Abstract
To clarify the role of protein Z (PZ) in children with stroke/thromboembolism (TE), the present haplotype (HT)–based family study was performed. We genotyped 365 pediatric stroke/TE families (stroke n = 216; TE n = 149) for 4 single nucleotide polymorphisms (SNPs; rs3024718, rs3024731, rs3024772, and rs3024778) to assess the association between genetic variation within a conserved block of linkage disequilibrium harboring the PZ gene and pediatric TE. Association was assessed with use of the transmission disequilibrium test (TDT), corrected for multiple testing (permutation testing: HAPLOVIEW). In addition, PZ antigen was determined and correlated with carriership of PZ haplotypes and the FV G1691A mutation. Rs3024718, rs3024731, and rs3024772 are in tight linkage disequilibrium (LD) and define 4 haplotypes, capturing 97% of the genetic variation for this LD block. HT1 (ATG) was significantly overtransmitted from parents to affected offspring (HT frequency 73.5%, T:U 122:80, χ2 = 8.791, P = .003). The ATG risk haplotype was significantly correlated with greater PZ antigen levels. Multivariate analysis adjusted for age, sex, established thrombophilias, smoking, fibrinogen, and PZ levels revealed a significant association of the ATG haplotype and TE in children (odds ratio [OR] 1.4; 95% confidence interval [95% CI] 1.08-1.93). Our results suggest that the ATG haplotype of the PZ gene is a genetic marker for symptomatic TE in white German children.
Introduction
Stroke in children is a heterogeneous disorder associated with significant morbidity and mortality. The incidence has been estimated at 1 per 4000 neonates and has ranged from 1 per 7000 to 1 per 70 000 for older children.1 The most frequently reported risk factors for stroke in neonates, children, and adolescents include underlying medical conditions such as cardiac disorders, metabolic diseases, cerebrovascular pathologies, and infections diseases.2 In addition, within the last decade, many genetic and acquired prothrombotic abnormalities have been evaluated in children with cerebrovascular disease and have been found more common in children with stroke compared with healthy children.3
tk;4The gene for human protein Z (PZ) is localized to chromosome 13q34, where the genes for factors VII and X exist side by side, and it spans approximately 14 kb, consisting of 9 exons, including 1 alternative exon.4 PZ is a single-chain glycoprotein of 62 kDa and is synthesized in the liver.5 It was first purified from bovine plasma and later also found in humans.6 PZ is a vitamin K–dependent protein such as factors II, VII, IX, and X and the inhibitors protein C and S.7 The complete amino acid sequence has been described by Ichinose et al8 in 1990 and Sejima et al,9 and the age-dependent plasma levels in healthy subjects ranged from 1.4 μg/mL to 2.9 μg/mL.10,11 Initially the physiologic function of PZ was unclear: In vitro studies showed that bovine PZ could promote the assembly of thrombin with phospholipid surfaces, thus enhancing coagulation. In contrast, the human form of PZ binds thrombin poorly with very little impact on the association of thrombin binding with phospholipids. More recent studies have shown that PZ forms a calcium ion–dependent complex with factor Xa on the phospholipid surface and thereby serves as a cofactor for the inhibition of factor Xa by a PZ-dependent protease inhibitor (ZPI).12,13
PZ phenotypes are considered to be influenced by genes, by acute-phase reactions,14 and by environmental factors. Thus, in a continuation of our previous finding that the phenotypic variation of PZ is significantly attributable to heritability and shared environmental effects in pediatric stroke families, the present haplotype (HT)–based family study was performed to identify additive genetic effects influencing the variability of the PZ phenotype.15
Methods
Patients and methods
In the Department of Pediatric Hematology/Oncology Study Center (University Clinics, Münster, Germany), plasma and DNA samples of 365 families from children with nonvascular stroke (n = 216) or symptomatic thromboembolism (TE; n = 149), that is, samples of the propositus, nonaffected brothers or sisters, and biological parents, were collected between January 2000 and December 2007 (Figure 1). The present study was performed in accordance with the ethical standards established in the updated version of the 1964 Declaration of Helsinki and was approved by the medical ethics committee of the University of Münster (Münster, Germany). With written parental consent, term neonates and children with confirmed diagnosis of TE who were not older than 18 years at onset, biological brothers and sisters, and available parents were enrolled. Stroke subtypes of the children enrolled were reclassified according to explicit predefined criteria based on the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria modified for children by substituting vasculopathy for large vessel atherosclerosis.16-18 Premature birth (< 36 gestational weeks), patients older than 18 years at study onset, and children with a first stroke of vascular origin, for example, moyamoya disease, vasculitis, dissection, fibromuscular dysplasia, and focal stenosing arteriopathy, were excluded. In addition, children with missing parents, families with genetically implausible paternity, children lost to follow-up, and patients without parental consent were not ascertained. Further exclusion criteria were ongoing liver, renal, or inflammatory diseases; malignancies; and concurrent treatment regimens known to influence PZ levels. Inclusion and exclusion criteria are shown in Figure 1. In addition, the role of elevated PZ antigen levels in patients and healthy unrelated pediatric control subjects are evaluated (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Flow chart of patient-parent trio selection. Inclusion and exclusion criteria for patients enrolled in the study are shown.
Flow chart of patient-parent trio selection. Inclusion and exclusion criteria for patients enrolled in the study are shown.
Genetic analysis
We identified 4 haplotype-tagging SNPs (htSNPs) with a minor allele frequency of greater than 1% capturing 97.5% of the genetic variation in PZ genes19 by using the genotype information from the Center d'Etude du Polymorphisme Humain (CEPH) families available from HAPMAP (www.hapmap.org) and the SNPtagger tool as implemented in HAPLOVIEW.20 These 4 htSNPs located in PZ are rs3024718, rs3024731, rs3024772, and rs3024778. Rs3024718 and rs3024772 are in accordance with the polymorphism recently reported by Staton et al21 and by Kemkes-Mattes et al.22 Genotyping was performed with the TaqMan allelic discrimination method on a 384-well HT7900 (Applied Biosystems, Foster City, CA) with 2 ng genomic DNA. For quality control each plate contained 8 positive CEPH controls and 8 empty wells (no template controls [NTCs]) to ensure genotyping accuracy. Genotyping efficiency was greater than 99.5%. DNA extraction was performed by a spin column procedure (QIAGEN, Hilden, Germany) as previously described.
Blood sample collection
For the genotype-phenotype correlation study, blood sample collection from patients and relatives was performed in the morning after a 12-hour fasting period (4-6 hours for infants); samples were drawn by peripheral venipuncture into plastic tubes containing 1/10 by volume of 3.8% trisodium citrate (Sarsted, Nümbrecht, Germany) and were placed immediately on melting ice. The blood samples from patients were collected 6 to 12 months after the acute thrombotic event and at least 6 weeks apart from anticoagulation. Platelet-poor plasma was prepared by centrifugation at 3000g and 4°C for 2 × 20 minutes, aliquoted in polystyrene tubes, stored at −70°C, and thawed immediately before assay.23,24 For the present PZ phenotype, study blood samples were drawn from otherwise-healthy relatives with normal hemograms and no evidence of further diseases.
Laboratory analysis
Total PZ concentrations were measured along with fibrinogen and D-Dimer25 levels with the enzyme-linked immunosorbent assay (ELISA) technique (Asserachrom protein Z; Diagnostica Stago, Asnieres, France) 6 to 12 months after the acute TE onset and after withdrawal of oral anticoagulation.24-26 Pediatric reference values previously reported were used for comparison.24-26 As an acute-phase reactant, fibrinogen was measured according to the Clauss method (Dade Behring BCS analyzer; Marburg, Germany). Quantitative D-dimer levels were measured by a latex-enhanced turbimetric test (D-dimer plus; Dade-Behring) with use of the Dade Behring BCS analyzer. Further evaluation included testing for the FII G20210A variant, the FV G1691A mutation, antiphospholipid antibodies (including the lupus anticoagulant at minimum), levels of antithrombin, protein C, free protein S-antigen, and lipoprotein (a) in all cases.
Statistical analysis
The Hardy-Weinberg equilibrium for each htSNP was tested by the use of χ2 analysis across all samples. Family-based association was determined with the transmission disequilibrium test (TDT) in 365 trios comprising unaffected parents and the affected child27 ; haplotypes were inferred with the expectation maximization algorithm (EM algorithm) as implemented in Haploview (version 4.0; Broad Institute, Cambridge, MA).20 Significance of association was assessed with a Pearson χ2 test and corrected for multiple testing with use of 10 000 permutations as implemented in HAPLOVIEW. Association was assessed with the TDT and corrected for multiple testing with permutation testing.
For correlation analyses between risk haplotype and total PZ levels in the affected subjects, the individual haplotypes were reconstructed based on phase because genotype information was available for both parents and the affected child. Distribution of total PZ was assessed with the Kolmogorov-Smirnoff test for normalcy to ensure a normal distribution as a prerequisite for parametric analyses (Student t test: values are shown as mean and standard deviation [SD]). Correlation analyses was performed by the use of 1-way analysis of variance (ANOVA), followed by the appropriate post-hoc test (Student t test). Furthermore, associations between risk haplotypes and established risk factors for pediatric TE (FII G120210A variant, FV G1691A mutation, antiphospholipid antibodies, antithrombin, protein C, free protein S-antigen, elevated lipoprotein [a]) were compared with the χ2 analysis or by the Fisher exact test, if appropriate. Multivariate analysis (logistic regression) adjusted for possible confounders, such as age, sex, smoking (yes/no), established inherited thrombophilias, including antiphospholipid antibodies, fibrinogen, and PZ antigen levels, was performed to investigate the association between PZ risk haplotypes and nonvascular stroke/TE compared with healthy relatives: in this multivariate model, variables known a priori to be important covariates based on previous work and variables that have shown a P value less than .2 in univariate analysis were incorporated into the final statistical model.28 Results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Distribution of htSNPs in the cohorts investigated
The distribution of the 4 htSNPs is shown in Table 1: no statistical significant differences were found between nonvascular stroke trios and trios with TE with respect to observed/predicted heterozygosity, nonmissing genotypes, and minor allele frequencies; thus, to increase statistical power both pediatric cohorts were analyzed together. Data of the nonvascular stroke and TE cohorts were additionally shown.
Distribution of htSNPs in pediatric trios with nonvascular stroke (n = 216) and TE (n = 149)
| htSNP* . | ID no. rs3024718 . | ID no. rs3024731 . | ID no. rs3024772 . | ID no. rs3024778 . | χ2P . |
|---|---|---|---|---|---|
| Observed heterozygosity | .95 | ||||
| Stroke | 0.3 | 0.35 | 0.062 | 0.012 | |
| TE | 0.33 | 0.342 | 0.066 | 0.0 | |
| Σ stroke and TE | 0.31 | 0.382 | 0.062 | 0.006 | |
| Predicted heterozygosity | .96 | ||||
| Stroke | 0.317 | 0.381 | 0.064 | 0.12 | |
| TE | 0.305 | 0.382 | 0.061 | 0.0 | |
| Σ stroke and TE | 0.311 | 0.382 | 0.061 | 0.006 | |
| Nonmissing genotype | .89 | ||||
| Stroke | 99.2 | 98.5 | 100 | 99.9 | |
| TE | 99.4 | 97.6 | 99.0 | 99.6 | |
| Σ stroke and TE | 99.7 | 98.8 | 99.8 | 99.9 | |
| Minor allele frequency | .12 | ||||
| Stroke | 0.197 | 0.256 | 0.033 | 0.006 | |
| TE | 0.188 | 0.256 | 0.029 | 0 | |
| Σ stroke and TE | 0.192 | 0.257 | 0.031 | 0.003 |
| htSNP* . | ID no. rs3024718 . | ID no. rs3024731 . | ID no. rs3024772 . | ID no. rs3024778 . | χ2P . |
|---|---|---|---|---|---|
| Observed heterozygosity | .95 | ||||
| Stroke | 0.3 | 0.35 | 0.062 | 0.012 | |
| TE | 0.33 | 0.342 | 0.066 | 0.0 | |
| Σ stroke and TE | 0.31 | 0.382 | 0.062 | 0.006 | |
| Predicted heterozygosity | .96 | ||||
| Stroke | 0.317 | 0.381 | 0.064 | 0.12 | |
| TE | 0.305 | 0.382 | 0.061 | 0.0 | |
| Σ stroke and TE | 0.311 | 0.382 | 0.061 | 0.006 | |
| Nonmissing genotype | .89 | ||||
| Stroke | 99.2 | 98.5 | 100 | 99.9 | |
| TE | 99.4 | 97.6 | 99.0 | 99.6 | |
| Σ stroke and TE | 99.7 | 98.8 | 99.8 | 99.9 | |
| Minor allele frequency | .12 | ||||
| Stroke | 0.197 | 0.256 | 0.033 | 0.006 | |
| TE | 0.188 | 0.256 | 0.029 | 0 | |
| Σ stroke and TE | 0.192 | 0.257 | 0.031 | 0.003 |
htSNP indicates haplotype-tagging single nucleotide polymorphism; and TE, thromboembolism.
Single point association between PZ htSNPs and nonvascular stroke/TE
Single-point TDT in 365 nonvascular stroke/TE trios did not identify significant associations between rs3024718, rs3024731, rs3024772, or rs3024778 and pediatric nonvascular stroke/TE, respectively; for further details, see Table 2.
Single-point association (TDT) between variants in PZ and nonvascular stroke, TE, and combined data
| htSNP ID no. . | Minor allele frequency . | Overtransmitted . | T:U . | χ2 . | P . |
|---|---|---|---|---|---|
| rs3024718 | |||||
| Stroke | 0.197 | A | 83:63 | 2.74 | .097 |
| TE | 0.188 | A | 49:46 | 0.10 | .75 |
| Σ stroke and TE | 0.192 | A | 132:109 | 2.2 | .139 |
| rs3024731 | |||||
| Stroke | |||||
| TE | 0.265 | T | 62:56 | 0.40 | .581 |
| Σ stroke and TE | 0.257 | T | 154:128 | 2.40 | .122 |
| rs3024772 | |||||
| Stroke | 0.033 | G | 17:12 | 0.86 | .353 |
| TE | 0.029 | A | 10:04 | 2.57 | .108 |
| Σ stroke and TE | 0.064 | A | 22:21 | 0.02 | .878 |
| rs3024778 | |||||
| Stroke | 0.006 | G | 03:02 | 0.20 | .654 |
| TE | |||||
| Σ stroke and TE | 0.003 | G | 03:02 | 0.20 | .654 |
| htSNP ID no. . | Minor allele frequency . | Overtransmitted . | T:U . | χ2 . | P . |
|---|---|---|---|---|---|
| rs3024718 | |||||
| Stroke | 0.197 | A | 83:63 | 2.74 | .097 |
| TE | 0.188 | A | 49:46 | 0.10 | .75 |
| Σ stroke and TE | 0.192 | A | 132:109 | 2.2 | .139 |
| rs3024731 | |||||
| Stroke | |||||
| TE | 0.265 | T | 62:56 | 0.40 | .581 |
| Σ stroke and TE | 0.257 | T | 154:128 | 2.40 | .122 |
| rs3024772 | |||||
| Stroke | 0.033 | G | 17:12 | 0.86 | .353 |
| TE | 0.029 | A | 10:04 | 2.57 | .108 |
| Σ stroke and TE | 0.064 | A | 22:21 | 0.02 | .878 |
| rs3024778 | |||||
| Stroke | 0.006 | G | 03:02 | 0.20 | .654 |
| TE | |||||
| Σ stroke and TE | 0.003 | G | 03:02 | 0.20 | .654 |
TDT indicates transmission disequilibrium test; PZ, protein Z; TE, thromboembolism; and T:U, the ratio of transmissions to nontransmissions of the overtransmitted allele.
Association between PZ haplotypes and nonvascular stroke/TE
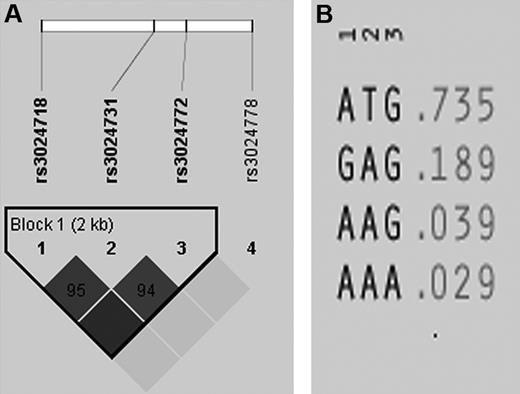
The pooled cohort genotyping of 365 family trios for TE showed that the 4 selected htSNPs identified the 2 most common haplotypes in PZ. Rs3024718, rs3024731, and rs3024772 are in tight linkage disequilibrium (LD) and define 4 haplotypes, capturing 97% of the genetic variation for this single LD block capturing the entire PZ gene (Figure 2A,B). Whereas single-point TDT identified no significantly associated SNPs with pediatric nonvascular stroke/TE, haplotype-based association analysis revealed that haplotype HT1 of the PZ gene (ATG) was significantly overtransmitted from parents to affected children (HT1, ratio of transmissions to nontransmissions of the overtransmitted allele: T:U = 122:80, χ2 = 8.79, P = .003). The same was true in the cohort of 216 trios with nonvascular stroke (T:U = 73.2:42.2 χ2 = 8.35, P = .004) and a trend in 149 trios with symptomatic TE (T:U = 49.5:32.2, χ2 = 3.66, P = .055).
LD structure and haplotypes in PZ genes. (A) Depicted is the LD structure between the haplotype tagging SNPs: rs3024718, rs3024731, and rs3024772 are in tight LD. (B) Shown are the 4 haplotypes defined by 3 tagging SNPs capturing 97% of the genetic variation for this single LD block within the entire PZ gene.
LD structure and haplotypes in PZ genes. (A) Depicted is the LD structure between the haplotype tagging SNPs: rs3024718, rs3024731, and rs3024772 are in tight LD. (B) Shown are the 4 haplotypes defined by 3 tagging SNPs capturing 97% of the genetic variation for this single LD block within the entire PZ gene.
In contrast, in the entire cohort, haplotype HT2 (GAG) was significantly undertransmitted to affected offsprings (HT2, T:U = 64.5:94.5, χ2 = 5.66, P = .017; for detailed information, see Table 3). The association for HT1 in PZ remained significant after permutation testing that used 10 000 permutations (HT1, χ2 = 8.35, P = .018), whereas significance was lost for HT2 (HT2, χ2 = 3.05, P = .253), indicating that the observed undertransmission is likely a statistical fluctuation rather than a true association.
Association between PZ haplotypes and nonvascular stroke, TE, and combined data
| Block . | Haplotype . | Frequency . | T:U . | χ2 . | P . |
|---|---|---|---|---|---|
| HT1 | |||||
| Stroke | ATG | 0.735 | 73.2:42.2 | 8.35 | .004 |
| TE | AT | 0.735 | 49.5:32.2 | 3.66 | .055 |
| Σ stroke and TE | ATG | 0.735 | 122:80 | 8.79 | .003 |
| HT2 | |||||
| Stroke | GAG | 0.189 | 39:56 | 3.04 | .081 |
| TE | GA | 0.183 | 24.5:34.5 | 1.69 | .193 |
| Σ stroke and TE | GAG | 0.189 | 64.5:94:5 | 5.66 | .017 |
| HT3 | |||||
| Stroke | AAG | 0.034 | 10.2:12.2 | 0.19 | .667 |
| TE | AA | 0.078 | 9.7:19 | 3.00 | .08 |
| Σ stroke and TE | AAG | 0.039 | 20.5:25.7 | 0.57 | .449 |
| HT4 | |||||
| Stroke | AAA | 0.031 | 6:13 | 2.57 | .10 |
| TE | |||||
| Σ stroke and TE | AAA | 0.029 | 14.0:17.0 | 0.29 | .59 |
| Block . | Haplotype . | Frequency . | T:U . | χ2 . | P . |
|---|---|---|---|---|---|
| HT1 | |||||
| Stroke | ATG | 0.735 | 73.2:42.2 | 8.35 | .004 |
| TE | AT | 0.735 | 49.5:32.2 | 3.66 | .055 |
| Σ stroke and TE | ATG | 0.735 | 122:80 | 8.79 | .003 |
| HT2 | |||||
| Stroke | GAG | 0.189 | 39:56 | 3.04 | .081 |
| TE | GA | 0.183 | 24.5:34.5 | 1.69 | .193 |
| Σ stroke and TE | GAG | 0.189 | 64.5:94:5 | 5.66 | .017 |
| HT3 | |||||
| Stroke | AAG | 0.034 | 10.2:12.2 | 0.19 | .667 |
| TE | AA | 0.078 | 9.7:19 | 3.00 | .08 |
| Σ stroke and TE | AAG | 0.039 | 20.5:25.7 | 0.57 | .449 |
| HT4 | |||||
| Stroke | AAA | 0.031 | 6:13 | 2.57 | .10 |
| TE | |||||
| Σ stroke and TE | AAA | 0.029 | 14.0:17.0 | 0.29 | .59 |
PZ indicates protein Z; TE, thromboembolism; and T:U, the ratio of transmissions to nontransmissions of the overtransmitted allele.
Correlation between ATG haplotype and PZ concentrations
For this analysis, the complete data for 1116 subjects derived from nonvascular stroke/TE families (index patient and healthy relatives) were available, with blood samples drawn clearly beyond the acute TE onset. In subjects with the PZ ATG risk haplotype (n = 632) PZ levels (mean + SD) were significantly greater compared with those without this haplotype (n = 484: 1.64 +0.69 μg/mL vs 1.27 + 0.60 μg/mL; P < .001). In contrast, mean + SD fibrinogen (274 + 72.3 vs 273 + 76.4 mg/dL; P = .61) and D-dimer levels (0.15 + 0.22 vs 0.18 + 0.45 mg/L; P = .41) were no different between subjects of the ATG haplotype and those without. The role of elevated PZ antigen levels greater than 90th age-dependent percentiles (Table S1) is shown in Table S2: elevated PZ antigen levels greater than 90th age-dependent percentiles in children with nonvascular stroke or venous TE were significantly more common in patients compared with healthy control subjects.
No statistical significant difference, however, was found for PZ antigen concentrations in index patients compared with healthy relatives (P = .56). Heterozygosity of FV G1691A was equally distributed in patients/relatives carrying the ATG haplotype compared with those without, and PZ levels were not statistically different between FV G1691A heterozygotes versus wild-type carriers (1.46 + 0.64 μg/mL vs 1.49 + 0.69 μg/mL; P = .675).
Interaction of PZ ATG haplotype with established thrombophilia and further possible cofactors (data for combined analyses, nonvascular stroke, and TE patients are shown separately)
In univariate analysis the PZ risk haplotype (ATG) did not show any statistically significant positive association with the overall rate of established thrombophilic risk factors (FII G120210A variant, FV G1691A mutation, antiphospholipid antibodies, antithrombin, protein C, free protein S-antigen, lipoprotein (a) (combined data [P = .29], nonvascular stroke [P = .38], TE [P = .11]): factor II G20210A variant (combined data [P = .71], nonvascular stroke [P = .98], TE [P = .53]), factor V G1691A mutation (combined data [P = .18], nonvascular stroke [P = .09], TE [P = .07]) and, interestingly, elevated lipoprotein (a) was found less commonly in subjects carrying the risk haplotype compared with those without (combined data [P = .038], nonvascular stroke [P = .031], TE [P = .07]). In subsequent multivariate analysis (logistic regression) adjusted for age, sex, smoking, presence of established thrombophilias, PZ, and fibrinogen levels the HT1 haplotype (ATG) was significantly more common in nonvascular stroke/TE patients compared with healthy relatives (combined data [OR 1.4; 95% CI 1.08-1.93], nonvascular stroke [OR 1.46; 95% CI 1.02-2.1], TE [OR 1.54; 95% CI 1.01-2.35]).
Discussion
The definition of haplotype blocks of SNPs has been proposed as markers in association studies to efficiently describe human genetic variation.29 In addition, it has been proposed that to distinguish between block haplotypes, a minimal set of HT-tagging SNPs should be used, which would then efficiently describe the variation in the human genome by allowing for the genotyping of only a subset of SNP loci.30 In the present candidate gene association study, whose cohorts comprised white German pediatric nonvascular stroke or TE patients and their biological parents (nuclear families), we have used the latter approach. In the present cohorts we found that the PZ ATG haplotype is a genetic marker for nonvascular stroke/TE in children and also that the PZ or a neighboring gene is a susceptibility gene for pediatric nonvascular stroke/TE. In addition, in the present study the ATG risk haplotype was significantly correlated with greater PZ antigen levels compared with subjects not carrying the risk haplotype.
In the first step, we performed single-point TDT followed by HT-based association analysis. Our observation that single-point TDT did not identify significantly associated SNPs with pediatric nonvascular stroke/TE is in line with observations of Cesari et al31 and Sofi et al32 that the intron F G79A polymorphism (rs3024718 SNP) is not associated with acute coronary syndrome or ischemic stroke in adults. In the second step, we performed a HT-based association study, demonstrating that this HT-based analysis revealed the PZ ATG haplotype is associated with pediatric nonvascular stroke/TE. In the third step, we further investigated this association with pediatric nonvascular stroke/TE in multivariate analysis adjusted for possible confounders such as age, sex, smoking habits, inherited thrombophilias, and fibrinogen levels.15
A smaller but identical HT subset has been observed in the TE cohort (AT) or nonvascular stroke (ATG) alone, which is likely a consequence of the inaccuracy of the EM algorithm in smaller study samples. However, the T allele of rs3024731 uniquely tags HT1 (Figure 2) and is be sufficient to correctly call this haplotype. Along with the observed identical distribution of observed/predicted heterozygosity, the percentage of nonmissing genotype, and the missing statistical difference between minor allele frequencies, we were confident that haplotypes were similarly distributed between the 2 pediatric cohorts and could thus be pooled. In addition to our finding that the ATG HT is a risk marker for nonvascular stroke/TE in white German children, we found that the GAG haplotype was undertransmitted, which either might constitute an independent protective HT or, more likely, a statistical fluctuation mirroring the overtransmission of HT1.
Contradictory data have been reported for PZ plasma levels associated with coagulation disorders: on the one hand, many disease states have been reported in relation to PZ deficiency, ie, ischemic stroke,33 unexplained early fetal loss,34 and deep venous thrombosis related to factor V Leiden35 but, on the other hand, ischemic stroke also has been discussed with normal or elevated PZ levels in adults and children.21,26,36,37 Although in the present study greater PZ levels (determined in plasma samples clearly collected beyond the acute disease onset concomitant with normal fibrinogen and D-dimer levels) are correlated with the PZ ATG risk haplotype, these values are within the pediatric reference ranges previously reported.26 Thus, from the family-based association study presented here we only can conclude that the PZ ATG haplotype is not associated with PZ deficiency. In the case-control study performed (see Document S1), however, we were able to demonstrate that elevated PZ antigen levels greater than 90th age-dependent percentiles are found more often in patients with nonvascular stroke or venous TE compared with normal control subjects. Contradictory associations between plasma PZ plasma levels and deep venous thrombosis also have been reported in adults. Although Vasse et al33 did not observe an association between low PZ levels and deep venous thrombosis, Kemkes-Matthes et al35 could clearly demonstrate an aggravation of deep venous thrombosis, that is, an earlier manifestation with more severe thromboembolic complications in adults with PZ deficiency and FV Leiden carrier status. In our analysis, however, a statistically significant correlation between lower PZ antigen levels and FV Leiden carrier status could not be shown. Apart from our findings that the ATG haplotype itself is associated with the outcome of interest, eg, pediatric nonvascular stroke or TE, we speculate that the observed greater PZ antigen levels in our study might be caused by an interaction with the ZPI. Here we suggest the hypothesis that in children with persistently elevated PZ antigen levels PZ is not completely bound to ZPI as the result of either acquired or inherited ZPI deficiency.38 ZPI is a hemostatic serpin with anticoagulant activity which, in its deficient state, comparable with antithrombin deficiency, could have thromboembolic consequences.7 Genetic variations in the ZPI gene have been described recently,39,40 and in the present cohorts only preliminary data with respect to ZPI gene analyses were available: 4 (1.9%) of 216 patients with nonvascular stroke, 3 (2.0%) of 149 children with TE, and 9 (1.2%) of 751 unaffected relatives were heterozygous for the silent ZPI gene mutation in exon 3 (1276C>T: rs2232707). Interestingly, in 9 of these cases this ZPI gene mutation was combined with the PZ ATG risk haplotype (personal communication). The ZPI 728C>T mutation and other polymorphisms have not been investigated by us.40
Limitations of the present family-based cohort study include (1) the nonavailability of PZ values in the entire study cohort and (2) the fact that children in this study were principally white. Thus, at the present stage the association study between PZ haplotypes and phenotypes is preliminary and should be confirmed by future cohort studies. In addition, because populations differ in prevalence of many complex genetic diseases, such as cardiovascular disease, stroke, and deep venous thrombosis, the data presented here cannot be extrapolated to pediatric nonvascular stroke/TE patients of other ethnicities.
In conclusion, our results suggest that the ATG haplotype of the PZ gene is a genetic marker for symptomatic nonvascular stroke/TE in white children and also that the PZ or a neighboring gene is a susceptibility gene for TE in the German family-based cohort investigated. Our observation that the HT-based study design is superior to the single-point approach underlines the necessity to overcome the limitations of single-point association studies in diseases that do not underlie a monogenetic Mendelian inheritance. Finally, our study highlights the complex nature of pediatric nonvascular stroke/TE and the importance of evaluating gene-gene interactions in association studies of complex, polygenic diseases. Further studies in the present cohort are ongoing to investigate the associations between PZ haplotypes and PZ phenotypes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We acknowledge Fenella Kirkham for helpful discussions and help in editing the manuscript.
Authorship
Contribution: Along with the principal study investigators, for example, M.S. and U.N.-G. who act as the guarantors, all other investigators had full access to the data (B.F., S.T., A.H.) and took part in the design, execution, and data analysis, and in writing the report, and M.S. and U.N.-G. were responsible for the statistical analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulrike Nowak-Göttl, Pediatric Hematology & Oncology, University of Münster, Albert-Schweitzer-Str. 33, 48149 Münster, Germany; e-mail: leagottl@uni-muenster.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal