Abstract
Granule proteins play a major role in bacterial killing by neutrophils. Serglycin proteoglycan, the major intracellular proteoglycan of hematopoietic cells, has been proposed to play a role in sorting and packing of granule proteins. We examined the content of major neutrophil granule proteins in serglycin knockout mice and found neutrophil elastase absent from mature neutrophils as shown by activity assay, Western blotting, and immunocytochemistry, whereas neutrophil elastase mRNA was present. The localization of other neutrophil granule proteins did not differ between wild-type and serglycin knockout mice. Differential counts and neutrophil ultrastructure were unaffected by the lack of serglycin, indicating that defective localization of neutrophil elastase does not induce neutropenia itself, albeit mutations in the neutrophil elastase gene can cause severe congenital neutropenia or cyclic neutropenia. The virulence of intraperitoneally injected Gram-negative bacteria (Klebsiella pneumoniae) was increased in serglycin knockout mice compared with wild-type mice, as previously reported for neutrophil elastase knockout mice. Thus, serglycin proteoglycan has an important role in localizing neutrophil elastase in azurophil granules of neutrophils, while localization of other granule proteins must be mediated by other mechanisms.
Introduction
Neutrophils contain several subsets of granules characterized by their content of proteins and antimicrobial peptides.1,2 The sequential up-regulation of membrane proteins at the surface of neutrophils and the extracellular release of proteins during diapedesis, chemotaxis, and phagocytosis depends on this granule heterogeneity.2,3 The bactericidal activity of neutrophils depends partly on granule proteins with antimicrobial activity and partly on the oxidative burst, as seen from the severe immunodeficiency of individuals with neutrophil-specific granule deficiency4 or chronic granulomatous disease,5 respectively. The localization of proteins to the different subsets of granules is determined by their time of synthesis, as described by the “targeting by timing” hypothesis.6 However, the mechanisms for granule assembly and sorting of proteins from the trans-Golgi network to granules as opposed to constitutive secretion have not been elucidated for neutrophils.3
Formation of granules and targeting of proteins from the trans-Golgi network is managed differently in different cell types and even varies for different granule proteins in the same subset of granules (eg, only one of several granule proteins depends on serglycin for proper localization in cytotoxic lymphocytes).7 Major mechanisms for protein targeting and granule formation involve mannose-6-phosphate receptors and adaptor proteins (AP) 1 and 2.8 None of these proteins has proven to be implicated in granule formation or protein targeting in neutrophils. However, AP-3 is implicated in targeting proteins to lysosome-related organelles—and probably azurophil (primary) granules of neutrophils—as seen by the phenotype of patients with Hermansky-Pudlak syndrome 2, caused by mutations in AP-3,9 by the canine cyclic hematopoiesis dog model likewise caused by mutations in AP-3,10 and by the mocha and pearl mouse models of the Hermansky-Pudlak syndrome.11,12 Also, the product of the LYST gene is involved in structuring azurophil granules of neutrophils, as seen in the Chediak-Higashi syndrome and in the beige mouse model where disturbances in the size of azurophil granules and the processing, secretion, and activity of neutrophil elastase is observed.13 These effects of mutations in the LYST gene seem, at least in part, to be mediated through abnormal down-regulation of protein kinase C activity.14 Still, the mechanisms for protein targeting to granules during myeloid differentiation of neutrophils remain unresolved.
Serglycin proteoglycan, the major intracellular proteoglycan of hematopoietic cells,15 has been suggested to play a role in localization and packing of granule proteins in neutrophils.16 In mast cells, serglycin, with heparin side chains, is needed for correct localization of granule proteins.17–19 In cytotoxic lymphocytes, serglycin is present in a macromolecular complex with perforin and granzyme B,20 and serglycin is necessary for granule maturation and storage of granzyme B.7 We have previously shown that serglycin is localized to the trans-Golgi network and immature granules of myeloid progenitor cells from human bone marrow, whereas serglycin proteoglycan is absent from mature neutrophils,21 indicating a possible role for serglycin in granule formation and protein localization but not in storage of granule contents.
To further investigate the role of serglycin in neutrophil granule formation and protein localization, we studied the granules of neutrophils from serglycin knockout mice.17 We show here that neutrophils from serglycin−/− mice lack neutrophil elastase whereas all other tested granule proteins are unaffected by the lack of serglycin.
Materials and methods
Serglycin knockout mice
Generation of the serglycin−/− mice is previously described.17 N6 or further backcrosses to C57BL/6 were used; breeding and experiments were according to permissions and guidelines from the Danish Animal Experiments Inspectorate.
Isolation and separation of bone marrow cells
Femora, tibiae, and humeri were dissected from mice; bone marrow cells were flushed out by a total of 10 mL PBS, 1 mM EDTA per animal. Erythroid cells were lysed by hypotonic lysis. Human bone marrow aspirates and blood samples were obtained from healthy volunteers with informed consent according to the permission from the local ethics committee. Cells from different stages of myeloid differentiation were isolated as described.22,23
Northern blotting
Total RNA was extracted with Trizol (Invitrogen, San Diego, CA). Northern blotting and hybridization were performed as described.23,24 The full-length murine serglycin probe was purified from a pcDNA3/MMSG plasmid (kindly provided by Dr Urban Gullberg, University of Lund, Sweden). Probes for murine neutrophil elastase and cathepsin G were polymerase chain reaction (PCR) amplified with the primers 5′-CAGACTATCCAGCCGGACT-3′ and 3′-AATGGGTAGGGTTTCTGGCA-5′ (neutrophil elastase) and 5′-TCACACGAGCAGGAAAATAGA-3′ and 3′-GGTATCGGGAATAATTACGGA-5′ (cathepsin G) from cDNA from wild-type murine bone marrow cells. Sequences were verified by sequencing. Probes were α-32P labeled with the Random Primers DNA Labeling System (Invitrogen).
Antibody generation, cloning, and purification of recombinant murine serglycin
The full-length coding sequence of murine serglycin (without the signal peptide) including an N-terminal His tag was expressed in the pTrcHis B bacterial vector (Invitrogen) in Escherichia coli (BL21(DE3)pLysS; Invitrogen). Induction and purification were as previously described.21 The size and identity were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry (kindly performed by Dr Anders H. Johnsen, Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark). Immunization (DakoCytomation, Glostrup, Denmark) was with the purified recombinant murine serglycin (nonglycosylated). The IgG fraction was isolated on a HiTrap Protein A HP column (Amersham Biosciences, Buckinghamshire, United Kingdom).
Immunocytochemistry and Western blotting
Cytospins of bone marrow cells were treated as previously described.21 Detection was with Envison+ System-HRP (K4010; DakoCytomation) using an Olympus BX51 microscope (60×/1.40 PlanApo oil objective) with an Olympus DP70 camera and the analySIS B 5.0 software package (Olympus, Hamburg, Germany) and Photoshop 7.0 (Adobe Systems, San Jose, CA) for adjustment of brightness and contrast (Figures 2A-B, 3D-E, 4A-J, 5D-E, and 7A-B). Antibodies used included rabbit anti–murine serglycin immunoglobulin 1:5000, rabbit anti–murine 24p3 antiserum 1:1000 (kindly provided by Dr Jack B. Cowland, Rigshospitalet),25 rabbit antisera for murine neutrophil elastase 1:500 and cathepsin G 1:200 (kindly provided by Dr Jürgen Roes),26 rabbit anti–murine BPI 1:250 (kindly provided by Dr Urban Gullberg),27 rabbit anti–murine MPO 1:500 (Ab-1; NeoMarkers, Fremont, CA), rabbit anti–murine MMP-9 1:40000 (M2425-36; Nordic BioSite, Täby, Sweden), rabbit anti–human lactoferrin 1:500 (A0186; DakoCytomation), monoclonal mouse anti–β-actin 1:5000 (clone AC-15; Sigma, Steinheim, Germany), or control normal rabbit IgG (X0903; DakoCytomation). Western blotting conditions were as described21 except that the blots in Figures 1C and 3B were developed with SuperSignal (Pierce, Rockford, IL) and detected with a ChemiDoc XRS (Bio-Rad, Hercules, CA).
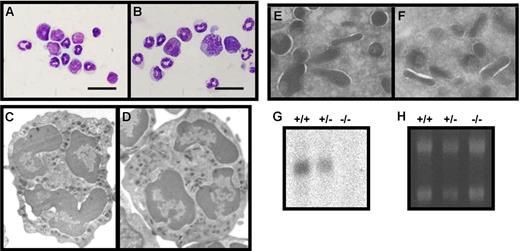
Characterization of bone marrow cells from wild-type (+/+), heterozygous (+/−), and serglycin knockout (−/−) mice. (A-B) Giemsa staining of bone marrow cells from wild-type (A) and serglycin−/− mice (B). Murine neutrophils are characterized by donut-shaped nuclei with increasing lobulation during final maturation; bar, 20 μm. (C-D) Ultrastructure by electron microscopy of neutrophils from wild-type (C) and serglycin−/− mice (D). Higher magnifications of areas with granules from wild-type (E) and serglycin−/− neutrophils (F) show the same electron density of granules from the 2 genotypes. (G) Northern blotting with full-length serglycin probe. (H) Ethidium bromide staining of the gel before blotting, for control of equal loading; 5 μg total RNA from bone marrow cells per lane.
Characterization of bone marrow cells from wild-type (+/+), heterozygous (+/−), and serglycin knockout (−/−) mice. (A-B) Giemsa staining of bone marrow cells from wild-type (A) and serglycin−/− mice (B). Murine neutrophils are characterized by donut-shaped nuclei with increasing lobulation during final maturation; bar, 20 μm. (C-D) Ultrastructure by electron microscopy of neutrophils from wild-type (C) and serglycin−/− mice (D). Higher magnifications of areas with granules from wild-type (E) and serglycin−/− neutrophils (F) show the same electron density of granules from the 2 genotypes. (G) Northern blotting with full-length serglycin probe. (H) Ethidium bromide staining of the gel before blotting, for control of equal loading; 5 μg total RNA from bone marrow cells per lane.
Immunostaining for serglycin in bone marrow cells from wild-type and serglycin knockout mice and detection of 35S-labeled macromolecules. (A-B) Immunocytochemistry for serglycin; bar, 20 μm; neutrophils and precursor cells from wild-type mouse (A) and serglycin knockout mouse (B). (C) Western blotting of 1 ng recombinant murine serglycin per lane; detection with rabbit anti–murine serglycin IgG, blocked with 0 to 100 ng/mL murine serglycin as indicated above each lane. As seen, the immunoreactivity of the antibody is blocked in a dose-dependent manner by addition of the antigen in solution to the antibody. (D) Immunoelectron microscopy showing serglycin immunoreactivity (10 nm gold particles) in a myeloid cell from wild-type bone marrow; Golgi stacks are indicated by arrows. (E-F) Biosynthesis of 35S-labeled macromolecules in bone marrow cells and SDS-PAGE of cell lysates with (+) or without (−) cABC treatment. (E) Murine bone marrow cells from wild-type (+/+) and serglycin knockout mice (−/−). (F) Myeloid cells from human bone marrow and peripheral blood separated according to maturational stage; the designation of cell populations indicates the major cell types in each group (PMN, peripheral blood neutrophils; BC+SC, band cells and segmented neutrophils; MC+MM, myelocytes and metamyelocytes; MB+PM, myeloblasts and promyelocytes).
Immunostaining for serglycin in bone marrow cells from wild-type and serglycin knockout mice and detection of 35S-labeled macromolecules. (A-B) Immunocytochemistry for serglycin; bar, 20 μm; neutrophils and precursor cells from wild-type mouse (A) and serglycin knockout mouse (B). (C) Western blotting of 1 ng recombinant murine serglycin per lane; detection with rabbit anti–murine serglycin IgG, blocked with 0 to 100 ng/mL murine serglycin as indicated above each lane. As seen, the immunoreactivity of the antibody is blocked in a dose-dependent manner by addition of the antigen in solution to the antibody. (D) Immunoelectron microscopy showing serglycin immunoreactivity (10 nm gold particles) in a myeloid cell from wild-type bone marrow; Golgi stacks are indicated by arrows. (E-F) Biosynthesis of 35S-labeled macromolecules in bone marrow cells and SDS-PAGE of cell lysates with (+) or without (−) cABC treatment. (E) Murine bone marrow cells from wild-type (+/+) and serglycin knockout mice (−/−). (F) Myeloid cells from human bone marrow and peripheral blood separated according to maturational stage; the designation of cell populations indicates the major cell types in each group (PMN, peripheral blood neutrophils; BC+SC, band cells and segmented neutrophils; MC+MM, myelocytes and metamyelocytes; MB+PM, myeloblasts and promyelocytes).
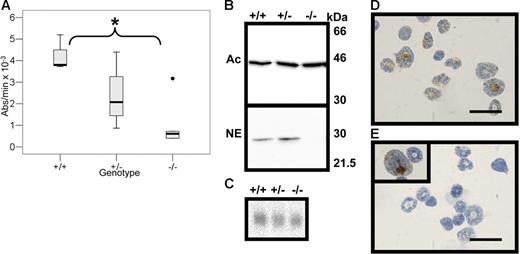
Neutrophil elastase in bone marrow cells from wild-type (+/+), heterozygous (+/−), and serglycin knockout (−/−) mice. (A) Elastase activity measured by spectrophotometry following the degradation rate for methoxysuccinyl-Ala-Ala-Pro-Val-P-nitroanilide. The SLPI-inhibitable activity is expressed as absorbance change per minute at 405 nm. Median is indicated by bold line and range by whiskers. The asterisk signifies statistically significant difference at the 5% level, log10-transformed data, independent t test; +/+, n = 3; +/−, n = 4; −/−, n = 6. (B) Western blotting of bone marrow cell lysates. The 2 blots have been loaded and processed in parallel; the lower blot has been incubated with rabbit anti–murine neutrophil elastase (NE), and the upper blot has been incubated with monoclonal mouse anti–murine β-actin (Ac) for loading control. Molecular weight markers are indicated. (C) Northern blotting with a full-length probe for murine neutrophil elastase; the blot is the same as in Figure 1 after stripping and reprobing with the neutrophil elastase probe. (D-E) Immunocytochemistry for neutrophil elastase in murine bone marrow cells from wild-type mice (D) and serglycin−/− mice (E); bar, 20 μm. Rarely a promyelocyte from serglycin−/− mice stains positive for neutrophil elastase (E, inset).
Neutrophil elastase in bone marrow cells from wild-type (+/+), heterozygous (+/−), and serglycin knockout (−/−) mice. (A) Elastase activity measured by spectrophotometry following the degradation rate for methoxysuccinyl-Ala-Ala-Pro-Val-P-nitroanilide. The SLPI-inhibitable activity is expressed as absorbance change per minute at 405 nm. Median is indicated by bold line and range by whiskers. The asterisk signifies statistically significant difference at the 5% level, log10-transformed data, independent t test; +/+, n = 3; +/−, n = 4; −/−, n = 6. (B) Western blotting of bone marrow cell lysates. The 2 blots have been loaded and processed in parallel; the lower blot has been incubated with rabbit anti–murine neutrophil elastase (NE), and the upper blot has been incubated with monoclonal mouse anti–murine β-actin (Ac) for loading control. Molecular weight markers are indicated. (C) Northern blotting with a full-length probe for murine neutrophil elastase; the blot is the same as in Figure 1 after stripping and reprobing with the neutrophil elastase probe. (D-E) Immunocytochemistry for neutrophil elastase in murine bone marrow cells from wild-type mice (D) and serglycin−/− mice (E); bar, 20 μm. Rarely a promyelocyte from serglycin−/− mice stains positive for neutrophil elastase (E, inset).
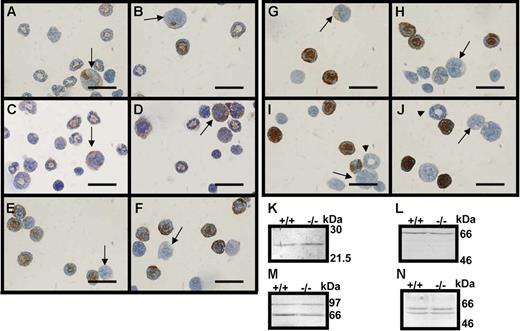
Immunocytochemistry and Western blotting for granule proteins. (A-J) Immunocytochemistry for neutrophil granule proteins; bar, 20 μm; arrows point toward promyelocytes and arrowheads toward myelocytes. (A,C,E,G,I) Bone marrow cells from wild-type mice. (B,D,F,H,J) Bone marrow cells from serglycin knockout mice. (A-B) MPO and (C-D) BPI protein, azurophil granule proteins, (E-F) 24p3 and (G-H) lactoferrin (specific granule proteins), and (I-J) gelatinase B (MMP-9) (gelatinase granule protein). (K-N) Western blotting for 24p3 (K), lactoferrin (L), MMP-9 (M), and MPO (N), lysates of bone marrow cells from wild-type (+/+) and serglycin knockout mice (−/−); molecular weight markers are indicated.
Immunocytochemistry and Western blotting for granule proteins. (A-J) Immunocytochemistry for neutrophil granule proteins; bar, 20 μm; arrows point toward promyelocytes and arrowheads toward myelocytes. (A,C,E,G,I) Bone marrow cells from wild-type mice. (B,D,F,H,J) Bone marrow cells from serglycin knockout mice. (A-B) MPO and (C-D) BPI protein, azurophil granule proteins, (E-F) 24p3 and (G-H) lactoferrin (specific granule proteins), and (I-J) gelatinase B (MMP-9) (gelatinase granule protein). (K-N) Western blotting for 24p3 (K), lactoferrin (L), MMP-9 (M), and MPO (N), lysates of bone marrow cells from wild-type (+/+) and serglycin knockout mice (−/−); molecular weight markers are indicated.
The serine proteases cathepsin G and proteinase 3 in wild-type (+/+) and serglycin knockout mice (−/−) bone marrow cells. (A) Proteinase 3 activity (+/+, n = 3; −/−, n = 6) was measured spectrophotometrically by the degradation rate for methoxysuccinyl-Ala-Ala-Pro-Val-P-nitroanilide. The activity expressed as absorbance change per minute at 405 nm is the activity not inhibitable by SLPI. (B) Cathepsin G activity (+/+, n = 3; −/−, n = 3) was measured spectrophotometrically by dissociation rate for N-succinyl-Ala-Ala-Pro-Phe-P-nitroanilide at 410 nm. No statistically significant differences (5% level, log10-transformed data, independent t test) in serine protease activities could be detected between wild-type and serglycin−/− bone marrow cells. Median is indicated by bold line and range by whiskers. (C) Western blotting for cathepsin G (CG); MPO is used as an internal loading control. The 2 blots represent the upper and lower part of the same blot cut into 2 before antibody incubation. Molecular weight markers are indicated. (D-E) Immunocytochemistry for cathepsin G on bone marrow cells from wild-type (D) and serglycin−/− mice (E); bar, 20 μm.
The serine proteases cathepsin G and proteinase 3 in wild-type (+/+) and serglycin knockout mice (−/−) bone marrow cells. (A) Proteinase 3 activity (+/+, n = 3; −/−, n = 6) was measured spectrophotometrically by the degradation rate for methoxysuccinyl-Ala-Ala-Pro-Val-P-nitroanilide. The activity expressed as absorbance change per minute at 405 nm is the activity not inhibitable by SLPI. (B) Cathepsin G activity (+/+, n = 3; −/−, n = 3) was measured spectrophotometrically by dissociation rate for N-succinyl-Ala-Ala-Pro-Phe-P-nitroanilide at 410 nm. No statistically significant differences (5% level, log10-transformed data, independent t test) in serine protease activities could be detected between wild-type and serglycin−/− bone marrow cells. Median is indicated by bold line and range by whiskers. (C) Western blotting for cathepsin G (CG); MPO is used as an internal loading control. The 2 blots represent the upper and lower part of the same blot cut into 2 before antibody incubation. Molecular weight markers are indicated. (D-E) Immunocytochemistry for cathepsin G on bone marrow cells from wild-type (D) and serglycin−/− mice (E); bar, 20 μm.
Immunoelectron microscopy
Neutrophils were fixed with 4% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) and embedded in 16% gelatin before processing as previously described28 with rabbit anti–murine serglycin 1:2000 as primary antibody and 10 nm colloidal gold–coupled goat antirabbit secondary antibody (BioCell, Cardiff, United Kingdom) imaged with a Philips (Copenhagen, Denmark) CM 100 electron microscope and Photoshop 7.0 for adjustment of brightness and contrast (Figures 2D and 7C-F).
Biosynthesis
Cells were washed in RPMI 1640 medium (Invitrogen), incubated in the same medium at 3 × 107/mL with 0.1 mCi (3.7 × 106 Bq) 35S-sulfate (35S) (Amersham Biosciences) for 2 hours, and processed directly for SDS-PAGE or initially treated with chondroitinase ABC (cABC).21 SDS-PAGE 5%/20% gradient gels, at 106 cells per lane, were stained and soaked in amplifier (NAMP100V; Amersham Biosciences) before exposure to a Fuji (Vedbaek, Denmark) BAS2500 PhosphorImager.
Enzyme activity assays
Bone marrow cells (107/mL) were lysed in PBS, 0.2% Triton X-100 and diluted in phosphate buffer (pH 6.2) with or without secretory leukocyte protease inhibitor (SLPI) (1274-Pi; R&D Systems, Minneapolis, MN) treatment for inhibition of elastase.29 Methoxysuc-AAPV-p-nitroanilide, 0.25 mg/mL (M4765; Sigma), was used as substrate for elastase and N-suc-AAPF-p-nitroanilide, 1 mg/mL (S7388; Sigma), for cathepsin G30 ; the reaction was measured at 405 nm in an ultrospec 3300 pro (Biochrom, Cambridge, United Kingdom).
Molecular modeling
The 3-dimensional models for the corresponding human proteins 1h1b (neutrophil elastase, 75% identities),31 1cgh (cathepsin G, 70% identities),32 and 1fuj (proteinase 3 [myeloblastin], 68% identities)33 were obtained from the Protein Data Bank (accessed November 2005) and used to construct homology models for the mouse proteins using SOD34 and the molecular graphics program O.35 Side chain rotamers were adjusted where necessary to avoid steric clashes and to maximize rotamer overlap for “mutated” residues. Surface representations were generated using PyMOL.36
Intraperitoneal bacterial challenge
Mice were inoculated intraperitoneally with Klebsiella pneumoniae (10031; American Type Culture Collection [ATCC], Manassas, VA). Colony-forming unit (CFU) counts were performed for axillary blood samples (sampled under CO2 anesthesia) and peritoneal fluid (collected immediately after humane killing) at 1 and 5 hours after inoculation. Sterile saline (2 mL) was injected intraperitoneally, and the abdomen was gently massaged before peritoneal fluid sampling. CFU counts were determined by plating dilution series of blood and peritoneal fluid.
Statistics
All statistic calculations were performed with SPSS 13.0 for Windows (SPSS, Chicago, IL).
Results
Characterization of neutrophils from serglycin knockout mice
The distribution of neutrophils and myeloid progenitors in bone marrow from serglycin knockout mice was indistinguishable from wild-type bone marrow as illustrated by Giemsa staining (Figure 1A-B). Also, differential counts of peripheral blood and bone marrow revealed no statistically significant differences between the genotypes: peripheral blood neutrophils/total leukocytes: wild-type and heterozygous mice, 19.03% (12.20 to 25.86), n = 6, and serglycin−/− mice, 17.74% (11.07 to 24.41), n = 4; bone marrow band cells and neutrophils/total leukocytes: wild-type mice, 18.20% (13.20 to 23.20), n = 3, and serglycin−/− mice, 14.08% (11.82 to 16.33), n = 4 (c.i.).
The overall structure of neutrophils at the level of resolution by light microscopy was unaffected by the lack of serglycin (Figure 1A-B). The ultrastructure of neutrophils from wild-type and serglycin−/− bone marrow was further studied by electron microscopy. As seen in Figures 1C-F, no differences could be detected: Lobulated nuclei and granules with varying shapes and electron densities were observed in both genotypes. To exclude the possibility that a truncated transcript or a cryptic splice site gave rise to some serglycin expression in neutrophils from the knockout mice, we performed Northern blotting with both the full-length serglycin probe and probes specific for exon 3 or exon 2 (the promoter region and exon 1 are deleted in the serglycin−/− mouse; the glycosaminoglycan-binding region is in exon 3). All serglycin probes gave similar results. A representative blot showing lack of serglycin mRNA in serglycin−/− mice, reduced serglycin mRNA in heterozygous mice, and a clear signal in wild-type mice is shown in Figure 1G.
Serglycin is localized to the Golgi area and granules of murine neutrophil precursors
To compare the localization of serglycin immunoreactivity in murine neutrophils with our previous work on serglycin in human myeloid cells,21 we raised a polyclonal rabbit antibody against full-length recombinant murine serglycin. We found that the immunoreactivity for serglycin in murine bone marrow was more widespread both inside the cells and among the cells than in the human counterpart21 (Figure 2A). Serglycin localized to the perinuclear region of the cells as well as to the entire cytoplasm of promyelocytic cells and more mature myeloid cells. However, as seen also in human neutrophils, serglycin immunoreactivity was absent from some of the mature polymorphonucleated neutrophils (irregularly ring-shaped nuclei in mice).
To further localize serglycin at the subcellular level, we performed immunoelectron microscopy with the same antibody. Serglycin was localized primarily in the Golgi area (Figure 2D, arrows) of promyelocytes and myelocytes (Figure 2D); rarely, labeling was seen over some granules of more mature myeloid cells (data not shown).
Serglycin is the major sulfated macromolecule in murine bone marrow cells
To evaluate whether other sulfated macromolecules than serglycin are present in murine bone marrow cells, we performed pulse labeling of bone marrow cells from wild-type and serglycin−/− mice with 35S. As seen in Figure 2E, almost no macromolecules with 35S incorporation were detected in the cells from serglycin−/− mice, whereas a diffuse, high molecular weight band that was degradable by chondroitinase ABC (cABC) was seen in the cells from wild-type mice. To further define the time of synthesis for sulfated macromolecules during myeloid differentiation, we separated myeloid cells from human bone marrow according to their stage of maturation and evaluated the incorporation of 35S. A high level of 35S incorporation into macromolecules of the most immature cells (myeloblasts and promyelocytes) is seen in Figure 2F (MB+PM), whereas the more mature myeloid cells incorporated much less 35S. This correlates with the expression profile of serglycin mRNA and protein in myeloid cells from human bone marrow.21 The sulfated macromolecules were degradable by cABC as also seen in mice. Similar experiments could not be performed on mouse cells due to lack of sufficient amounts of bone marrow.
Neutrophil elastase, a serine protease of azurophil granules in neutrophils, is absent from serglycin−/− neutrophils
To evaluate granule protein localization in neutrophils from serglycin knockout mice, we tested elastase activity as measured by degradation of N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide, a substrate specific for elastase and proteinase 3 but not cathepsin G.30 To distinguish between elastase and proteinase 3 activity, we used SLPI that specifically inhibits elastase but not proteinase 3 activity.37 We found that elastase activity (SLPI-inhibitable activity) in bone marrow cells from serglycin−/− mice was significantly below the elastase activity of wild-type bone marrow cells (P < .01) (Figure 3A). We confirmed the absence of neutrophil elastase from serglycin−/− neutrophils by Western blotting with a primary rabbit anti–murine neutrophil elastase antibody (Figure 3B). The statistically insignificant decrease in elastase activity in bone marrow cells from serglycin+/− mice compared with wild-type mice (Figure 3A) was not seen by Western blotting (Figure 3B).
To ascertain that the lack of neutrophil elastase was not due to transcriptional regulation, we performed Northern blotting of bone marrow cells from wild-type, heterozygous, and serglycin knockout mice. Equal levels of elastase mRNA were found in all 3 genotypes (Figure 3C). Finally, neutrophil elastase immunoreactivity was localized as a granular cytoplasmic stain of bone marrow neutrophils and myeloid precursors from wild-type mice, whereas no immunoreactivity was seen in serglycin−/− bone marrow cells except from very rare promyelocytes with a disperse staining (Figures 3D-E).
No other proteins resident to azurophil granules, specific (secondary) granules, or gelatinase (tertiary) granules of neutrophils were influenced by lack of serglycin
Serglycin has previously been claimed as necessary for proper localization of granule proteins in neutrophils in general.16,38 To explore whether granule proteins other than neutrophil elastase are dependent on serglycin for correct localization, we performed Western blotting and immunocytochemistry (Figure 4) of bone marrow cells from wild-type and serglycin−/− mice for myeloperoxidase (MPO) and bacterial permeability-increasing (BPI) protein (azurophil granules), 24p3 (the murine ortholog of NGAL) and lactoferrin (specific granules), and gelatinase B (MMP-9) (gelatinase granules). All 5 granule proteins, representing all 3 known classes of neutrophil granules, showed similar levels of immunoreactivity in serglycin−/− bone marrow cells compared with wild-type bone marrow cells by both Western blotting and immunocytochemistry. Notably, the promyelocytes (arrows, Figure 4A-J) were positive only for MPO and BPI, the markers of azurophil granules, while the more mature myeloid cells with donut-shaped nuclei were positive for markers of all granule classes except some of the myelocytes (arrowheads, Figure 4I-J) that were negative for MMP-9, the marker of gelatinase granules. This is in accordance with the previously published “targeting by timing” hypothesis of granule protein targeting in human neutrophils6 and the mRNA profiles of granule proteins in human.24,39
Proteinase 3 and cathepsin G, 2 other serine proteases from azurophil granules of neutrophils, are independent of serglycin for proper localization
Even though neutrophil elastase was the only granule protein that depends on serglycin for proper localization among those tested, we speculated that other serine proteases with structure and sequence homology to elastase might also depend on serglycin for localization to azurophil granules. We therefore measured the proteinase 3–specific activity (as absorbance per minute evolved with N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide that could not be inhibited by SLPI29 ) and the cathepsin G–specific activity (as absorbance per minute evolved with N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as substrate30 ) of bone marrow cells from wild-type and serglycin−/− mice. We found no statistically significant differences between the 2 genotypes (Figure 5A-B). We further tested the presence of cathepsin G by Western blotting and immunocytochemistry of bone marrow cells from wild-type and serglycin−/− mice with a rabbit anti–murine cathepsin G antibody. Equal levels of cathepsin G immunoreactivity were detected by Western blotting in the 2 genotypes (Figure 5C), and the immunoreactivity was localized to neutrophils and myeloid cells as a cytoplasmic staining in both wild-type and serglycin−/− bone marrow specimens (Figure 5D-E).
A putative serglycin-binding surface of neutrophil elastase is absent from proteinase 3 and cathepsin G
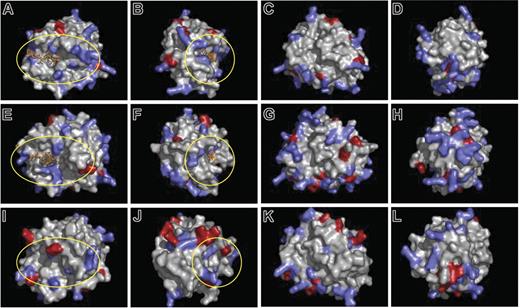
The striking serglycin dependency for localization of neutrophil elastase in azurophil granules, despite serglycin independency for localization of the highly similar serine proteases cathepsin G and proteinase 3, prompted us to look for a putative glycosaminoglycan-binding surface in the 3-dimensional structure of murine neutrophil elastase (modeled upon the crystal structure of human neutrophil elastase31 ). We identified a horseshoe-shaped surface with basic amino acids surrounding the active site of neutrophil elastase (encircled in Figure 6A-B), the same region that was previously proposed to interact with sulfated glycosaminoglycans.40 The corresponding region of murine cathepsin G (modeled upon the human cathepsin G crystal structure32 ) is encircled (Figures 6E-F); only 3 of 6 basic amino acids were present in this region of cathepsin G despite the almost identical theoretical isoelectric point (pI) of neutrophil elastase and cathepsin G (10.16 and 10.84, respectively). In the proteinase 3 model (based upon human proteinase 3 crystal structure33 ), the corresponding area of basic amino acids was interposed with acidic amino acids (encircled in Figure 6I-J), and the overall charge of the molecule differed markedly from that of neutrophil elastase (theoretical pI, 6.24).
Three-dimensional molecular models of neutrophil elastase, proteinase 3, and cathepsin G. The basis for modeling is described in “Materials and methods.” Residues with positively charged side chains are indicated by blue; those with negatively charged side chains are indicated by red. Neutrophil elastase and cathepsin G are modeled with an inhibitor in the active site (orange). The putative glycosaminoglycan-binding regions around the active sites of the enzymes are encircled (yellow). (A-D) Murine neutrophil elastase, (E-F) murine cathepsin G, (I-L) murine proteinase 3. For each protein model, 4 different views separated by sequential 90-degree clockwise rotations around the vertical axis are shown.
Three-dimensional molecular models of neutrophil elastase, proteinase 3, and cathepsin G. The basis for modeling is described in “Materials and methods.” Residues with positively charged side chains are indicated by blue; those with negatively charged side chains are indicated by red. Neutrophil elastase and cathepsin G are modeled with an inhibitor in the active site (orange). The putative glycosaminoglycan-binding regions around the active sites of the enzymes are encircled (yellow). (A-D) Murine neutrophil elastase, (E-F) murine cathepsin G, (I-L) murine proteinase 3. For each protein model, 4 different views separated by sequential 90-degree clockwise rotations around the vertical axis are shown.
The lack of serglycin, and thereby neutrophil elastase, impairs bactericidal activity in vivo
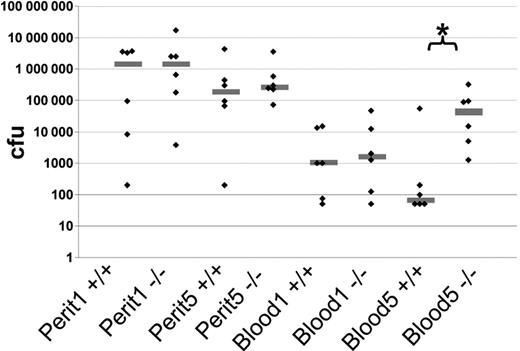
To determine the biologic significance of serglycin, we challenged wild-type and serglycin-deficient mice with the Gram-negative bacteria K pneumoniae in a peritonitis/sepsis model.41 This microorganism was chosen based on the previous report on impaired bactericidal activity toward Gram-negative bacteria such as K pneumoniae and E coli but not toward the Gram-positive bacteria Staphylococcus aureus in the neutrophil elastase knockout mouse.42 The number of CFUs from the peritoneal cavity 1 and 5 hours after inoculation showed too high variance to make conclusions on any differences between wild-type and serglycin−/− mice. CFU counts of peripheral blood from serglycin knockout mice at 1 hour showed a tendency to higher numbers than the wild-type mice; this difference was increased thereafter with a statistically significant higher CFU count of serglycin knockout mice compared with wild-type mice 5 hours after inoculation (P < .02) (Figure 7). We could not detect any statistically significant differences in peritoneal neutrophil counts at either time point between the 2 genotypes (data not shown).
In vivo virulence of K pneumoniae in a peritonitis/sepsis model in wild-type (+/+) and serglycin knockout (−/−) mice. Mice were challenged with K pneumoniae intraperitoneally at 0 hours. Six animals from each genotype were killed at 1 hour and 5 hours after bacterial injection, and CFU counts were determined for blood and peritoneal fluid (perit) samples for each time point (1 and 5). Gray line shows the median; ♦, single experiments; *, statistically significant difference at the 5% level, Mann-Whitney nonparametric test.
In vivo virulence of K pneumoniae in a peritonitis/sepsis model in wild-type (+/+) and serglycin knockout (−/−) mice. Mice were challenged with K pneumoniae intraperitoneally at 0 hours. Six animals from each genotype were killed at 1 hour and 5 hours after bacterial injection, and CFU counts were determined for blood and peritoneal fluid (perit) samples for each time point (1 and 5). Gray line shows the median; ♦, single experiments; *, statistically significant difference at the 5% level, Mann-Whitney nonparametric test.
Discussion
We analyzed neutrophils from serglycin−/− mice17 to elucidate the role of serglycin proteoglycan in formation of granules in neutrophils. We found no differences in the distribution of neutrophils or their precursors in blood or bone marrow; nor did we find any structural differences at the cellular or subcellular level (Figure 1). This is in contrast with mast cells where serglycin−/− mice completely lack normal granulated mast cells in peripheral tissues and where differentiating mast cells from bone marrow show empty-looking vesicles instead of granules.17 However, our findings are in some respects in line with the recent findings for cytotoxic lymphocytes where only minor alterations in granule ultrastructure are observed in serglycin−/− lymphocytes.7 Yet, serglycin proteoglycan is the major cell-associated proteoglycan in all 3 cell types (Figure 2C).7,17 The different structural effects of serglycin in different hematopoietic cells relate to differences in glycosaminoglycan side chains as well as to cell type–specific differences in granule formation.43,44
On the basis of previous hypotheses of the function of serglycin during granule formation in neutrophils and other hematopoietic cells,15 we speculated that localization of proteins in granules of neutrophils would be influenced by lack of serglycin proteoglycan. To our surprise, only a single of the 8 tested neutrophil granule proteins was affected by the lack of serglycin. Neutrophil elastase was absent from neutrophils and their precursors in bone marrow cells from serglycin−/− mice except from some rare promyelocytes showing a disperse immunoreactivity for neutrophil elastase, while neutrophil elastase mRNA was present at normal levels as expected (Figure 3). This indicates that neutrophil elastase is transcribed and probably translated, at least to some degree, at the promyelocyte stage. The absence of neutrophil elastase from mature neutrophils due to lack of serglycin is most likely a result of either degradation or secretion of neutrophil elastase at the earlier stages of myelopoiesis. Studies of biosynthesis to explore the fate of neutrophil elastase in wild-type and serglycin knockout neutrophils were not conclusive due to insufficient signal and fragility of murine neutrophils under in vitro culture conditions (data not shown).
We found a normal expression and localization of the other granule proteins tested in bone marrow cells from serglycin−/− mice. As marker proteins for azurophil granules we used MPO and BPI, for specific granules we used lactoferrin and 24p3, and for gelatinase granules we used MMP-9. None of these proteins showed differences in localization or level of immunoreactivity between bone marrow cells from wild-type and serglycin−/− mice. Localization to specific and gelatinase granules is expected to be independent of serglycin, because serglycin is expressed at the promyelocyte stage, the stage of azurophil granule formation, whereas cells in the later stages of myeloid differentiation that form specific and gelatinase granules are devoid of serglycin expression or immunoreactivity.21 The proper localization of MPO despite the lack of serglycin contrasts with a previous report on correlation of serglycin and MPO transport in myeloid HL-60 cells and the binding of MPO and other cationic granule proteins to chondroitin sulfate, the constituent of serglycin side chains in neutrophils.45 This illustrates the limitations of studies based on cell lines and assessment of binding from affinity chromatography data.
The different effects of serglycin proteoglycan for granules and granule proteins in neutrophils compared with mast cells are likely due to differences in the composition of the glycosaminoglycan side chains of serglycin in the different cell types. In connective tissue-type mast cells, serglycin side chains consist of heparin, the most heavily sulfated glycosaminoglycan.18,19 The more negative charge of heparin compared with chondroitin-4-sulfate side chains of neutrophil serglycin can explain the binding to serglycin of a wider range of granule proteins with less specific binding motifs or less positive charge at physiological pH in mast cells. This is in line with the stronger binding and inhibition of neutrophil elastase by heparin compared with chondroitin sulfate in vitro.46–48 In cytotoxic lymphocytes, like in neutrophils, serglycin has chondroitin sulfate side chains attached, and only a single granule protein has been found to be dependent on serglycin for proper localization here,7 indicating that selectivity in serglycin-mediated localization in granules is associated with the nature of glycosaminoglycan side chains.
Glycosaminoglycans bind neutrophil elastase in a partly inhibitory way49,50 involving arginine residues close to the active site of neutrophil elastase in the binding,40 although not directly influencing the active site.51 The ability of chondroitin sulfate to bind and inhibit cathepsin G in vitro is markedly lower than the corresponding binding and inhibition of neutrophil elastase.48 This may explain the observed serglycin-independent localization of cathepsin G (Figure 5) despite almost the same pI as neutrophil elastase. The cleaved C-terminal 20–amino acid prodomain of neutrophil elastase, which contains 5 basic residues, has previously been shown not to be necessary for granule localization.52 A putative glycosaminoglycan-interacting region of granzyme B, which is dependent on serglycin for localization in granules of cytotoxic lymphocytes, was recently proposed.7 This region of granzyme B has some resemblance with the herein proposed glycosaminoglycan-interacting region of neutrophil elastase. Although other possible glycosaminoglycan-binding sites can be pointed out in the model of neutrophil elastase (eg, the lower surface in Figure 6C), we found that neutrophil elastase displays a unique region among these 3 homologous serine proteases of neutrophils that may explain the dependency on serglycin for localization in granules of neutrophil elastase.
We were surprised to find that among the azurophil granule proteins tested, only neutrophil elastase depends on serglycin for proper localization in granules. However, neutrophil elastase is the major serine protease of azurophil granules known to be sorted as an active protease,53 thereby raising the possibility that serglycin is necessary for neutrophil elastase targeting to prevent untoward proteolytic activity during transport to granules.
Cyclic neutropenia (CN) and severe congenital neutropenia (SCN) are associated with mutations in the gene encoding neutrophil elastase.54–56 There have been several contradicting reports on disturbances in the subcellular localization of neutrophil elastase caused by mutations associated with CN or SCN.10,57 It has particularly been suggested that mutations in neutrophil elastase affect the sorting to granules via disorganization of putative transmembrane and cytosolic domains.58 Our finding that lack of serglycin completely prevents granule localization of elastase argues against a significant role of putative transmembrane and cytosolic domains of neutrophil elastase in granule targeting.
The increased susceptibility to Gram-negative infections of mice lacking neutrophil elastase42 was paralleled in our challenge of serglycin−/− mice with the Gram-negative bacteria K pneumoniae in a peritonitis/sepsis model (Figure 7). This indicates that mislocalization of neutrophil elastase due to lack of serglycin results in a functional neutrophil elastase knockout phenotype. However, serglycin−/− mice are devoid of normal mast cells in peripheral tissues,7 and mast cells are implicated in recruitment of neutrophils to sites of inflammation.59,60 Although our results do not show any differences between the 2 genotypes in numbers of neutrophils recruited to the peritoneal cavity during K pneumoniae infection, we cannot rule out the possibility that the mast cell defects of serglycin knockout mice are also implicated in the increased susceptibility to Gram-negative infections.
Sorting of proteins to storage organelles such as secretory granules is assumed to be an active process that diverts proteins at the level of the trans-Golgi network away from the default route, which leads to constitutive secretion.61 In cells such as promyelocytes and myelocytes, which generate huge numbers of granules, it could be expected that some sorting to granules occurs by simple bulk flow, thus partially rendering active sorting mechanisms to granules superfluous. The observation here that elastase is completely absent from granules of serglycin knockout neutrophils argues against bulk flow and strongly indicates a need for active sorting mechanisms yet to be discovered.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carsten Utoft Niemann, Rigshospitalet, Department of Hematology, The Granulocyte Research Laboratory, Bldg 9322, Blegdamsvej 9, DK-2100 Copenhagen Ø, Denmark; e-mail: niemann@dadlnet.dk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Danish Medical Research Council (N.B.) and a grant from H:S, Copenhagen University Hospital (C.U.N.).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal