered for 2 hours in polystyrene plates (column 2). (E) IL-1β and pro-interleukin-1β (proIL-1β) Western blots of lysates of monocyte-derived macrophages before and after stimulation for 24 hours with 100 ng/mL LPS.
mRNA levels and processing of IL-1β in monocytes versus macrophages. (A) Steady-state levels of IL-1β and TNF-α in freshly isolated PBMCs or monocyte-derived macrophages after 4 hours of incubation with, LPS (100 ng/mL), Pam3Cys (10 μg/mL), or S epidermidis (106 organisms/mL). (B) Intracellular levels of precursor IL-1β in monocyte-derived macrophages stimulated for 24 hours with the various stimuli as in panel A. (C) Western blot of caspase-1 peptides in monocytes (Mo), macrophages (Mf), or THP-1 cells after 2 hours with and without LPS (100 ng/mL). (D) Western blot of the active p10 fragment of caspase-1 (sc515 antibody from Santa Cruz Biotechnology) in freshly isolated monocytes (column 1) and in monocytes that have adhered for 2 hours in polystyrene plates (column 2). (E) IL-1β and pro-interleukin-1β (proIL-1β) Western blots of lysates of monocyte-derived macrophages before and after stimulation for 24 hours with 100 ng/mL LPS.
To test the hypothesis that a processing defect of the IL-1β precursor accounts for the differences between the monocyte and macrophage, Western blots of the inactive p45 caspase-1 and the active p10 caspase-1 form were performed in both cell types. As shown in Figure 4C, after 2 hours of incubation, monocytes display clear activation of caspase-1 regardless of LPS stimulation. The constitutive presence of both p45 and the active p10 caspase-1 can be observed in freshly isolated mononuclear cells that, to avoid stimulation, were directly lysed after isolation at 4°C (Figure 4D). In contrast to monocytes, macrophages have significant amounts of the inactive p45 caspase-1 form, but less of the active p10 caspase-1 fragment, even after stimulation with LPS (Figure 4C).
THP-1 cells exhibit no activation of caspase-1 (Figure 4C), and there was no release of active IL-1β when stimulated with LPS alone (data not shown). However, when primed for 3 days with 10 μg/mL PMA, THP-1 cells released significant amounts of IL-1β into the supernatant after stimulation for 24 hours with 100 ng/mL LPS (1.4 ± 0.4 ng/mL, n = 5). This was the result of a moderate activation of caspase-1 after PMA priming, as well as the spontaneous release of endogenous ATP (1.4 ± 0.4 nM vs < 0.2 nM in nonprimed cells), followed by the production of IL-1β on stimulation with LPS.
Despite the relatively ineffective activation of caspase-1 by LPS in macrophages, Western blots of cell lysates reveal the intracellular presence of the precursor as well as the mature forms of IL-1β (Figure 4E). These findings suggest that the defect in the release of mature IL-1β is partially at the level of inflammasome activation and partially at the level of IL-1β secretion.
The role of the inflammasome components for the stimulation and release of IL-1β in primary blood monocytes
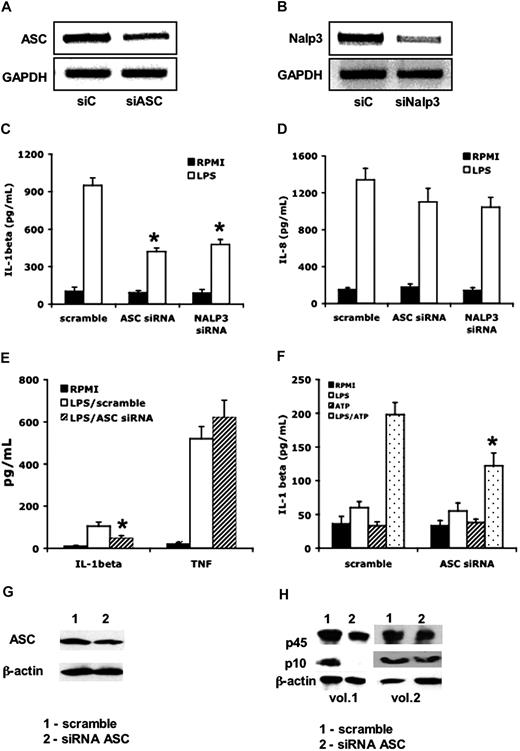
To assess the role of the inflammasome components ASC and NALP3 for the activation of caspase-1 and IL-1β release, we transfected PMA-primed THP-1 cells with siRNA against either ASC or NALP3. The transfection of PMA-primed THP-1 cells with siRNA decreased the steady-state levels of ASC by 48% plus or minus 13% (Figure 5A), and of NALP3 by 67% plus or minus 19% (Figure 5B). These reductions were accompanied by a significant inhibition of IL-1β release that was induced by LPS (Figure 5C). No effects on IL-8 production were apparent (Figure 5D). Similarly, freshly isolated PBMCs were transfected with siRNA specific for ASC as well as a control of scrambled siRNA. As depicted in Figure 5E, decreasing ASC expression in primary monocytes was associated with a decrease in IL-1β release, which was induced after a 24-hour exposure to LPS (single stimulation; Figure 5E). A similar reduction was observed after a short 4-hour exposure to LPS followed by a second stimulation with ATP (double stimulation; Figure 5F). There was no effect on the production of the caspase-1–independent cytokine TNF-α in these cultures (Figure 5E). Moreover, inhibition of ASC expression by siRNA (Figure 5G) in primary cells resulted in a significantly decreased activation of caspase-1: the p10/p45 ratio was decreased from 0.37 plus or minus 0.19 to 0.14 plus or minus 0.09 (P < .05; Figure 5H).
The role of ASC and NALP3 in the production and release of IL-1β. THP-1 cells were transfected with siRNA against ASC or NALP3 or control scramble (siC). RT-PCR of lysates after overnight incubation for ASC (A) or NALP3 (B). Cells were transfected with scrambled or target siRNA, then primed for 24 hours with PMA (100 ng/mL), and thereafter stimulated for 24 hours with LPS (1 μg/mL). After 24 hours, IL-1β (C) or IL-8 (D) was assessed in the supernatants (*P < .05 compared with the stimulation with LPS in cells treated with scramble siRNA). (E) Levels of supernatant IL-1β and TNF-α in primary PBMCs after transfection with scramble or siRNA for ASC and subsequent stimulation with LPS for 24 hours (n = 5, mean ± SEM, *P < .05 compared with LPS/scramble). (F) Levels of supernatant IL-1β in PBMCs stimulated for 3 hours with LPS (100 ng/mL), after which ATP (1 mM) or a combination of LPS and ATP was added for an additional 15 minutes. PBMCs were stimulated that were transfected with either scramble or siRNA against ASC (n = 5, means ± SEM, *P < .05 compared with LPS/ATP in the cells transfected with scramble siRNA). (G) Western blot of ASC in PBMCs transfected with either scramble or siRNA against ASC. (H) Western blot of the p10 and p45 caspase-1 in PBMCs transfected with either scramble (column 1) or siRNA (column 2) against ASC.
The role of ASC and NALP3 in the production and release of IL-1β. THP-1 cells were transfected with siRNA against ASC or NALP3 or control scramble (siC). RT-PCR of lysates after overnight incubation for ASC (A) or NALP3 (B). Cells were transfected with scrambled or target siRNA, then primed for 24 hours with PMA (100 ng/mL), and thereafter stimulated for 24 hours with LPS (1 μg/mL). After 24 hours, IL-1β (C) or IL-8 (D) was assessed in the supernatants (*P < .05 compared with the stimulation with LPS in cells treated with scramble siRNA). (E) Levels of supernatant IL-1β and TNF-α in primary PBMCs after transfection with scramble or siRNA for ASC and subsequent stimulation with LPS for 24 hours (n = 5, mean ± SEM, *P < .05 compared with LPS/scramble). (F) Levels of supernatant IL-1β in PBMCs stimulated for 3 hours with LPS (100 ng/mL), after which ATP (1 mM) or a combination of LPS and ATP was added for an additional 15 minutes. PBMCs were stimulated that were transfected with either scramble or siRNA against ASC (n = 5, means ± SEM, *P < .05 compared with LPS/ATP in the cells transfected with scramble siRNA). (G) Western blot of ASC in PBMCs transfected with either scramble or siRNA against ASC. (H) Western blot of the p10 and p45 caspase-1 in PBMCs transfected with either scramble (column 1) or siRNA (column 2) against ASC.
Double stimulation is necessary for the release of IL-1β by macrophages
We investigated whether inflammasome stimuli can reverse the defect of IL-1β release in macrophages and increase release of mature IL-1β from monocytes. The costimulation of macrophages with LPS plus MDP did not result in the release of IL-1β, demonstrating that activation of the inflammasome alone is not sufficient for the release of IL-1β (Figure 6A). In contrast, the costimulation of LPS-primed cells with ATP (which induces both caspase-1 activation and IL-1β secretion) induced the release of IL-1β from both monocytes and macrophages (Figure 6B). As shown in Figure 6C, stimulation of monocytes with LPS plus ATP induced a significant decrease in intracellular caspase-1 p10 and p45, consistent with a release of the inflammasome components into the supernatant (Figure 6C).
ATP induces IL-1β secretion in both monocytes and macrophages. (A) Human monocytes and macrophages were incubated with RPMI, LPS (1 μg/mL), or a combination of LPS and MDP (10 μg/mL) for 24 hours. Intracellular pro-IL-1β or secreted mature IL-1β were measured by specific ELISA kits (n = 6, mean ± SEM). (B) Monocytes or macrophages were incubated with RPMI or LPS for 4 hours, followed by ATP (1 mM) for 15 minutes (RPMI/ATP or LPS/ATP). Mean levels of mature IL-1β secreted in the supernatant were measured by ELISA (n = 6, mean ± SEM). (C) Western blots of caspase-1 in lysates after stimulation of monocytes with RPMI, LPS (1 μg/mL), ATP (1 mM), or a combination of LPS and ATP. (D) Monocytes and macrophages were incubated with RPMI or LPS (1 μg/mL) for 4 hours, and ATP was measured in the supernatant using a luciferase assay (n = 6, mean ± SEM, *P < .05 compared with monocytes). (E) Human monocytes and macrophages were incubated with RPMI or LPS (1 μg/mL) for 4 hours, and P2X7 receptors were blocked by adding oxidized ATP (oATP, 300 μM). Concentrations of mature IL-1β were measured in the supernatants by ELISA (n = 6, mean ± SEM, *P < .05 compared with RPMI). (F) Macrophages (Mf) differentiated in the presence of 10 ng/mL IFN-γ were stimulated for 24 hours with LPS (1 μg/mL), MDP (10 μg/mL), or heat-killed S epidermidis (106 organisms/mL). IL-1β in the supernatant was measured by ELISA (n = 6, mean ± SEM). (G) Macrophages differentiated for 5 days in RPMI with 10% plasma while in rotating (4 rpm) in 50-mL polypropylene tubes were stimulated for 24 hours with LPS, MDP, or heat-killed S epidermidis. IL-1β in the supernatant was measured by ELISA (n = 6, mean ± SEM). (H) Western blot of the active caspase-1 p10 fragment in macrophages differentiated in the absence (RPMI) or presence (IFN-γ) of IFN-γ. Data from 2 volunteers (1 and 2) are presented. (I) ATP release from macrophages differentiated in the absence or presence of IFN-γ (n = 5, mean ± SEM, *P < .05 compared with macrophages without IFN-γ).
ATP induces IL-1β secretion in both monocytes and macrophages. (A) Human monocytes and macrophages were incubated with RPMI, LPS (1 μg/mL), or a combination of LPS and MDP (10 μg/mL) for 24 hours. Intracellular pro-IL-1β or secreted mature IL-1β were measured by specific ELISA kits (n = 6, mean ± SEM). (B) Monocytes or macrophages were incubated with RPMI or LPS for 4 hours, followed by ATP (1 mM) for 15 minutes (RPMI/ATP or LPS/ATP). Mean levels of mature IL-1β secreted in the supernatant were measured by ELISA (n = 6, mean ± SEM). (C) Western blots of caspase-1 in lysates after stimulation of monocytes with RPMI, LPS (1 μg/mL), ATP (1 mM), or a combination of LPS and ATP. (D) Monocytes and macrophages were incubated with RPMI or LPS (1 μg/mL) for 4 hours, and ATP was measured in the supernatant using a luciferase assay (n = 6, mean ± SEM, *P < .05 compared with monocytes). (E) Human monocytes and macrophages were incubated with RPMI or LPS (1 μg/mL) for 4 hours, and P2X7 receptors were blocked by adding oxidized ATP (oATP, 300 μM). Concentrations of mature IL-1β were measured in the supernatants by ELISA (n = 6, mean ± SEM, *P < .05 compared with RPMI). (F) Macrophages (Mf) differentiated in the presence of 10 ng/mL IFN-γ were stimulated for 24 hours with LPS (1 μg/mL), MDP (10 μg/mL), or heat-killed S epidermidis (106 organisms/mL). IL-1β in the supernatant was measured by ELISA (n = 6, mean ± SEM). (G) Macrophages differentiated for 5 days in RPMI with 10% plasma while in rotating (4 rpm) in 50-mL polypropylene tubes were stimulated for 24 hours with LPS, MDP, or heat-killed S epidermidis. IL-1β in the supernatant was measured by ELISA (n = 6, mean ± SEM). (H) Western blot of the active caspase-1 p10 fragment in macrophages differentiated in the absence (RPMI) or presence (IFN-γ) of IFN-γ. Data from 2 volunteers (1 and 2) are presented. (I) ATP release from macrophages differentiated in the absence or presence of IFN-γ (n = 5, mean ± SEM, *P < .05 compared with macrophages without IFN-γ).
A pioneering study published by Ferrari et al has demonstrated that human monocytes can release endogenous ATP.29 We compared the release of ATP into the supernatant of PBMC or macrophages. As shown in Figure 6D, monocytes from the PBMCs, but not macrophages, release endogenous ATP. Similarly, purified monocytes had an identical effect on ATP release (1.8 ± 0.4 nM). Because monocytes cultured with LPS for 4 hours spontaneously release ATP, we blocked the ATP interaction with P2X7 receptor with oxidized ATP (oATP).27 As shown in Figure 6E, inhibition of endogenous ATP binding to the P2X7 receptor in the presence of oATP resulted in near-total reduction in the release of IL-1β release.
IFN-γ stimulation or prevention of adherence affects IL-1β release
We assessed the effect of the macrophage-activating cytokine interferon-γ (IFN-γ) on macrophages differentiated from blood monocytes. Incubation of macrophages differentiated with IFN-γ resulted in substantial IL-1β release after stimulation with TLR ligands or heat-killed S epidermidis for an additional 24 hours (Figure 6F). In addition, the differentiation of macrophages in the presence of IFN-γ led to the activation of caspase-1 (Figure 6H) and the release of endogenous ATP (Figure 6I). This suggests that IFN-γ–activated macrophages have a more proinflammatory phenotype, capable of releasing active IL-1β.
We also exploited a simple but effective method to prevent the adherence of monocytes to the polystyrene plate during the differentiation process. By slowly rotating PBMCs in polypropylene tubes, one prevents adherence, which also partially reversed the near-total lack of IL-1β release from macrophages adhering to the polystyrene plates during differentiation (Figure 6G).
Alveolar macrophages also require double stimulation for release of IL-1β
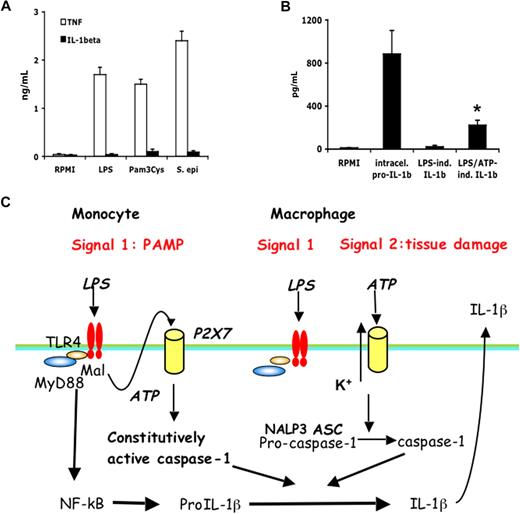
We sought to assess whether naturally occurring macrophages are also deficient in the release of IL-1β and are similar to the in vitro differentiated macrophages in requiring 2 stimuli for production of IL-1β. Although in alveolar macrophages LPS, Pam3Cys, or heat-killed S epidermidis stimulated high levels of TNF-α, there was no release of IL-1β after 24 hours of incubation with these stimuli alone (Figure 7A). There were, however, substantial amounts of intracellular precursor IL-1β (884 ± 219 pg/mL) in alveolar macrophages after LPS. Although LPS did not induce IL-1β release from the alveolar macrophages, the addition of ATP induced partial release of the IL-1β into the extracellular compartment (223 ± 45 pg/mL), similar to its effects in monocyte-derived macrophages (Figure 7B).
Alveolar macrophages require the second stimulation by ATP to release mature IL-1β after LPS. (A) Human alveolar macrophages were stimulated for 24 hours with RPMI, LPS (100 ng/mL), Pam3Cys (10 μg/mL), or S epidermidis (106 organisms/mL). (B) Alveolar macrophages were stimulated with LPS (1 μg/mL) or a combination of LPS and ATP (1 mM). Intracellular pro-IL-1β after LPS stimulation after 4 hours, and the extracellular IL-1β release after stimulation with LPS or LPS/ATP, was assessed by ELISA. The stimulation experiments were performed in cells harvested from 5 volunteers (means ± SEM, *P < .05 compared with LPS stimulation alone). (C) Diagram representing the IL-1β activation pathways in monocytes and macrophages. Caspase-1 is constitutively activated in monocytes, and these cells release mature IL-1β after single stimulation with TLR ligands. IL-1β secretion is induced by endogenously released ATP. In contrast, macrophages need a double stimulation: one stimulus (TLR ligands) induces transcription, and a second stimulus (ATP) induces IL-1β secretion.
Alveolar macrophages require the second stimulation by ATP to release mature IL-1β after LPS. (A) Human alveolar macrophages were stimulated for 24 hours with RPMI, LPS (100 ng/mL), Pam3Cys (10 μg/mL), or S epidermidis (106 organisms/mL). (B) Alveolar macrophages were stimulated with LPS (1 μg/mL) or a combination of LPS and ATP (1 mM). Intracellular pro-IL-1β after LPS stimulation after 4 hours, and the extracellular IL-1β release after stimulation with LPS or LPS/ATP, was assessed by ELISA. The stimulation experiments were performed in cells harvested from 5 volunteers (means ± SEM, *P < .05 compared with LPS stimulation alone). (C) Diagram representing the IL-1β activation pathways in monocytes and macrophages. Caspase-1 is constitutively activated in monocytes, and these cells release mature IL-1β after single stimulation with TLR ligands. IL-1β secretion is induced by endogenously released ATP. In contrast, macrophages need a double stimulation: one stimulus (TLR ligands) induces transcription, and a second stimulus (ATP) induces IL-1β secretion.
Discussion
The controversy
The well-known capacity of human blood monocytes to release IL-1β after a single stimulation with TLR ligands such as LPS has been recently challenged by studies using the THP-1 leukemic cell line16,30 or mouse peritoneal macrophages.17,20 In human cell lines and mouse macrophages, LPS fails to activate caspase-1 and release mature IL-1β. Those reports propose that release of IL-1β by LPS requires an additional stimulation of the inflammasome with MDP or ATP.19,31 It has been claimed that the large number of published research on LPS stimulation of the synthesis and release of IL-1β is based on contamination of LPS with bacterial peptidoglycans.16 However, this claim contradicts a large body of evidence that human blood monocytes release bioactive IL-1β on stimulation by purified LPS or other (sometimes synthetic) TLR ligands.32-34
If human blood monocytes were indeed unable to respond to LPS with release of IL-1β, a large body of clinical studies using freshly obtained human blood and their conclusions would be brought into question. Therefore, the purpose of the present study was to assess whether a sole TLR ligand can induce the synthesis, processing, and release of IL-1β from blood monocytes and whether primary cells differ from THP-1 cells. We demonstrate that synthesis and release of IL-1β differ between human monocytes and macrophages. Monocytes have constitutively activated caspase-1, leading to release of active IL-1β after a single stimulation event with bacterial ligands such as LPS, whereas macrophages need 2 distinct stimuli: one stimulus induces transcription and translation, and a second stimulus is needed for caspase-1 activation with subsequent IL-1β processing and secretion.
The major differences between the various studies that assessed IL-1β production and release are represented by the type of cell studied and by the stimulation model used. Practically all studies challenging the capacity of TLR ligands alone to induce IL-1β release have used either cell lines (especially THP-1) or mouse macrophages stimulated for short intervals (2-4 hours) with LPS followed by ATP. In contrast, the “classic” IL-1β stimulation is performed in primary human monocytes in which release of the processed cytokine takes place over 24 hours. The first aim of our study was to verify the validity of classic 24-hour IL-1β stimulation. Using several approaches, we demonstrate that the purified LPS used in the present study is a TLR4 agonist not contaminated with peptidoglycans or bacterial lipopeptides. This highly purified LPS preparation induced the release of mature IL-1β from human monocytes in the routine 24-hour stimulation assay.
The inflammasome needs a second signal in macrophages and dendritic cells
A strikingly different response occurred when monocytes were differentiated into macrophages or dendritic cells. When stimulated with LPS, the TLR2 ligand Pam3Cys, or even whole S epidermidis microorganisms, macrophages or dendritic cells did not release IL-1β. The lack of IL-1β release was observed not only for the in vitro–differentiated monocyte-derived macrophages, but also for alveolar macrophages isolated from healthy volunteers, the latter observation being consistent with earlier studies.35,36 Nevertheless, macrophages and monocytes produced comparable amounts of the caspase-1–independent TNF-α.
To investigate the molecular mechanisms responsible for these differences, we dissected the steps of IL-1β production and release. LPS induced marked increases in steady-state levels of IL-1β mRNA as well as of intracellular levels of the IL-1β precursor, clearly demonstrating that the defect in IL-1β release is posttranslational. Thereafter, we performed Western blots of the inactive and cleaved caspase-1 fragments in unstimulated or stimulated monocytes and macrophages. Unexpectedly, freshly isolated primary blood monocytes contained the activated form of caspase-1, even when lysed immediately after the isolation procedure without adherence to plastic. Moreover, stimulation with LPS had little additional effect (Figure 4). In contrast, macrophage expression of caspase-1 was much lower, and activation by IFN-γ was required for the induction of active p10 fragments. The presence of both the IL-1β precursor and mature IL-1β in the macrophage lysate is consistent with the lack of IL-1β release despite LPS stimulation, and only a second stimulation event with ATP was able to induce IL-1β secretion. In addition, ATP also induces a decrease in intracellular caspase-1 components.8
Why are the monocytes capable of IL-1β secretion, whereas macrophages are not? An earlier study has reported that monocytes can release endogenous ATP.29 In the present study, we confirm the importance of endogenous ATP for IL-1β secretion by monocytes and show that macrophages are not capable of ATP release (Figure 6). It is, therefore, the defective activation of caspase-1, coupled with the lack of endogenous ATP release, that renders human macrophages incapable of secretion of bioactive IL-1β.
IL-1β release from monocytes of patients with autoinflammatory diseases
Although the human monocytic leukemia cell-line THP-1 is studied to investigate inflammasome activation, we think that these cells are inappropriate for extrapolating to responses of primary cells. In contrast to primary monocytes, THP-1 cells did not express constitutively activated caspase-1 and responded poorly to LPS-induced IL-1β synthesis and release. To release IL-1β, THP-1 cells requires either double stimulation with LPS and ATP16 or priming with PMA, leading to constitutive release of endogenous ATP.
Although they are highly responsive to TLR stimulation and they release IL-1β resulting from constitutively active caspase-1, blood monocytes also respond to ATP challenge, leading to a greater release of IL-1β.8 This is highly relevant to the role of NALP3 in monocytes isolated from patients with autoinflammatory diseases. Monocytes from patients with Muckle-Wells syndrome and NALP3 mutations did not require additional ATP for the release of IL-1β.37 Similarly, 24-hour stimulation with LPS of monocytes isolated from patients with NOMID38 or hyper IgD syndrome39 also exhibit increased release of IL-1β compared with unaffected volunteers. Thus, the NALP3 mutations result in an inflammasome that is already maximally stimulated without the need for a second signal from ATP.37 Having a maximally activated inflammasome that efficiently processes IL-1β even after minimal stimulation may explain the inflammatory attacks in these syndromes, which are induced by the most trivial of stimuli.2,40
Alveolar macrophages
Consistent with the failure of in vitro differentiated macrophages to release IL-1β are the alveolar macrophages. Wewers et al obtained similar results and proposed a posttranscriptional defect in freshly obtained alveolar macrophages.41 Recently, they reported differences in pyrin expression between monocytes and macrophages and suggested that pyrin induces IL-1β release.42 However, it is unclear how pyrin modulates the inflammasome, as other studies propose inhibitory effects of pyrin on the inflammasome.1,43 Monocytes from patients with FMF and mutations in the carboxy terminal domain of pyrin release more IL-1β than cells from control subjects, suggesting a failure to suppress the activation of caspase-1.1
Importance of ASC in monocytes and macrophages
It is clear from the studies in mice deficient in ASC and NALP3 that the inflammasome contributes to LPS responses in vivo.44,45 However, we are not aware of data showing the importance of inflammasome components for the release of IL-1β after LPS stimulation in primary cells from healthy subjects or mouse monocytes. We showed here that when ASC or NALP3 expression was inhibited in primed THP-1 cells or primary monocytes by specific siRNA, caspase-1 activation, and IL-1β release were significantly reduced, even after stimulation with LPS alone. Moreover, as expected, these inhibitory effects by specific siRNAs were observed in cells stimulated with LPS for 4 hours followed by ATP. We also observed a similar reduction in caspase-1 activation and release of IL-1β in primary monocytes stimulated only with LPS for 24 hours. Our data in primary human cells are consistent with in vivo LPS responses in mice deficient in ASC and NALP-3,44,45 which support a role of ASC and Nalp3 in the responses to TLR ligands through their capacity to control the constitutive caspase-1 activation and IL-1β processing. On the other hand, the caspase-1 activation in unstimulated cells is also consistent with data showing that activation of the inflammasome is independent of TLRs,20 but rather dependent on the hemichannel protein pannexin.20
These data imply a paradigm shift in our understanding of the inflammasome. The demonstration of a role of ASC and Nalp3 in the constitutive activation of caspase-1, independent of stimulation by TLRs or inflammasome ligands, uncouples caspase-1 activation from pathogen-associated molecular pattern recognition in human primary monocytes. This new model, in which the inflammasome components ASC and Nalp3 form a protein platform responsible for the constitutive activation of caspase-1, explains why the IL-1β induced in monocytes by a very diverse panel of stimuli (including TLR ligands) is caspase-1 dependent, as well as the resistance to experimental endotoxemia in ASC−/− and Nalp-3−/− mice,44,45 and the cytokine production in patients with autoinflammatory disorders. The rate-limiting step is represented in macrophages by the presence of danger signals, such as ATP, that induce both an increase in IL-1β processing, but especially secretion. A common pathway in IL-1β secretion is represented by decreased intracellular potassium concentrations,46 induced in monocytes by P2X7 engagement by endogenous ATP release, and in macrophages by exogenous ATP released from tissue damage.
Relevance for the role of IL-1β in host responses to exogenous challenges
The single (TLR only) stimulation in monocytes compared with the double (TLR/ATP) stimulation in macrophages (schematic diagram in Figure 7C) probably represents an adaptation of each cell type to their respective environments. Circulating monocytes function in the surveillance of an essentially pathogen-free environment, so they must respond promptly to any danger signal (especially of microbial origin). Our experiments showing the importance of adhesion for the inhibitory effects on IL-1β production suggest that the migration and homing of monocyte into the tissues are essential for the phenotypic loss of IL-1β production capacity. On the other hand, macrophages are confined to an environment (eg, alveolar space, mucosal surfaces) in which they are constantly exposed to microbial stimuli but also to carcinogenic substances. A sensitive response in macrophages to such stimuli for the release of IL-1β for each encounter with such exogenous stimuli would result in recurring and deleterious inflammatory reactions. Thus, repeated bouts of inflammation are probably reduced by the requirement of a second stimulus for the activation of the inflammasome and release of active IL-1β. Such second stimuli would be available at sites of infection, trauma, or necrosis where ATP levels are elevated and can trigger the P2X7 receptor.47 In addition, second signals can come from the cathelicin-derived peptide LL37 from infiltrating neutrophils,48 IFN-γ from a lymphocytic infiltrate (Figure 6), or the release of bacterial toxins.17
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants AI-15614 and CA-046934; C.A.D.) and the Netherlands Organization for Scientific Research (a Vidi grant; M.G.N.).
National Institutes of Health
Authorship
Contribution: M.G.N. designed and performed the research, analyzed the data, and wrote the manuscript; C.A.N.-P., M.F.N., B.O., F.L.v.d.V., B.H., J.H.M.v.d.M., L.A.B.J., I.D., G.F., and C.J.F. performed research and corrected the manuscript; R.J.M., B.J.K., A.R., and J.W.M.v.d.M. designed the research and corrected the manuscript; and C.A.D. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mihai G. Netea, Department of Medicine, Radboud University Nijmegen Medical Center, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: m.netea@aig.umcn.nl.
References
Author notes
*C.A.N.-P. and M.F.N. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal