Abstract
The impact of imatinib mesylate (IM) treatment for chronic myeloid leukemia (CML) on subsequent allogeneic transplantation is uncertain. To better understand this relationship, we retrospectively compared 145 patients with CML receiving IM for a minimum of 3 months before allogeneic hematopoietic cell transplantation (HCT) to 231 patients with CML who did not. IM treatment was associated with no increase in early hepatotoxicity or engraftment delay after HCT compared with the historical cohort. In addition, there was no statistically significant difference in the IM-treated cohort compared with the historical cohort with regard to overall survival, disease-free survival, relapse, and nonrelapse mortality. For chronic-phase (CP) patients, IM response prior to HCT was associated with post-HCT outcome. Patients who underwent transplantation in CP with a suboptimal response or a loss of response on IM had a statistically significant higher hazard of mortality when compared with CP patients who achieved a complete cytogenetic response (CCR) or major cytogenetic response (MCR) on IM (HR = 5.31, 95% confidence interval [CI] 1.13-25.05, P = .03). These data indicate that pre-HCT IM is not associated with increased transplant-related morbidity (TRM) or poorer outcomes. However, patients with a suboptimal or loss of IM response before HCT do worse, suggesting a more aggressive disease course for these patients.
Introduction
The Abl tyrosine kinase inhibitor, imatinib mesylate (IM), has become first-line therapy for chronic myeloid leukemia (CML) patients. The landmark phase 3 International Randomized Interferon vs STI571 (IRIS) trial of newly diagnosed patients with chronic-phase (CP) CML randomized to IM, now at 54 months follow-up, had complete hematologic responses (CHRs) in 98% of patients, major cytogenetic responses (MCRs) in 92%, and complete cytogenetic responses (CCRs) in 84%.1 Outcomes on IM are poorer in advanced-phase patients where disease progression is seen in 40% of accelerated phase (AP) patients and 80% of blast crisis (BC) patients in phase 2 clinical trials with a median follow-up of 18 months.2-7 However, approximately 10% of CP patients have poor responses to IM and relapse after initial response does occur. For advanced-phase patients treated with IM, relapse is, unfortunately, quite common. A main mechanism of relapse is the acquisition of point mutations in the ABL tyrosine kinase domain (TKD).8-13
Allogeneic hematopoietic cell transplantation (HCT) for CML is a potentially curative therapy. For matched related transplantation, survival for CP CML patients using current preparative and supportive measures is greater than 85% at selected institutions at 3 years after HCT,14 with similar results for younger matched unrelated donors.15 Additionally, HCT yields complete molecular responses that are associated with a lower risk of relapse after HCT and a potential cure of CML disease. Such responses occur in up to 75% of HCT patients, as compared with IM therapy where complete molecular responses are unusual.16-18 Results for AP or BC disease are significantly poorer as a consequence of increased transplant-related mortality (TRM) and higher posttransplantation relapse rates of 50% or more.19,20
HCT is increasingly chosen as “salvage” therapy for patients with CML who are intolerant of IM, fail to achieve a CCR, or relapse. This change in treatment strategy raises several issues. First, does treatment with IM prior to HCT result in increased regimen-related toxicities or affect posttransplantation outcomes? Second, does a poor response or a loss of response on IM negatively impact transplantation outcomes, particularly in CP patients? Finally, as ABL TKD mutations are associated with aggressive disease, is the presence of an ABL TKD mutation prior to HCT associated with poorer outcome? To address these questions, we retrospectively compared 145 patients who received IM for a minimum of 3 months before allogeneic HCT to 231 historical cohort patients who did not receive IM.
Patients, materials, and methods
Patient characteristics and definitions of phase
We examined a cohort of 145 patients who were treated with IM and received a first full allogeneic HCT between 10/27/2000 and 4/19/2005. Seventy-seven patients underwent transplantation at Fred Hutchinson Cancer Research Center (FHCRC), 51 patients at City of Hope National Medical Center (COHNMC), and 17 patients at Stanford University Medical Center (SUMC). This group was compared with an historical cohort of 231 consecutively treated FHCRC CML patients who did not receive IM and who received a first full allogeneic transplantation between 1/5/1999 and 10/9/2004. Approval for this retrospective study protocol was obtained from the FHCRC, COHNMC, and SUMC institutional review boards. Informed consent was provided in accordance with the Declaration of Helsinki. Accelerated phase was defined by any of the following: the presence of blasts in the bone marrow (BM) or peripheral blood (PB) of at least 15% but less than 30%, the presence of blasts and promyelocytes of more than 30% in BM or PB, or basophils in the PB of more than 20%. Blast crisis was defined as at least 30% blasts in the BM or PB. Chronic phase 2 (CP 2) was defined as a return to CP after treatment for AP or BC disease. European Group Bone and Marrow Transplantation (EBMT) scores were calculated based on stage of disease (0 for first CP, 1 for AP or CP2, and 2 for BC), age (0 for < 20 years, 1 for 20-40 years, and 2 for > 40 years), interval from diagnosis to transplantation (0 for ≤ one year and 1 for > one year), donor type (0 for an HLA-identical sibling and 1 for an unrelated donor), and donor-recipient sex match (1 for female donor for male recipient and 0 for all others).21,22
Definitions of IM response and treatment
Documentation of IM administration, duration, dosing, and response were obtained from the clinical records of patients at each center. Response to IM was characterized as follows. A CHR was defined as less than 5% BM blasts and no immature myeloid cells seen in the PB, platelets greater than 100 000/μL, and neutrophils greater than 1500/μL. Cytogenetic response was assessed based on a minimum of 20 metaphase preparations and was graded as complete (0% Philadelphia [Ph]–positive cells), major (1%-34% Ph-positive cells), minor (35%-65% Ph-positive cells), or minimal (66%-95% Ph-positive cells). For patients treated with IM for advanced-phase disease, response was considered a return to CP. Additionally, based on the protocols of each center, a subset of patients was monitored for cytogenetic response by fluorescence in situ hybridization (FISH) and for molecular response by qualitative reverse transcriptase polymerase chain reaction (RT-PCR). Sequencing for ABL TKD point mutations was performed at each center as previously described.9,10
Transplantation regimens
One hundred sixty-four patients received 1 mg/kg busulfan orally in 4 doses daily for 4 days (total dose 16 mg/kg) and 60 mg/kg cyclophosphamide intravenously daily for 2 days (total dose 120 mg/kg). Busulfan was targeted at 900 ng/mL as previously described.23 One hundred sixty-eight patients received cyclophosphamide and total body irradiation (total dose 1200 cGy or 1320 cGy). Nine patients received total body irradiation (total dose 1200 cGy) and etoposide (total dose 60 mg/kg). Fourteen patients received other regimens. Standard techniques for marrow and peripheral blood stem cell collection were used.24 Infection prophylaxis was administered according to each center's policies for prevention of bacterial, fungal, and viral infections. Graft-versus-host disease (GVHD) prophylaxis with cyclosporin (CSP) and methotrexate was as previously described.25,26 Acute GVHD was treated with prednisone, antithymocyte globulin, monoclonal antibodies, and other investigational agents at the discretion of each center. Chronic GVHD was treated with prednisone alone or with CSP. Secondary chronic GVHD therapy was initiated for progression of symptoms after at least 2 weeks of therapy, the absence of improvement after one month of therapy, persistent symptoms after 9 to 12 months of therapy after an initial improvement, or the recurrence of symptoms after cessation of immunosuppressive therapy, as previously documented.27
Definition of posttransplantation outcomes
The date of neutrophil engraftment was defined as the first of 3 consecutive days where the absolute neutrophil count (ANC) was greater than or equal to 500/μL and the date of platelet engraftment was defined as the first of 7 days where the untransfused platelet count was greater than or equal to 50 000/μL. The grading of GVHD was as previously described.27,28 Disease relapse after transplantation was monitored by pathology, cytogenetics, and by qualitative RT-PCR on days 28 and 80, at 6-month intervals up to 2 years, and then on a yearly basis after 2 years. FHCRC patients were also monitored by quantitative PCR. The techniques of qualitative and quantitative RT-PCR for bcr-abl messenger RNA have been previously published.18 Relapse was defined as hematologic relapse or as cytogenetic relapse characterized by the presence of 5 or more Ph chromosomes on a single cytogenetic analysis, or the presence of any Ph chromosomes on at least 2 consecutive cytogenetic evaluations.18,24
Statistical analysis
Kaplan-Meier estimates were used to summarize the probability of overall and disease-free survival.29 Cumulative incidence estimates were used to summarize the probability of relapse and nonrelapse mortality (NRM).30 Relapse was regarded as a competing risk for NRM, and death without relapse a competing risk for relapse. Cox regression was used to compare relevant groups for each of the time-to-event outcomes including overall survival (OS), disease-free survival (DFS), NRM, relapse, and chronic GVHD. Logistic regression was used for grades 2 to 4 and grades 3 to 4 acute GVHD. Linear regression was used to compare bilirubin parameters between groups and to compare time to engraftment among patients who engrafted. Both adjusted and unadjusted models are presented. The adjusted models contained EBMT score modeled as a continuous variable in all cases, and source of stem cells when assessing engraftment and GVHD.
Results
Patient demographics and characteristics
Patient and transplant characteristics are reported in Tables 1 and 2, respectively. Among patients who received IM, the median age at transplantation was 40.1 years compared with 40.6 years among the historical cohort patients. Forty percent of the IM-treated patients received a transplant from a matched-sibling donor, compared with 45% of historical cohort patients. The mean time to transplantation among IM-treated patients was 1.70 years and for the historical cohort patients it was 0.94 years (P < .001). At the time of HCT there was a higher proportion of patients with advanced-phase disease among the IM-treated cohort compared with the historical cohort. This difference in the distribution of phase between the 2 cohorts at the time of HCT was also reflected in EBMT scores.
Patient demographics and characteristics
| . | IM cohort . | Historical cohort . |
|---|---|---|
| Sex, no. (%) | ||
| Male | 94 (64) | 141 (61) |
| Female | 51 (36) | 90 (39) |
| Age, y, median (mean) | 40.1 (40.6) | 40.6 (39.0) |
| Disease phase at diagnosis, no. (%) | ||
| Chronic phase | 117 (81) | — |
| Accelerated phase and higher CP | 22 (15) | — |
| Blast crisis | 6 (4) | — |
| Disease phase at transplantation, no. (%) | ||
| Chronic phase or better* | 72 (50) | 183 (79) |
| Accelerated phase and higher CP | 60 (41) | 38 (17) |
| Blast crisis | 13 (9) | 10 (4) |
| Interval from diagnosis to transplantation, y | 1.70 | 0.94 |
| IM therapy duration, y, median (range) | 0.83 (0.25-3.92) | — |
| Interval from IM cessation to transplantation, wk EBMT score, no. (%) | 2-4 | — |
| 0 and 1 | 10 (7) | 29 (13) |
| 2 | 28 (19) | 65 (28) |
| 3 and 4 | 73 (50) | 111 (48) |
| 5+ | 34 (24) | 17 (7) |
| Unknown | — | 9 (4) |
| . | IM cohort . | Historical cohort . |
|---|---|---|
| Sex, no. (%) | ||
| Male | 94 (64) | 141 (61) |
| Female | 51 (36) | 90 (39) |
| Age, y, median (mean) | 40.1 (40.6) | 40.6 (39.0) |
| Disease phase at diagnosis, no. (%) | ||
| Chronic phase | 117 (81) | — |
| Accelerated phase and higher CP | 22 (15) | — |
| Blast crisis | 6 (4) | — |
| Disease phase at transplantation, no. (%) | ||
| Chronic phase or better* | 72 (50) | 183 (79) |
| Accelerated phase and higher CP | 60 (41) | 38 (17) |
| Blast crisis | 13 (9) | 10 (4) |
| Interval from diagnosis to transplantation, y | 1.70 | 0.94 |
| IM therapy duration, y, median (range) | 0.83 (0.25-3.92) | — |
| Interval from IM cessation to transplantation, wk EBMT score, no. (%) | 2-4 | — |
| 0 and 1 | 10 (7) | 29 (13) |
| 2 | 28 (19) | 65 (28) |
| 3 and 4 | 73 (50) | 111 (48) |
| 5+ | 34 (24) | 17 (7) |
| Unknown | — | 9 (4) |
IM indicates imatinib mesylate; —, data not available or not applicable.
“Better” indicates patients in chronic phase with a major or complete cytogenetic response on IM at the time of transplantation.
Transplant characteristics
| . | IM cohort . | Historical cohort . |
|---|---|---|
| Donor type, no. (%) | ||
| HLA-identical sibling | 58 (40) | 103 (45) |
| Matched unrelated donor | 81 (56) | 120 (52) |
| HLA mismatch | 1 (1) | 5 (2) |
| Unknown | 5 (3) | 3 (1) |
| Gender mismatch, no. (%) | ||
| Female donor, male recipient | 41 (28) | 63 (27) |
| Other | 104 (72) | 168 (73) |
| Conditioning regimen, no. (%) | ||
| Cy/TBI | 54 (37) | 114 (49) |
| Bu/Cy | 75 (52) | 89 (39) |
| Etoposide/TBI | 9 (6) | 0 |
| Other | 7 (5) | 7 (3) |
| Unknown | 0 | 21 (9%) |
| Stem-cell source, no. (%) | ||
| BM | 55 (38) | 178 (77) |
| PBSC | 90 (62) | 53 (23) |
| . | IM cohort . | Historical cohort . |
|---|---|---|
| Donor type, no. (%) | ||
| HLA-identical sibling | 58 (40) | 103 (45) |
| Matched unrelated donor | 81 (56) | 120 (52) |
| HLA mismatch | 1 (1) | 5 (2) |
| Unknown | 5 (3) | 3 (1) |
| Gender mismatch, no. (%) | ||
| Female donor, male recipient | 41 (28) | 63 (27) |
| Other | 104 (72) | 168 (73) |
| Conditioning regimen, no. (%) | ||
| Cy/TBI | 54 (37) | 114 (49) |
| Bu/Cy | 75 (52) | 89 (39) |
| Etoposide/TBI | 9 (6) | 0 |
| Other | 7 (5) | 7 (3) |
| Unknown | 0 | 21 (9%) |
| Stem-cell source, no. (%) | ||
| BM | 55 (38) | 178 (77) |
| PBSC | 90 (62) | 53 (23) |
Bu indicates busulfan; Cy, cyclophosphamide; TBI, total body irradiation; BM, bone marrow; PBSC, peripheral blood mobilized stem cells.
Response to IM and indications for transplantation
Among patients receiving IM prior to HCT, the median duration of drug therapy was 0.83 years (range, 0.25-3.92 years). The duration of IM use and time from diagnosis to transplantation were correlated with an estimated correlation coefficient of R = 0.60. The median IM dose was 500 mg (range, 300-800 mg). Fifty-five patients received therapies in addition to IM. Twenty-three patients received interferon-α (IFN) or IFN in combination with cytarabine (Ara-C), 18 patients received hydrea, 13 patients received chemotherapy with various agents, and one patient received a vaccine. The time from IM cessation to HCT ranged from 2 to 4 weeks. Indications for HCT in the IM-treated cohort varied. Among patients who underwent transplantation in first CP, indications included no response to IM (15 patients), a suboptimal (minor or minimal) response to IM (7 patients), and loss of initial IM response (9 patients). However, there was also a sizeable group of CP patients who underwent transplantation while still achieving an MCR or CCR on IM (38 patients, or 55%). Among patients with advanced-phase disease, 42 patients underwent transplantation as a consequence of progression from CP to advanced-phase disease while on IM. Twenty-seven patients were in AP, 4 in CP2, and 11 in BC at the time of HCT.
Engraftment and treatment-related toxicity
As IM therapy is associated with adverse hematologic and hepatic effects, we looked specifically at the effect of IM on engraftment (Table 3)and liver toxicity. Ninety percent of patients in the historical cohort achieved an ANC of more than 500/μL, and 88% of IM-treated patients achieved this level. Eighty-one percent of the historical cohort patients reached an unsupported platelet count of 50 000/μL for 7 consecutive days, and 78% of IM-treated patients achieved this level. After adjusting for both EBMT score and source of stem cells, patients who received IM reached an ANC of more than 500/μL, an estimated 1.5 days earlier than patients who did not receive IM (P = .009).
Outcomes for the IM-treated cohort as compared with the historical cohort for all centers and for Fred Hutchinson Cancer Research Center (FHCRC) patients only
| . | All centers; OR or HR (95% CI; P)* . | FHCRC; OR or HR, (95% CI; P)* . |
|---|---|---|
| Acute GVHD | ||
| Grades 2-4 | OR = 0.67 (0.40-1.12; .13) | OR = 0.87 (0.46-1.65; .68) |
| Grades 3-4 | OR = 0.99 (0.53-1.83; .97) | OR = 0.53 (0.23-1.27; .15) |
| Extensive chronic GVHD | HR = 0.33 (0.22-0.48; < .0001) | HR = 0.65 (0.45-0.95; .03) |
| NRM | HR = 0.73 (0.44-1.20; .22) | HR = 0.49 (0.26-0.95; .03) |
| Relapse | HR = 0.56 (0.27-1.13; .10) | HR = 0.51 (0.21-1.23; .13) |
| Relapse or mortality | HR = 0.66 (0.44-1.00; .05) | HR = 0.50 (0.30-0.85; .01) |
| Mortality | HR = 0.71 (0.46-1.11; .13) | HR = 0.49 (0.28-0.87; .01) |
| Engraftment | All centers | FHCRC |
| Days to ANC > 500 | 1.5 days earlier; .009 | 1.9 days earlier; .005 |
| Days to Plts > 50 000 | 4.7 days longer; .06 | 0.6 days earlier; .77 |
| . | All centers; OR or HR (95% CI; P)* . | FHCRC; OR or HR, (95% CI; P)* . |
|---|---|---|
| Acute GVHD | ||
| Grades 2-4 | OR = 0.67 (0.40-1.12; .13) | OR = 0.87 (0.46-1.65; .68) |
| Grades 3-4 | OR = 0.99 (0.53-1.83; .97) | OR = 0.53 (0.23-1.27; .15) |
| Extensive chronic GVHD | HR = 0.33 (0.22-0.48; < .0001) | HR = 0.65 (0.45-0.95; .03) |
| NRM | HR = 0.73 (0.44-1.20; .22) | HR = 0.49 (0.26-0.95; .03) |
| Relapse | HR = 0.56 (0.27-1.13; .10) | HR = 0.51 (0.21-1.23; .13) |
| Relapse or mortality | HR = 0.66 (0.44-1.00; .05) | HR = 0.50 (0.30-0.85; .01) |
| Mortality | HR = 0.71 (0.46-1.11; .13) | HR = 0.49 (0.28-0.87; .01) |
| Engraftment | All centers | FHCRC |
| Days to ANC > 500 | 1.5 days earlier; .009 | 1.9 days earlier; .005 |
| Days to Plts > 50 000 | 4.7 days longer; .06 | 0.6 days earlier; .77 |
OR indicates odds ratio; HR, hazard ratio; NRM, nonrelapse mortality; GVHD, graft-versus-host disease; ANC, first of 3 consecutive days where the absolute neutrophil count was ≥ 500/μL; Plts, first of 7 days where the untransfused platelet count was ≥ 50 000/μL.
Ratios adjusted for EBMT score and/or stem-cell source; data reported for the IM-treated cohort relative to the historical cohort.
Among IM-treated patients, there were only 2 reported cases of veno-occlusive disease (VOD). As a measure of early posttransplantation liver function, we used posttransplantation bilirubin levels as a surrogate for hepatic toxicity. The median and maximum bilirubin values within the first 20 days after HCT were obtained for each patient. The average median bilirubin in the first 20 days was 1.66 and 1.19 in the historical and IM-treated groups, respectively (P = .008). After adjusting for EBMT score, the average of the median bilirubin values was estimated to be 0.72 units lower in the IM-treated cohort compared with the historical cohort (P < .001). The average maximum bilirubin in the first 20 days was 2.96 and 2.33 in the historical and IM-treated groups, respectively (P = .004). After adjusting for EBMT score, the difference between groups was increased to 1.18 units (P < .001). These data suggest that pretransplantation IM does not generally predispose patients to major hepatic dysfunction early after transplantation. One patient, however, died from fulminate liver failure on day 160 after failing 2 courses of denileukin diftitox for chronic GVHD. The patient had documented IM-induced subacute hepatic necrosis and fibrosis prior to HCT and had been switched from IM to IFN prior to HCT. The initial post-HCT course had been uneventful until day 80 when the patient presented with very high bilirubin values.
Graft-versus-host disease
Outcomes for acute and chronic GVHD are reported in Table 3. These data were adjusted for EBMT score and stem-cell source. Among all patients, the adjusted odds of grades 2 to 4 acute GVHD were slightly lower in patients who received IM compared with those who did not (odds ratio [OR] = 0.67, 95% CI 0.40-1.12, P = .13) and the odds of grades 3 to 4 acute GVHD were very similar in the 2 groups (OR = 0.99, 95% CI 0.53-1.83, P = .97). The hazard of clinical extensive chronic GVHD was statistically significantly less in patients who received IM compared with those who did not (HR = 0.33, 95% CI 0.22-0.48, P < .001). As the historical cohort consisted of patients who underwent transplantation at FHCRC only, it was possible that any association between IM prior to HCT and GVHD was partly confounded by the “center effect.” Among all patients (ie, those who received IM and those who did not), the rate of grades 2 to 4 acute GVHD was 70% (216/308) for those who underwent transplantation at FHCRC compared with 54% (37/68) for patients at non-FHCRC sites. When the analysis was restricted to grades 3 to 4 GVHD, the rate of acute GVHD was 18% (56/308) for FHCRC patients as compared with 29% (20/68) for patients at non-FHCRC sites. Clinical extensive chronic GVHD was seen in 55% (170/308) of FHCRC patients compared with 31% (21/68) of non-FHCRC patients. GVHD analyses were then restricted to FHCRC patients. We found no statistically significant difference in the odds of acute GVHD when comparing the IM-treated cohort to the historical cohort. For grades 2 to 4 acute GVHD, the OR was 0.87 (95% CI 0.46-1.65, P = .68) and for grades 3 to 4 acute GVHD, OR was 0.53 (95% CI 0.23-1.27, P = .15). The hazard of clinical extensive chronic GVHD was statistically significantly less in the IM-treated cohort, HR = 0.65 (95% CI 0.45-0.95, P = .03), a result consistent with the analysis from all centers.
Nonrelapse mortality
Among the 145 patients in the IM-treated cohort, 31 died without relapse. The main causes of death included chronic GVHD of the liver and GI tract (7 patients), acute GVHD (7 patients), sepsis and multiorgan failure (5 patients), and pulmonary causes including diffuse alveolar damage and idiopathic pneumonitis (5 patients). Other causes included VOD (1 patient), brain injury from insulin overdose accompanied by pericardial effusion (1 patient), idiopathic thrombocytopenia (1 patient), CNS white matter disease (1 patient), toxoplasmosis (1 patient), and cytomegalovirus (CMV) enteritis (1 patient). CMV infection was present in one fatal case of acute and one fatal case of chronic GVHD. Fungal infections with Aspergillus and Scedosporium were present in 2 separate cases of fatal chronic GVHD. The estimated probability of NRM at 100 days, 1 year, and 3 years was 0.11, 0.20, and 0.24, respectively, in the historical cohort and 0.08, 0.19, and 0.30, respectively, in the IM-treated cohort. The unadjusted hazard of NRM was similar in the IM-treated group as compared with the historical cohort (HR = 1.11, 95% CI 0.72-1.72, P = .63) and the adjusted hazard was less, but not statistically significantly so (HR = 0.73, 95% CI 0.44-1.20, P = .22). When limited to FHCRC patients only, the adjusted hazard reached statistical significance (HR = 0.49, 95% CI 0.26-0.95, P = .03).
Disease-free survival and relapse
The estimated 1- and 3-year DFS rates were 69% and 59%, respectively, for the patients in the historical cohort and 71% and 57%, respectively, in the IM-treated group. The unadjusted hazard of failure (relapse or death) for DFS was similar among IM-treated and historical cohort patients (HR = 0.96, 95% CI 0.68-1.36, P = .82). After adjusting for EBMT score, the hazard was decreased in IM-treated patients compared with the historical cohort in the analysis using all centers (HR = 0.66, 95% CI 0.44-1.00, P = .05) and in the analysis limited to FHCRC patients (HR = 0.50, 95% CI 0.30-0.85, P = .01). The probability of relapse at 1 and 3 years was estimated to be 12% and 17%, respectively, in the historical cohort, and 9% and 13%, respectively, for IM-treated patients. The unadjusted hazard ratio for relapse was 0.73 (95% CI 0.40-1.34, P = .31) and the adjusted hazard ratio was 0.56 (95% CI 0.27-1.13, P = .10). The impact of IM use on outcome does not appear to depend on stage of disease for any of these end points (data not shown).
Overall survival
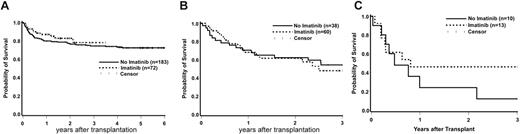
Estimates of OS are shown by phase in Figure 1A-C. The 3-year estimates for historical cohort patients with CP or AP/CP2 disease are 74% and 54%, respectively, and 78% and 48%, respectively, among patients who received IM. Eight of 10 historical cohort patients who underwent transplantation in BC died (follow-up in the 2 survivors, 152 and 2352 days), whereas 6 of 12 IM-treated patients who underwent transplantation in BC died (follow-up among survivors, 542 to 1593 days). These results, along with the data presented in Figure 1A-C, suggest that the impact of IM on OS following HCT is not dependent on stage of disease. A formal statistical test of interaction between stage and use of IM supports this observation (P > .58 for each relevant comparison). The unadjusted hazard of mortality among IM-treated patients was slightly higher than that among patients not receiving IM, but the difference was not statistically significant (HR = 1.17, 95% CI 0.80-1.71, P = .43). After adjusting for EBMT score, the hazard of mortality in the IM-treated group was decreased relative to the group that did not receive IM, but the difference between groups was not statistically significant (HR = 0.71, 95% CI 0.46-1.11, P = .13). When limited to FHCRC patients only, the adjusted hazard of mortality reached statistical significance (HR = 0.49, 95% CI 0.28-0.87, P = .01).
Overall survival in the IM-treated and historical cohort patients undergoing transplantation, by disease phase. (A) Chronic phase. (B) Accelerated phase or second chronic phase. (C) Blast crisis.
Overall survival in the IM-treated and historical cohort patients undergoing transplantation, by disease phase. (A) Chronic phase. (B) Accelerated phase or second chronic phase. (C) Blast crisis.
Previous studies have shown an impact of time from diagnosis to transplantation on OS, particularly in CP patients.15,21,31 In the current study, CP patients who did not receive IM and who underwent transplantation more than 1 year after diagnosis had a hazard of mortality of 1.54 (95% CI 0.82-2.87, P = .18) times that of CP patients not receiving IM who underwent transplantation within 1 year of diagnosis. Patients who received IM and who underwent transplantation in CP more than 1 year after diagnosis had a hazard of mortality of 1.29 (95% CI 0.42-4.01, P = .66) times that of IM patients who underwent transplantation in CP within a year of diagnosis. These data suggest that the deleterious effect of time from diagnosis to transplantation is not magnified by the use of IM among patients who undergo transplantation in CP. Additionally, we did not find that the impact of IM on overall survival was altered by either hematopoietic-cell source (P = .42) or conditioning regimen (P = .88).
Overall survival by response to IM at the time of HCT
Data on each patient's best response and ultimate response to IM at the time of HCT were obtained from all centers. Patients were categorized by cytogenetic and hematologic response as documented in “Patients, materials, and methods.” For analysis purposes, patients who underwent transplantation in CP were divided into 2 groups. Patients achieving and maintaining a CCR or MCR response on IM therapy (good response) were compared with patients who achieved a minor, minimal, or no response (suboptimal response) and patients who had lost their response to IM. Sixty-nine of 72 patients who underwent transplantation in CP had available cytogenetic and hematologic response data on IM.
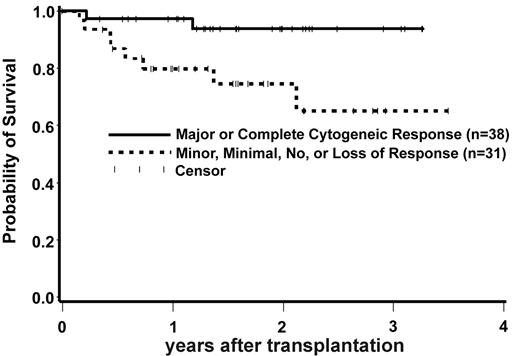
As indicated in Figure 2, pretransplantation IM response affected posttransplantation OS for patients who underwent transplantation in CP. Patients who underwent transplantation in CP who had a suboptimal response or lost their response to IM had a statistically significantly higher hazard of mortality compared with CP patients who achieved and maintained a CCR or MCR on IM at the time of HCT. Eight deaths occurred among 31 patients with a suboptimal or loss of response, whereas 2 deaths occurred among 38 patients with a good response (HR = 5.31, 95% CI 1.13-25.05, P = .03). This result was virtually unchanged after adjusting for EBMT score (data not shown). There were only 4 relapses among the patients who underwent transplantation in CP. Two of these relapses occurred among the 38 patients with a good response (neither of these 2 patients have died as of last contact) and 2 relapses occurred among the 31 patients who had a suboptimal or loss of response to IM (2 deaths). Six patients in the suboptimal or loss of response group died from nonrelapse causes after HCT compared with only 2 nonrelapse deaths among those with a good response.
Overall survival in IM-treated CP patients by IM response at the time of transplantation. Patients who underwent transplantation in CP with a suboptimal response or a loss of response on IM had a statistically significantly higher hazard of mortality when compared with CP patients who achieved and maintained a complete cytogenetic response or major cytogenetic response on IM (HR = 5.31, 95% CI 1.13-25.05, P = .03).
Overall survival in IM-treated CP patients by IM response at the time of transplantation. Patients who underwent transplantation in CP with a suboptimal response or a loss of response on IM had a statistically significantly higher hazard of mortality when compared with CP patients who achieved and maintained a complete cytogenetic response or major cytogenetic response on IM (HR = 5.31, 95% CI 1.13-25.05, P = .03).
We then divided patients into 3 groups: patients who achieved and maintained a CCR or MCR; patients who achieved only minor, minimal, or no responses on IM (suboptimal response); and patients who lost their IM response. Patients with a suboptimal response to IM had a suggestively worse OS when compared with those who underwent transplantation with a CCR or MCR (HR = 3.97, 95% CI 0.99-15.99, P = .05). For patients who lost their response to IM there was no statistically significant difference in OS compared with patients who underwent transplantation with a CCR or MCR. There were only 9 patients within the group that lost IM response. Among these patients 8 had cytogenetic relapse, whereas only one had hematologic relapse in addition to cytogenetic relapse. We also looked at outcomes based on best response to IM (rather than response at the time of HCT). We found that those patients whose best response to IM was a CCR or MCR had a suggestive decrease in the hazard of mortality when compared with patients with a suboptimal response (HR = 0.46, P = .05).
Among patients with more advanced disease, overall survival was similar among those whose disease progressed from CP to advanced-phase disease while on IM (19 deaths among 42 patients [45%]), those whose disease returned back to CP or AP on IM (6 deaths among 13 patients [46%]), and those who didn't respond to IM for treatment of advanced-phase disease (6 deaths among 17 patients [35%]). Evidence of clonal evolution on IM was documented in 23 patients. At the time of diagnosis 22 patients were in CP and 1 was in AP. At the time of HCT 2 patients were in CP, 17 patients were in AP, 1 patient was in CP2, and 3 patients were in BC. Patients with clonal evolution had an increased hazard of mortality compared with those without clonal evolution, although the difference was not statistically significant after adjustment using a modified EBMT score that excluded the disease evolution–related variable disease phase (HR = 1.37, 95% CI 0.67-2.79, P = .39).
TKD point mutations and outcome
Point mutation analysis of the ABL TKD was performed retrospectively on archival samples obtained prior to HCT. Samples were available and successfully sequenced for 103 patients. Among the 103 patients, 91 had no ABL TKD mutations and 12 patients had point mutations. A sizeable number of patients underwent transplantation in CCR or MCR (38 patients) and, as expected, had no ABL TKD mutations. Among patients with available samples who were treated with IM for CP disease at diagnosis, 31 patients had a suboptimal or absent response and are best characterized as patients with primary resistance where the incidence of point mutations is lower. Mutations detected included M244V (1 patient), L248V (1 patient), G250E (1 patient), Y253H (2 patients), D276G (1 patient), T315I (1 patient), F317L (1 patient), E355G (1 patient), F359V (2 patients), and H396R (1 patient). ABL TKD point mutations were detected more frequently in advanced-disease patients: 6 in AP and CP2 patients and 3 in BC patients compared with 3 in CP patients. All 6 patients with P-loop and T315I mutations underwent transplantation with more advanced-phase disease. Twenty-four of 91 patients (26%) without a point mutation died and 4 of 12 patients (33%) with a mutation died. Relapse occurred in 2 of 12 patients with TKD mutations on days 112 and 873, respectively. One patient with persistent disease after HCT and an ABL TKD mutation died on day 294. Three of 6 patients (50%) with a P-loop or T315I mutation died, compared with 1 of 6 patients (17%) with other mutations. Among these 3 patients with P-loop or T315I mutations, 2 died from relapse.
Monitoring and interventions for persistent or recurrent CML
Seventeen patients in the IM-treated cohort relapsed. Twelve patients have died from relapse-associated causes: 6 patients who underwent transplantation in BC, 4 patients in CP2 or higher, 1 patient in AP, and 1 patient in CP. Five patients are alive and are receiving various salvage therapies. Two patients within this group have achieved a CCR and complete molecular response (CMR) on IM-containing salvage therapies.
Discussion
Our data provide no evidence that the use of IM before HCT results in increased transplant-related toxicity or a detrimental effect on post-HCT outcomes. Despite the fact that the IM-treated group had, on average, a higher proportion of patients with adverse risk factors, the unadjusted results, which did not consider the difference in risk characteristics, were quite similar between the groups. After controlling for the difference in risk factors, modeled by EBMT score, any advantages seen in the historical cohort disappeared. However, there was one finding of potential consequence. We observed that 31 CP patients who achieved only a suboptimal response or who lost their response to IM had a statistically significantly higher hazard of mortality when compared with 38 CP patients who underwent transplantation while achieving and maintaining a CCR or MCR on IM at the time of HCT (HR = 5.31, 95% CI 1.13-25.05, P = .03).
As a consequence of the widespread use of IM as first-line treatment for CML, the population of patients undergoing allogeneic transplantation has changed. Most patients receive IM prior to HCT and typically undergo transplantation in CP if they are intolerant of or failing IM. Increasingly, patients undergo transplantation with more advanced disease after failing IM. Given that HCT is potentially curative and has a very high survival rate in CP disease, the strategy of IM treatment until failure is a reasonable approach if pretransplantation IM therapy does not adversely impact transplantation outcomes.
Several small retrospective studies of TRM associated with IM use prior to allogeneic HCT found a higher incidence of liver toxicity.32,33 Two larger studies that compared IM-treated and historical cohorts found no significant increase in hepatic toxicity after HCT.34,35 Furthermore, in studies of patients receiving IM for relapse after HCT, hepatic toxicity appears to be minimal.36-38 Similar to both larger studies of patients receiving IM prior to HCT, we found no overall increase in hepatotoxicity. However, the observation of fatal posttransplantation hepatotoxicity in a patient with a history of IM-induced subacute hepatic necrosis and fibrosis suggests that caution should be taken in this particular clinical situation. To date there are no reports of delayed engraftment in the myeloablative and nonmyeloablative setting for CML and Ph+ ALL patients.34,35,39-41 After adjusting for EBMT score and stem-cell source, we found that patients who received IM had slightly faster myeloid engraftment.
With regard to the incidence and severity of acute and chronic GVHD, reports have been mixed, with some studies suggesting more GVHD in IM-treated patients,32 while other studies have demonstrated no such increase in either acute or chronic GVHD.33-35,39 We found no statistically significant difference in either grades 2 to 4 or grades 3 to 4 acute GVHD between the IM-treated cohort and the historical cohort. Similar to the larger cohort study reported by Deininger et al,35 we found a lower incidence of chronic GVHD, specifically clinically extensive chronic GVHD in the IM-treated cohort. The hazard of clinical extensive chronic GVHD in patients who received IM was 0.33 times (95% CI 0.22-0.48, P < .001) that of those who did not. The mechanism of the effect of IM on subsequent GVHD is speculative. IM inhibits T-cell proliferation, TCR-mediated T-cell activation, and CMV and Epstein-Barr virus (EBV) CD8+ T-cell responses.42 Other recent reports indicate that imatinib may be immunosuppressive as it inhibits dendritic-cell development and function, resulting in cells that do not respond to maturation stimulus and do not elicit primary T-cell responses or T-cell responses to a recall antigen.43 However, it is unclear why these effects would persist in patients who are no longer taking IM, especially given the extremely large number of T cells of donor origin infused.
There are few data currently available with regard to OS, DFS, NRM, and relapse in patients who received IM prior to HCT. In smaller series of patients there have been reports of poorer outcomes associated with IM prior to HCT; however, these studies were too small to address these outcomes fully.32 Two larger studies have compared IM-treated and historical cohorts. The earlier study assessed 30 patients with CML who received IM prior to HCT.34 A more recent and larger study analyzed a retrospective cohort of 70 patients with CML and 21 patients with Ph+ ALL who underwent transplantation at 24 centers and who received IM prior to HCT.35 The larger study by Deininger et al34 reported a trend toward an increase in relapse in the IM-treated CML group, whereas Zaucha et al35 reported no difference in OS between the IM-treated and historical cohorts.35 In our study, we found no detrimental effect of IM for any end point and in analysis limited to FHCRC patients there was a suggestion that the hazards of NRM and overall survival were decreased in IM-treated patients. However, CP patients with suboptimal or loss of IM response at the time of HCT had inferior outcomes after transplantation when compared with patients achieving and maintaining a CCR or MCR. This result is consistent with a recent study of 27 patients with CML who underwent transplantation in late CP.44
ABL TKD mutations are a common mechanism of IM resistance. Both clinical and molecular studies have documented the aggressive nature of the P-loop and T315I mutations.45-47 We found ABL TKD mutations in 12 of 103 cases studied, but found no obvious association between mutation status and outcome. All 6 patients with P-loop and T315I mutations had advanced-phase disease, and while 3 of these patients died, the rate of death is not unexpected for this group. This finding is consistent with a recent report of 10 patients with ABL TKD mutations who received myeloablative or nonmyeloablative HCT where outcomes did not appear to be adversely affected by mutation status prior to HCT.48 Overall, these studies suggest that transplantation is an effective salvage strategy for patients with acquired ABL TKD point mutations.
In conclusion, we found that IM prior to HCT did not result in increased TRM or worse outcomes following HCT. However, the observations that CP patients with suboptimal or loss of IM response at the time of HCT have inferior outcomes and that most patients with Abl TKD point mutations underwent transplantation with more advanced-phase disease, where outcomes are poorer, underscores the importance of prospective cytogenetic and molecular monitoring on IM therapy49 and moving to alternative treatments, including HCT, as early as possible in patients with poor response or acquired mutations on IM therapy.
Authorship
Contribution: V.G.O. initiated the study, collected and analyzed data, and wrote the manuscript; J.P.R. helped organize the study and contributed to the writing of the manuscript; T.G. conducted the statistical analysis and contributed to the writing of the manuscript; D.S.S. helped organize the study and collected data; L.J. helped organize the study and collected data; A.L. helped collect data; C.C.C., S.C., and R.B. performed PCR and sequencing of the ABL TKD; S.J.F., R.S.N., and F.R.A. contributed to the organization and design of the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vivian G. Oehler, 1100 Fairview Ave N, D4-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: voehler@u.washington.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank the clinical staff at the Fred Hutchinson Cancer Research Center, City of Hope National Medical Center, and Stanford University Medical Center for their excellent patient care. This work was supported in part by National Institutes of Health Grants CA-106796 (V.G.O.) and CA-18029 (J.P.R.) and an American Society of Hematology Clinical/Translational Research Fellow Scholars Award (V.G.O.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal