Abstract
The immunosurveillance of Hodgkin lymphoma (HL) by cytotoxic T lymphocytes (CTLs) is insufficient, and the clinical experience with adoptive transfer of CTLs is limited. We have previously reported that defects in mitochondrial apoptotic pathways and elevated XIAP expression confer resistance to different apoptotic stimuli in HL cells. Here, we aimed to develop molecular strategies to overcome the resistance of HL cells against CTL-mediated killing via granzyme B (grzB). In HL cells, grzB-induced mitochondrial release of proapoptotic Smac is blocked, which results in complete abrogation of cytotoxicity mediated by CTLs. Cytosolic expression of recombinant mature Smac enhanced caspase activity induced by grzB and restored the apoptotic response of HL cells. Similarly, down-regulation of XIAP by RNA interference markedly enhanced the susceptibility of HL cells for CTL-mediated cytotoxicity. XIAP gene knockdown sensitized HL cells for killing by antigen-specific CTLs redirected by grafting with a chimeric anti-CD30scFv-CD3zeta immunoreceptor. The results suggest that XIAP targeting by Smac agonists or XIAP-siRNA can be used as a synergistic strategy for cellular immunotherapy of Hodgkin lymphoma.
Introduction
Apoptosis is a highly regulated cellular response that ultimately results in the elimination and disposal of unwanted or damaged cells. Apoptosis is brought about by a family of proteases known as the caspases, the activity of which is responsible for the organized destruction of the cell.1 At least 2 distinct major apoptotic signaling pathways initiating caspase activity can be distinguished. The triggering of death domain-containing cell surface receptors of the TNF receptor superfamily results in the activation of the initiator caspase, caspase-8.2 A second apoptotic signaling pathway involves mitochondria and results in the release of cytochrome c, which subsequently initiates the activation of another initiator caspase, caspase-9.3 The regulatory mechanisms of caspase activation require, in general, activation of the zymogens of initiator caspase-8 or -9. Once active, these initiator caspases cleave and activate the zymogens of executioner caspases such as caspases-3 and -7, which in turn are responsible for the majority of proteolytic events that ultimately result in the destruction of the cell.
Caspase activation and activity can be modulated by direct interaction with members of the IAP (inhibitor of apoptosis protein) family, such as XIAP (X-chromosome-linked IAP).4-6 XIAP binds to and inhibits caspases-3, -7, and -9.7 The inhibitory action of XIAP, in turn, is regulated by Smac (second mitochondria-derived activator of caspases). In intact cells, Smac is a mitochondrial protein that is proteolytically processed and released during apoptosis along with cytochrome c and other mitochondrial proapoptotic factors. Once in the cytosol, Smac protein binds to XIAP and disrupts its activity.8
Cytotoxic T lymphocytes (CTLs) are known to initiate target cell death via 2 pathways: CD95 ligand and perforin/granzyme (granule-mediated killing). Granzyme B (grzB) is a major effector molecule of granule-mediated killing that rapidly induces cell death after entering the cytoplasm of the target cell.9 The enzymatic activity of grzB is the key to its ability to induce cell death. The executioner caspase-3 has been shown to be proteolytically processed and activated by grzB.10 In addition, mitochondrial perturbation in response to grzB seems to be essential for caspase activity to reach a threshold sufficient to kill the cell.11 In cells with dysfunctional mitochondria, the initial direct cleavage of caspase-3 by grzB occurs, resulting in production of the p20 fragment of caspase-3.12 Further processing, however, becomes stalled due to the caspase inhibitory effect of XIAP.13-15 Obviously, processing of procaspases by grzB alone is not sufficient to induce a level of caspase activation that can kill the target cell. This is dependent on additional release of proapoptotic factors, such as Smac from mitochondria displacing XIAP from partially processed caspases to permit completion of caspase autoactivation.
Resistance to apoptotic stimuli is one of the main mechanisms of cancer cell survival. Hodgkin and Reed-Sternberg (H-RS) cells are derived mainly from germinal center or post-germinal center B cells, while a very small minority (< 2%) is derived from T cells. H-RS cells lack specific functional markers of mature B or T cells, seem to be arrested in maturation, and therefore should be physiologically prone to undergo apoptosis. The mechanisms of apoptotic resistance in Hodgkin lymphoma (HL) cells have been intensively investigated. It has been shown that HL cells are resistant to CD95-mediated apoptosis16 due to the constitutive expression of cFLIP.17 In addition, HL cells display a defective mitochondrial apoptotic pathway18 and uniformly show up-regulated XIAP expression.19 In the present study, we demonstrate that up-regulated XIAP together with dysfunctional mitochondria provide an effective barrier against CTL-mediated cytotoxicity, which can be overcome by functional and physical XIAP depletion.
Materials and methods
Cell culture
The establishment and culturing of the Hodgkin lymphoma and control cell lines have been previously described.18,20 GrzB/Ad (adenovirus)-mediated cell death was carried out as described previously by Goping et al.14 In brief, 1 × 106 cells/mL were treated with 600 ng isolated human grzB (Alexis, Lausen, Switzerland) and 100 pfu Ad (a gift from Dr U. Protzer, University of Cologne/Germany) in serum-free media supplemented with 0.1% (wt/vol) BSA. Cells were incubated for 4 to 5 hours at 37°C, then harvested and assessed for cell death. Protein transfection was carried out as described previously.19 Briefly, cells (106/mL) were transfected with Smac N-terminal peptide, H-AVPIAQK-OH (200 ng; Calbiochem, Mannheim, Germany), murine recombinant grzB (200 ng; Sigma, Deisenhofen, Germany), or β-galactosidase (119-kDa subunit; 100-300 ng) as a control using the Chariot protein transfection kit according to the instructions of the manufacturer (Activemotif, Brussels, Belgium).
Sample preparation and immunoblotting
Whole cell extracts were prepared by lysing cells in CHAPS lysis buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 1% CHAPS, protease complete cocktail) on ice for 20 minutes. Cytosolic extracts were prepared in buffer A (20 mM PIPES, pH 7.0, 50 mM KCl, 2 mM MgCl2, 5 mM EGTA, 1 mM dithiothreitol), and mitochondria were isolated as described previously.21 Mitochondria (300 mg) were treated with recombinant murine Bax protein (200 nM)22 or Bax storage buffer in 50 μL MSB supplemented with 5 mM succinate, 5 mM rotenone, and 4 mM MgCl2 at 30°C for 30 minutes. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Rabbit polyclonal antisera specific for human caspase-9, mouse anti-XIAP mAb, and mouse anti-cytochrome c mAb were obtained from (BD-laboratories, Heidelberg, Germany). Polyclonal antiserum specific for caspase-3 was obtained from Cell Signaling (Beverly, MA). Polyclonal antiserum specific for Smac/DIABLO was obtained from Alexis.
Caspase activation
For initiating caspase activation, 20 μL cell extracts (10 μg/mL) were treated with either 10 μM horse heart cytochrome c and 1 mM dATP (Sigma) or 20 ng recombinant murine grzB (Sigma)23 with or without Smac N-terminal peptide, H-AVPIAQK-OH (10 μM; Calbiochem) for 1 hour at 30°C. Caspase-3 activity was assayed by using 100 μM Ac-DEVD-AFC and presented as arbitrary fluorescence units per minute (FU/min); 1 FU is equivalent to 0.65 pmol released AFC.19
siRNA and lentiviral gene transfer
To silence XIAP expression, double-stranded (ds) small-interfering RNAs (siRNAs) of XIAP (XIAP-siRNA1, ID: 2553; XIAP-siRNA2, ID: 2645; XIAP-siRNA3, ID: 2733) and control (scrambled) siRNA were obtained from Ambion Europe (Huntingdon, United Kingdom). The most efficient siRNA (XIAP-siRNA3) down-regulating XIAP was identified by transient transfection of HeLa and 293HEK cells (data not shown).
pENTR construct was generated with a pair of oligonucleotides derived from XIAP mRNA (XIAP-siRNA3) that includes the unique N-19 target as described in pSUPER RNAi System Manual.24 The vector uses the polymerase-III H1-RNA gene promoter. After generating an entry clone, the pLenti6/V5DEST XIAP-siRNA3-expressing vector was created using LR recombination. The viral particles were produced according to the instructions of the manufacturer (pLenti-Dest Gateway system; Invitrogen, Karlsruhe, Germany). The recombinant lentiviral constructs were transduced into the cells and stable cell lines were generated using Blasticidin (Invitrogen) selection.
Anti-CD30 immunoreceptor and engraftment of T cells
The generation of the CD30-specific HRS3-scFv-Fc-zeta immunoreceptor and of the retroviral vector was previously described.25 To generate GALV-pseudotyped retrovirus, 293T cells were cotransfected with the recombinant vector DNA (6 μg) and with DNA of the retroviral helper plasmids pHIT and pCOLT (each 6 μg DNA/mL), encoding the Moloney murine leukemia virus (MMLV) gag and pol genes and the GALV envelope gene, respectively. Peripheral blood lymphocytes were isolated by density centrifugation and cultured for 48 hours in RPMI 1640 medium, 10% (vol/vol) FCS in the presence of 400 U/mL IL-2 (Endogen, Woburn, MA), and 100 ng/mL anti-CD3 mAb (OKT3). CD3+ T cells were isolated by magnetic cell sorting procedures using the AutoMACS facility (Miltenyi Biotec, Bergisch Gladbach, Germany). For retroviral transduction, cells were harvested, resuspended in medium with 400 U/mL IL-2, and cocultured for 48 hours with transfected 293T cells. The number of receptor-expressing T cells was determined by flow cytometry. T cells were simultaneously incubated with the HRS3 anti-idiotypic mAb 9G10 (IgG1, 10 μg/mL) directed against the scFv binding domain of the immunoreceptor and with the anti-CD3 mAb OKT3 (IgG2a, 5 μg/mL). Bound antibodies were subsequently detected by an FITC-conjugated F(ab′)2 anti-mouse IgG1 antibody and a PE-conjugated F(ab′)2 anti-mouse IgG2a antibody (each 1 μg/mL; both from Southern Biotechnology, Palo Alto, CA). Dead cells were stained by propidium iodide (Sigma). Cells were analyzed by flow cytometry.
Cytotoxicity assay
Specific cytotoxicity of receptor-grafted T cells against target cells was monitored after 48 hours by an XTT-based colorimetric assay according to the method described by Jost et al.26 Maximal reduction of XTT was determined as the mean of 6 wells containing tumor cells only, and the background as the mean of 6 wells containing RPMI 1640 medium, 10% (vol/vol) FCS. The nonspecific formation of formazan due to the presence of effector cells was determined from triplicate wells containing effector cells in the same number as in the corresponding experimental wells. The number of viable tumor cells was calculated as follows: viability (%) = [OD(experimental wells - corresponding number of effector cells)]/[OD(tumor cells without effectors-medium)] × 100.
Redirected cytotoxicity assay
Redirected cytotoxicity was determined according to standard procedures. Briefly, peripheral blood mononuclear cells (PBMCs) from healthy volunteer donors were activated at a density of 5 × 106 cells/mL by concanavalin A (ConA; 2 μg/mL) in complete RPMI medium for 5 days. Redirected CTL activity was assayed by coincubating 51Cr-labeled target cells at graded effector-target ratios with activated PBMCs in the presence of ConA (5 μg/mL) for 4 hours before measuring the activity of 51Cr in the cell-free supernatants of these cocultures in a gamma counter.
Results
Defective grzB-mediated cytotoxicity in HL B cells
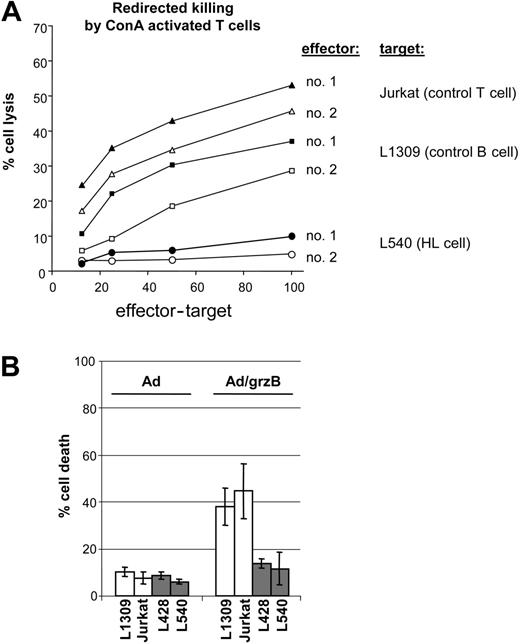
Hodgkin lymphoma is mostly associated with an ineffective CTL response against the tumor, suggesting immune evasion strategies by tumor cells.27 As shown in Figure 1A, the L540 HL cell line was almost completely resistant to cytolysis by ConA-activated CTLs from 2 healthy volunteers, while control target cells, L1309 B cells, and Jurkat T cells were readily lysed by the same effector cells. In order to unravel the molecular mechanism of grzB resistance, HL cells were exposed to grzB in the presence of replication-deficient adenovirus (Ad). Like the granular pore-forming protein perforin, Ad facilitates the entry of grzB into the cytoplasm of target cells.28,29 L428 and L540 HL cells displayed only a minor response to grzB/Ad treatment (14% and 12% cell death, respectively, after 4-hour treatment), whereas grzB/Ad induced marked cytotoxicity in L1309 B cells and Jurkat T cells (38% and 45%, respectively) (Figure 1B). These data suggest that inefficient CTL-mediated cytotoxicity is brought about by resistance of HL cells against grzB action.
Defective grzB/CTL-mediated cytotoxicity in HL cells. (A) PBMCs from healthy volunteer donors were activated at a density of 5 × 106 cells/mL by ConA (2 μg/mL) in complete RPMI medium for 5 days. Redirected CTL activity was assayed by coincubating for 4 hours 51Cr-labeled target cells at graded effector-target ratios with activated PBMCs in the presence of ConA (5 μg/mL). Thereafter, the activity of 51Cr was measured in the cell-free supernatants using a gamma counter. Jurkat T-cell as well as L1309 B-cell lines were used as positive controls for CTL-mediated target cells lysis. (B) Control L1309 B cells and Jurkat T cells (□) or L428 and L540 HL cells ( ) (all 106) were treated with adenovirus at an moi of 100 with or without 600 ng/mL isolated human grzB and incubated for 4 hours at 37°C. Data are mean ± SD values from 3 different individual experiments performed in triplicate.
) (all 106) were treated with adenovirus at an moi of 100 with or without 600 ng/mL isolated human grzB and incubated for 4 hours at 37°C. Data are mean ± SD values from 3 different individual experiments performed in triplicate.
Defective grzB/CTL-mediated cytotoxicity in HL cells. (A) PBMCs from healthy volunteer donors were activated at a density of 5 × 106 cells/mL by ConA (2 μg/mL) in complete RPMI medium for 5 days. Redirected CTL activity was assayed by coincubating for 4 hours 51Cr-labeled target cells at graded effector-target ratios with activated PBMCs in the presence of ConA (5 μg/mL). Thereafter, the activity of 51Cr was measured in the cell-free supernatants using a gamma counter. Jurkat T-cell as well as L1309 B-cell lines were used as positive controls for CTL-mediated target cells lysis. (B) Control L1309 B cells and Jurkat T cells (□) or L428 and L540 HL cells ( ) (all 106) were treated with adenovirus at an moi of 100 with or without 600 ng/mL isolated human grzB and incubated for 4 hours at 37°C. Data are mean ± SD values from 3 different individual experiments performed in triplicate.
) (all 106) were treated with adenovirus at an moi of 100 with or without 600 ng/mL isolated human grzB and incubated for 4 hours at 37°C. Data are mean ± SD values from 3 different individual experiments performed in triplicate.
GrzB fails to initiate the release of mitochondrial proapoptotic factors. (A) Control L1309 B cells, Jurkat T cells, and L428 or L540 HL cells (all 106) were treated with adenovirus at an moi of 100 with or without 600 ng/mL grzB and incubated for 2 hours at 37°C. Smac and cytochrome c were detected in cytosolic extracts by respective specific antibodies. Reprobing for actin ensured equal loading of cytosolic extracts. (B) Isolated postnuclear fractions (cytoplasm and mitochondria) from Jurkat T cells and L540 and L428 HL cells were treated for 30 minutes at 30°C with grzB (200 ng) or 200 nM recombinant Bax. After centrifugation, supernatants were analyzed by Western blotting with monoclonal antibodies specific for cytochrome c, Smac, and actin.
GrzB fails to initiate the release of mitochondrial proapoptotic factors. (A) Control L1309 B cells, Jurkat T cells, and L428 or L540 HL cells (all 106) were treated with adenovirus at an moi of 100 with or without 600 ng/mL grzB and incubated for 2 hours at 37°C. Smac and cytochrome c were detected in cytosolic extracts by respective specific antibodies. Reprobing for actin ensured equal loading of cytosolic extracts. (B) Isolated postnuclear fractions (cytoplasm and mitochondria) from Jurkat T cells and L540 and L428 HL cells were treated for 30 minutes at 30°C with grzB (200 ng) or 200 nM recombinant Bax. After centrifugation, supernatants were analyzed by Western blotting with monoclonal antibodies specific for cytochrome c, Smac, and actin.
GrzB fails to induce mitochondrial release of cytochrome c and Smac in HL cells
One hallmark of grzB cytotoxicity is a proapoptotic amplification loop involving mitochondrial release of Smac to overcome the inhibitory effect of XIAP.13,14 We, therefore, investigated the capability of grzB to initiate mitochondrial release of proapoptotic proteins, Smac and cytochrome c. As shown in Figure 2A, grzB/Ad treatment of HL cells did not result in efflux of Smac and cytochrome c into the cytoplasm, whereas Smac and cytochrome c were detected in cytosolic extracts of grzB/Ad-treated L1309 B cells and Jurkat T cells. These results suggest a defective grzB-initiated mitochondrial pathway in HL cells. In order to test the general capacity of mitochondria to respond to apoptotic signals, we used a cell-free system using grzB or recombinant Bax protein and isolated postnuclear fractions containing cytosolic and mitochondrial fractions. As shown in Figure 2B, grzB induced release of cytochrome c and Smac only from mitochondria isolated from Jurkat T cells but not from HL cells. Similarly, recombinant Bax initiated the efflux of cytochrome c and Smac from mitochondria isolated from Jurkat T cells, but not from L428- and L540-derived mitochondria, indicating a defect of HL-derived mitochondria to receive and/or transduce proapoptotic signals.
Cytosolic Smac restores grzB-mediated cytotoxicity in HL B cells
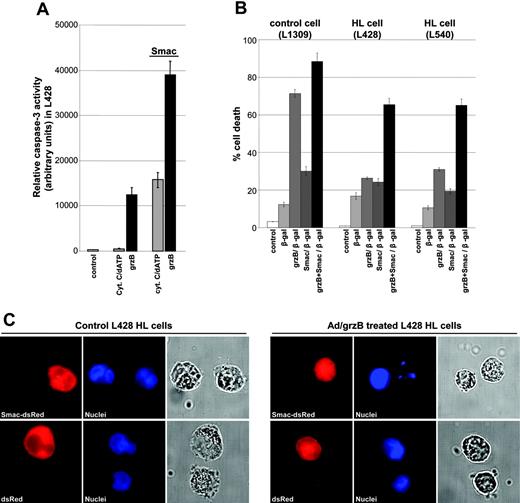
In order to further define the role of mitochondria in resistance against grzB-mediated cytotoxicity, we selectively bypassed the mitochondria by ectopic cytosolic expression of mature Smac. Once released into the cytosol, Smac interacts with XIAP to release XIAP-mediated inhibition of caspase-3. In cytosolic extracts of untreated L428 cells, cytochrome c/dATP or grzB only partially initiated caspase-3 activity (Figure 3A). Addition of the Smac N7 peptide restored caspase-3 activity initiated by cytochrome c/dATP treatment. Similarly, grzB-induced caspase-3 activity was significantly increased upon Smac treatment (Figure 3A). Correspondingly, transfection of intact HL cells with grzB and Smac proteins showed that Smac is able to restore cytotoxicity of grzB in L540 and L428 cells (Figure 3B). The potency of Smac to support grzB-mediated cell death was further examined by expression of cytosolic mature Smac lacking the mitochondrial targeting sequence, which was accomplished by using the ubiquitin (Ub) fusion system previously described by Hunter et al.30 The Ub-Smac fusion protein contains an amino terminal human Ub fused to human mature Smac, which when expressed in the cytosol will be immediately cleaved by a Ub-specific protease to generate the correct AVPI amino terminus of Smac critical for interaction with XIAP. In addition, a dsRed fluorescence tag was added to the carboxyl terminus to allow distinction of the transfected cells. When L428 HL cells were transiently transfected with DNA constructs expressing Ub-Smac-dsRed or Ub-dsRed as a control, all fusion proteins displayed diffused, cytoplasmic distribution patterns (Figure 3C). The apoptotic capability of grzB was analyzed by staining of nuclei using Hoechst (blue), visualizing nuclear fragmentation as a sign of apoptosis. Only expression of mature Smac contributed to nuclear fragmentation in grzB/Ad-treated L428 HL cells. No nuclear fragmentation was observed in HL cells expressing Ub-dsRed lacking Smac sequences. These observations underscore the notion that defective mitochondrial release of Smac determines the resistance of HL cells against grzB-mediated caspase-3 activation.
Smac enhances grzB-induced caspase activity and cell death. (A) Cytosolic extracts of L428 HL cells were prepared, and the protein content was normalized (10 μg/μL). Caspase activation was initiated by addition of cytochrome c/dATP or recombinant grzB (20 ng) in the absence or presence of Smac N7 peptide (10 μM). After incubation for 1 hour at 30°C, relative caspase-3 activity was measured by using DEVD-AFC as substrate. (B) Cells (5 × 105) were transfected using Chariot as vehicle, with β-galactosidase alone, in combination with either recombinant grzB or Smac N7 peptide, or in combination with recombinant grzB and Smac N7 peptide. Cell death was determined after 2 hours. Data are mean ± SD values from 3 individual experiments performed in triplicate. (C) L428 HL cells were transiently transfected with Ub-Smac-dsRed or Ub-dsRed (red). Cells were left untreated or treated with grzB/Ad. After fixation, nuclei were stained (Hoechst 33258) (blue) and analyzed by a motorized inverted microscope (Olympus Ix81; Tokyo, Japan) using a 63×/1.40 numerical aperture Planapo oil objective. Images were acquired using analy-SIS software (Soft Imaging System, Münster, Germany) and were further processed and assembled using Power-Point (Microsoft, Redmond, WA).
Smac enhances grzB-induced caspase activity and cell death. (A) Cytosolic extracts of L428 HL cells were prepared, and the protein content was normalized (10 μg/μL). Caspase activation was initiated by addition of cytochrome c/dATP or recombinant grzB (20 ng) in the absence or presence of Smac N7 peptide (10 μM). After incubation for 1 hour at 30°C, relative caspase-3 activity was measured by using DEVD-AFC as substrate. (B) Cells (5 × 105) were transfected using Chariot as vehicle, with β-galactosidase alone, in combination with either recombinant grzB or Smac N7 peptide, or in combination with recombinant grzB and Smac N7 peptide. Cell death was determined after 2 hours. Data are mean ± SD values from 3 individual experiments performed in triplicate. (C) L428 HL cells were transiently transfected with Ub-Smac-dsRed or Ub-dsRed (red). Cells were left untreated or treated with grzB/Ad. After fixation, nuclei were stained (Hoechst 33258) (blue) and analyzed by a motorized inverted microscope (Olympus Ix81; Tokyo, Japan) using a 63×/1.40 numerical aperture Planapo oil objective. Images were acquired using analy-SIS software (Soft Imaging System, Münster, Germany) and were further processed and assembled using Power-Point (Microsoft, Redmond, WA).
XIAP down-regulation in HL B cells restores grzB-mediated caspase activity
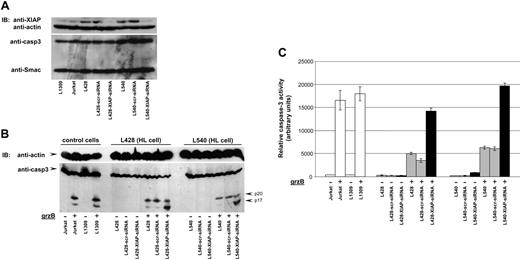
Our finding that cytosolic Smac expression suffices to restore HL cell susceptibility to grzB-induced apoptosis suggested that XIAP is a key inhibitor of grzB-mediated apoptosis in HL cells. If so, down-regulation of XIAP expression should also sensitize HL cells to grzB-induced apoptosis. In order to address this issue, we specifically down-regulated XIAP expression by using small hairpin RNA (shRNA) targeting XIAP mRNA. Three shRNA sequences were designed to down-regulate XIAP expression. All shRNAs were first examined in HeLa and HEK293 cells. The most potent shRNA down-regulating XIAP was identified (data not shown) and stably expressed in L540 as well as in L428 cells using lentiviral gene transfer. As shown in Figure 4A, only HL cells expressing shRNA against XIAP (L428-XIAP-shRNA and L540-XIAP-shRNA) displayed down-regulated XIAP expression, while scrambled (scr) shRNA (L428-scr-shRNA and L540-scr-shRNA) remained ineffective. The specificity of XIAP knockdown was revealed by unaltered expression of caspase-3, Smac, and actin.
We next examined whether XIAP down-regulation results in increased grzB-mediated caspase-3 activation/activity in cytosolic extracts of HL cells. GrzB treatment of L428-XIAP-shRNA and L540-XIAP-shRNA depleted of XIAP resulted in caspase-3 auto-catalytic activity yielding p17 active caspase-3 (Figure 4B). Addition of grzB to cytosolic extracts from L1309 B and Jurkat T cells also led to initial cleavage of pro-caspase-3, producing the characteristic p20 fragment, which was followed by autocatalytic activity of caspase-3, eventually generating the p17 mature caspase-3. In contrast, grzB induced only the initial cleavage of caspase-3, generating the p20 fragment of caspase-3 in cytosolic extracts from parental L428 and L540 or from their derivatives expressing scrambled shRNA. Of importance, further autocatalytic processing was blocked. Consistent with these results, grzB-induced caspase-3 activity was significantly increased in L428-XIAP-shRNA and L540-XIAP-shRNA cells compared with L428, L428-scr-shRNA, L540, and L540-scr-shRNA (Figure 4C).
The potential relevance of these observations using cellular lysates was revealed by analyzing grzB-mediated cytotoxicity in intact HL cells expressing XIAP shRNA. Depletion of XIAP in HL cells resulted in an increased caspase-3 activity after grzB/Ad treatment in L428-XIAP-shRNA and L540-XIAP-shRNA cell lines. Concomitantly, down-regulation of XIAP restored grzB-mediated cytotoxicity in XIAP-shRNA-L428 and XIAP-shRNA-L540 cell lines (43% and 51%, respectively), whereas grzB/Ad treatment in L428, scr-shRNA-L428, L540, and scr-shRNA-L540 produced only minor cytotoxicity (9%, 14%, 24%, and 20%, respectively) (Figure 5).
XIAP knockdown restores caspase activity. (A) Cell lysates of L1309 B cells, Jurkat T cells, and HL cells L428, L428-scr-shRNA, L428-XIAP-shRNA, L540, L540-scr-shRNA, and L540-XIAP-shRNA were prepared, and equal amounts of protein were examined for XIAP, Smac, and caspase-3 expression using respective specific antibodies. Reprobing for actin ensured equal loading of cell extracts. (B) Cytosolic extracts of L1309 B cells, Jurkat T cells, and HL cells L428, L428-scr-shRNA, and L428-XIAP-shRNA were prepared, and equal amounts of protein were incubated with grzB (20 ng) for 1 hour at 30°C. Cytosolic extracts were resolved by SDS-PAGE and subjected to Western blot analysis for caspase-3 by polyclonal rabbit anti-caspase-3 antibody. Reprobing for actin ensured equal loading. (C) Relative caspase-3 activity was analyzed in the cytosolic extracts after grzB treatment using DEVD-AFC as substrate. Data are mean ± SD values from 3 individual experiments performed in triplicate.
XIAP knockdown restores caspase activity. (A) Cell lysates of L1309 B cells, Jurkat T cells, and HL cells L428, L428-scr-shRNA, L428-XIAP-shRNA, L540, L540-scr-shRNA, and L540-XIAP-shRNA were prepared, and equal amounts of protein were examined for XIAP, Smac, and caspase-3 expression using respective specific antibodies. Reprobing for actin ensured equal loading of cell extracts. (B) Cytosolic extracts of L1309 B cells, Jurkat T cells, and HL cells L428, L428-scr-shRNA, and L428-XIAP-shRNA were prepared, and equal amounts of protein were incubated with grzB (20 ng) for 1 hour at 30°C. Cytosolic extracts were resolved by SDS-PAGE and subjected to Western blot analysis for caspase-3 by polyclonal rabbit anti-caspase-3 antibody. Reprobing for actin ensured equal loading. (C) Relative caspase-3 activity was analyzed in the cytosolic extracts after grzB treatment using DEVD-AFC as substrate. Data are mean ± SD values from 3 individual experiments performed in triplicate.
XIAP down-regulation restores apoptotic capability of grzB in HL cells. L1309 B cells, Jurkat T cells, or HL cells L428, L428-scr-shRNA, L428-XIAP-shRNA, L540, L540-scr-shRNA, L540-XIAP-shRNA (all 106) were left untreated or treated with adenovirus (moi of 100), isolated human grzB (600 ng), or adenovirus (moi of 100) plus isolated human grzB (600 ng). (A) Cell death was determined after 4 hours. (B) After 2 hours, cytosolic extracts were isolated and caspase-3 activity was measured using DEVD-AFC as substrate. Cell death was assessed after 4 hours.
XIAP down-regulation restores apoptotic capability of grzB in HL cells. L1309 B cells, Jurkat T cells, or HL cells L428, L428-scr-shRNA, L428-XIAP-shRNA, L540, L540-scr-shRNA, L540-XIAP-shRNA (all 106) were left untreated or treated with adenovirus (moi of 100), isolated human grzB (600 ng), or adenovirus (moi of 100) plus isolated human grzB (600 ng). (A) Cell death was determined after 4 hours. (B) After 2 hours, cytosolic extracts were isolated and caspase-3 activity was measured using DEVD-AFC as substrate. Cell death was assessed after 4 hours.
HL cells with decreased XIAP expression are more efficiently lysed by antigen-specific CTLs
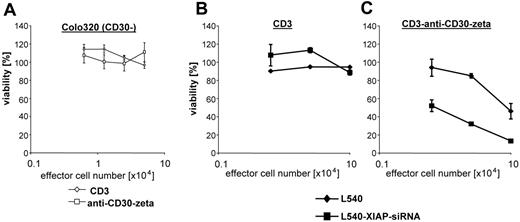
T cells can be specifically redirected against defined antigen by expression of a recombinant receptor with an antibody-like specificity (immunoreceptor). The immunoreceptor consists of an extracellular scFv domain as antigen receptor and of an intracellular CD3zeta signaling domain for cellular activation. CD3+ T cells from the peripheral blood equipped with an anti-CD30 immunoreceptor bind specifically CD30+ Hodgkin lymphoma cells and become activated to secrete IFN-γ and to lyse CD30+ target cells.25 Cytolysis mediated by anti-CD30 immunoreceptor-grafted T cells is antigen specific because CD30+ L540 Hodgkin lymphoma cells are lysed, whereas CD30- Colo320 cells are not (Figure 6A-B). CD3+ T cells without immunoreceptor do not lyse L540 cells (Figure 6B). It is important to note that anti-CD30 immunoreceptor CTL-mediated cytolysis of Hodgkin lymphoma cells is poor and requires large numbers of effector T cells (Figure 6B). We therefore tested whether L540 Hodgkin lymphoma cells with decreased XIAP expression (L540-XIAP-shRNA) are more susceptible to an antigen-specific CTL attack. L540-XIAP-shRNA cells are lysed by CD30-specific T cells at markedly reduced effector-target cell ratios when compared with wild-type L540 cells (Figure 6B). Cytolysis is specifically mediated by receptor-grafted T cells because CD3+ T cells without immunoreceptor do not lyse L540 or L540-XIAP-shRNA cells (Figure 6B).
Discussion
In Hodgkin lymphoma, many defects in apoptotic pathways have been described that may establish resistance to chemotherapeutic agents and contribute to cell survival in general. Here, we show that dysfunctional mitochondria in conjunction with up-regulated XIAP expression generate a robust antiapoptotic barrier protecting HL cells against CTL-induced cytotoxicity. The results of our study indicate that grzB-induced caspase-3 activation is blocked in HL cells at 3 distinct levels including (1) lack of mitochondrial release of cytochrome c needed for the activation of caspase-9, (2) lack of release of Smac needed for disruption of XIAP interaction with caspase-3, and (3) up-regulation of XIAP, the combination of which results in abrogation of grzB-induced caspase-3 activation and CTL-mediated cell death.
Enhancement of CD30-specific CTL cytotoxicity in XIAP down-regulated H-RS cells. Specificity of target cell lysis mediated by CD30-specific T cells. Isolated peripheral blood CD3+ T cells grafted by retroviral gene transfer with the CD30-specific HRS3scFv-Fc-zeta immunoreceptor and nontransduced T cells were incubated with CD30- Colo320 (A) or CD30+ L540 (B) cells (each 5 × 105 cells/well) for 24 hours. Viability of target cells was monitored by an XTT-based colorimetric assay. XIAP down-regulation improves antigen-specific CTL cytotoxicity against HL cells. Nontransduced and HRS3scFv-Fc-zeta receptor-grafted CD3+ T cells were incubated for 24 hours with L540-scr-shRNA or L540-XIAP-shRNA target cells (each 5 × 105 cells/well). Viability of target cells was monitored by an XTT-based colorimetric assay. Data are mean ± SD values from 3 individual experiments performed in triplicate.
Enhancement of CD30-specific CTL cytotoxicity in XIAP down-regulated H-RS cells. Specificity of target cell lysis mediated by CD30-specific T cells. Isolated peripheral blood CD3+ T cells grafted by retroviral gene transfer with the CD30-specific HRS3scFv-Fc-zeta immunoreceptor and nontransduced T cells were incubated with CD30- Colo320 (A) or CD30+ L540 (B) cells (each 5 × 105 cells/well) for 24 hours. Viability of target cells was monitored by an XTT-based colorimetric assay. XIAP down-regulation improves antigen-specific CTL cytotoxicity against HL cells. Nontransduced and HRS3scFv-Fc-zeta receptor-grafted CD3+ T cells were incubated for 24 hours with L540-scr-shRNA or L540-XIAP-shRNA target cells (each 5 × 105 cells/well). Viability of target cells was monitored by an XTT-based colorimetric assay. Data are mean ± SD values from 3 individual experiments performed in triplicate.
GrzB-mediated cytotoxicity by CTLs is principally associated with proteolysis of caspase-3 in the effector stage of apoptosis.31 The activation of caspase-3 by grzB is known to be a 2-step process. Initial cleavage by grzB results in the production of a p20 fragment, which is then ultimately autocatalytically processed to p17, resulting in a fully active caspase-3.10 However, caspase-3 is physiologically guarded by IAPs establishing a threshold for its activation. In particular, XIAP binds to the p20 subunit of caspase-3,32,33 thereby inhibiting its further maturation to p17.34,35 As previously reported, strong up-regulation of XIAP expression in HL cell lines and in primary H-RS cells diminished grzB-induced processing of caspase-3 and reduced caspase-3 proteolytic activity.19 The results of the present study suggest that mitochondrial dysfunction in HL cells (Figure 2) adds to the inhibitory action of XIAP. In HL cells, mitochondria fail to release cytochrome c essentially required for the caspase amplification cycle involving apoptosome formation and caspase-9 activation, which subsequently would support grzB-mediated caspase-3 activation. In addition, mitochondria of HL cells do not release Smac in response to apoptotic stimuli leaving alone the XIAP blockade of caspase-3. This is in line with recent reports suggesting that mitochondria are intimately involved in the grzB pathway. GrzB is known to modulate the action of Bid and Bax, proapoptotic members of the Bcl2 protein family, resulting in efflux of mitochondrial proapoptotic factors such as cytochrome c and Smac.11-15 In HL cells, the grzB-induced caspase amplification cycle is obviously blocked at the level of mitochondria.
Strikingly, the multifaceted antiapoptotic barrier of HL cells could be disrupted by single-step anti-XIAP strategies. Defective mitochondria could be functionally replaced by expression of cytosolic mature Smac, which completely restored grzB-mediated caspase-3 activity and cell death (Figure 3). This observation underlines the role of mitochondria, in particular, the mitochondrial release of Smac as an important regulator of grzB-induced caspase-3 activation. In the absence of Smac, the disruption of the inhibitory XIAP/caspase-3 interaction occurs at only suboptimal levels. Correspondingly, apoptotic resistance of HL cells was also abrogated by depletion of XIAP via specific siRNA. XIAP knockdown in HL cells not only sufficed to restore grzB-mediated caspase-3 activation and cell death but also sensitized HL cells for CTL-mediated cytotoxicity, indicating that XIAP is the main determinant of HL cell resistance to CTL-mediated cytolysis. It should be noted that the CD95-induced apoptotic pathway is completely blocked in HL cells, which has been suggested to be the result of constitutive expression of cFLIP.17 Therefore, CTL-mediated cytotoxicity toward HL cells is presumably brought about by grzB and related cytotoxic effector molecules.
As shown with activated CTLs redirected to HL cells via expression of a chimeric anti-CD30 immunoreceptor, HL cells are for the most part resistant to CTL-mediated killing (Figure 6). CTL-mediated lysis of malignant cells is crucial for the control of tumor surveillance.36 Ineffectivity of CTL action can be the result of active and passive immune evasion strategies by tumor cells. By expression of apoptosis-inducing ligands such as CD95L and secretion of inhibitory cytokines, tumor cells can counteract CTL attacks. On the other hand, tumor cells may evade or protect themselves against CTL, respectively, by down-regulation of immunodominant proteins or by expression of antiapoptotic proteins such as XIAP. Obviously, in HL cells down-regulation of XIAP appears to be especially important for the sensitization of the malignant cells for antigen-specific CTL attack.
Multiple studies have demonstrated that overexpression of XIAP confers resistance to multiagent chemotherapy including stimuli of the mitochondrial and death receptor pathways of caspase activation.37,38 Knocking down XIAP with siRNA or antisense oligonucleotides restores chemosensitivity to a variety of malignant cells. Strikingly, Smac promotes apoptosis by releasing the inhibitory action of IAPs only in the presence of apoptotic stimuli30,39 and thus may not be toxic for nonmalignant cells. In fact, the XIAP knock-out mouse lacks overt significant pathology, highlighting XIAP as a potential therapeutic target for the treatment of malignancies.40 The finding that Smac agonistic peptides or XIAP-siRNA sensitizes HL cells for grzB-induced apoptosis adds to the therapeutic implications of XIAP targeting in malignancies. Sensitization of neoplastic cells for CTL-mediated cell death by XIAP targeting might be instrumental for the improvement of adoptive cellular immunotherapy.
Prepublished online as Blood First Edition Paper, July 25, 2006; DOI 10.1182/blood-2006-05-021675.
Supported by grants from Deutsche Krebshilfe (M.K. and H.A.) and Cologne Fortune Program/Faculty of Medicine, University of Cologne (H.K.).
The authors declare no competing financial interests.
H.K. designed, performed, and analyzed the data and wrote the paper; J.-M.S., A.H., A.D., O.U., B.Y., and G.H. participated in performing the research; H.A. and M.K. designed, controlled, and analyzed the data; and all authors checked the final version of the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal