Abstract
To determine whether the leukemia-associated Wilms tumor antigen (WT1) contributes to a graft-versus-leukemia (GVL) effect after allogeneic stem-cell transplantation (SCT) for acute lymphoblastic leukemia (ALL), we studied CD8+ T-cell responses to WT1 in 10 human lymphocyte antigen (HLA)–A*0201–positive ALL patients during the early phase of immune recovery after SCT (days 30-120). Seven of 10 patients had detectable WT1 expression in their peripheral blood (PB) before SCT by quantitative reverse-transcription polymerase chain reaction. Using WT1/HLA-A*0201 tetramers and intracellular interferon-γ (IFN-γ) staining, WT1+ CD8+ T-cell responses after SCT were found only in patients with detectable WT1 expression before SCT (5 of 7 vs. 0 of 3; P < .05). To monitor the kinetics of WT1+ CD8+ T-cell responses and disease regression after SCT, absolute WT1+ CD8+ T-cell numbers and WT1 expression were studied for each time point. The emergence of WT1+ CD8+ T cells was associated with a decrease in WT1 expression, suggesting a WT1-driven GVL effect. Loss of WT1+ CD8+ T-cell responses was associated with reappearance of WT1 transcripts, consistent with a molecular relapse (P < .001). WT1+ CD8+ T cells had a predominantly effector–memory phenotype (CD45RO+ CD27−CD57+) and produced IFN-γ. Our results support the immunogenicity of WT1 after SCT for ALL and highlight the potential for WT1 vaccines to boost GVL after SCT for ALL.
Introduction
There is abundant evidence for the presence of a graft-versus-leukemia (GVL) effect after allogeneic stem-cell transplantation (SCT) for acute lymphoblastic leukemia (ALL). Relapse rates are lower after allogeneic SCT compared with autologous SCT, especially in patients in whom acute graft-versus-host disease (GVHD), chronic GVHD, or both, develops.1 However, patients with ALL respond poorly to conventional donor lymphocyte infusion (DLI), with reported remission rates between 0% and 18%.2–4 Nevertheless, although response rates are low, durable remissions are possible. One of the first recipients of DLI to treat relapsed ALL had a sustained remission for more than 8 years at the time of last report.5 Further support for a T-cell–mediated GVL effect in ALL comes from the finding of T cells specific for minor histocompatibility antigens, which are reactive to ALL cells after allogeneic SCT.6,7 A role for donor-driven GVL responses against self-antigens such as the Wilms tumor antigen-1 (WT1) in ALL has not yet been explored. The WT1 gene is implicated in leukemogenesis and is commonly expressed in ALL. WT1 overexpression occurs in 70% to 90% of ALL patients, with an even higher frequency at relapse.8–11 In vitro and murine data suggest that WT1 could serve as a useful and broadly expressed antigenic target for immunotherapy of leukemia.12–17 We and others18–20 have shown the presence of WT1-specific CD8+ T cells in patients with myeloid leukemias and healthy volunteers,18,19 suggesting that WT1 expression readily induces T-cell responses. More recently, small clinical studies have demonstrated the feasibility and potential efficacy of WT1 peptide vaccination in humans.21–23
The profoundly lymphopenic milieu immediately after transplantation reduces the activation threshold of antigen-specific T cells and promotes homeostatic proliferation. This allows donor-derived alloantigen and self antigen–specific T-cell clones to participate in rapid and extensive antigen-driven T-cell expansions.24–27 We hypothesized that WT1 presented by ALL cells persisting after SCT might drive such lymphocyte expansion in the lymphopenic period early after allogeneic transplantation, leading to an effective GVL response.
We report here that memory CD8+ T-cell responses against WT1 occur in patients with ALL after allogeneic SCT. WT1-specific CD8+ T cells were only found in patients who had WT1 gene expression in their peripheral blood (PB) before transplantation. The emergence of WT1-specific CD8+ T cells was associated with a reduction in leukemia load as assessed by WT1 expression, supporting the immunogenicity of WT1 as a potential target for immunotherapeutic approaches in ALL.
Patients, materials, and methods
Subjects studied
All HLA-A*0201–positive patients with ALL who received a T-cell–depleted SCT between September 1998 and February 2006 in the Hematology Branch, National Heart, Lung, and Blood Institute (Bethesda, MD) were eligible for study inclusion, provided before-SCT and after-SCT cells were available. Patients and their HLA-identical sibling donors were treated on National Heart, Lung, and Blood Institute SCT protocols approved by the National Heart, Lung, and Blood Institute's Institutional Review Board. Patients and donors gave written informed consent in accordance with the Declaration of Helsinki for laboratory-based studies on blood samples obtained before and after SCT at various time points. Ten human lymphocyte antigen (HLA)-A*0201+ALL patients and their respective stem cell donors had adequate numbers of pre-SCT and post-SCT samples to be studied. Clinical characteristics of these 10 patients and their respective donors are presented in Table 1. Samples were analyzed before SCT and at regular intervals (days 30, 45, 60, 90, and 120) after transplantation to study the kinetics of WT1-specific CD8+ T-cell responses. For long-term survivors, a sample from their most recent outpatient visit was also analyzed. Cells obtained from leukapheresis products or from peripheral blood (PB) were separated using Ficoll-Hypaque density gradient centrifugation (Organon Teknika, Durham, NC) and subsequently frozen in RPMI 1640 (Life Technologies, Gaithersburg, MD) supplemented with 20% heat-inactivated fetal calf serum and 10% dimethyl sulfoxide according to standard protocols. Before use, frozen cells were thawed, washed, and suspended in RPMI+ 10% pooled human AB serum (Sigma Chemical, St Louis, MO). High-resolution HLA class I genotyping was performed by sequence-specific polymerase chain reaction (PCR) using genomic DNA (HLA Laboratory, Department of Transfusion Medicine, Warren G. Magnusson Clinical Center, NIH, Bethesda, MD). The presence of IgG and IgM cytomegalovirus (CMV) antibodies in the donors was analyzed by passive latex agglutination (CMVSCAN kit; Becton Dickinson Microbiology System, Cockeysville, MD).
Patient characteristics
| Patient . | Diagnosis . | Age, y . | Sex, P/D . | CMV serostatus, P/D . | Disease status at SCT . | DFS, d . | DLI days after SCT . | Relapse . | aGVHD, day, grade (site) . | WT1 expression before SCT, WT1/ABL . | Maximal WT1 CD8+ T-cell response after SCT, % (time after SCT) . | ALC D30 . | T-cell chimerism D30 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B-ALL | 20 | M/M | +/+ | CR2 | 780 | 60 | N | 47, II (S,G) | 0.0028 | 0.57 (30 d) | 3295 | 100 |
| 2 | Ph+ B-ALL | 33 | F/F | +/+ | CR2 | 120 | 45 | Y | 82, II (S) | 0.0107 | 0.12 (60 d) | 458 | 97 |
| 3 | B-ALL | 27 | F/F | +/+ | Primary refractory | 75 | 45 | Y | 48, II (S) | 0.1882 | 0.63 (60 d) | 926 | 100 |
| 4 | B-ALL | 33 | F/M | +/+ | CR2 | 339 | 45 | Y | 34, II (S) | 0.0024 | 0.12 (30 d) | 755 | 97 |
| 5 | B-ALL | 15 | M/M | +/+ | Primary refractory | 154 | 60 | Y | N | 0.0015 | 0.13 (14 mo) | 612 | 99 |
| 6 | B-ALL | 28 | F/F | +/+ | CR2 | 97 | N | N | 33, IV (S, G) | 0.0007 | 0 | 1792 | 100 |
| 7 | B-ALL | 37 | M/F | +/+ | CR3 | 374 | N | N | 38, II (S) | 0.0031 | 0 | 1320 | 100 |
| 8 | B-ALL | 10 | F/F | +/+ | CR2 | 2809 | 58 | N | 88–100, II (G) | 0 | 0 | 54 | N/A |
| 9 | B-ALL | 22 | F/F | +/+ | CR2 | 1508 | 38 | N | 46–80, II (S,G) | 0 | 0 | 34 | N/A |
| 10 | B-ALL | 36 | F/F | +/+ | Primary refractory | 192 | N | N | 32, II (S,G) | 0 | 0 | 1156 | 98 |
| Patient . | Diagnosis . | Age, y . | Sex, P/D . | CMV serostatus, P/D . | Disease status at SCT . | DFS, d . | DLI days after SCT . | Relapse . | aGVHD, day, grade (site) . | WT1 expression before SCT, WT1/ABL . | Maximal WT1 CD8+ T-cell response after SCT, % (time after SCT) . | ALC D30 . | T-cell chimerism D30 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B-ALL | 20 | M/M | +/+ | CR2 | 780 | 60 | N | 47, II (S,G) | 0.0028 | 0.57 (30 d) | 3295 | 100 |
| 2 | Ph+ B-ALL | 33 | F/F | +/+ | CR2 | 120 | 45 | Y | 82, II (S) | 0.0107 | 0.12 (60 d) | 458 | 97 |
| 3 | B-ALL | 27 | F/F | +/+ | Primary refractory | 75 | 45 | Y | 48, II (S) | 0.1882 | 0.63 (60 d) | 926 | 100 |
| 4 | B-ALL | 33 | F/M | +/+ | CR2 | 339 | 45 | Y | 34, II (S) | 0.0024 | 0.12 (30 d) | 755 | 97 |
| 5 | B-ALL | 15 | M/M | +/+ | Primary refractory | 154 | 60 | Y | N | 0.0015 | 0.13 (14 mo) | 612 | 99 |
| 6 | B-ALL | 28 | F/F | +/+ | CR2 | 97 | N | N | 33, IV (S, G) | 0.0007 | 0 | 1792 | 100 |
| 7 | B-ALL | 37 | M/F | +/+ | CR3 | 374 | N | N | 38, II (S) | 0.0031 | 0 | 1320 | 100 |
| 8 | B-ALL | 10 | F/F | +/+ | CR2 | 2809 | 58 | N | 88–100, II (G) | 0 | 0 | 54 | N/A |
| 9 | B-ALL | 22 | F/F | +/+ | CR2 | 1508 | 38 | N | 46–80, II (S,G) | 0 | 0 | 34 | N/A |
| 10 | B-ALL | 36 | F/F | +/+ | Primary refractory | 192 | N | N | 32, II (S,G) | 0 | 0 | 1156 | 98 |
ALC indicates absolute lymphocyte count (× 103/mL); aGVHD, acute graft-versus-host disease; CR, complete remission; D, donor; DFS, disease-free survival; F, female; G, gut; M, male; N/A, not applicable; N, no; Ph, Philadelphia; P, patient; S, skin; and Y, yes.
Transplantation approach
The conditioning regimen was 12 Gy total body irradiation, fludarabine (125 mg/m2) and cyclophosphamide (Cy, 120 mg/kg). Patients underwent a T-cell–depleted granulocyte colony-stimulating factor–mobilized peripheral blood stem-cell transplantation from their HLA-identical sibling. CD34+ cells were positively selected using anti-CD34 beads (Isolex 300i immunomagnetic cell selection system, Nexell Therapeutics, Irvine, CA), and residual T cells were removed with a cocktail of anti-CD2, anti-CD6, and anti-CD7 antibody-coated beads (a kind gift from Dr Ronald Gress, National Cancer Institute, NIH, Bethesda, MD). The CD34+ cell dose ranged from 3.2-15.9 (median, 6.4) × 106/kg; the T-cell dose was fixed at 2 × 104 CD3+ T cells/kg recipient weight by adding back lymphocytes to the stem-cell fraction where necessary. Patients received low-dose cyclosporine A (target plasma level 100-200 μg/mL), starting on day − 4. Cyclosporine A was continued for at least 6 months if indicated for chronic GVHD treatment. In the absence of acute GVHD, donor T cells (107/kg) were added back preemptively on days 45 to 100 after transplantation. Standard prophylaxis against infection included fluconazole and bactrim for at least 6 months after transplantation, and weekly surveillance for CMV antigenemia as described previously.28,29
Determination of donor-recipient chimerism after SCT
A quantitative PCR–based analysis of short tandem repeats was used to measure donor-recipient chimerism separately in lymphoid and myeloid lineages.30 DNA was extracted from donor and pretransplantation patient blood samples, and PCR using fluorescent primer sets flanking 8 different short tandem repeats and amelogenin was performed using the manufacturer's conditions (Powerplex 1.2; Promega, Madison, WI) to identify an informative allele. Ficoll-hypaque–fractionated mononuclear cells were collected on posttransplantation days 14, 30, 60, and 100, and sorted into CD14+/CD15+myeloid and CD3+ T cells using immunomagnetic beads (Dynal, Oslo, Norway). DNA was extracted and lineage-specific chimerism was performed by PCR using fluorescent primers flanking a single short tandem repeat previously identified as polymorphic between the patient and donor. Predetermined mixtures of pretransplantation patient and donor DNA were run in conjunction with test samples as quantitative controls (sensitivity for minor populations, 2%-3%). The PCR products were analyzed with a 310 ABI PRISM sequencer with the chimerism percentage determined by comparing the ratio of donor and patient DNA bands (Genescan software; both from PE Applied Biosystems, Foster City, CA).
Peptide synthesis
Flow cytometric detection of functional antigen-specific CD8+ T cells
Intracellular cytokine detection was performed as described previously.32 In brief, peripheral blood mononuclear cells (PBMCs) (106) were loaded with or without test peptides (0.1 μM). The response to CMV pp65 495-503 was used as positive control. After 2 hours, 10 μg/mL Brefeldin A (Sigma, St Louis, MO) was added. After an additional 4 hours, CD3+ CD8+ T cells were stained with an anti-CD3 peridinin chlorophyll protein (PerCP)–conjugated antibody and anti-CD8 phycoerythrin–conjugated antibody, fixed/permeablized, and then stained with an anti-interferon (IFN)-γ fluorescein isothiocyanate, anti-IL-4, anti-IL-10 allophycocyanin conjugate (all BD Pharmingen, San Jose, CA).
Peptide–HLA class I tetrameric complexes and immunophenotyping
Tetramers were constructed as described previously with minor modifications.18,33 Once prepared, tetramers were stored in the dark at 4°C. CMVpp65495/HLA-A*0201 and HIV-1 Gag/HLA-A*0201 tetramers conjugated to allophycocyanin were used as positive and negative controls, respectively. Sample staining was performed using 2 × 106 PBMCs in 50 μL 1% fetal calf serum/phosphate-buffered saline. Tetramers (1-2 μg per test with respect to the peptide–MHC class I component) were added for 20 to 30 minutes at 37°C. Cells were washed once in 1% fetal calf serum/phosphate-buffered saline and then stained with a titrated panel of directly conjugated antibodies to CD3, CD8, CD27, CD57, and CD45RO (all Beckman Coulter, Miami, FL). Fluorescein isothiocyanate, phycoerythrin, PercP, allophycocyanin Cy7, and Cy7 phycoerythrin were used as fluorophores. The lymphocytes were then washed in 1% bovine serum albumin in phosphate-buffered saline, and resuspended in 1% paraformaldehyde in phosphate-buffered saline. A minimum of 0.5 × 106 gated cells were acquired. Flow cytometry was performed on an LSR II flow cytometer (BD Biosciences, San Jose, CA) using FacsDiva software (BD Biosciences).
Measurement of WT1 and BCR-ABL by real-time quantitative reverse-transcription PCR
All samples for quantitative reverse-transcription PCR were blinded. RNA was isolated from a minimum of 106 PBMCs using RNeasy mini kits (Qiagen, Valencia, CA). cDNA was synthesized using the Advantage RT-for-PCR kit (Clontech, Mountain View, CA). ABL expression was used as the endogenous cDNA quantity control for all samples34 ; its expression was measured using 300 nM primers and 200 nM probe.35 Expression of WT1 was measured using 500 nM primers and 200 nM probe.36 All reactions by quantitative reverse-transcription PCR using the ABI PRISM 7900 sequence detection system (Applied Biosystems) were performed in triplicate in 10 μL volume using standard conditions with 40 cycles of amplification. For correlation of ALL disease load with WT1 expression, informative patients with known minor breakpoint BCR-ABL fusion gene transcripts were assessed according to an established minimal residual disease detection assay using recommended standardized probe and primers.37 Both WT1 and minor breakpoint BCR-ABL quantitative reverse-transcription PCR reactions could consistently detect 1 leukemic cell in 1 000 000 nonleukemic cells.
Statistical analysis
Data were analyzed by χ2 test for categorical data and Mann-Whitney test for continuous data with the use of SPSS 14 for Windows software (SPSS, Chicago, IL) and Prism 4.00 for Windows software (GraphPad Software, San Diego, CA). P values were from 2-sided tests, with values less than .05 considered statistically significant.
Results
WT1-specific CD8+ T cells are present in ALL patients after SCT
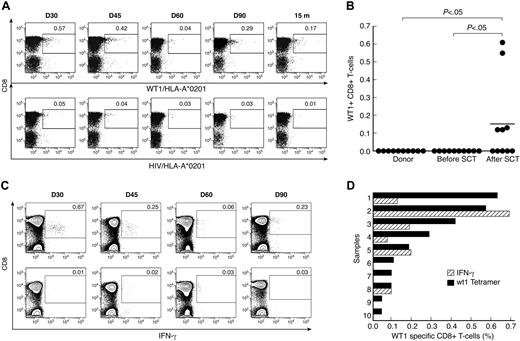
Unstimulated PBMC samples from 10 HLA-A*0201–positive patients with ALL and their respective donors were analyzed for circulating CD8+ T cells specific for WT1 using FACS staining with WT1/HLA-A*0201 tetramer (Figure 1A). Clinical data are given in Table 1. A CD8+ T-cell response to WT1 was observed in 5 of 10 patients after SCT, with frequencies between 0.05% and 0.63% of the CD8+ T-cell subpopulation. In contrast, WT1+ CD8+ T cells were not detected by tetramer staining in any of the donors or patients before SCT (Table 1, Figure 1B; P < .05). We next analyzed CD8+ T-cell responses to WT1 by intracellular IFN-γ staining in 6 patients in whom sufficient material for intracellular IFN-γ was available. A response was considered positive if the percentage of peptide-specific IFN-γ producing CD8+ T cells was 2-fold or higher compared with the percentage of IFN-γ producing CD8+ T cells in the absence of peptide and if there was a minimum of 0.05% peptide-specific IFN-γ producing CD8+ T cells in 106 PBMCs (after subtracting the percentage of IFN-γ producing CD8+ T cells in the absence of peptide; Figure 1C). CD8+ T cells specifically producing IFN-γ when exposed to WT1 were detected in 3 of 6 tested patients at frequencies between 0.05% and 0.69% of CD8+ T cells. These IFN-γ responders (patients 1, 3, and 4) also had CD8+ T-cell responses to WT1 detected by WT1/HLA-*0201 tetramer staining, whereas nonresponders (patients 7, 8, and 10) were also negative for WT1 specificity by tetramer. In samples from 4 time points, no WT1-specific CD8+ T-cell responses could be detected by intracellular IFN-γ, whereas low-frequency responses ranging from 0.05% to 0.11% were detected by WT1/HLA-A*0201 staining (Figure 1D). Using intracellular cytokine staining, we also analyzed the Th2 cytokine profile (IL-4 and IL-10 production) after stimulation with WT1 peptide in 7 samples shown to have detectable WT1-specific CD8+ T cells by HLA-A2/WT1 tetramer staining. No significant IL-4 or IL-10 responses were seen in the samples tested (data not shown).
CD8+ T-cell responses to WT1 in donors and patients with ALL after SCT. (A) Tetramer analysis of PBMCs was performed by 6-color flow cytometry. Longitudinal data from patient 1 are presented. WT1/HLA-A*0201+ CD8+ T cells were gated on CD3+ events after passing through a small lymphocyte gate; HIV/HLA-A*0201 tetramer was used similarly as a negative control. (B) Comparison of frequency of WT1/HLA-A*0201+ CD8+ T cells in samples before SCT and after SCT. The values represent the maximal WT1/HLA-A*0201+ CD8+ T-cell response for each patient before and after SCT. Bars represent means. (C) IFN-γ production by CD8+ T cells in PBMC samples from patient 1, cultured for 6 hours with (top panel) or without (negative control; bottom panel) WT1 peptide. Results are expressed as percentages of CD8+ T cells. (D) Frequencies of WT1-specific CD8+ T cells by tetramer analysis ( ) and WT1-specific IFN-γ producing CD8+ T cells (
) and WT1-specific IFN-γ producing CD8+ T cells ( ).
).
CD8+ T-cell responses to WT1 in donors and patients with ALL after SCT. (A) Tetramer analysis of PBMCs was performed by 6-color flow cytometry. Longitudinal data from patient 1 are presented. WT1/HLA-A*0201+ CD8+ T cells were gated on CD3+ events after passing through a small lymphocyte gate; HIV/HLA-A*0201 tetramer was used similarly as a negative control. (B) Comparison of frequency of WT1/HLA-A*0201+ CD8+ T cells in samples before SCT and after SCT. The values represent the maximal WT1/HLA-A*0201+ CD8+ T-cell response for each patient before and after SCT. Bars represent means. (C) IFN-γ production by CD8+ T cells in PBMC samples from patient 1, cultured for 6 hours with (top panel) or without (negative control; bottom panel) WT1 peptide. Results are expressed as percentages of CD8+ T cells. (D) Frequencies of WT1-specific CD8+ T cells by tetramer analysis ( ) and WT1-specific IFN-γ producing CD8+ T cells (
) and WT1-specific IFN-γ producing CD8+ T cells ( ).
).
WT1 expression in patients with ALL and their donors
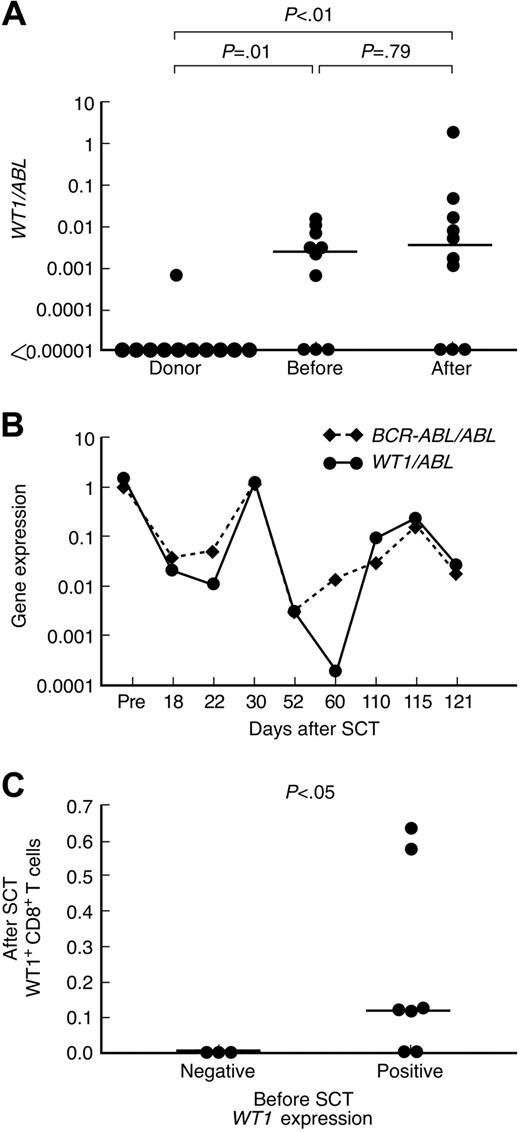
WT1 expression was detected in 1 of 10 PB samples from healthy donors (median, 0; range, 0-.0007 WT1/ABL) and 7 of 10 samples from patients with ALL before SCT (median, 0.0026; range, 0-0.0107 WT1/ABL). In patients with ALL after SCT, WT1 expression in PB samples varied within a wide range of more than 4 logs (median, 0.0036; range, 0-1.993 WT1/ABL). WT1 expression was significantly higher in PB from patients with ALL (before and after SCT) compared with healthy donors (Figure 2A; P < .01). Of note, WT1 expression was detected after SCT only in patients who had detectable WT1 expression before SCT (7 of 7 compared with 0 of 3 with undetectable WT1 expression before SCT). To further investigate the reliability of WT1 expression as a marker of minimal residual disease (MRD), we analyzed the correlation of expression levels between WT1 and a leukemia-specific fusion transcript. In this study cohort, only 1 of the 7 patients who had WT1 expression (patient 2) had a leukemia-specific fusion transcript (minor breakpoint BCR-ABL). MRD in PB samples at different time points after transplantation was analyzed using real-time PCR for WT1 and BCR-ABL. Among the non-HLA-A*0201 ALL patients from our center, only one other BCR-ABL–positive ALL patient had cells available for assessment of concurrent WT1 and BCR-ABL expression. A significant correlation between WT1 and BCR-ABL was seen in both patients. The time courses of the gene expression levels of WT1 and BCR-ABL in patient 2 are shown in Figure 2B.
Relationship between WT1-specific CD8+ T-cell responses and WT1 gene expression in peripheral blood of patients with ALL and donors. (A) WT1 gene expression in peripheral blood samples from healthy donors, patients with ALL before SCT and after SCT. (B) Correlation between WT1 and BCR-ABL expression in PB samples from patient 2. (C) Relationship between WT1-specific CD8+ T-cell frequencies in PB samples after SCT and WT1 gene expression in PB samples obtained before SCT. Bars represent medians.
Relationship between WT1-specific CD8+ T-cell responses and WT1 gene expression in peripheral blood of patients with ALL and donors. (A) WT1 gene expression in peripheral blood samples from healthy donors, patients with ALL before SCT and after SCT. (B) Correlation between WT1 and BCR-ABL expression in PB samples from patient 2. (C) Relationship between WT1-specific CD8+ T-cell frequencies in PB samples after SCT and WT1 gene expression in PB samples obtained before SCT. Bars represent medians.
WT1 gene expression in PB samples before SCT strongly correlated with detection of WT1+ CD8+ T-cell responses after SCT. Five of 7 patients with detectable WT1 gene expression in PB samples collected before SCT had WT1+ CD8+ T-cell responses after SCT, whereas none of the 3 patients whose PB samples tested negative for WT1 gene expression before transplantation had detectable WT1+ CD8+ T-cell responses after SCT (Table 1, Figure 2C; P < .05).
Phenotype of WT1-specific CD8+ T cells
Antigen-specific CD8+ T cells identified by HLA-A*0201 tetramers were analyzed in 2 patients (patients 1 and 3) for expression of CD45RO, CD27, and CD57 to characterize naive, memory, and effector phenotype. The longitudinal phenotypic analysis of patient 1, with a follow-up of 15 months, is illustrated in Figure 3. CMV-specific CD8+ T cells were used as a positive control. CMVpp65495/HLA-A*0201+ CD8+ T cells exhibited a predominant phenotype of effector memory cells (CD45RO+ CD27-CD57+) and terminally differentiated effector memory cells (CD45RO-CD27-CD57+) with a smaller population of central memory cells (CD45RO+ CD27+ CD57−; Figure 3A). WT1/HLA-A*0201+ CD8+ T cells, however, had a predominantly effector phenotype early after SCT suggesting an ongoing immune response against WT1, with a shift toward a more central memory phenotype later after SCT (Figure 3B).
Longitudinal phenotypic characterization of tetramer-positive CD3+ CD8+ T cells. Analysis of PBMCs was performed by 6-color flow cytometry in 2 ALL patients at defined time points after SCT. CD45RO, CD27, and CD57 phenotype of CD3+ CD8+ T-cell gated tetramer positive lymphocytes on samples from patient 1 is presented here. (A) CMVpp65495/HLA-A*0201+ CD8+ T cells. (B) WT1/HLA-A*0201+ CD8+ T cells
Longitudinal phenotypic characterization of tetramer-positive CD3+ CD8+ T cells. Analysis of PBMCs was performed by 6-color flow cytometry in 2 ALL patients at defined time points after SCT. CD45RO, CD27, and CD57 phenotype of CD3+ CD8+ T-cell gated tetramer positive lymphocytes on samples from patient 1 is presented here. (A) CMVpp65495/HLA-A*0201+ CD8+ T cells. (B) WT1/HLA-A*0201+ CD8+ T cells
Kinetics of CD8+ T-cell responses to WT1 and disease activity after SCT
To monitor the kinetics of WT1-specific CD8+ T-cell responses and disease regression, absolute WT1/HLA-A*0201+ CD8+ T-cell numbers and WT1 gene expression in PB were calculated for each posttransplant sample time point. As shown in Figure 4, WT1/HLA-A*0201+ CD8+ T-cell emergence (blue symbols and line) coincided with a decrease in leukemic load as measured by WT1 gene expression (red symbols and line). Similarly, loss of WT1/HLA-A*0201+ CD8+ T-cell responses was followed by a reemergence of leukemia as measured by WT1 gene expression (P < .001). In 3 patients (patients 1, 2, and 3; Figures 4A-C, respectively), the initiation of immunosuppressive therapy for the treatment of acute GVHD led to abrogation of the WT1+ CD8+ T-cell response and resultant molecular relapse; in one patient (patient 7; Figure 4G), cessation of immunosuppressive therapy led to the detection of WT1/HLA-A*0201+ CD8+ T cells and disappearance of WT1 gene transcripts.
WT1-specific CD8+ T-cell responses in peripheral blood in relation to disease response as measured by WT1/ABL gene expression. Results in 7 individual patients with detectable WT1 gene expression before SCT are shown (A, patient 1; B, patient 2; C, patient 3; D, patient 4; E, patient 5; F, patient 6; G, patient 7). The number of days after transplantation is shown on the x-axis. WT1/HLA-A*0201+ CD8+ T cells are expressed as absolute numbers/mL of peripheral blood (left, y-axis; dark blue); the shaded area represents absolute numbers of CMVpp65495/HLA-A*0201+ CD8+ T cells. WT1 gene expression in peripheral blood is expressed as the ratio of WT1/ABL (right, y-axis; red). Times of DLI (black arrow), GVHD (med blue), and relapse (green) are depicted on each graph. G indicates GVHD grade; S, skin; P, prednisone; m, months; CyA, cyclosporine A; D, dacluzimab; I, infliximab.
WT1-specific CD8+ T-cell responses in peripheral blood in relation to disease response as measured by WT1/ABL gene expression. Results in 7 individual patients with detectable WT1 gene expression before SCT are shown (A, patient 1; B, patient 2; C, patient 3; D, patient 4; E, patient 5; F, patient 6; G, patient 7). The number of days after transplantation is shown on the x-axis. WT1/HLA-A*0201+ CD8+ T cells are expressed as absolute numbers/mL of peripheral blood (left, y-axis; dark blue); the shaded area represents absolute numbers of CMVpp65495/HLA-A*0201+ CD8+ T cells. WT1 gene expression in peripheral blood is expressed as the ratio of WT1/ABL (right, y-axis; red). Times of DLI (black arrow), GVHD (med blue), and relapse (green) are depicted on each graph. G indicates GVHD grade; S, skin; P, prednisone; m, months; CyA, cyclosporine A; D, dacluzimab; I, infliximab.
Patient 1 was a 20-year-old man with ALL in second remission (CR2). WT1 gene transcripts were present before SCT (0.0028 WT1/ABL). After SCT, the number of WT1+ CD8+ T cells rapidly increased to reach a peak of more than 13 000 cells/mL by day 30 (Figure 4A). Immediately after the WT1+ CD8+ T-cell peak, no WT1 transcripts were detectable and complete donor chimerism developed. Grade 2 acute GVHD of skin developed and the patient was treated with 1 mg/kg prednisolone for 7 days. Although testing revealed persistent complete donor chimerism, during immunosuppressive treatment WT1+ CD8+ T cells disappeared from the blood, coinciding with reappearance of WT1 transcripts compatible with molecular relapse. On discontinuation of immunosuppressive therapy, the patient received a DLI at day 60 and WT1+ CD8+ T cells were once again detectable, associated with disappearance of WT1 transcripts by day 90. The patient remains in complete remission 2 years after SCT with persistent WT1+ CD8+ T-cell responses.
Patient 2 was a 33-year-old woman with Philadelphia-positive ALL in CR2. WT1 gene transcripts were present before SCT (0.0107 WT1/ABL; Figure 4B). No WT1+ CD8+ T cells were detected in the peripheral blood for the first 45 days after SCT. This was associated with detectable WT1 gene transcripts compatible with persistent molecular disease. The patient received a DLI at day 45. This was followed by an increase in WT1+ CD8+ T cells to more than 500 cells/mL by day 60 after SCT (2 weeks after DLI). The peak WT1+ CD8+ T-cell response was associated with disappearance of WT1 gene transcripts from peripheral blood. The patient remained WT1 transcript–negative at day 90 but grade 2 acute GVHD of the skin developed on day 82, requiring treatment with oral prednisone (1 mg/kg) and cyclosporine. This was followed by a decrease in the number of detectable WT1+ CD8+ T cells and reappearance of WT1 gene transcripts at day 120 in the peripheral blood. The patient then progressed to clinical relapse and died soon after.
Patient 3 was a 27-year-old woman with primary refractory ALL. WT1 gene transcripts were present before SCT (0.1882 WT1/ABL). By day 45 after SCT, WT1+ CD8+ T cells increased rapidly to more than 5500 cells/mL (Figure 4C) and the patient was 100% donor chimeric. This was associated with the disappearance of WT1 gene transcripts in the peripheral blood. She received a preemptive DLI on day 45 after SCT, and within a few days grade 2 acute GVHD of the skin developed, necessitating prolonged treatment with oral prednisone and cyclosporine. During this period, the patient became progressively mixed donor chimeric (84% donor T cells). WT1+ CD8+ T-cell numbers decreased to as low as 100 cells/mL, coinciding with reappearance and progressive increase in WT1 transcripts. The patient then progressed to overt disease relapse and died soon after.
Patient 4 was a 33-year-old woman with ALL in CR2. WT1 gene transcripts were present before SCT (0.0024 WT1/ABL; Figure 4D). Grade 2 acute GVHD of skin developed at day 34, requiring a short course of oral prednisone (1 mg/kg). During this time, WT1 gene transcripts were detectable in the peripheral blood. The patient was 97% donor T-cell chimeric. She then received a DLI on day 45 and soon after WT1+ CD8+ T cells were detected, reaching a peak of 500 cells/mL by day 60. This was associated with disappearance of WT1 gene transcripts in the peripheral blood. The patient survived disease-free for 339 days before dying of relapsed disease 357 days after SCT.
Patient 5 was a 15-year-old boy with primary refractory ALL. WT1 gene transcripts were present before SCT (0.0015 WT1/ABL; Figure 4E). By day 45, low frequencies of WT1+ CD8+ T cells were detected, reaching a peak of 400 cells/mL after a DLI on day 60. However, the WT1+ CD8+ T-cell response was short-lived. The patient lost this response by day 120, experienced relapse clinically 5 months after SCT, and died soon after.
Patient 6 was a 28-year-old woman with ALL in CR2. WT1 gene transcripts were present before SCT (0.0007 WT1/ABL; Figure 4F). The patient achieved 100% donor T-cell chimerism at day 14 after SCT. Grade 4 acute GVHD of skin and gut developed, necessitating treatment with intravenous methylprednisone (1 mg/kg) and a course of daclizumab and infliximab. No WT1+ CD8+ T cells were detectable in this patient and she remained persistently positive for WT1 gene transcripts. She died of complications of acute GVHD 97 days after SCT.
Patient 7 was a 37-year-old man with ALL in CR3. WT1 gene transcripts were present before SCT (0.0031 WT1/ABL; Figure 4G). The patient achieved 100% donor T-cell chimerism at day 14 after SCT. On day 38, grade 2 acute GVHD of skin developed, necessitating treatment with prednisone (1 mg/kg) and infliximab. While receiving immunosuppression, no WT1+ CD8+ T cells were detectable in his peripheral blood. Interestingly, however, he was intermittently negative for WT1 gene transcripts, suggesting the presence of other GVL responses, such as minor histocompatibility antigen-driven T-cell responses. Of note, this patient received a graft from a female donor and, among others, Y-chromosome–encoded minor histocompatibility antigens may possibly explain this GVL effect.38,39 Chronic GVHD developed, affecting his skin. He remains disease-free and off all immunosuppression for more than 1 year after SCT with detectable WT1+ CD8+ T cells at frequencies more than 2000 cells/mL.
CD8+ T-cell responses to CMV in patients and donors
To ascertain that the post-SCT immune response is antigen-driven and not a consequence of nonspecific lymphopenia-driven homeostasis, the CD8+ T-cell response to the HLA-A*0201–restricted CMVpp65495 peptide was analyzed by tetramer staining and intracellular IFN-γ assay in patients and their respective donors. All patients and their respective donors were CMV seropositive before transplantation. We estimated the expansion of CMVpp65495/HLA-A*0201+ CD8+ T cells, compared with the expansion of the CD3+and CD8+ T-cell compartments as a whole. All patients in our study received a fixed CD3+ T-cell dose of 2 × 104/kg at the time of transplantation. It is possible to estimate the number of CD3+lymphocytes left in the peripheral blood of each patient after infusion of the donor graft using kinetic models of biodistribution. After infusion, more than 90% of the infused CD3+ T-cell dose is retained in other tissues, especially the lungs, liver, and spleen such that less than 10% of the initial cell dose remains in the peripheral blood circulation.40 From this model, we extrapolated that once steady state is achieved, at best only 10% of the infused CD3+ T-cell dose (ie, 2 × 103/kg) will remain in the circulation. Using this assumption, we estimated the absolute CD3+ T-cell blood count at day 0 using the formula:
The fold expansion in CD3+ T cells, CD8+ T cells, and CMV+ CD8+ T cells at day 30 after SCT was then calculated using the following formula:
Absolute CD8+ T-cell count (cells/mL) at day 30 ÷ Absolute CD8+ T cells at day 0
Absolute CMV+ CD8+ T-cell count (cells/mL) at day 30 ÷ Absolute CMV+ CD8+ T cells at day 0
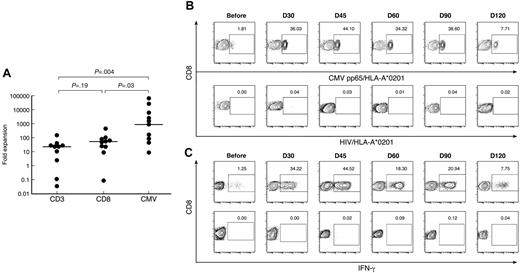
CMVpp65495/HLA-A*0201+ CD8+ T cells expanded to a considerably greater extent (median, 951 × 103 fold expansion; range, 0.009-65.15 × 103), equivalent to 18 to 19 doublings, compared with the total CD3+(median, 23 × 103 fold expansion; range, 0.03-154 × 103; P = .004), and CD8+ T cells (median, 55 × 103 fold expansion; range, 0.09-464 × 103; P = .03), equivalent to 14 to 15 doublings (Figure 5A). There was no significant difference in fold expansion between the CD3+and the CD8+ T-cell compartments (P = .19). In 3 patients the fold expansion of CMV-specific CD8+ T cells was 2 logs or more greater than that of the CD3+or CD8+ T-cells compartments (patients 2, 3, and 4). Two of these 3 patients had documented early CMV reactivation (before day 30), providing further evidence for an antigen-driven expansion of CMV-specific CD8+ T cells (Figure 5B).
CMV-specific CD8+ T cells in patients and donors. (A) After SCT, patients have significantly greater frequencies of CMVpp65495/HLA-A*0201+ CD8+ T cells than donors and patients before SCT. Data from multiple time points after SCT are presented. (B) Longitudinal analysis of CMVpp65495/HLA-A*0201+ CD8+ T cells after SCT in patient 4. HIV/HLA-A*0201 was used as a negative control for tetramer staining. Results are expressed as percentages of CD8+ T cells. (C) Longitudinal analysis of IFN-γ production by CD8+ T cells in post-SCT PB samples from patient 4 cultured for 6 hours with CMV peptide (top panel) or without peptide (negative control, bottom panel). Results are expressed as percentages of CD8+ T cells.
CMV-specific CD8+ T cells in patients and donors. (A) After SCT, patients have significantly greater frequencies of CMVpp65495/HLA-A*0201+ CD8+ T cells than donors and patients before SCT. Data from multiple time points after SCT are presented. (B) Longitudinal analysis of CMVpp65495/HLA-A*0201+ CD8+ T cells after SCT in patient 4. HIV/HLA-A*0201 was used as a negative control for tetramer staining. Results are expressed as percentages of CD8+ T cells. (C) Longitudinal analysis of IFN-γ production by CD8+ T cells in post-SCT PB samples from patient 4 cultured for 6 hours with CMV peptide (top panel) or without peptide (negative control, bottom panel). Results are expressed as percentages of CD8+ T cells.
Discussion
This study demonstrates that CD8+ T-cell responses against the leukemia-associated antigen WT1 develop in patients with ALL receiving a T-depleted SCT. The absence of WT1-specific CD8+ T-cell responses in patients with ALL before SCT19,41 suggests that the early post-SCT immune environment facilitates T-cell immune responses and results in T-cell proliferation directed against self-antigens. To our knowledge, WT1 immunity in GVL responses against ALL has not been previously described. However, data from patients with myeloid leukemias suggest that WT1 is naturally immunogenic and that healthy individuals can have immunologic memory for WT1.18–20,41,42
Animal experiments have shown that lymphopenia reduces the activation threshold of specific T-cells and creates a homeostatic drive to stimulate lymphocyte recovery driven by IL-2,43 IL-7,44 IL-12,45 IL-15,46,47 and Flt3 ligand.48 Lymphopenia allows donor T cells to be more easily recruited into specific immune responses, including those directed against self-antigens.24–27 A similar homeostatic drive favoring lymphocyte expansion occurs after SCT. T-cell receptor V-β spectratyping reveals that in the first few months after transplantation, the T-cell repertoire is oligoclonal because of selective expansion of antigen-stimulated T cells reactive against host, leukemia, and viral antigens (with the potential to cause GVHD and exert GVL and antiviral activity), despite global immune deficiency.49–52 In our study, WT1-specific CD8 + T-cell responses were only detected after SCT in cases in which the WT1 gene was expressed before SCT. The massive apoptosis of leukemia cells and release of antigenic cellular components during the conditioning regimen may promote antileukemia effects by making leukemia antigens available for antigen presentation to incoming donor T cells. Similarly, as we confirm here, patients who experienced CMV reactivation very early after SCT had massive expansions of CMV-specific CD8+ T cells (> 40% of the circulating CD8+ T cell compartment). This expansion appeared specific to the CMV antigen and was not a consequence of nonspecific lymphopenia-driven expansions of the CD3+and CD8+ T-cell compartments. We therefore propose that clonal expansions of leukemia-specific T cells may occur spontaneously in vivo, provided that a sufficient amount of leukemia-associated antigens are presented under conditions of lymphopenia.
The ability of WT1-specific CD8 + T cells detected by tetramer staining and intracellular IFN-γ production to mediate in vivo antileukemic cytotoxicity was assessed indirectly by correlating the emergence of WT1-specific CD8+ T cells with the leukemia load in the PB. Emergence of WT1-specific CD8+ T cells coincided with reduction or complete disappearance of WT1 gene expression in PB, whereas loss of WT1 response, for example after the initiation of immunosuppressive therapy, was associated with an increase in WT1 expression in PB. WT1 appeared to be an adequate marker of MRD. We found very low levels of WT1 in the PB of healthy donors, often below the level of detection of quantitative reverse-transcription PCR (10−6), whereas WT1 expression was significantly higher in patients with ALL. The detection of WT1 in post-SCT samples appeared to be disease specific and not merely caused by a regenerating hemopoietic system. WT1 was not expressed in the regenerating PB of 3 patients who did not have detectable WT1 expression in their PB before SCT. This is in keeping with work performed by others11,53,54 in which no significant difference was seen between normal and regenerating bone marrow, suggesting that the WT1 expression in the post-SCT samples was indeed a marker of MRD. To further ascertain the reliability of WT1 expression as a marker of MRD, we quantitated WT1 transcripts in samples that expressed BCR-ABL in Ph+ ALL patients. A strong correlation in the levels of gene expression between WT1 and BCR-ABL was thereby observed, again supporting the reliability and validity of WT1 as a marker of MRD.
To better characterize the functional status of these cells in ALL patients after SCT, we used multiparametric flow cytometry to study the surface phenotype of WT1/HLA-A*0201 tetramer-binding CD8+ T cells. Overlapping markers such as CD45RA, CD45RO, CD28, CD27, CD57, and CCR7 have been used to identify the differentiation state of antigen-specific CD8+ T cells.55–58 Ex vivo phenotypic characterization of WT1-specific CD8+ T cells identified these cells as mostly effector memory (CD27−CD57+) early after SCT, suggesting an ongoing immunologic response, with a trend toward more central memory phenotype later after SCT (CD27+ CD57−). Whereas CD57+ T cells are capable of rapid secretion of effector cytokines after antigenic stimulation, they undergo apoptosis after stimulation and fail to proliferate.55,59 This was further demonstrated by Gattinoni et al,60 who showed that adoptively transferred T-cell clones with a terminally differentiated effector phenotype were less successful at eradicating established tumors, partly because of their rapid disappearance after adoptive transfer. Similarly, we have recently demonstrated the marked expansion of CD8+ CD57+ T cells within the PR1/HLA-A*0201 tetramer–positive population in a patient with CML early after SCT and after a subsequent DLI. Within a few weeks, however, this terminally differentiated memory population had significantly diminished.61 In our study, insufficient material was available to perform direct ex vivo cytotoxicity against ALL, and repeated attempts at in vitro expansion to generate sufficient quantities of WT1-specific cytotoxic T-lymphocytes were unsuccessful (data not shown). Our inability to expand these cells in vitro is further testament to the proliferative senescence of these WT1-specific CD8+ CD57+ T cells. The observation that 15 months after SCT the WT1 response was still detectable but at much lower frequencies is consistent with the interpretation that WT1-specific CD8+ CD57+ T cells had undergone activation-induced cell death. The central memory phenotype appeared to be relatively diminished after transplantation but was nevertheless identifiable, suggesting that under favorable conditions CD57+ cells could be renewed from a central memory pool.
Therefore, it appears that in this small cohort of poor-risk ALL patients, evidence for a GVL effect exists and this GVL response is at least partly driven by WT1. However, immune responses against leukemia are often naturally directed against multiple antigens and it is very likely that WT1 is only one of many targets of this GVL response.18,20,62 The role of minor histocompatibility antigens in GVL and GVHD is well established.63,64 GVHD developed in 5 of 6 patients in our study, and in patient 7, the recipient of a female-into-male graft, GVHD was associated with intermittent reductions in WT1 expression without evidence of a WT1 CD8+ T-cell response, suggesting a minor histocompatibility antigen-driven GVL response. In the remaining 4 patients, however, an inverse relationship between GVHD and WT1 expression was not seen. GVHD and the initiation of immunosuppressive therapy were associated with an increase in WT1 gene expression and subsequent molecular relapse, making it unlikely that in this cohort of patients the GVL response as determined by a reduction in WT1 expression was driven by GVHD.
The prompt CD8+ T-cell response to WT1 and the association with suppression of the molecular marker of leukemia, WT1, suggest that WT1 could be a useful target for posttransplantation immunotherapy of ALL. Furthermore, the vigorous homeostatic proliferation of donor T cells after allogeneic SCT, especially in a T-depleted setting, may represent a hitherto unconsidered window of opportunity for posttransplantation vaccination and exploitation of the preferential proliferation of lymphocytes recognizing leukemia-associated antigens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We would like to thank Mrs Faith Williams for her technical assistance in the presentation of figures.
National Institutes of Health
Authorship
Contribution: K.R. conceived and designed the study, processed samples, performed experiments, analyzed data, and wrote the report. A.S.M.Y. performed experiments, advised on study design, and commented on manuscript. B.N.S. collected patient data, provided clinical care, advised on statistical analysis, and commented on the manuscript. S.M. advised on statistical analysis and commented on the manuscript. K.K. advised on FACS analysis and commented on the manuscript. E.G. provided tetramers. D.A.P. provided tetramers and commented on the manuscript. D.C.D. provided tetramers and commented on the manuscript. A.J.B. supervised the study, provided clinical care, and wrote the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katayoun Rezvani, National Heart, Lung, and Blood Institute, Building 10, Hatfield CRC, Rm 3-5410, 10 Center Drive, MSC 1202, Bethesda, MD 20892-1201; email: rezvanik@nhlbi.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal