Abstract
Multiple myeloma (MM) plasma cells, but not those from healthy donors and patients with monoclonal gammopathy of undetermined significance or other plasma cell dyscrasias involving the bone marrow, express the Wnt-signaling antagonist DKK1. We previously reported that secretion of DKK1 by MM cells likely contributes to osteolytic lesions in this disease by inhibiting Wnt signaling, which is essential for osteoblast differentiation and survival. The mechanisms responsible for activation and regulation of DKK1 expression in MM are not known. Herein, we could trace DKK1 expression changes in MM cells to perturbations in the JNK signaling cascade, which is differentially modulated through oxidative stress and interactions between MM cells with osteoclasts in vitro. Despite its role as a tumor suppressor and mediator of apoptosis in other cell types including osteoblasts, our data suggest that DKK1, a stress-responsive gene in MM, does not mediate apoptotic signaling, is not activated by TP53, and its forced overexpression could not inhibit cell growth or sensitize MM cells to apoptosis following treatment with thalidomide or lenalidomide. We conclude that specific strategies to modulate persistent activation of the JNK pathway may be beneficial in preventing disease progression and treating myeloma-associated bone disease by inhibiting DKK1 expression.
Introduction
Multiple myeloma (MM), a tumor of terminally differentiated plasma cells, depends on the bone marrow microenvironment for growth and survival.1,2 MM is characterized by extensive bone loss3 and osteolytic lesions located at sites of medullary plasmacytomas, suggesting that myeloma cells secrete factors that alter the biology of bone remodeling.4,5 Wnt signaling is essential for maintenance of osteoblast and osteoclast homeostasis and, thus, coupled bone turnover.6–8 Emerging evidence suggests that production of Wnt-signaling inhibitors DKK1, sFRP2, and sFRP3 by MM plasma cells contributes to development of osteolytic lesions through the direct suppression of osteoblast differentiation.9–11 Recently, we demonstrated a consistent, close correlation between DKK1 expression by myeloma cells and the occurrence of focal lytic bone lesions in patients with MM.9
Although the biologic function of DKK1 in MM pathophysiology has recently begun to be elucidated,9,12 it is currently not known how DKK1 expression is activated and regulated in MM plasma cells during different stages of disease. Our previous study showed that DKK1 expression was very high in nearly 80% of newly diagnosed patients but was essentially undetectable in plasma cells from control subjects and those with Waldenström macroglobulinemia and monoclonal gammopathy of undetermined significance (MGUS), 2 plasma cell dyscrasias involving the bone marrow not associated with lytic bone disease.9 In addition, we found that DKK1 is also low in cells from secondary plasma cell leukemia and pleural effusions at the end stage of MM.9 Gene expression profiling-defined classification of MM has revealed that DKK1 expression and osteolysis is significantly elevated in hyperdiploid disease with characteristics of microenvironment-dependent growth.13 These data suggest that DKK1 activation, leading to alterations in the functional activity of osteoblasts and osteoclasts, could be an essential event in disease progression, especially in forms of the disease lacking oncogene-activating translocations that are presumed most dependent of these changes.
Recently, a variety of mechanisms has been proposed to explain induction of DKK1 expression in healthy cells and cancer cells in humans. DKK1 is reportedly a direct transcriptional target of the β-catenin/T-cell factor (TCF) complex, suggesting the existence of a negative regulation loop in Wnt signaling.14–16 Furthermore, DKK1 can be activated in response to proapoptotic stimuli induced by different types of genotoxic stress or DNA damage, and this activation is regulated by transcription factors p53 and c-Jun.17–21 Interestingly, during apoptotic signaling, activity of both of these transcription factors can be modulated through posttranslational modifications by the Jun N-terminal kinase (JNK) cascade.22–24
Recent studies demonstrated that constitutive activation of the JNK pathway modulates the cell cycle and proliferation of MM plasma cells25 and participates in the cellular response to proapoptotic stimuli induced by treatment with therapeutic agents.26–29 Activated JNKs can translocate to mitochondria, inducing the release of proapoptotic proteins such as cytochrome c and Smac, and to the nucleus where they phosphorylate and stabilize the transcriptional activity of c-Jun, ATF2, p53, and Elk-1.30 Different forms of stress mediate JNK activation via various cellular pathways31 ; the sustained activation of JNKs induced by oxidative stress is mainly mediated by the apoptosis signal-regulating kinase 1 (ASK1), a mitogen-activated protein (MAP) kinase, which activates the MKK4/MKK7-JNK pathway by direct site-specific Ser/Thr phosphorylation.32
Understanding the shift in DKK1 expression from absence in MGUS to universal expression in MM may provide tools for preventing disease progression from MGUS to MM. Here, we present evidence that DKK1 expression in MM plasma cells is, in part, dependent on JNK signaling, suggesting that modulating the JNK pathway may have an important role in severing the link between the MM cell and its soil.
Materials and methods
Purification of primary myeloma plasma cells
Plasma cells were obtained from heparinized bone marrow aspirates of patients with active myeloma during scheduled clinic visits. The Institutional Review Board of the University of Arkansas of Medical Sciences (Little Rock, AR) approved the studies. Patient samples were obtained with signed informed consent, in accordance with the Declaration of Helsinki. Myeloma plasma cells were isolated by automated CD138 immunomagnetic bead selection (Miltenyi Biotec, Auburn, CA). CD38/CD45 flow cytometry determined that purity of plasma cells was routinely 85% or greater.
Preparation of osteoclasts
Cultures of multinucleated bone-resorbing osteoclasts were prepared as previously described.33 Briefly, peripheral blood mononuclear cells were obtained from patients with MM. The cells were cultured at 2.5 × 106 cells/mL in α-MEM supplemented with 10% fetal bovine serum, antibiotics, RANKL (50 ng/mL), and macrophage colony-stimulating factor (25 ng/mL) for 10 to 14 days, at which time they contained large numbers of multinucleated osteoclasts with bone-resorbing activity.
Cocultures of osteoclasts with myeloma plasma cells
Osteoclasts were washed 3 times with phosphate-buffered saline (PBS) to detach and remove nonadherent cells. Purified myeloma plasma cells (0.5-1 × 106 cells/mL) were added to osteoclasts in 6-well plates (3 mL/well) in osteoclast medium. After 2 days of culturing, viable myeloma cells were counted by trypan blue staining and analyzed.
Gene-expression profiling
Gene-expression profiling using the Affymetrix U95Av2 microarray, which contains approximately 12 000 genes (Affymetrix, Santa Clara, CA), was performed on CD138-purified plasma cells after coculturing with osteoclasts and receiving treatment with relevant agents, as previously described.9
Cell treatments
Lenalidomide and thalidomide (Celgene, Summit, NJ) were dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO) at a concentration of 200 mM and were stored at −20°C until use. Immediately before use, each agent was diluted in culture medium (100 μM) with less than 0.1% DMSO; an equivalent amount of DMSO was added to all control samples. For inhibition experiments, myeloma plasma cells were pretreated with JNK inhibitor SP600125 (10 μM; Calbiochem, San Diego, CA) or phenyl N-t-butylnitrone (PBN; 2 mM; Sigma) for 1 hour. After 24 hours, myeloma plasma cells were analyzed for DKK1 expression, JNK phosphorylation, and c-Jun activity.
Real-time reverse transcriptase-polymerase chain reaction
RNA was extracted using the RNeasy Kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from 1 μg purified RNA using MMLV reverse transcriptase III (Invitrogen, Carlsbad, CA) and random hexamers provided by the manufacturer. After reverse transcription, cDNA samples were diluted to a final volume of 480 μL and 5 μL for each quantitative polymerase chain reaction (PCR) analysis. Primers and probes for DKK1 amplification were obtained from Applied Biosystems (20× Assays-on-Demand Gene Expression Assay Mix, Applied Biosystems, Foster City, CA). Real-time PCR was performed on an ABI PRISM 7900 analytical thermal cycler (Applied Biosystems) following the manufacturer's recommendations. The glyceraldehyde-3-phosphate dehydrogenase housekeeping gene was used to normalize samples for RNA quality and quantity. The ΔΔCt approach was used for relative quantification of DKK1 mRNA, as suggested by Applied Biosystems. The amount of DKK1 in each sample of plasma cells from an individual patient was expressed as a relative value normalized to the sample with lower expression. Each sample was analyzed in duplicate and the results were expressed as the mean ± SEM.
Western blotting
Cytosolic and nuclear fractions were isolated using the Nuclear/Cytosol Fractionation Kit (BioVision Research Products, Mountain View, CA). Equal amounts of lysates were separated by electrophoresis on 4% to 12% sodium dodecyl sulfate-polyacrylamide gels, and Western blotting was carried out using the Western Breeze Chemiluminescent Immunodetection protocol as described (Invitrogen). The following primary antibodies were used: anti–c-Jun (Zymed Laboratories, South San Francisco, CA), anti-p53 (Chemicon International, Temecula, CA), anti-JNK and anti–phospho JNK (Thr183/Tyr185; Cell Signaling Technology, Danvers, MA), anti-DKK1 (R&D Systems, Minneapolis, MN), and anti–β-tubulin and anti–histone 1 (Upstate Biotechnology, Charlottesville, VA).
Evaluation of DNA binding activity of c-Jun by ELISA
The DNA binding activity of c-Jun was quantified by enzyme-linked immunosorbent assay (ELISA) using the Trans-AM AP-1 transcription factor assay kit (Active Motif North America, Carlsbad, CA), according to the instructions of the manufacturer. Briefly, nuclear extracts were prepared as previously described and incubated in 96-well plates coated with immobilized oligonucleotide (5′-CGCTTGATGAGTCAGCCGGAA-3′) containing a consensus-binding site for c-Jun. c-Jun binding to the target oligonucleotide was detected by incubation with primary antibody specific for the activated form of c-Jun (phospho-Ser 73; Active Motif North America), visualized with anti-IgG horseradish peroxidase conjugate and developing solution, and quantified at 450 nm with a reference wavelength of 655 nm. Background binding was subtracted from the value obtained for binding to the consensus DNA sequence. Each sample was analyzed in duplicate and the results were expressed as the mean ± SEM.
Transfection of myeloma cell lines
DKK1, p53, and c-Jun cDNA sequences were derived by PCR amplification and cloned into the pWPI lentiviral vector (generous gift from Dr Didier Trono, School of Life Science, Lausanne, Switzerland). Recombinant lentivirus was produced by transient transfection of 293T cells following a standard protocol.34 Crude virus was concentrated by ultracentrifugation at 90 000g for 90 minutes. Viral titers were determined by measuring the amount of HIV-1 p24 antigen by ELISA (NEN Life Sciences, Boston, MA). A 99% transduction efficiency of myeloma cells was achieved with 3 μg lentiviral p24 particles/106 cells. All experiments of transfection were performed in duplicate.
Apoptosis assay
Cells (1 × 106) of each sample were fixed in 75% ethanol at −20°C overnight. The following day, cells were washed with cold PBS, treated with 100 μg RNase A (Qiagen), and stained with 50 μg propidium iodide (Roche, Indianapolis, IN). Flow cytometric acquisition was performed using a 3-color FACScan flow cytometer and CellQuest software (Becton Dickinson, San Jose, CA). For each sample, 10 000 events were gated. Data analysis was performed using Modfit LT software (Verify Software House, Topsham, ME). The apoptotic cell fraction was determined as mean ± standard error of the percent of cells with sub-G0 DNA content of 2 independent experiments.
Statistical analysis
Student paired t test was performed to test changes in DKK expression by real-time PCR and changes in c-Jun activity by ELISA.
Results
Osteoclasts down-regulate DKK1 expression and decrease JNK pathway activation in myeloma plasma cells
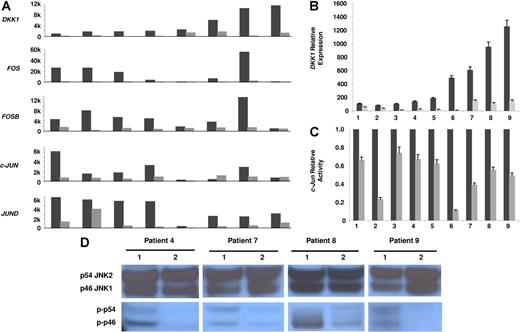
Our first evidence of the potential mechanisms underlying the regulation of DKK1 expression in myeloma plasma cells has arisen from analyzing their gene expression profiling following 2 days of coculture with osteoclasts. In 14 of 19 myeloma plasma cell samples cocultured with osteoclasts, we identified 97 probe sets that changed 2- to 50-fold (89 up-regulated and 8 down-regulated genes; data not shown). Surprisingly, DKK1 signal decreased from 2- to 10-fold in all 8 samples with high baseline expression (Affymetrix signal > 1000), as compared to the controls in culture without osteoclasts (Figure 1A); this down-regulation was more pronounced when myeloma plasma cells were cocultured in direct contact with osteoclasts (data not shown). DKK1 inhibition strongly correlated with the simultaneous down-regulation of AP-1 transcription factors FOS, FOSB, c-JUN, and JUND (Figure 1A). In all experiments the viability of MM plasma cells recovered from cocultures was consistently > 95% and their apoptotic rate was lower than those of MM plasma cells cultured alone (data not shown), confirming that osteoclasts can support MM plasma cell survival, as we have previously demonstrated.33
Osteoclasts down-regulate DKK1 and JNK pathway components in myeloma plasma cells. Primary myeloma plasma cells were cultured in the presence (⊡) or absence (▪) of osteoclasts for 2 days. (A) Microarray analysis was used to determine expression of DKK1, FOS, FOSB, c-JUN, and JUND in 8 samples with high baseline DKK1 expression (expressed as Affymetrix signal, 1000-unit increments). (B) DKK1 expression was evaluated by real-time PCR in another 9 samples cultured with (⊡) or without osteoclasts (▪), expressed as relative values using ΔΔCt method. (C) c-Jun–binding activity was evaluated in nuclear extracts of the same 9 samples of myeloma plasma cells shown in panel B, using an ELISA-based assay (expressed as relative value compared to each control). In panels B and C, each sample is expressed as the mean ±SEM of 2 duplicates. (D) The level of activation of the JNK pathway was determined by Western blot analysis for the phosphorylated isoforms of p46 JNK1 (p-p46) and p54 JNK2 (p-p54) in cytosolic extracts of 4 samples shown in panel C that had sufficient quantities of protein extracts available. Number 1 represents control samples; number 2 represents samples after osteoclast cocultures. Note that the modulation in JNK pathway activation is not due to variation in total levels of the JNK isoforms.
Osteoclasts down-regulate DKK1 and JNK pathway components in myeloma plasma cells. Primary myeloma plasma cells were cultured in the presence (⊡) or absence (▪) of osteoclasts for 2 days. (A) Microarray analysis was used to determine expression of DKK1, FOS, FOSB, c-JUN, and JUND in 8 samples with high baseline DKK1 expression (expressed as Affymetrix signal, 1000-unit increments). (B) DKK1 expression was evaluated by real-time PCR in another 9 samples cultured with (⊡) or without osteoclasts (▪), expressed as relative values using ΔΔCt method. (C) c-Jun–binding activity was evaluated in nuclear extracts of the same 9 samples of myeloma plasma cells shown in panel B, using an ELISA-based assay (expressed as relative value compared to each control). In panels B and C, each sample is expressed as the mean ±SEM of 2 duplicates. (D) The level of activation of the JNK pathway was determined by Western blot analysis for the phosphorylated isoforms of p46 JNK1 (p-p46) and p54 JNK2 (p-p54) in cytosolic extracts of 4 samples shown in panel C that had sufficient quantities of protein extracts available. Number 1 represents control samples; number 2 represents samples after osteoclast cocultures. Note that the modulation in JNK pathway activation is not due to variation in total levels of the JNK isoforms.
Because expression of the AP-1 family is primarily regulated by phosphorylation of c-Jun by JNK, we investigated whether osteoclast coculture could modify c-Jun and JNK activity in myeloma plasma cells. In another 9 samples in which real-time PCR revealed that DKK1 expression was significantly down-regulated after 2 days of coculture (range, 2- to 47-fold; mean, 10.9 ± 0.5; P < .01; Figure 1B), we also observed a significant decrease in c-Jun–binding activity (range, 1.5- to 9-fold; mean, 2.85 ± 0.15; P < .01; Figure 1C), based on an ELISA-based assay for JNK phosphorylated c-Jun (phospho-Ser73). Western blot analysis of the active phosphorylated forms of JNK revealed that the reduction in c-Jun–binding activity was due to decreased levels of activated JNK isoforms (p46 and p54) in the 4 patients whose samples contained sufficient protein quantities for analysis (Figure 1D). Collectively, these data suggest that DKK1 expression in myeloma plasma cells can be regulated by AP-1 transcription factors through modulations of the JNK pathway.
Thalidomide and lenalidomide up-regulate DKK1 expression through JNK pathway activation in myeloma plasma cells
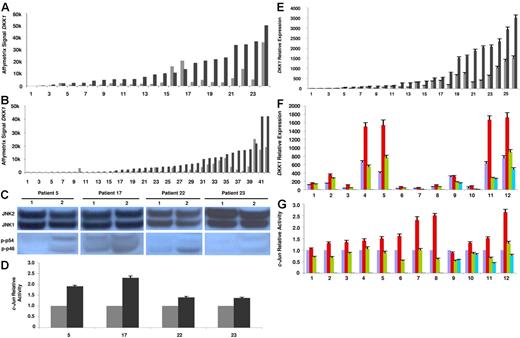
Our attempt to understand the regulation of DKK1 continued when we observed that DKK1 expression could be modulated in myeloma plasma cells after treatment with therapeutic agents in vivo. Indeed, analyzing gene expression profiles of myeloma plasma cells from patients receiving single agent lenalidomide, we found that DKK1 transcription was up-regulated 4.7-fold (range, 2.4-8.6) in 19 of 24 samples following 48 hours of in vivo exposure (Figure 2A) and 7.3-fold (range, 2.2-32.9) in 21 of 42 samples following 48 hours of in vivo exposure to thalidomide (Figure 2B). The strong association between absence of treatment-induced DKK1 hyperactivation and low or absent DKK1 prior to treatment (Figure 2A-B) suggests an inherent inability of DKK1 expression in these cases.
Lenalidomide up-regulates DKK1 in myeloma plasma cells through JNK. Microarray analysis of primary myeloma plasma cell samples showed up-regulation of DKK1 expression after 48 hours of in vivo exposure to lenalidomide (A) or thalidomide (B) used as single agents. ⊡ represents pretreatment values; ▪, posttreatment results. DKK1 mRNA level in each sample before and after treatment is expressed as Affymetrix signal (1000-unit increments). (C) JNK activation was evaluated by Western blot analysis of phosphorylated isoforms p46 JNK1 (p-p46) and p54 JNK2 (p-p54) in 4 of the 24 myeloma plasma cell samples shown in panel A. Number 1 represents samples before lenalidomide exposure; number 2, samples after lenalidomide exposure. (D) c-Jun activity was evaluated in nuclear extracts of the same 4 samples shown in panel C and expressed as relative value compared to each control. ⊡ represents samples before lenalidomide exposure; ▪, samples after lenalidomide exposure. (E) DKK1 relative expression was evaluated by real-time PCR in another 26 myeloma plasma cell samples after 24 hours of in vitro treatment with lenalidomide (100 μM) and compared to each sample with no treatment. DKK1 mRNA level in each sample before (⊡) and after (▪) lenalidomide is expressed as relative value using the ΔΔCt approach. (F) The effect of pretreating cells with the JNK inhibitor SP600125 (10 μM) on lenalidomide-induced up-regulation of DKK1 expression was evaluated by real-time PCR (expressed as relative value). Violet bars represent control samples; red bars, samples after lenalidomide treatment; green bars, samples treated with lenalidomide in the presence of the JNK-specific inhibitor SP600125; and blue bars, samples treated with the JNK inhibitor alone. (G) The inhibition of JNK activation induced by SP600125 was confirmed in all 12 samples shown in panel F by analyzing c-Jun–binding activity expressed in relative units. In panels D-G each sample is expressed as the mean ± SEM of 2 duplicates.
Lenalidomide up-regulates DKK1 in myeloma plasma cells through JNK. Microarray analysis of primary myeloma plasma cell samples showed up-regulation of DKK1 expression after 48 hours of in vivo exposure to lenalidomide (A) or thalidomide (B) used as single agents. ⊡ represents pretreatment values; ▪, posttreatment results. DKK1 mRNA level in each sample before and after treatment is expressed as Affymetrix signal (1000-unit increments). (C) JNK activation was evaluated by Western blot analysis of phosphorylated isoforms p46 JNK1 (p-p46) and p54 JNK2 (p-p54) in 4 of the 24 myeloma plasma cell samples shown in panel A. Number 1 represents samples before lenalidomide exposure; number 2, samples after lenalidomide exposure. (D) c-Jun activity was evaluated in nuclear extracts of the same 4 samples shown in panel C and expressed as relative value compared to each control. ⊡ represents samples before lenalidomide exposure; ▪, samples after lenalidomide exposure. (E) DKK1 relative expression was evaluated by real-time PCR in another 26 myeloma plasma cell samples after 24 hours of in vitro treatment with lenalidomide (100 μM) and compared to each sample with no treatment. DKK1 mRNA level in each sample before (⊡) and after (▪) lenalidomide is expressed as relative value using the ΔΔCt approach. (F) The effect of pretreating cells with the JNK inhibitor SP600125 (10 μM) on lenalidomide-induced up-regulation of DKK1 expression was evaluated by real-time PCR (expressed as relative value). Violet bars represent control samples; red bars, samples after lenalidomide treatment; green bars, samples treated with lenalidomide in the presence of the JNK-specific inhibitor SP600125; and blue bars, samples treated with the JNK inhibitor alone. (G) The inhibition of JNK activation induced by SP600125 was confirmed in all 12 samples shown in panel F by analyzing c-Jun–binding activity expressed in relative units. In panels D-G each sample is expressed as the mean ± SEM of 2 duplicates.
The strong up-regulation of DKK1 after lenalidomide therapy in vivo provided a useful tool for exploring the signaling pathway involved in DKK1 expression. The observations of coordinated hyperexpression of DKK1 and JNK activation in myeloma plasma cells after coculture with osteoclasts and lenalidomide-induced increased AP-1–binding activity36 and JNK activation27 prompted us to evaluate whether lenalidomide-associated up-regulation of DKK1 in vivo correlated with an increase in JNK and c-Jun activities. Indeed, increased levels of JNK phosphorylation (Figure 2C) and activation of c-Jun binding (Figure 2D) were demonstrated in 4 available samples of myeloma plasma cells (patients no. 5, 17, 22, and 23, shown in Figure 2A; P < .01).
Supporting a direct up-regulation of DKK1 after lenalidomide treatment in vivo was the observation of DKK1 activation in 19 of 26 primary MM samples 24 hours after lenalidomide exposure in vitro, using real-time PCR (range, 2- to 6-fold; mean, 2.92 ± 0.12; P < .01; Figure 2E). Finally, to evaluate whether DKK1 up-regulation depended on JNK activation, myeloma plasma cells were treated with lenalidomide for 24 hours in the presence or absence of the JNK-specific inhibitor SP600125. SP600125 effectively prevented DKK1 overexpression by lenalidomide in 10 of 12 experiments (P < .01; Figure 2F). Inhibition in JNK activation was confirmed by analyzing c-Jun–binding activity (Figure 2G) and JNK phosphorylation (data not shown). Interestingly, in 4 experiments, preincubating cells with the JNK inhibitor reduced lenalidomide-induced DKK1 expression to levels below those present in controls. This effect could be attributed to inhibition of the constitutive activation of JNK in myeloma plasma cells because the JNK inhibitor alone could reduce the basal level of both DKK1 expression and c-Jun activity (Figure 2F-G).
Oxidative stress is responsible for lenalidomide-induced up-regulation of DKK1
To investigate the upstream stimuli responsible for JNK pathway activation and DKK1 up-regulation by lenalidomide, we analyzed changes in gene expression that correlated with DKK1 up-regulation following lenalidomide treatment in vivo. Microarray analysis showed a clear indication of the activation of genes involved in the cellular response to oxidative stress, which is in line with previous reports indicating that thalidomide could induce oxidative injury in the rabbit model system by forming free radical-initiated reactive oxygen species.37 Interestingly, in the 40 most highly correlated genes, we identified genes encoding caspase 4 (CASP4, 8.7-fold increase; most up-regulated gene) and calpain 2 (CAPN2, 5.3-fold increase), which are involved in endoplasmic reticulum stress-induced apoptosis,38,39 and glutaredoxin (GLRX, 7.45-fold increase), glutathione peroxidase (GPX1, 5.7-fold increase), and glutathione S-transferase (GSTO1, 5-fold increase), which play important roles in cellular detoxification, suppressing activities of JNK and its upstream kinases through direct interactions.32
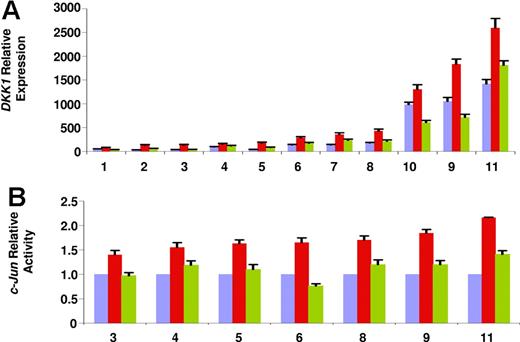
Therefore, to determine whether oxidative stress plays a role in mediating lenalidomide-induced DKK1 up-regulation, we administered the drug along with the antioxidant agent PBN. As shown in Figure 3A, pretreating cells with PBN significantly reduced lenalidomide-induced DKK1 up-regulation (expression reduced 1.5- to 3.3-fold; mean: 2 ± 0.1; P < .01). Interestingly, preincubating cells with PBN alone could reduce the basal level DKK1 expression (data not shown). Furthermore, in line with previous reports,40,41 PBN counteracted c-Jun–binding activation (P < .01; Figure 3B) and JNK phosphorylation (data not shown). Taken together, these data demonstrate that oxidative stress is an upstream stimulus responsible for JNK pathway activation and DKK1 up-regulation induced by lenalidomide.
Oxidative stress regulates lenalidomide-induced up-regulation of DKK1. (A) DKK1 relative expression was evaluated by real-time PCR in 11 samples of myeloma plasma cells cultured in the presence (red bars) or absence (violet bars) of treatment with lenalidomide (100 μM, 24 hours) alone (red bars) or combined with pretreatment with antioxidant agent PBN (2 mM; green bars). (B) In 7 parallel experiments, c-Jun–binding activity was determined. Each sample is expressed as the mean ± SEM of 2 duplicates.
Oxidative stress regulates lenalidomide-induced up-regulation of DKK1. (A) DKK1 relative expression was evaluated by real-time PCR in 11 samples of myeloma plasma cells cultured in the presence (red bars) or absence (violet bars) of treatment with lenalidomide (100 μM, 24 hours) alone (red bars) or combined with pretreatment with antioxidant agent PBN (2 mM; green bars). (B) In 7 parallel experiments, c-Jun–binding activity was determined. Each sample is expressed as the mean ± SEM of 2 duplicates.
Osteoclasts counteract lenalidomide-induced up-regulation of DKK1 by inhibiting JNK pathway
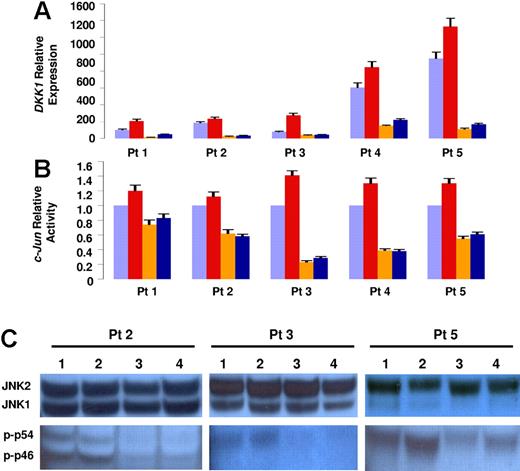
To further clarify the role of osteoclasts in the regulation of DKK1 expression, we evaluated their capacity to inhibit lenalidomide-induced DKK1 activation. When myeloma plasma cells were cocultured with osteoclasts for 2 days in the presence of lenalidomide, DKK1 up-regulation induced in the control cells was lost and DKK1 expression was comparable to the levels present in myeloma plasma cells cocultured without drug (Figure 4A). Consistently, both c-Jun–binding activity (Figure 4B) and JNK phosphorylation (Figure 4C) were not increased in myeloma plasma cells cocultured with osteoclasts and decreased below basal levels of control cells not cocultured with osteoclasts.
Osteoclasts counteract lenalidomide-induced DKK1 up-regulation. Five primary myeloma plasma cell samples were cultured with or without osteoclasts for 2 days in the absence (orange and violet bars, respectively) or in the presence of lenalidomide (blue and red bars, respectively). (A) DKK1 relative expression was evaluated by real-time PCR; (B) c-Jun binding activity was evaluated in nuclear extracts. In panels A and B each sample is expressed as the mean ± SEM of 2 duplicates. (C) The level of JNK pathway activation was determined by Western blot analysis of JNK phosphorylated isoforms in cytosolic extracts of 3 representative samples. Number 1 represents samples cultured without osteoclasts; number 2, samples treated with lenalidomide; number 3, samples cultured with osteoclasts; and number 4, samples cultured with osteoclasts in the presence of lenalidomide.
Osteoclasts counteract lenalidomide-induced DKK1 up-regulation. Five primary myeloma plasma cell samples were cultured with or without osteoclasts for 2 days in the absence (orange and violet bars, respectively) or in the presence of lenalidomide (blue and red bars, respectively). (A) DKK1 relative expression was evaluated by real-time PCR; (B) c-Jun binding activity was evaluated in nuclear extracts. In panels A and B each sample is expressed as the mean ± SEM of 2 duplicates. (C) The level of JNK pathway activation was determined by Western blot analysis of JNK phosphorylated isoforms in cytosolic extracts of 3 representative samples. Number 1 represents samples cultured without osteoclasts; number 2, samples treated with lenalidomide; number 3, samples cultured with osteoclasts; and number 4, samples cultured with osteoclasts in the presence of lenalidomide.
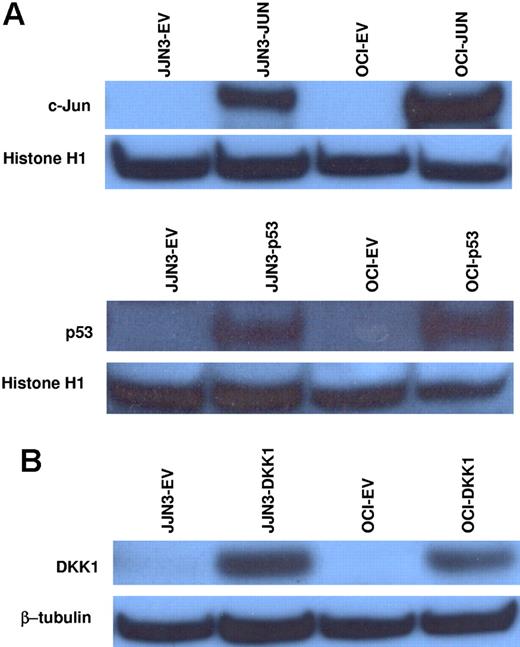
c-Jun and p53 cannot activate DKK1 transcription in myeloma cells and DKK1 overexpression does not induce effects on myeloma cell apoptosis and drug response
Human DKK1 can be a direct transcriptional target of c-Jun and p53, and both of these transcription factors can be activated in response to oxidative stress; therefore, we evaluated whether lentiviral overexpression of c-Jun or p53 could induce DKK1 up-regulation in JJN3 and OCI-MY5 myeloma cell lines, which have very low mRNA and protein levels of c-Jun, p53, and DKK1. Overexpression of both c-Jun and p53 (Figure 5A) did not activate DKK1 expression, based on real-time PCR performed 6 and 24 hours after lentiviral infections (data not shown).
c-Jun and p53 overexpression do not activate DKK1 transcription in myeloma cells, and overexpression of DKK1 does not affect myeloma cell apoptosis and drug response. (A) c-Jun or p53 proteins were overexpressed in JJN3 and OCI-MY5 cell lines using a lentiviral system. Western blot analysis of the nuclear extracts confirmed c-Jun or p53 up-regulation, as compared to each control transfected with empty vector. DKK1 expression, evaluated in the same samples by real-time PCR, was not activated. (B) JJN3 and OCI-MY5 cell lines were transfected with DKK1 overexpression vector using a lentiviral system. DKK1 protein was confirmed by Western blot analysis of cytosolic fractions (normalized to β-tubulin internal control). JJN3 and OCI-MY5 cell lines transfected with DKK1 or empty vector were treated with thalidomide or lenalidomide; after 48 hours of treatment, the percent of apoptotic cells was determined using flow cytometry (Table 1).
c-Jun and p53 overexpression do not activate DKK1 transcription in myeloma cells, and overexpression of DKK1 does not affect myeloma cell apoptosis and drug response. (A) c-Jun or p53 proteins were overexpressed in JJN3 and OCI-MY5 cell lines using a lentiviral system. Western blot analysis of the nuclear extracts confirmed c-Jun or p53 up-regulation, as compared to each control transfected with empty vector. DKK1 expression, evaluated in the same samples by real-time PCR, was not activated. (B) JJN3 and OCI-MY5 cell lines were transfected with DKK1 overexpression vector using a lentiviral system. DKK1 protein was confirmed by Western blot analysis of cytosolic fractions (normalized to β-tubulin internal control). JJN3 and OCI-MY5 cell lines transfected with DKK1 or empty vector were treated with thalidomide or lenalidomide; after 48 hours of treatment, the percent of apoptotic cells was determined using flow cytometry (Table 1).
Induction of DKK1 expression in response to apoptotic stimuli can modulate programmed cell death,21 and DKK1 expression in myeloma plasma cells can be up-regulated by genotoxic stress and down-regulated after osteoclast coculture, which supports survival of myeloma plasma cells.33 Therefore, we investigated whether DKK1 overexpression in myeloma cell lines directly induces apoptosis or sensitizes myeloma cells to drug-induced apoptosis. DKK1 overexpression, confirmed by Western blot analysis of cytosolic fractions (Figure 5B), did not suppress the growth of JJN3 or OCI-MY5 myeloma cell lines (data not shown). Moreover, when compared to control cells infected with the empty vector, cells overexpressing DKK1 showed no significant differences in apoptotic responses induced by 48 hours of treatment with lenalidomide or thalidomide (P > .05; Table 1). Together these results suggest that, although DKK1 can be considered a stress-responsive gene, it does not mediate apoptotic signaling in myeloma plasma cells.
DKK1 overexpression does not affect myeloma cell apoptosis and drug response
| Cell line . | Apoptotic cells with empty vector, % . | Apoptotic cells overexpressing DKK1, % . | ||||
|---|---|---|---|---|---|---|
| Control . | Lenalidomide . | Thalidomide . | Control . | Lenalidomide . | Thalidomide . | |
| JJN3 | 2.2 ± 0.4 | 8.7 ± 0.5 | 7.3 ± 0.1 | 3.9 ± 0.3 | 10.5 ± 0.6 | 8.5 ± 0.2 |
| OCI-MY5 | 3.3 ± 0.1 | 7.9 ± 0.5 | 6.3 ± 0.3 | 3.5 ± 0.1 | 9.9 ± 0.7 | 8.4 ± 0.4 |
| Cell line . | Apoptotic cells with empty vector, % . | Apoptotic cells overexpressing DKK1, % . | ||||
|---|---|---|---|---|---|---|
| Control . | Lenalidomide . | Thalidomide . | Control . | Lenalidomide . | Thalidomide . | |
| JJN3 | 2.2 ± 0.4 | 8.7 ± 0.5 | 7.3 ± 0.1 | 3.9 ± 0.3 | 10.5 ± 0.6 | 8.5 ± 0.2 |
| OCI-MY5 | 3.3 ± 0.1 | 7.9 ± 0.5 | 6.3 ± 0.3 | 3.5 ± 0.1 | 9.9 ± 0.7 | 8.4 ± 0.4 |
Plus-minus values indicate___.
Discussion
Emerging evidence9,12 emphasizes that the Wnt-signaling inhibitor DKK1 plays a role in the pathogenesis of osteolytic lesions in MM. Although DKK1 is rarely expressed in MGUS, it is found in plasma cells of virtually all cases of myeloma (our unpublished data, J.D.S., January 2006). Thus, understanding the mechanisms responsible for DKK1 regulation in myeloma could provide the basis for novel therapeutic strategies to prevent MM-associated bone disease and possibly progression of MGUS to MM. Our previous study9 showed that DKK1 expression in myeloma plasma cells is associated with particular stages of the disease, suggesting that DKK1 could be regulated by specific stimuli, extent of genomic instability, or interactions between myeloma plasma cells and the bone marrow microenvironment.
In this study, we demonstrated that DKK1 expression in myeloma plasma cells can be regulated through the JNK-signaling cascade, initiated by oxidative stress conditions following lenalidomide treatment or by interactions between myeloma plasma cells and osteoclasts. DKK1 activation in myeloma plasma cells following thalidomide or lenalidomide exposure in vivo is consistent with in vitro studies of other cancer cell types exposed to alkylating agents, ionizing radiation, and hydrogen peroxide18 and of osteoblasts exposed to dexamethasone.42 Although DKK1 has been shown to be a target of TP53,17 we did not observe this in myeloma cells. In this study, we found that DKK1 is responsive to JNK signaling but overexpression of c-Jun alone was not sufficient to activate DKK1 expression in vitro. It is possible that other signaling pathways are important for regulating DKK1 expression in vivo. Indeed, it has been demonstrated that DKK1 can be a direct target for β-catenin/TCF transcriptional regulation via LEF/TCF-binding sites present in its promoter region14–16 and we have supporting evidence demonstrating that treating myeloma plasma cells with Wnt-3a, which is known to stabilize β-catenin, can weakly activate DKK1 transcription (data not shown). The transcription factors and cis elements of the DKK1 promoter required for its expression in myeloma cells in response to lenalidomide, oxidative stress, and JNK activation are currently being investigated.
Sensing of cell damage induced by oxidative stress and the consequent triggering of the JNK apoptotic pathway can occur through different cellular organelles, including not only cell surface receptors and mitochondria but also the endoplasmic reticulum.43 In myeloma plasma cells, lenalidomide treatment can significantly up-regulate caspase 4 expression, which is specifically implicated in endoplasmic reticulum stress-induced apoptosis.38 The endoplasmic reticulum serves as a primary sensor of environmental stress and can determine cell fate by triggering apoptosis through JNK activation in response to diverse pathophysiologic conditions and a large number of physical and chemical insults, including disruption of calcium homeostasis, inhibition of protein glycosylation, hypoxia, ischemia, and oxidative stress.
Interestingly, recent evidence demonstrated that endoplasmic reticulum stress is implicated in various types of neurodegenerative diseases, such as β-amyloid– or τ-induced Alzheimer disease, and in development of ischemic neuronal death. Surprisingly, in all these pathologic conditions, DKK1 expression was required for the development of neuronal degeneration and apoptosis.19,44 In line with these reports, other recent studies have strongly suggested that restoration of DKK1 expression in tumor cells may have proapoptotic effects mediated through mechanisms dependent on17,18,21 or independent of45–48 canonical Wnt signaling inhibition, supporting the role of DKK1 as a tumor suppressor. Conversely, in this study, we demonstrated that DKK1 overexpression in myeloma cell lines did not induce direct growth inhibition or apoptosis sensitization following drug treatment. A possible explanation is that myeloma plasma cells are intrinsically incapable of producing canonical Wnt factors,49 which suggests that, whereas DKK1 plays an important role in suppressing Wnt-mediated tumor formation, it has no effect on proliferation of cells not transformed by Wnt. Moreover, DKK1 expression is absent in normal plasma cells and those from patients with MGUS, and its production by myeloma plasma cells in the early stage of disease could simply be a consequence of their sensitivity to apoptotic stimuli; this differs from other cancers, in which DKK1 expression is lost in malignant cells but present in the normal counterparts.15,47,50
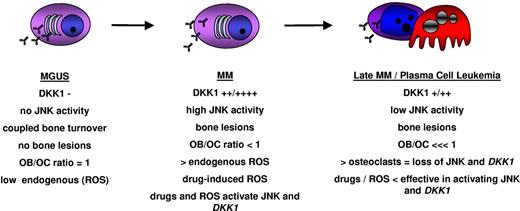
Based on the results of this study, we propose a model to explain DKK1 regulation in myeloma plasma cells during the different stages of MM disease (Figure 6). In the early stages, during the transition from MGUS to MM, myeloma plasma cells could be more sensitive to different forms of stress arising in the bone marrow microenvironment, triggering specific responses such as activation of JNK signaling, which results in DKK1 expression. The high levels of DKK1 secreted by the plasma cells do not influence their survival but result in profound defects in osteoblast function which, combined with osteoclast hyperactivation, cause uncoupling of bone turnover and development of osteolytic bone lesions. The altered balance of the bone marrow milieu may also promote further disease progression. Increased numbers of osteoclasts that support MM plasma cell survival counteracting the oxidative stress by inhibiting JNK pathway and the accumulation of genetic alterations may be responsible for the resistance of plasma cells to apoptotic programs and eventual independence from the microenvironment. Indeed, the low level DKK1 expression in late-stage disease, as seen in plasma cell leukemia and pleural effusions, may reflect an impaired ability of these cells to respond to oxidative stresses.
A hypothetical model of DKK1 regulation in myeloma plasma cells during different stages of MM disease progression. ROS indicates reactive oxygen species.
A hypothetical model of DKK1 regulation in myeloma plasma cells during different stages of MM disease progression. ROS indicates reactive oxygen species.
The up-regulation of DKK1 in plasma cells, and possibly the microenvironment, by drugs commonly used to treat myeloma poses several questions. Does the drug-induced activation of DKK1 exacerbate the bone loss phenotype or does DKK1 activation have antitumor effects? It is feasible that DKK1 activation could suppress canonical Wnt signaling in the tumor cells. However, although active in myeloma cells and suppressible by DKK1, as previously reported,51 in our hands canonical Wnt signaling does not induce proliferation of myeloma cells.49 Moreover, the high levels of DKK1 seen in patients with active and progressive disease would argue that DKK1 does not have direct antitumor activity. Moreover, DKK1 does not promote apoptosis in myeloma cells in vitro and does not increase the cell kill activity of lenalidomide in vitro. If DKK1 indeed lacks antitumor activity, then methods to combat its activation or neutralize its activity during treatment may eliminate potential side effects of therapy-induced activation. In addition to directly interfering with its expression by regulating JNK activity another alternative is to neutralize its activity with monoclonal antibody therapy. We recently showed that this approach is feasible in that administration of a DKK1-neutralizing antibody to mice with myeloma could prevent bone loss and tumor growth in vivo.12 It will be extremely informative to see the effects of combining anti-DKK1 therapy with chemotherapy.
In conclusion, in the current study, we demonstrated that DKK1 expression can be induced in myeloma plasma cells by oxidative stress through activation of the JNK pathway and can be regulated by specific interactions between myeloma plasma cells and cells predominating in the bone marrow microenvironment of patients with myeloma. Future efforts to identify new potential targets for treating MM-associated bone disease should be directed toward understanding specific factors or conditions that stimulate the oxidative stress response in myeloma plasma cells and are responsible for DKK1 activation in early-stage MM.
Authorship
Contribution: J.D.S. conceived the work and experiments, supervised work, analyzed data, and wrote the paper; S.C. and F.Z. conceived experiments, performed experiments, analyzed data, and wrote the paper; X.W., W.X., H.X., O.S., S.Y., and J.C. performed research studies included in this report; and B.B. performed clinical research, provided clinical samples, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
S.C. and F.Z. contributed equally to this work.
Correspondence: John D. Shaughnessy Jr, Donna D. and Donald M. Lambert Laboratory of Myeloma Genetics at the Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, L301 West Markham, Little Rock, AR; e-mail: shaughnessyjohn@uams.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants CA55819 (J.D.S., F.Z., J.E., B.B.) and CA97513 (J.D.S.) and by the Fund to Cure Myeloma.
The authors would like to thank David R. Williams, Christopher Randolph, Yan Xiao, and Yongsheng Huang for assistance. We would also like to recognize Margaret E. Brenner and Kahla Hebert for help with manuscript preparation and editing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal