Abstract
Acute megakaryoblastic leukemia (AMKL) is a subtype of acute myeloid leukemia associated with a poor prognosis. However, there are relatively few insights into the genetic etiology of AMKL. We developed a screening assay for mutations that cause AMKL, based on the hypothesis that constitutive activation of STAT5 would be a biochemical indicator of mutation in an upstream effector tyrosine kinase. We screened human AMKL cell lines for constitutive STAT5 activation, and then used an approach combining mass spectrometry identification of tyrosine phosphorylated proteins and growth inhibition in the presence of selective small molecule tyrosine kinase inhibitors that would inform DNA sequence analysis of candidate tyrosine kinases. Using this strategy, we identified a new JAK2T875N mutation in the AMKL cell line CHRF-288-11. JAK2T875N is a constitutively activated tyrosine kinase that activates downstream effectors including STAT5 in hematopoietic cells in vitro. In a murine transplant model, JAK2T875N induced a myeloproliferative disease characterized by features of AMKL, including megakaryocytic hyperplasia in the spleen; impaired megakaryocyte polyploidization; and increased reticulin fibrosis of the bone marrow and spleen. These findings provide new insights into pathways and therapeutic targets that contribute to the pathogenesis of AMKL.
Introduction
Acute megakaryoblastic leukemia (AMKL) is a heterogeneous subtype of acute myeloid leukemia (AML) with diverse genetic and morphologic characteristics. The estimated frequency of AMKL varies between studies, perhaps due to a reliance on morphology for diagnostic criteria, but ranges from 3% to 14% of AML, and is more frequent in children than in adults.1-3 In adults, AMKL is also frequently observed as secondary leukemia after chemotherapy or leukemic transformation of several chronic myeloproliferative syndromes including chronic myelogenous leukemia (CML), polycythemia vera (PV), essential thrombocytosis (ET), and idiopathic myelofibrosis (IMF).4-7 Approximately 65% of AMKLs are associated with myelofibrosis.2 Our understanding of the molecular basis of AMKL has progressed over the past several years. In adults, cytogenetic abnormalities are diverse and not restricted to AMKL, including abnormalities of 3p21-3p26; partial or total deletion of chromosomes 5 and 7; or gain of chromosome 19.2,8,9 In childhood AMKL, 2 major subgroups have been described that include patients with constitutional trisomy 21 associated with GATA1 mutations, and those with the t(1;22)(p13;q13) translocation encoding the OTT-MAL (RBM15-MKL1) fusion protein.10-12 Several observations suggest that these latter genetic mutations may not be sufficient to cause an AMKL phenotype. For example, although most Down syndrome patients with constitutional trisomy 21 and GATA1 mutations present with a transient myeloproliferative disorder (TMD) at or around birth, there is spontaneous remission of the TMD and absence of further malignant disease in most instances. However, in approximately 20% of cases, AMKL will develop in the first 4 years of life.10,13-15 In addition, expression of a mutant “GATA-1s” protein in a knock-in mouse model is able to induce a transient hyper-proliferation of yolk sac and fetal liver megakaryocyte progenitors, but is not sufficient to induce AMKL leukemogenesis per se.16 Also, t(1;22)-positive AMKL has been found in monozygotic twins, indicating that the translocation can arise early in development, even though signs and symptoms of disease do not manifest until later in life.17 Together, these observations indicate that there are multigenic contributions to the development of AMKL.
Constitutive tyrosine phosphorylation of STAT5 has been described in a significant proportion of cases of AML.18-20 In several instances, the molecular basis for the constitutive activation of STAT5 is known to be due to activating mutations in tyrosine kinases, including internal tandem duplication (ITD) and activation loop mutations in FLT3, as well as activating mutations in c-KIT.21-23 Although FLT3 mutations have been identified in a broad spectrum of AML, with higher frequencies observed in patients with acute promyelocytic leukemia (APL) with t(15;17) and in AML with normal karyotype,24 mutations in tyrosine kinases are only rarely reported in AMKL. However, a report of a single case of AMKL with an activating mutation in FLT3 suggested to us that other tyrosine kinase mutations might exist in AMKL patients.25 Access to primary AMKL cells is extremely limited due in part to severe myelofibrosis in many cases that precludes bone marrow aspiration, and to prevalence in pediatric populations. In this study, we screened AMKL cell lines for evidence of activation of STAT5, and then used an approach combining mass spectrometry and selective small molecule tyrosine kinase inhibitors as a strategy for identifying novel activating tyrosine kinase mutations in AMKL.
Materials and methods
Cell culture
CHRF-288-11, HEL, and K562 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY). For growth of Ba/F3, 32D, M07e, UT7, Ba/F3 EpoR,26 Ba/F3 TpoR,2 and Ba/F3 granulocyte macrophage-colony-stimulating factor receptor (GMCSFR),28 medium was supplemented with 10 ng/mL mouse interleukin 3 (IL-3). When culture conditions were changed, cells were washed 3 times in phosphate buffered saline (PBS) 1 × and resuspended in the appropriate media: RPMI 1640 with 1% bovine serum albumin (BSA) for 4 hours for serum starvation, RPMI 1640 with 10% FBS and 10 ng/mL of the appropriate cytokine to assess responsiveness to these cytokines. For inhibition studies, cells were resuspended in serum starvation medium with JAK inhibitor I (Calbiochem, San Diego, CA) for 4 hours. For dose response assays, cells were cultured in regular media with various concentrations of the JAK inhibitor I for 72 hours, and the number of viable cells was assessed with the CellTiter 96 Aqueous One Cell Proliferation Assay (Promega, Madison, WI). For each individual cell line, growth in the presence of an increasing amount of inhibitor was normalized to the vehicle-control-only (0 nM) growth. Unless specifically mentioned, fresh media was added every other day according to cell growth. For cytokine independent growth assays, cells were sorted for green fluorescent protein (GFP) expression 24 hours after infection and seeded at 0.1 × 106 cells per mL RPMI 1640 with 10% FBS. Viable cells were counted every day using trypan blue staining. For ploidy analysis, bone marrow cells were cultured 4 days in RPMI 1640 supplemented with 10% FBS, 10 ng/mL mouse stem cell factor (SCF), and mouse thrombopoietin (TPO) prior to analysis.
Analysis by liquid chromatography tandem mass spectrometry (LC-MS/MS)
CHRF-288-11 cells were grown in RPMI 1640 plus 10% FBS and phosphopeptides were prepared using a PhosphoScan Kit (Cell Signaling Technology, Beverly, MA). Peptides in the immunoprecipitation eluate (40 μL) were concentrated using Stop and Go extraction tips (StageTips; Proxcon, Odense, Denmark) and were eluted with 1 μL of 60% MeCN, 0.1% TFA into 7.6 μL of 0.4% acetic acid/0.005% heptafluorobutyric acid. The sample was loaded onto a 10 cm × 75 μm PicoFrit capillary column (New Objective, Woburn, MA) packed with Magic C18 AQ reversed-phase resin (Michrom Bioresources, Auburn, CA) and the column was developed with a 45-minute linear gradient of acetonitrile in 0.4% acetic acid, 0.005% HFBA delivered at 280 nL/min (Ultimate, Dionex, Sunnyvale, CA). Tandem mass spectra were collected in a data-dependent manner with an LTQ ion trap mass spectrometer (ThermoFinnigan, Woburn, MA).
Cloning, immunoprecipitation, and Western blotting
The MSCV JAK2T875N IRES GFP (MIG JAK2T875N) vector was obtained by mutagenesis of the MIG JAK2WT described in Wernig et al29 and was confirmed by sequencing of the entire JAK2 coding region. MIG JAK2V617F was as described.29 For immunoprecipitation and Western blot (WB) analysis, Ba/F3 cells were sorted for GFP expression, and cultured 8 days in RPMI 1640 with 10% FBS and 10 ng/mL of IL-3 to obtain a sufficient amount of cells prior to biochemical analysis. In all cases, 20 × 106 cells were serum starved for 4 hours in RPMI plus 1% BSA, then washed in PBS 1× and lyzed in 20 mM Tris pH 7.4, 1% TritonX-100, 150 mM NaCl, 1 mM EDTA, 10% Glycerol Complete (Roche, Milan, Italy), 1 mM PMSF, 1 mM sodium orthovanadate, 0.1 mM phenylarsine oxide. Lysates were normalized for protein content (BCA protein assay; Pierce, Rockford, IL), immunoprecipitated overnight, incubated with protein G sepharose (Amersham, Arlington Heights, IL) for 1 hour, washed 3 times in lysis buffer, and eluted from the sepharose beads with 2× Laemmli. Direct Western blotting was performed as described before using 50 μg protein.30 Phospho-JAK2(Tyr1007/1008), phospho-STAT5(Y694), phospho-AKT-(Ser473), phospho-ERK1/2(Thr202/Tyr204), AKT, ERK1/2 were obtained from Cell Signaling. STAT5(C17), JAK2(C20) were obtained from Santa Cruz (Santa Cruz, CA), and phospho-tyrosine (4G10) was obtained from Upstate (Lake Placid, NY).
Cell staining, antibodies, and flow cytometry
Staining for flow cytometry was performed in PBS 1× with 2% FBS. All antibodies were obtained from BD Pharmingen (San Diego, CA), except the CD42b antibody obtained from Emfret (Eibelstadt, Germany). Apoptosis was detected 72 hours after addition of the JAK inhibitor I or carrier alone (DMSO) in media using the annexin V-FITC Apoptosis Detection Kit I (BD Pharmingen) and following manufacturer recommendations. For ploidy analysis, cells were first stained with CD41 antibody, followed by staining with APC-conjugated secondary antibody. Cells were then incubated 30 minutes in a 0.1% sodium citrate solution containing 50 μg/mL of RNAseA and 50 μg/mL propidium iodide. Analysis of ploidy was realized on 2 × 104 CD41+ cells. Acquisition of the data was performed on a FACSCalibur (BD Biosciences) and analyzed with CellQuest software. Staining of paraffin-embedded tissue sections was realized using the von Willebrand factor antibody from Dakocytomation (Glostrup, Denmark) as primary antibody. Images of histologic slides were obtained using a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) equipped with a SPOT RT color digital camera (model 2.1.1; Diagnostic Instruments, Sterling Heights, MI). The microscope was equipped with a 10×/22 numeric aperture (NA) ocular lens. Low-power images (magnification × 100) were obtained using a 10×/0.25 NA objective lens; intermediate-power images (magnification × 400), using a 40×/1.3 NA objective lens with oil; and high-power images (magnification × 1000), using a 100×/1.4 NA objective lens with oil (Trak 300; Richard-Allan Scientific, Kalamazoo, MI). Images were analyzed using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). To compare the number of megakaryocytes in spleen from animals that underwent transplantation with JAK2WT and JAK2T875N, von Willebrand factor-positive megakaryocytes were counted under the microscope using a × 400 magnification, in 25 randomly chosen fields. Average values plus or minus the standard error of the mean (SEM) were: 0.88 ± 0.18 (JAK2WT) and 7.8 ± 0.9 (JAK2T875N) megakaryocytes per field (P < .001). For colony-forming assays, primary mouse bone marrow or spleen cells were collected from 5-month-old animals that underwent transplantation, and spleen cells were subsequently treated with red blood cell lysis buffer (Puregene, Gentra Systems, Minneapolis, MN). For myeloid colony assays, 20 000 cells were plated in MethoCult 3434 (StemCell Technologies, Vancouver, BC, Canada) and colonies were scored after 7 days. For megakaryocyte colony assays, 220 000 cells were mixed with MegaCult-C (StemCell Technologies) containing Tpo, IL-11, IL-3, and IL-6, and plated onto 2 double-chamber culture slides; colonies were enumerated after 8 days.
Bone marrow transplantation and animal analysis
Viral supernatants were obtained as described before.31 Briefly, 8- to 10-week-old C57BL/6 or Balb/C donor mice were injected with 5-FU 5 days prior to bone marrow collection. On day 0, primary bone marrow cells were obtained from femurs and tibiae and cultured overnight in RPMI 1640 supplemented with 10% FBS and IL-3, IL-6, and SCF after lysis of red blood cells. Cells were mixed with identical titer viral supernatants 2 times on day 1 and day 2 and spinfected for an hour and a half at 1800g each time. After the second spinfection, 1 × 106 cells were injected in the tail veins of lethally irradiated C57BL/6 or Balb/C recipients. At the time of injection, efficiency of retroviral transduction of bone marrow cells, as assessed by GFP expression by flow cytometry, was about 7% to 10% in both JAK2WT and JAK2T875N transduced cells. At the time of final analysis (150 days after transplantation), the percentage of GFP+ cells in the blood was about the same for JAK2WT but increased to 30% to 55% in JAK2T875N animals. Eye-bleeds were realized using EDTA or heparin-coated capillary tubes and blood counts were performed within 30 minutes on a HemaVet HV950FS (Drew Scientific, Dallas, TX). Erythropoietin concentration was determined on plasma, obtained by centrifugation of the blood (2000g for 20 minutes) using the Mouse Epo Immunoassay (R&D Systems, Minneapolis, MN) and following the manufacturer's recommendations. Approval for the use of animals in this study was granted by the Children's Hospital Boston Animal Care and Use Committee.
Crystal structure and statistical analysis
The Jak2 kinase domain crystal structure solved by Levine et al32 was used, Protein Data Bank (PDB) accession code 2B7A. The model of substitution shown in Figure 2F was made by simple substitution of the side-chain of threonine-875 with an asparagine in the program O,33 followed by rotation away from potential clashes with the crystallographically observed residues of the β2-β3 loop. Figure 2C-F was made using the program PYMOL (www.pymol.org). The Student t test was performed using the program Prism.
Results
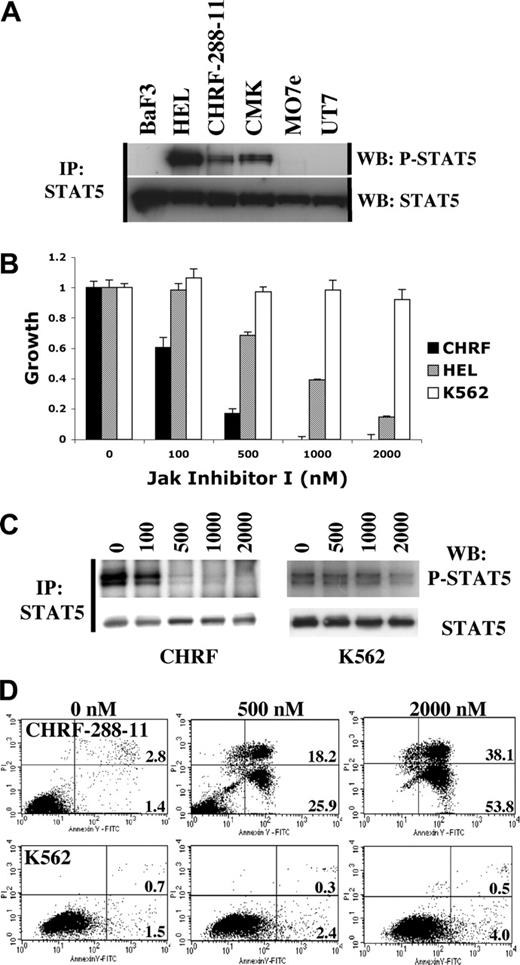
Tyrosine phosphorylation of STAT5 in megakaryocytic cell lines and inhibitory effect of a small molecule JAK kinase inhibitor
AMKL cell lines were screened for constitutive STAT5 phosphorylation in the absence of cytokine or serum stimulation. CHRF-288-11 and CMK, but not M07e or UT7, showed evidence of constitutive STAT5 phosphorylation as assessed by tyrosine phosphorylation (Figure 1A). HEL cells containing the JAK2V617F point mutation were used as a positive control. In order to further characterize the spectrum of kinases that were responsible for constitutive STAT5 phosphorylation, we used a phosphoproteomic approach34 in which phosphopeptides, obtained from immunoprecipitation of trypsin-digested CHRF-288-11 cell lysates, were analyzed by LC-MS/MS. We identified 346 nonredundant phosphopeptides (Table S1, available at the Blood website; see the Supplemental Materials link at the top of the online article) including 41 corresponding to tyrosine kinases. Among these were peptides corresponding to each of the 4 members of the JAK family. These included phosphorylation of 2 tyrosine residues of the activation loop of JAK1 and JAK2, Y1022/Y1023 and Y1007/Y1008 respectively, that are essential for kinase activation. We next tested the growth inhibitory activity of several small molecule tyrosine kinase inhibitors, including the JAK inhibitor I on CHRF-288-11 cells. Growth of CHRF-288-11 cells was potently inhibited by the JAK inhibitor I (Figure 1B). As reported previously, growth of HEL cells, but not K562 cells expressing the BCR-ABL fusion protein, was also inhibited by the JAK inhibitor I.32 In addition, culture of CHRF-288-11 cells in the presence of the JAK inhibitor I resulted in a substantial reduction in tyrosine phosphorylation content of STAT5 (Figure 1C) and induction of apoptosis within 72 hours (Figure 1D). These results indicated that constitutive activation of STAT5 in CHRF-288-11 cells was associated with phosphorylation of JAK kinases and was due to expression of an activated tyrosine kinase that was essential for proliferation and survival of the cells, and sensitive to inhibition by the JAK inhibitor I.
Constitutive STAT5 activation in AMKL cell lines and inhibitory effect of the JAK inhibitor I on CHRF-288-11 cells. (A) Constitutive phosphorylation of STAT5 in AMKL cell lines. (B) Dose-dependent growth inhibition of CHRF-288-11 and HEL cells, but not K562 cells, with increasing concentrations of JAK inhibitor I. The MTT assay was performed at 72 hours. Mean value plus or minus SD of experiments performed in triplicate is represented. For each individual cell line, growth in the presence of an increasing amount of inhibitor was normalized to the vehicle control (0 nM) growth. (C) Inhibition of STAT5 phosphorylation by JAK inhibitor I in CHRF-288-11 cells but not in K562 cells. (D) Induction of apoptosis (annexin V+/PI- cells, bottom right quadrant) and cell death (annexin V+/PI+ cells, top right quadrant) in CHRF-288-11 cells treated by JAK inhibitor I, at 72 hours. Percentage of total population is indicated.
Constitutive STAT5 activation in AMKL cell lines and inhibitory effect of the JAK inhibitor I on CHRF-288-11 cells. (A) Constitutive phosphorylation of STAT5 in AMKL cell lines. (B) Dose-dependent growth inhibition of CHRF-288-11 and HEL cells, but not K562 cells, with increasing concentrations of JAK inhibitor I. The MTT assay was performed at 72 hours. Mean value plus or minus SD of experiments performed in triplicate is represented. For each individual cell line, growth in the presence of an increasing amount of inhibitor was normalized to the vehicle control (0 nM) growth. (C) Inhibition of STAT5 phosphorylation by JAK inhibitor I in CHRF-288-11 cells but not in K562 cells. (D) Induction of apoptosis (annexin V+/PI- cells, bottom right quadrant) and cell death (annexin V+/PI+ cells, top right quadrant) in CHRF-288-11 cells treated by JAK inhibitor I, at 72 hours. Percentage of total population is indicated.
DNA sequence analysis identifies a T875N substitution in JAK2
The JAK inhibitor I is a JAK kinase-specific inhibitor that inhibits the catalytic activity of all 4 JAK family members (JAK1, JAK2, JAK3, and TYK2). Therefore, we screened for sequence variation in all coding exons of these 4 kinases using DNA derived from CHRF-288-11 cells. A single homozygous JAK2C2624A allele was identified in CHRF-288-11 DNA. This allele encodes for a substitution of a threonine for an asparagine at position 875 (T875N) of the JAK2 JH1 kinase domain (Figure 2A).
Analysis of the crystal structure of JAK235 reveals that T875 falls within the loop between strands β2 and β3. This loop is conformationally distinct for the solved JAK kinase domain crystal structures of JAK2 and JAK3 when it is compared to all other solved tyrosine kinase domains.36 The β2-β3 loop is stabilized in a divergent conformation, which exposes a hydrophobic patch, by a conserved aspartate residue (Asp-842 in Jak3, Asp-869 in JAK2) that extensively hydrogen-bonds to backbone amides. This conserved conformation suggests a role for the β2-β3 loop in the formation of inter- or intramolecular interfaces.36 Substitution of threonine-875 for asparagine will alter the surface properties of the loop and could potentially disrupt a JH1-JH2 interface.
JAK2T875N is a constitutively activated tyrosine kinase that confers factor-independent growth to Ba/F3 cells transduced with EpoR or TpoR, but not IL-3R or GMCSFR
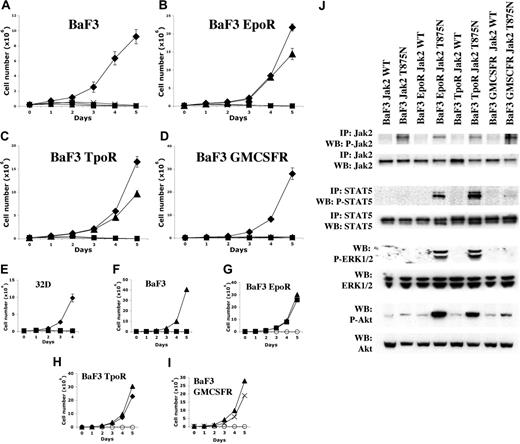
Culture of CHRF-288-11 cells in the absence of serum showed constitutive activation of JAK2 (Figure 2B) but not JAK1, JAK3, or Tyk2 (data not shown), suggesting that JAK2T875N was responsible for constitutive activation of JAK2 in CHRF-288-11 cells. To confirm this hypothesis, the JAK2T875N allele was cloned into the MSCV IRES GFP (MIG) backbone by site-directed mutagenesis of the MIG murine wild-type (WT) JAK2. MIG JAK2WT or MIG JAK2T875N were transfected into 293T cells to produce viral supernatant that was then used to transduce parental Ba/F3 cells expressing IL-3R; or Ba/F3 cells engineered to stably express either the erythropoietin receptor (Ba/F3-EpoR); the thrombopoietin receptor (Ba/F3-Mpl/TpoR); or the granulocyte-macrophage colony-stimulating factor receptor (Ba/F3-GMCSFR). At 24 hours after transduction, GFP-expressing cells were selected by flow cytometry and plated in liquid culture in the absence of IL-3 to assay for IL-3-independent growth. Alternatively, these cells were cultured for 8 days in the presence of IL-3 prior to biochemical analysis.
JAK2T875N conferred IL-3-independent growth to Ba/F3-EpoR or Ba/F3-TpoR cells but somewhat surprisingly, did not confer factor-independent growth to the respective parental Ba/F3 cells or Ba/F3-GMCSFR cells (Figure 3A-D). In addition, JAK2T875N did not confer IL-3-independent growth to the parental 32D myeloid cell line (Figure 3E). Functional expression of the respective cytokine receptors by Ba/F3 clones was confirmed by growth of cells in the presence of their respective ligands (Figure 3F-I). Biochemical analysis showed that although JAK2 was constitutively phosphorylated in all Ba/F3 clones, activation of downstream targets including STAT5, ERK1/2, and AKT was detected only in Ba/F3-EpoR or Ba/F3-TpoR cells, but not in parental Ba/F3 cells (Figure 3J). Weak phosphorylation of STAT5 and increased phosphorylation of AKT could be detected in Ba/F3-GMCSFR cells, although no detectable phosphorylation of ERK1/2 was observed by Western blot analysis. Finally, we used Ba/F3 EpoR cells to compare signaling from JAK2T875N mutant and the recently described JAK2V617F mutant found in patients with polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis.32,37-40 No significant difference was observed by Western blot analysis in the phosphorylation levels of JAK2, STAT5, ERK1/2, or AKT (Figure S1). Taken together these results indicated that JAK2T875N is sufficient to induce constitutive phosphorylation of the JAK2 kinase in the absence of serum or cytokine stimulation. Moreover, in this in vitro Ba/F3 cell culture system, coexpression of certain cytokine receptors including the EpoR or the TpoR resulted in activation of downstream signaling molecules that correlated with their ability to grow in the absence of IL-3.
JAK2 is mutated and constitutively activated in CHRF-288-11 cells. (A) Homozygous JAK2C2624A mutation encoding JAK2T875N in CHRF-288-11 cells. (B) Constitutive phosphorylation of JAK2 in CHRF-288-11 and HEL (positive control expressing JAK2V617F) cells but not in CMK cells. (C) Ribbon diagram representations of the JAK2 kinase domain crystal structure solved by Levine et al.32 β-Strands are shown as arrows, α-helices as coils. The activation loop is colored orange with the phosphorylated tyrosines shown in stick representation. The N-lobe, C-lobe, C-helix, glycine-rich P-loop, and catalytic cleft are all labeled. This crystal structure was solved in complex with a JAK-specific inhibitor, 2-tert-butyl-9-fluoro-3,6-dihydro-7H-benzo[h]imidazo-[4,5-f]isoquinoline-7-one, which is shown as space-filling spheres with carbon atoms colored yellow. The side-chain of threonine-875 is shown as red space-filling spheres and falls in the loop region between strands β2 and β3. (D) Ribbon diagram as in panel C but with the view rotated through 90 degrees. (E) Close up of the β2-β3 loop. Side-chains of residues comprising this loop are shown in stick format. Threonine-875 is additionally indicated with space-filling spheres and is labeled. (F) Same view as panel E, but illustrating the T875N point mutation with a model of the substitution. Modeling of the mutation of threonine-875 to asparagine suggests a change in the surface properties of this loop, which may disrupt potential JH1-JH2 interactions.
JAK2 is mutated and constitutively activated in CHRF-288-11 cells. (A) Homozygous JAK2C2624A mutation encoding JAK2T875N in CHRF-288-11 cells. (B) Constitutive phosphorylation of JAK2 in CHRF-288-11 and HEL (positive control expressing JAK2V617F) cells but not in CMK cells. (C) Ribbon diagram representations of the JAK2 kinase domain crystal structure solved by Levine et al.32 β-Strands are shown as arrows, α-helices as coils. The activation loop is colored orange with the phosphorylated tyrosines shown in stick representation. The N-lobe, C-lobe, C-helix, glycine-rich P-loop, and catalytic cleft are all labeled. This crystal structure was solved in complex with a JAK-specific inhibitor, 2-tert-butyl-9-fluoro-3,6-dihydro-7H-benzo[h]imidazo-[4,5-f]isoquinoline-7-one, which is shown as space-filling spheres with carbon atoms colored yellow. The side-chain of threonine-875 is shown as red space-filling spheres and falls in the loop region between strands β2 and β3. (D) Ribbon diagram as in panel C but with the view rotated through 90 degrees. (E) Close up of the β2-β3 loop. Side-chains of residues comprising this loop are shown in stick format. Threonine-875 is additionally indicated with space-filling spheres and is labeled. (F) Same view as panel E, but illustrating the T875N point mutation with a model of the substitution. Modeling of the mutation of threonine-875 to asparagine suggests a change in the surface properties of this loop, which may disrupt potential JH1-JH2 interactions.
JAK2T875N induces constitutive signaling leading to growth factor independence. JAK2T875N induces IL-3-independent growth of EpoR- or TpoR-expressing Ba/F3 cells (B, C) but not parental Ba/F3 cells (A), GMCSFR-expressing Ba/F3 cells (D) or parental 32D cells (E). Three independent experiments, each in triplicate, were realized and the mean plus or minus SD of one representative experiment is shown: MIG empty vector control +IL-3 (♦), MIG empty vector control -IL-3 (▪), MIG JAK2T875N -IL-3 (▴), MIG JAK2WT -IL-3 (×). Controls for functional expression of the different cytokine receptors were obtained by growing Ba/F3 EpoR, Ba/F3 TpoR, and Ba/F3 GMCSFR, respectively, in presence of EPO (squares; G), Tpo (♦; H), GMCSF (×; I) or in absence of cytokine (○). Parental Ba/F3 cells proliferate only in the presence of IL-3 (▴; F). (J) Studies of Jak2, STAT5, ERK1/2, and AKT phosphorylation in the different Ba/F3 clones overexpressing JAK2WT or mutant JAK2T875N.
JAK2T875N induces constitutive signaling leading to growth factor independence. JAK2T875N induces IL-3-independent growth of EpoR- or TpoR-expressing Ba/F3 cells (B, C) but not parental Ba/F3 cells (A), GMCSFR-expressing Ba/F3 cells (D) or parental 32D cells (E). Three independent experiments, each in triplicate, were realized and the mean plus or minus SD of one representative experiment is shown: MIG empty vector control +IL-3 (♦), MIG empty vector control -IL-3 (▪), MIG JAK2T875N -IL-3 (▴), MIG JAK2WT -IL-3 (×). Controls for functional expression of the different cytokine receptors were obtained by growing Ba/F3 EpoR, Ba/F3 TpoR, and Ba/F3 GMCSFR, respectively, in presence of EPO (squares; G), Tpo (♦; H), GMCSF (×; I) or in absence of cytokine (○). Parental Ba/F3 cells proliferate only in the presence of IL-3 (▴; F). (J) Studies of Jak2, STAT5, ERK1/2, and AKT phosphorylation in the different Ba/F3 clones overexpressing JAK2WT or mutant JAK2T875N.
Expression of JAK2T875N in a murine bone marrow transplantation model causes a myeloproliferative disease with certain features of AMKL
To assess in vivo effects of the JAK2T875N allele, JAK2T875N or JAK2WT were retrovirally transduced into primary bone marrow cells derived from C57Bl/6 or Balb/C mice after treatment with 5-fluorouracil (5-FU) and cells were transplanted into lethally irradiated recipient mice.
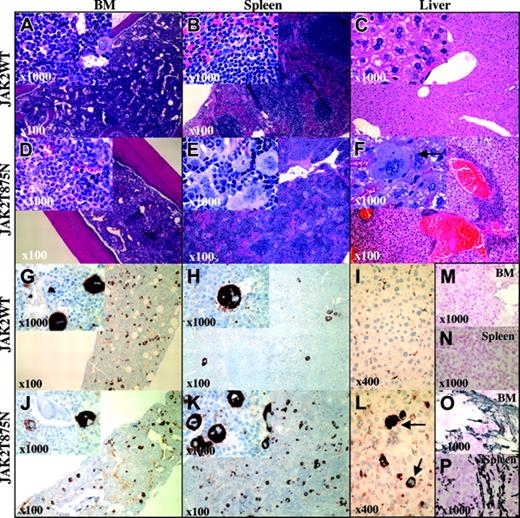
No animals that underwent transplantation with either JAK2T875N- or JAK2WT-transduced cells succumbed, with more than 6 months of follow-up. End-point analysis was therefore performed on a cohort of animals at 150 days after transplantation. In the Balb/C background, bone marrow from JAK2T875N animals exhibited marked myeloid hyperplasia comprised of maturing myeloid forms compared with normal maturing hematopoietic marrow elements in JAK2WT animals (Figure 4A,D). JAK2T875N induced marked splenomegaly (Figure 5A), and normal architecture was partially to completely disrupted with a significant expansion of the red pulp visible in histopathologic analysis, resulting from an infiltrate of admixed maturing myeloid, maturing erythroid elements, and dramatically increased numbers of atypical and dysplastic megakaryocytes (Figure 4B,E). Atypical morphologic features of megakaryocytes included the frequent presence of giant megakaryocytes that occurred singly or in clusters compared with the megakaryocytes that were observed in JAK2WT animals. Many JAK2T875N megakaryocytes contained abundant, mature cytoplasm, and often displayed abnormal patterns of chromatin clumping. In addition, emperipolesis of neutrophils in megakaryocyte cytoplasm was frequently observed. Immunochemistry using an anti-von Willebrand factor antibody to highlight megakaryocyte elements demonstrated an 8.8-fold increase (P < .001; see “Materials and methods”) in the average number of megakaryocytes present in the spleens from JAK2T875N versus JAK2WT animals (Figure 4H,K). In contrast, a slight decrease in the number of megakaryocytes was observed in the bone marrow of JAK2T875N animals compared with JAK2WT mice (Figure 4G,J). Evidence of extramedullary hematopoiesis (EMH) was observed in other organs in all animals examined, including perivascular infiltration of the liver with both atypical megakaryocytes and maturing myeloid cells (Figure 4C,F,I,L). Focal EMH was also observed in Peyer patches of the gastrointestinal tract as well as in lung parenchyma (data not shown).
Histopathology of representative Balb/C animals that underwent transplantation with JAK2WT and JAK2T875N. Panels display hematoxylin and eosin (H&E)-stained sections from bone marrow (BM: A,D), spleen (B,E), and liver (C,F) from Balb/C mice that underwent transplantation with bone marrow cells transduced with either JAK2WT or JAK2T875N. Immunohistochemical staining for von Willebrand factor is shown (G-L) as well as reticulin staining of BM (M,O) and spleen (N,P). Similar features were observed in C57Bl/6 animals, showing high hematocrit levels that underwent transplantation with JAK2T875N. Arrows indicate infiltrated megakaryocytes. Original magnifications are indicated in each panel and inset.
Histopathology of representative Balb/C animals that underwent transplantation with JAK2WT and JAK2T875N. Panels display hematoxylin and eosin (H&E)-stained sections from bone marrow (BM: A,D), spleen (B,E), and liver (C,F) from Balb/C mice that underwent transplantation with bone marrow cells transduced with either JAK2WT or JAK2T875N. Immunohistochemical staining for von Willebrand factor is shown (G-L) as well as reticulin staining of BM (M,O) and spleen (N,P). Similar features were observed in C57Bl/6 animals, showing high hematocrit levels that underwent transplantation with JAK2T875N. Arrows indicate infiltrated megakaryocytes. Original magnifications are indicated in each panel and inset.
JAK2T875N induces a myeloproliferative disease in a murine bone marrow transplant model. Spleen weight of Balb/C animals (A), hematocrit percentages (B), and white blood counts (C) of C57Bl/6 and Balb/C animals and platelet counts of Balb/C animals (D), analyzed at 150 days after transplantation are shown. Each histogram bar represents the mean value from 5 animals plus or minus SD. Student t test was performed and the P value is indicated when a significant difference (P < .05) between JAK2WT and JAK2T875N animals was observed.
JAK2T875N induces a myeloproliferative disease in a murine bone marrow transplant model. Spleen weight of Balb/C animals (A), hematocrit percentages (B), and white blood counts (C) of C57Bl/6 and Balb/C animals and platelet counts of Balb/C animals (D), analyzed at 150 days after transplantation are shown. Each histogram bar represents the mean value from 5 animals plus or minus SD. Student t test was performed and the P value is indicated when a significant difference (P < .05) between JAK2WT and JAK2T875N animals was observed.
As AMKL is often associated with abnormal fibrosis in patients, we performed reticulin staining on bone marrow and spleen from diseased and control animals. Both bone marrow and spleen of JAK2T875N animals showed a moderately increased reticulin staining compared with JAK2WT animals (Figure 4M-P). Although less penetrant, the disease induced by JAK2T875N was similar in the C57/Bl6 background (data not shown). In both strains, JAK2T875N mice also developed polycythemia (Figure 5B). In the Balb/C strain, there was 100% disease penetrance (10/10 mice), notable for extreme elevations of hematocrit (> .85 [85%]) and mild leukocytosis (Figure 5C), whereas in the C57Bl/6 strain, the disease penetrance was about 30% (3/10 mice), with an intermediate increase in hematocrit (range, .50-.85 [50%-85%]). In both strains, animals with elevated hematocrit levels also exhibited mildly to moderately increased white blood cell counts (range, 13 × 109-54 × 109/L [13 × 103-54 × 103/μL]. Polycythemia was stable for more than 5 months after transplantation in both strains (Figure S2). In order to exclude the unlikely possibility that the elevation in hematocrit was due to secondary causes, serum erythropoietin levels were measured. Very low concentrations (0-76 pg/mL) of erythropoietin were observed in JAK2T875N animals; low to intermediate concentrations (50-145 pg/mL) were observed in JAK2WT animals and positive control animals that were anemic after injection of 5-FU had the expected high concentrations of serum erythropoietin (2200-6100 pg/mL; Figure S3). There was no significant difference in platelet counts in either mouse strain that underwent transplantation with JAK2T875N or JAK2WT (Figure 5D).
Immunophenotypic analysis of JAK2T875N-induced myeloproliferative disease
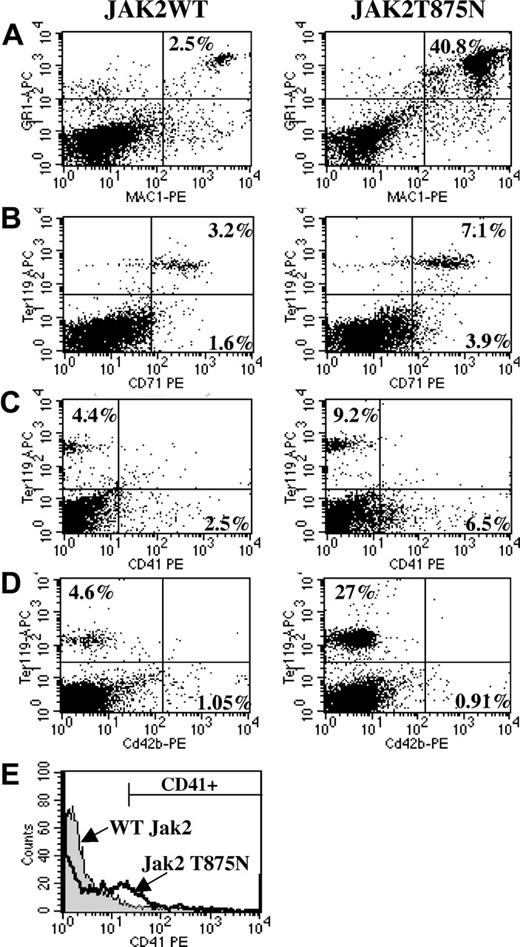
Immunophenotypic analysis showed a significant increase in the percentage of Mac1/Gr1 double-positive cells in spleen (Figure 6A, 40.6% ± 4.1% versus 5.2% ± 2.3%, P = .002, n = 3), peripheral blood, and bone marrow (data not shown) of JAK2T875N animals compared with those that underwent transplantation with JAK2WT. In addition, an increase (4.1% ± 0.3% in JAK2WT animals versus 15.2% ± 4.4% in JAK2T875N animals, P = .038, n = 5) in the percentage of Ter119-positive cells was observed in the spleen (Figure 6B-D), but not in the bone marrow (data not shown), in agreement with the histopathologic findings. Analysis of the megakaryocyte lineage was performed using antibodies directed against the adult megakaryocyte-specific markers CD41 and CD42b. During adult megakaryopoiesis, CD41 is expressed early during megakaryopoiesis whereas CD42b is expressed on more mature megakaryocytes.41,42 Consistent with histopathology and immunohistochemical studies, the percentage of CD41+ cells was increased in the spleens of JAK2T875N animals compared with JAK2WT animals (5.6% ± 0.5% versus 2% ± 0.2%, P = .002, n = 3; Figure 6C). This increase was even more pronounced (14.3% versus 2.4%) when the analysis was gated on GFP-positive cells only (Figure 6E). However, there was no significant difference in the percentage of CD42b-expressing cells (Figure 6D), nor was there a significant difference in the number of CD41- or CD42b-expressing cells in the bone marrow between JAK2T875N and JAK2WT animals (data not shown).
Taken together, these results confirmed the histopathologic analysis and indicate that JAK2T875N animals develop a myeloproliferative disease with a striking expansion of the megakaryocytic lineage that is most prominently observed in the spleen, as well as expansion of the erythroid and myeloid lineages.
Flow cytometric analysis of animals that underwent transplantation with JAK2WT and JAK2T875N. Following red blood cell lysis, total spleen cells from JAK2WT or JAK2T875N animals were analyzed by flow cytometry for expression of several lineage cell surface markers. Compared with cells from animals that underwent transplantation with JAK2WT, spleen cells from JAK2T875N mice exhibited significantly increased percentages of mature myeloid (A), erythroid (B), and megakaryocytic (C) elements. No increase in the percentage of CD42b-expressing cells was detected between JAK2WT and JAK2T875N animals (D). The increase in the number of CD41-expressing cells (megakaryocytes) in JAK2T875N mice was even more prominent when the analysis was performed on GFP-positive gated spleen cells (E).
Flow cytometric analysis of animals that underwent transplantation with JAK2WT and JAK2T875N. Following red blood cell lysis, total spleen cells from JAK2WT or JAK2T875N animals were analyzed by flow cytometry for expression of several lineage cell surface markers. Compared with cells from animals that underwent transplantation with JAK2WT, spleen cells from JAK2T875N mice exhibited significantly increased percentages of mature myeloid (A), erythroid (B), and megakaryocytic (C) elements. No increase in the percentage of CD42b-expressing cells was detected between JAK2WT and JAK2T875N animals (D). The increase in the number of CD41-expressing cells (megakaryocytes) in JAK2T875N mice was even more prominent when the analysis was performed on GFP-positive gated spleen cells (E).
Colony-forming assays and megakaryocyte differentiation of primary cells derived from animals with JAK2T875N-induced myeloproliferative disease
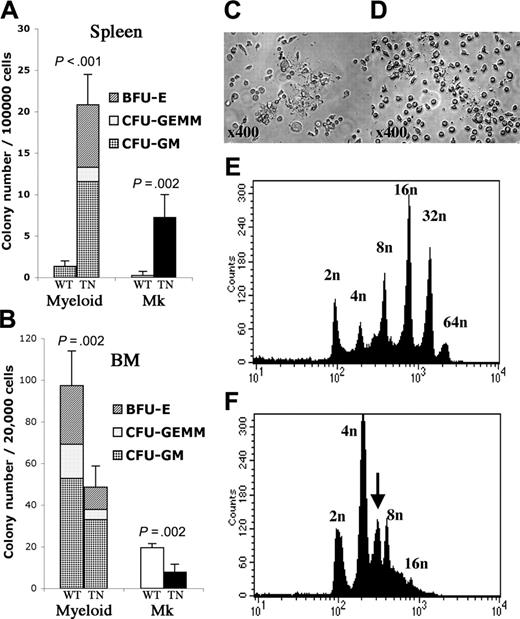
Myeloid and megakaryocyte colony-forming unit (CFU) assays were performed in methylcellulose containing IL-3, IL-6, SCF, and Epo, or in collagen-based media containing Tpo, IL-11, IL-3, and IL-6, respectively. Compared with JAK2WT mice, there was a significant increase in the number of CFU-megakaryocyte (CFU-Mk) and myeloid colonies (composed of erythroid burst-forming units [BFU-Es], granulocyte/macrophage/erythroid/megakaryocyte CFUs [CFU-GEMMs], and granulocyte-macrophage CFUs [CFU-GMs]) obtained from the spleens of JAK2T875N animals (Figure 7A). A consistent approximately 2-fold reduction in the number of myeloid or megakaryocyte progenitors was observed when cells were derived from bone marrow rather than spleen (Figure 7B). No significant difference was observed in the replating potential of JAK2T875N colonies compared with colonies derived from JAK2WT animals or when cells were plated in methylcellulose in the absence of cytokine (data not shown).
To further characterize the disparity between megakaryocyte hyperplasia in the spleen, but without an increase in peripheral blood platelet counts, we assessed terminal differentiation of megakaryocytes. Ploidy and ability to form platelets were assessed in bone marrow cells from animals that underwent transplantation, cultured ex vivo for 4 days in the presence of 10 ng/mL Tpo and SCF. In both JAK2WT and JAK2T875N cell cultures, mature proplatelet-forming megakaryocytes were observed, indicating that terminal differentiation was achieved (Figure 7C-D). Nonetheless, analysis of polyploidization of CD41+ cells showed a significant reduction in the median ploidy of JAK2T875N megakaryocytes compared with JAK2WT megakaryocytes (4 n versus 16 n; Figure 7E-F). Taken together, these results indicate that JAK2T875N induces an important increase in the number of megakaryocyte progenitors. In addition, although terminal differentiation of megakaryocytes can be achieved in vitro, expression of the JAK2T875N mutant induces a partial block in the megakaryocyte polyploidization process.
Discussion
AMKL is a subtype of AML that is frequently associated with a poor prognosis. This study was initiated to screen for novel tyrosine kinase mutations in AMKL that might contribute to disease pathogenesis, and provide novel targets for therapeutic intervention. We screened for AMKL cell lines in which STAT5 was constitutively activated, as assessed by tyrosine phosphorylation, and followed up with an approach combining mass spectrometry and inhibition of the cell lines with specific small-molecule tyrosine kinase inhibitors. Based on identification of JAK kinase phosphopeptides by mass spectrometry and inhibition of the CHRF-288-11 cell line growth with a small-molecule inhibitor of JAK kinases, we identified a new homozygous JAK2T875N activating mutation. CHRF-288-11 was originally established from a biopsy of a granulocytic sarcoma in a 17-month-old infant with AMKL and myelofibrosis.43 CHRF-288-11 differs from a number of other cell lines derived from patients with AMKL in its expression of megakaryocyte markers, but not erythroid markers.44 In addition to the JAK2T875N mutation described in this report, CHRF-288-11 presents several chromosomal abnormalities including several that are often identified in patients with AMKL, including trisomy 21 and trisomy 19. Although trisomy 21 is frequently found in association with GATA1 mutations in Down syndrome patients with AMKL, CHRF-288-11 does not contain mutations in exon 2 of GATA1 (T.M. and O.A.B., unpublished observation, November 2002). Also described here, the CMK cell line, derived from a patient with Down syndrome and AMKL, contains a GATA1 mutation10 and a constitutive STAT5 activation but no JAK2 mutation was detected. In addition, M07e and UT7 cell lines, respectively derived from 6-month-old and 64-year-old AMKL patients, do not present constitutive STAT5 activation. These observations confirm the molecular heterogeneity of AMKL and suggest that constitutive STAT5 activation, in some cases, likely results from mutations in other tyrosine kinases (eg, CMK) or, in some other cases, may not be exquisitely required for AMKL transformation.
Animals that underwent transplantation with JAK2T875N show increased spleen myeloid colony-forming progenitors and abnormal megakaryocyte ploidy content. Myeloid (CFU-GM, CFU-GEMM, BFU-E) and megakaryocyte colony-forming assays were performed, respectively, in methylcellulose supplemented with IL-3, IL-6, SCF, and EPO and collagen-based media supplemented with TPO, IL-11, IL-3, and IL-6. The experiment was performed in triplicate and the mean plus or minus SD colony number obtained from spleen cells (A) or bone marrow cells (B) is shown. For myeloid colonies, average ratio of BFU-Es, CFU-GEMMs, and CFU-GMs is represented. Of note, BFU-Es were identified in JAK2T875N-derived colonies but not in JAK2WT-derived colonies. WT: JAK2WT; TN: JAK2T875N; Mk: megakaryocyte colonies; BM: bone marrow. Student t test was performed and P value is indicated. For ploidy analysis, bone marrow cells from animals that underwent transplantation were cultured for 4 days in liquid media containing TPO and SCF. Terminal differentiation with proplatelet formation was achieved with both JAK2WT- and JAK2T875N-expressing megakaryocytes (C,D). Cells were stained with propidium iodide (PI) and CD41+ cells were analyzed for ploidy content. Analyses were performed in duplicate and representative PI histograms are shown. Compared with JAK2WT (E), ploidy content in JAK2T875N megakaryocytes (F) was markedly reduced and an abnormal 6 n peak (arrow) was observed, consistent with a partial block at the initiation of the polyploidization process.
Animals that underwent transplantation with JAK2T875N show increased spleen myeloid colony-forming progenitors and abnormal megakaryocyte ploidy content. Myeloid (CFU-GM, CFU-GEMM, BFU-E) and megakaryocyte colony-forming assays were performed, respectively, in methylcellulose supplemented with IL-3, IL-6, SCF, and EPO and collagen-based media supplemented with TPO, IL-11, IL-3, and IL-6. The experiment was performed in triplicate and the mean plus or minus SD colony number obtained from spleen cells (A) or bone marrow cells (B) is shown. For myeloid colonies, average ratio of BFU-Es, CFU-GEMMs, and CFU-GMs is represented. Of note, BFU-Es were identified in JAK2T875N-derived colonies but not in JAK2WT-derived colonies. WT: JAK2WT; TN: JAK2T875N; Mk: megakaryocyte colonies; BM: bone marrow. Student t test was performed and P value is indicated. For ploidy analysis, bone marrow cells from animals that underwent transplantation were cultured for 4 days in liquid media containing TPO and SCF. Terminal differentiation with proplatelet formation was achieved with both JAK2WT- and JAK2T875N-expressing megakaryocytes (C,D). Cells were stained with propidium iodide (PI) and CD41+ cells were analyzed for ploidy content. Analyses were performed in duplicate and representative PI histograms are shown. Compared with JAK2WT (E), ploidy content in JAK2T875N megakaryocytes (F) was markedly reduced and an abnormal 6 n peak (arrow) was observed, consistent with a partial block at the initiation of the polyploidization process.
Several lines of evidence suggest that the JAK2T875N mutation may play a role in the pathogenesis of AMKL. First, the mutation results in constitutive activation of the JAK2 kinase and its downstream effectors in cultured hematopoietic cell lines. Furthermore, JAK2T875N confers factor-independent growth to Ba/F3 cells that express either the TpoR or EpoR that are also expressed on early megakaryocyte-erythroid progenitors (MEPs). Interestingly, TpoR (Mpl) was also expressed and phosphorylated in CHRF-288-11 cells (Table S1). Second, expression of a mutant JAK2T875N in a murine bone marrow transplant model induces a myeloproliferative disease with a striking megakaryocyte phenotype, including increased number of megakaryocyte progenitors in the spleen, infiltration of several organs with atypical and dysplastic megakaryocytes, abnormal emperipolesis of neutrophils by megakaryocytes, and increased reticulin fibrosis of bone marrow and spleen. In addition, JAK2T875N expression induces abnormal megakaryopoiesis in vitro with a block of megakaryocyte polyploidization. The number of platelets produced per megakaryocyte has been reported to be proportional to the degree of polyploidization.45,46 This observation of decreased polyploidization may therefore explain why normal levels of platelets are observed in the blood of JAK2T875N animals in the context of megakaryocyte hyperplasia. Although there are several potential explanations for the apparent impairment of megakaryocyte maturation in JAK2T875N animals, it is possible that high JAK-STAT signaling is important in the early stages of erythroid and megakaryocytic progenitor proliferation, but that decreased JAK-STAT pathway activation is specifically required for terminal maturation of the megakaryocyte lineage. This hypothesis needs to be investigated in more detail but is consistent with our observation of an increased number of CD41+ immature megakaryocyte progenitors, but not of CD42b+ more mature megakaryocytes in the spleen of animals that underwent transplantation with JAK2T875N. Third, organomegaly and extramedullary hematopoiesis are frequently observed in patients with AMKL. One consistent observation in this study was the significant involvement of the spleen in disease pathogenesis, out of proportion to bone marrow involvement. In human AMKL, splenomegaly is generally thought to result from extramedullary hematopoiesis in the context of bone marrow infiltration and fibrosis. Nevertheless, it is also possible that this reduction in bone marrow myeloid progenitors results from cell-autonomous or nonautonomous contributions mediated by constitutive activation of JAK2. Several candidate pathways, regulated by JAK-STAT signaling, including the SDF1-CXCR4 axis important for interaction between stromal elements and hematopoietic progenitors and retention of the latter in the bone marrow,47,48 could potentially be implicated.
Additional support for a role of the JAK2T875N activating allele in AMKL includes the observation that JAK2V617F mutation, recently described in patients with polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis, has also been reported in 2 of 11 cases of AMKL.49 Although these cases may have been secondary to PV, ET, or IMF, this finding indicates that at least 2 JAK2 mutations that result in constitutive activation of JAK2 can be found in AMKL. It is worth noting that JAK2T875N shares several interesting features with the JAK2V617F allele. First, JAK2V617F also signals most efficiently in Ba/F3 cells transduced with EpoR or TpoR.32,37-40 This suggests that a cytokine receptor scaffold is specifically required for transformation mediated by JAK2 point mutants. Interestingly, this feature is not characteristic of other previously described activating alleles of tyrosine kinases (such as BCR-ABL, TEL-PDGFRβ, or even TEL-JAK2). Although the molecular mechanism for apparent cytokine receptor selectivity is not fully understood, these findings may provide insights into the myeloid lineage predilection of these mutant alleles. Second, bone marrow transplant models of disease with these 2 alleles present several common features, including striking erythrocytosis, megakaryocyte hyperplasia, impaired megakaryocyte polyploidization ex vivo, and increased bone marrow and spleen fibrosis.29 Many of these atypical megakaryocytes' morphologic features are reminiscent of those often found in association with the JAK2V617F associated chronic myeloproliferative disorders including essential thrombocythemia, polycythemia vera, and chronic idiopathic myelofibrosis, but unlike the small hypolobated megakaryocytes often found in BCR-ABL-associated chronic myelogenous leukemia (CML). Nevertheless, significant differences between JAK2T875N and JAK2V617F can also be noted. Interestingly, compared with JAK2V617F, JAK2T875N did not induce a striking leukocytosis, and disease was not lethal over a 5-month follow-up period in a Balb/C bone marrow transplantation model. In addition, the reduction in ploidy in megakaryocyte cultures was more important in JAK2T875N bone marrow-derived megakaryocytes compared with those derived from JAK2V617F bone marrow.29 These observations suggest that JAK2T875N may have a more prominent effect on the megakaryocyte lineage and that lethal leukocytosis and erythrocytosis can be uncoupled. The differences between the 2 models are likely not due to different bone marrow transplantation techniques, as these disparities have been replicated in multiple bone marrow transplantation experiments and were performed using identical protocols, murine background, retroviral backbones, and viral titers (data not shown).
Although we did not detect significant differences in STAT5, ERK, or AKT phosphorylation levels in a Ba/F3 EpoR in vitro culture system using conventional Western blot techniques, we cannot exclude subtle variations in activation of signaling pathways downstream of JAK2, and further investigation will therefore be required. Taken together, these results indicate that activation of the JAK/STAT pathway in AMKL warrants further study and that screening for JAK2 mutations should now include both the JH2 and JH1 domains. These data also annotate the value of mass spectrometric strategies as functional screens for mutant kinase alleles in human cancer.
Finally, although these lines of evidence support an important role for the JAK2T875N allele in pathogenesis of AMKL, it is equally clear that it is not sufficient per se in this model system for the development of AMKL. It is likely that JAK2T875N is one of several steps required for leukemogenesis and that additional mutations are required, with candidates that could include GATA1 mutations, genetic events that phenocopy trisomy 21 or trisomy 19, among others. Studies of genetic abnormalities found in the CHRF-288-11 cell line, primary AMKL samples, and murine models should help us to understand the nature of these required additional mutations as well as the target cell and order in which they need to occur.
Prepublished online as Blood First Edition Paper, June 27, 2006; DOI 10.1182/blood-2006-04-014712.
Supported in part by National Institutes of Health grants DK50 654 and CA66 996 (D.G.G.), the Leukemia and Lymphoma Society, grant FR 2113/1-1 from Deutsche Forschungsgemeinschaft (S.F.), and by an American Society of Hematology Basic Research Scholar Award (T.J.B.). D.G.G. is a Doris Duke Foundation Distinguished Clinical Scientist and an investigator of the Howard Hughes Medical Institute. T.M. is a recipient of a Special Fellow Award from the Leukemia and Lymphoma Society.
Two of the authors (T.-L.G. and R.D.P.) are employed by Cell Signalling Technology Inc, whose potential product was studied in the present work.
T.M. designed research, performed research, and wrote the manuscript; G.W. contributed vital reagents and performed research. S.A.M., R.L.L., T.-L.G., D.C., and R.D.P. performed research; S.F. provided patient samples; O.A.B. provided vital reagents; T.J.B. analyzed crystal structure; B.H.L. analyzed histopathology data; and D.G.G. designed research and revised the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Vainchenker, Dr D'Andrea, Dr Lodish, Dr Griffin, and Dr Nimer for providing CHRF-288-11 cells, Ba/F3-EpoR cells, Ba/F3-TpoR cells, Ba/F3-GMCSFR cells, and UT7 cells, respectively. We thank Jeffery L. Kutok and the Brigham and Women's Hospital Pathology Core Facility for histopathology analysis.

![Figure 2. JAK2 is mutated and constitutively activated in CHRF-288-11 cells. (A) Homozygous JAK2C2624A mutation encoding JAK2T875N in CHRF-288-11 cells. (B) Constitutive phosphorylation of JAK2 in CHRF-288-11 and HEL (positive control expressing JAK2V617F) cells but not in CMK cells. (C) Ribbon diagram representations of the JAK2 kinase domain crystal structure solved by Levine et al.32 β-Strands are shown as arrows, α-helices as coils. The activation loop is colored orange with the phosphorylated tyrosines shown in stick representation. The N-lobe, C-lobe, C-helix, glycine-rich P-loop, and catalytic cleft are all labeled. This crystal structure was solved in complex with a JAK-specific inhibitor, 2-tert-butyl-9-fluoro-3,6-dihydro-7H-benzo[h]imidazo-[4,5-f]isoquinoline-7-one, which is shown as space-filling spheres with carbon atoms colored yellow. The side-chain of threonine-875 is shown as red space-filling spheres and falls in the loop region between strands β2 and β3. (D) Ribbon diagram as in panel C but with the view rotated through 90 degrees. (E) Close up of the β2-β3 loop. Side-chains of residues comprising this loop are shown in stick format. Threonine-875 is additionally indicated with space-filling spheres and is labeled. (F) Same view as panel E, but illustrating the T875N point mutation with a model of the substitution. Modeling of the mutation of threonine-875 to asparagine suggests a change in the surface properties of this loop, which may disrupt potential JH1-JH2 interactions.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/8/10.1182_blood-2006-04-014712/4/m_zh80200602590002.jpeg?Expires=1769161304&Signature=E-6OE2BRbE0NhMrGOQ-MhQ~SMgHRowI8s2FrqVTWFjSY9P6OpM4rG1Q5g3ajOq70jglIwcXL8ePO9kpjeKJp-ocAgV1EClDj3b77xEP3VpH0FwDbb7wAAX~7RCRh~Vcdd4PDZ9g973Fo9tcdTyqCaJrMExQ6RCqDJSWnE6X2GweLwCN2QR23g1eWX9QyDyWMRsAuqgU2RV~TWRx1A5TOkprhrqmIV9bEUVYxNcRSyZHHeG-8GxXuDrkfvSvPv2ROkr3BGzryLJvxga7CBXv9xztuKW4eIQ0iypXvuoeqp8kJxFIux5TnDQVdrv7uZjOoCZntxg0WUeoPtqt7jU-UIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal