Abstract
The strength and duration of B-cell–receptor (BCR) signaling depends upon the balance between protein tyrosine kinase (PTK) activation and protein tyrosine phosphatase (PTP) inhibition. BCR-dependent activation of the SYK PTK initiates downstream signaling events and amplifies the original BCR signal. Although BCR-associated SYK phosphorylation is clearly regulated by PTPs, SYK has not been identified as a direct PTP substrate. Herein, we demonstrate that SYK is a major substrate of a tissue-specific and developmentally regulated PTP, PTP receptor–type O truncated (PTPROt). PTPROt is a member of the PTPRO family (also designated GLEPP, PTP-Ø, PTP-oc, and PTPu2), a group of highly conserved receptor-type PTPs that are thought to function as tumor suppressor genes. The overexpression of PTPROt inhibited BCR-triggered SYK tyrosyl phosphorylation, activation of the associated adaptor proteins SHC and BLNK, and downstream signaling events, including calcium mobilization and mitogen-activated protein kinase/extracellular signal–regulated kinase (MAPK/ERK) activation. PTPROt overexpression also inhibited lymphoma cell proliferation and induced apoptosis in the absence of BCR cross-linking, suggesting that the phosphatase modulates tonic BCR signaling.
Introduction
Protein tyrosine phosphatases (PTPs) regulate the amplitude and timing of tyrosine phosphorylation–based signaling events and modulate the growth, viability, and effector function of normal and malignant lymphocytes.1-3 In B cells, antigen receptor signaling induces receptor oligomerization and phosphorylation of immunoglobulin α (Igα) and β immunoreceptor tyrosine-based activation motifs (ITAMs) by SRC family kinases.4 ITAM phosphorylation results in the recruitment and activation of SYK, a protein tyrosine kinase (PTK) that initiates downstream events and amplifies the original B-cell–receptor (BCR) signal.4,5 The strength and duration of an antigen receptor signal depends upon the balance between PTK activation and PTP inhibition. Under basal conditions, SYK activity is tightly controlled by PTPs.3,6 However, BCR signaling leads to the local production of reactive oxygen species (ROSs), which inhibit PTP activity by oxidating the cysteine residue in the conserved catalytic domain ([I/V]XCXXGXXR[S/T]).2,6 Although BCR-dependent SYK activation is clearly regulated by PTPs,5 SYK is not known to be a direct PTP substrate.
In an earlier screen for genes that might contribute to the pathogenesis of diffuse large B-cell lymphoma (DLBCL), we identified and preliminarily characterized a lymphoid PTP called PTP receptor–type O truncated (PTPROt).7 PTPROt is a member of the PTPRO family (also designated GLEPP, PTP-Ø, PTP-oc, and PTPu28-11 ), a group of highly conserved receptor-type PTPs with a single catalytic domain, a transmembrane region, and an extracellular domain of variable length.7 The full-length PTPRO includes an extended extracellular domain with 8 fibronectin type III–like motifs, whereas PTPROt contains only an 8–amino acid extracellular region.7 Analysis of PTPRO genomic structure indicates that the unique 5′ untranslated region of PTPROT also functions as an intron that is spliced out of the larger PTPRO cDNA.7,12
The 2 major PTPRO isoforms exhibit tissue-specific patterns of expression; PTPRO is expressed predominantly in epithelial cells and most abundant in brain and kidney, whereas PTPROt is expressed primarily in B-lymphoid tissues, macrophages, and osteoclasts.7-11 PTPROt is also tightly regulated during normal B-cell development and more abundant in naive and memory B cells than in germinal center (GC) B cells.7 In earlier functional assays, lymphoma cells overexpressing PTPROt exhibited markedly increased G0/G1 arrest,7 providing the first functional evidence that this PTP regulated tumor growth.
More recent studies raise the possibility that PTPRO family members function as tumor suppressor genes.13-16 In a diet-induced model of rodent hepatocellular carcinoma, the full-length PTPRO gene was found to be heavily methylated in tumors but not in normal hepatic tissue.13 The observed differences in methylation were tightly associated with variations in PTPRO transcript abundance.13 In addition, treatment of hepatocarcinoma cell lines with a DNA hypomethylating agent markedly increased the transcription of PTPRO.13
In follow-up studies, PTPRO was found to be heavily methylated in primary human lung cancers but not in normal adjacent lung tissue.14 In human lung cancer cell lines, treatment with a demethylating agent also increased PTPRO expression.14 Of particular interest, the induction of PTPRO inhibited anchorage-dependent cell growth, delayed cell-cycle entry, and enhanced apoptosis of the lung cancer cells.14 In addition, PTPRO is localized to a chromosomal region, 12p12.3, that is frequently characterized by loss of heterozygosity in a variety of cancers, including B-cell malignancies.17-19
For these reasons, we postulated that the lymphoid-predominant PTPROt isoform might regulate signaling and growth in normal and malignant B cells. Herein, we identify SYK as a major lymphoid PTPROt substrate and determine the consequences of altered PTPROt expression and activity on BCR and SYK signaling.
Materials and methods
Cell culture
Human lymphoma cell lines DHL-4 and DHL-10 and the lymphoblastoid cell line CRL-8062 (ATCC, Manassas, VA) were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 2 mM glutamine and maintained at 37°C in 5% CO2.
GST pull-down assay
The catalytic domain of PTPROT–wild type (WT) (1006-1218 bp)7 was used as a template to generate 2 substrate-trapping mutants (PTPROT-CS [C325S] and –DA [D291A]) by site-directed mutagenesis (Stratagene, La Jolla, CA). The resulting WT and mutant catalytic domains were then cloned into pGEX vector (GE Healthcare Biosciences, Piscataway, NJ). Thereafter, glutathione S–transferase (GST) fusion proteins were expressed and affinity purified by glutathione-sepharose beads.
Lymphoma cell lines were treated with 100 μM pervanadate (100 μM sodium orthovanadate and 100 μM hydrogen peroxide in RPMI) for 15 minutes and subsequently lysed in NP-40 lysis buffer containing 5 mM iodoacetic acid and 1 mM sodium orthovanadate. After 20 minutes of incubation on ice, dithiothreitol was added to a 15 mM final concentration. Lysates (500 μg protein) were then precleared and incubated with GST-PTPROt-WT, GST-PTPROt-CS (C325S), or GST-PTPROt-DA (D291A) bound to glutathione-sepharose beads overnight at 4°C. The beads were subsequently washed with NP-40 lysis buffer containing 5 mM, 20 mM, or no sodium orthovanadate, centrifuged, and resuspended in loading buffer. GST-PTPROt–bound proteins were then size-fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), immunoblotted, and analyzed with the antiphosphotyrosine antibody 4G10.
Establishment of doxycycline-inducible PTPROt cell lines
The PTPROt-inducible lymphoma cell line was generated using a Tet-On Gene Expression System (BD Biosciences Clontech, Palo Alto, CA). In brief, the pTET-On regulatory plasmid was transfected into the lymphoma cell line, and several independent G418-resistant clones (0.75 mg/ml) were obtained. pTET-On–positive clones were screened for low background and high inducibility with pTRE2-Luc. Thereafter, selected clones were stably transfected with appropriate plasmids (pTRE2-PTPROT-WT, pTRE2-PTPROT-C325S, or pTRE2-PTPROT-D291A) and selected with puromycin (at 1 μg/mL). The resulting transfectants were treated with doxycycline (1 μg/mL) for 12 hours at 37°C to induce PTPROt expression.
Immunoprecipitation and immunoblotting
Cells were lysed in NP-40 lysis buffer (1% NP-40, 50 mM Tris-HCl [pH7.4], 150 mM NaCl, and 2 mM Na3VO4) containing protease inhibitors (Complete protease inhibitor cocktail; Roche Diagnostics, Indianapolis, IN). Following centrifugation, supernatants were incubated with 2 μg/mL primary antibody overnight at 4°C with rotation. Thereafter, 50 μL protein G–Sepharose beads (50% slurry in lysis buffer) was added, and samples were rotated for 4 additional hours. Immunocomplexes were then recovered by centrifugation, washed with cold lysis buffer, resuspended in sample buffer, and boiled at 95°C for 5 minutes and prior to SDS-PAGE.
After SDS-PAGE, proteins were transferred to Immunobilon-PVDF membranes (Millipore, Billerica, MA). Blots were first incubated in blocking buffer (5% milk, 0.1% Tween in phosphate-buffered saline [PBS]) at room temperature for 1 hour and subsequently incubated with primary antibodies (1 μg/mL) in blocking buffer for 2 hours at room temperature (for phospho-specific antibodies, overnight at 4°C). After sequential washes with 0.1% Tween/PBS, blots were incubated with horseradish peroxidase (HRP)–labeled secondary antibodies at room temperature for 1 hour, developed by enhanced chemiluminescence (GE Healthcare Biosciences) and visualized with Hyperfilm-ECL (GE Healthcare Biosciences).
To reprobe with another antibody, the blots were stripped with washing buffer (0.063 M Tris-HCl[pH 6.8], 2% SDS, 0.026 M DTT) at 50 °C for 40 minutes, and analyzed as described.
Antibodies used in immunoprecipitation and immunoblotting included mouse monoclonal antibody anti-GST, anti-SYK, and anti-BLNK, rabbit polyclonal antibody anti–extracellular signal–regulated kinase 1 (ERK1), (Santa Cruz Biotechnology, Santa Cruz, CA), anti–phospho-p44/42 mitogen-activated protein kinase (MAPK) antibody (Cell Signaling Technology, Danvers, MA), mouse monoclonal antiphosphotyrosine antibody (4G10; Upstate, Lake Placid, NY), rabbit polyclonal anti-SHC antibody (BD Biosciences, San Jose, CA), mouse monoclonal anti–β-actin, and anti-FLAG antibody (Sigma, St Louis, MO). Goat anti–human IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The PTPROt polyclonal antiserum was generated by repetitively immunizing rabbits with a PTPROt peptide (c-TNPVQLDDFDAYIKDMAKDS-n, 104-123 aa) and affinity-purifying the resulting serum using standard protocols.
Analysis of cellular proliferation and apoptosis
Cellular proliferation was determined by MTT assay (Roche Diagnostics) using standard protocols. Apoptosis was assessed using annexin V–FITC/propidium iodide (PI) staining (Annexin V–FITC apoptosis detection KIT I; BD Biosciences). In brief, cells were suspended in binding buffer, incubated with Annexin V–FITC and PI at room temperature in the dark, and subsequently analyzed by flow cytometry.
Dual-laser flow cytometric analysis of [Ca2+]i
Cells were loaded with Indo-1 am at 10 μg/mL and incubated at 37°C in the dark for 45 minutes. After washing with Dulbecco modified Eagle medium (DMEM)/1% fetal calf serum (FCS), the cells were resuspended in loading medium (RPMI, 1% FCS, 25 mM HEPES [pH 7.4]) at 3 × 106 cells/mL. Measurement of indo-1 fluorescence by flow cytometry was performed with an Epics V dual-laser flow cytometer (Beckman Coulter, Fullerton, CA) as described.20 After 30 seconds of baseline analyses, goat anti–human IgG (10 μg/mL) was added to the samples to cross-link BCR.
Results
SYK is an in vitro PTPROt substrate
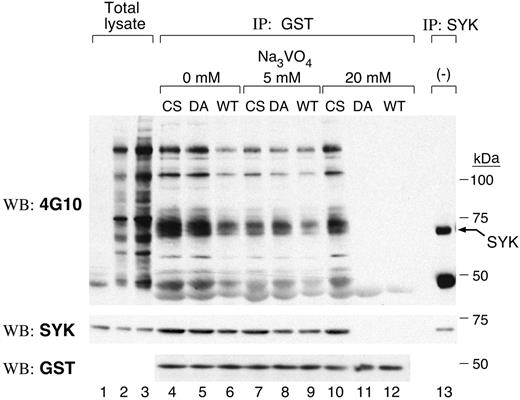
We initially used substrate-trapping mutants that lack catalytic activity but retain the ability to bind substrates to identify candidate PTPROt substrates.21-23 Two PTPROT mutants were generated: C325S (CS), which replaces the cysteine residue in the signature motif; and D291A (DA), which replaces an aspartic acid residue in the catalytic domain.22 GST-fusion proteins encoding the CS, DA, and WT PTPROt catalytic domains were generated and incubated with pervanadate-treated cell lysates; cellular proteins binding to the respective PTPROt catalytic domains were collected by centrifugation and analyzed by immunoblotting with antiphosphotyrosine antibody, 4G10 (Figure 1, top panel). A 72-kDa tyrosylphosphorylated protein was more abundant in PTPROt-CS and PTPROt-DA than in PTPROt-WT GST pull-downs (Figure 1, lanes 4-6, top panel). The molecular weight of this protein coincided with that of SYK (Figure 1, lane 13, top panel) and the 72-kDa tyrosyl-phosphorylated protein was recognized by the SYK antibody (Figure 1, middle panel), suggesting that this tyrosine kinase was a likely PTPROt substrate.
Vanadate forms a covalent bond with the catalytic cysteinyl residue of PTPs, and elution with vanadate-containing buffer results in the release of PTP substrates. Since vanadate elution releases PTP-bound substrates with differing efficiency (WT > DA mutant > CS mutant),23 we performed additional GST pull-down assays in which the bound proteins were eluted in 0 to 20 mM sodium orthovanadate (Figure 1, lanes 4-12). The amount of tyrosyl-phosphorylated SYK pulled down by GST-PTPROt-WT decreased after 5 mM vanadate elution (compare Figure 1, lanes 6 and 9). After high-concentration (20 mM) vanadate elution, almost no tyrosyl-phosphorylated SYK bound to either PTPROt-WT or the DA mutant (Figure 1, lanes 11 and 12), whereas a large amount of tyrosyl-phosphorylated SYK remained bound to CS mutant (Figure 1, lane 10). These data further support the notion that SYK is a substrate of PTPROt in vitro.
SYK is a substrate of PTPROt in vitro. GST fusion protein binding assays were carried out using GST-PTPROt-WT, -CS, or -DA proteins and pervanadate-treated lymphoma cell lysate. Bound proteins were washed with lysis buffer containing the indicated concentrations of sodium orthovanadate (0-20 mM). Thereafter, the remaining bound proteins were size fractionated, immunoblotted, and probed with the antiphosphotyrosine antibody 4G10 (top panel). The blot was stripped and reprobed with anti-SYK and anti-GST antibodies (middle and bottom panels). Lanes 1 to 3 are total lysates from untreated cells (lane 1, 25 μg protein) or pervanadate-treated cells (lane 2, 25 μg protein; lane 3, 50 μg protein). The last lane is an immunoprecipitation with anti-SYK antibody.
SYK is a substrate of PTPROt in vitro. GST fusion protein binding assays were carried out using GST-PTPROt-WT, -CS, or -DA proteins and pervanadate-treated lymphoma cell lysate. Bound proteins were washed with lysis buffer containing the indicated concentrations of sodium orthovanadate (0-20 mM). Thereafter, the remaining bound proteins were size fractionated, immunoblotted, and probed with the antiphosphotyrosine antibody 4G10 (top panel). The blot was stripped and reprobed with anti-SYK and anti-GST antibodies (middle and bottom panels). Lanes 1 to 3 are total lysates from untreated cells (lane 1, 25 μg protein) or pervanadate-treated cells (lane 2, 25 μg protein; lane 3, 50 μg protein). The last lane is an immunoprecipitation with anti-SYK antibody.
SYK is an in vivo PTPROt substrate
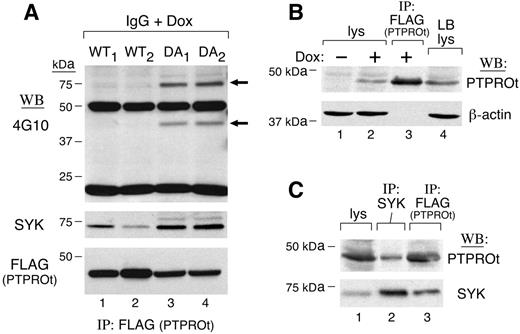
To characterize PTPROt substrates in vivo, we generated tet-inducible B-cell lines that expressed either WT or DA mutant FLAG-tagged PTPROt. If SYK is a PTPROt substrate, DA mutants should bind more tyrosyl-phosphorylated SYK than PTPROt-WT. To test this assumption, we induced FLAG-tagged PTPROt-WT or PTPROt-DA expression with doxycycline (Dox), triggered the BCR with anti–human IgG, and immunoprecipitated FLAG-tagged PTPROt-WT and PTPROt-DA proteins. Thereafter, we analyzed the tyrosyl-phosphorylated proteins bound to PTPROt in vivo by immunoblotting with 4G10 (Figure 2A, top panel; compare lanes 1-2 and 3-4). The DA-trapping mutants bound significantly more tyrosyl-phosphorylated and total SYK than PTPROt-WT (Figure 2A, top and middle panels), even though PTPROt-DA was less abundant than PTPROt-WT in Dox-induced cells (Figure 2A, bottom panel, compare lanes 1-2 and 3-4). Of interest, an approximately 42-kDa tyrosyl-phosphorylated protein that comigrated with PTPROt was significantly more abundant in PTPROt-DA immunoprecipitates.
After demonstrating that SYK was immunoprecipitated with PTPROt-GST fusion proteins in vitro and Dox-induced PTPROt in vivo, we next asked whether PTPROt was coprecipitated with endogenous SYK. For this analysis, we used a B-lymphoblastoid cell line with endogenous PTPROt levels comparable with those in Dox-induced PTPROt clones (Figure 2B; compare lanes 2 and 4). In the lymphoblastoid line, endogenous PTPROt was coprecipitated with SYK (Figure 2C, lane 2). Taken together, these in vivo and in vitro studies demonstrate that SYK is a bona fide substrate of PTPROt.
SYK is a substrate of PTPROt in vivo. (A) Coimmunoprecipitation of SYK and WT or mutant PTPROt in tet-inducible clones. Tet-inducible FLAG-tagged PTPROt WT (WT1,WT2) or mutant (DA1,DA2) clones were cultured with doxycycline (Dox) overnight. Thereafter, cells were serum starved and stimulated with goat anti–human IgG (10 μg/mL) for 8 minutes. Cells were then lysed and samples were immunoprecipitated with anti-FLAG (PTPROt) antibody. The tyrosyl-phosphorylated proteins pulled down by anti-FLAG (PTPROt) were detected by immunoblotting with the antiphosphotyrosine antibody 4G10 (top panel). The blot was then stripped and reprobed with anti-SYK (middle panel) and anti-FLAG (bottom panel). (B) PTPROt expression in lymphoblastoid B cells and tet-inducible PTPROt clones. Lanes 1, 2, and and 4 show total cell lysates from PTPROt WT cells without or with Dox induction (lanes 1 and 2, respectively) and CRL-8062 lymphoblastoid cells (lane 4). Lane 3 shows control anti-FLAG (PTPROt) immunoprecipitate from Dox-induced PTPROt WT cells. Samples were size-fractionated, blotted, and analyzed with anti PTPROt (top panel) or β-actin antibody (bottom panel). (C) Coimmunoprecipitation of SYK and PTPROt in lymphoblastoid cells. CRL-8062 cells were serum starved and subsequently stimulated with anti-IgG. Thereafter, cells were lysed and size-fractionated directly (lane 1) or following immunoprecipitation with anti-SYK antibody (lane 2). Anti-FLAG (PTPROt) immunoprecipitate from Dox-induced PTPROt-WT cells was analyzed simultaneously to serve as a positive control (lane 3). After size fractionation, the samples were immunoblotted and analyzed with PTPROt antiserum (top panel). Thereafter, the blot was stripped and reprobed with anti-SYK (bottom panel).
SYK is a substrate of PTPROt in vivo. (A) Coimmunoprecipitation of SYK and WT or mutant PTPROt in tet-inducible clones. Tet-inducible FLAG-tagged PTPROt WT (WT1,WT2) or mutant (DA1,DA2) clones were cultured with doxycycline (Dox) overnight. Thereafter, cells were serum starved and stimulated with goat anti–human IgG (10 μg/mL) for 8 minutes. Cells were then lysed and samples were immunoprecipitated with anti-FLAG (PTPROt) antibody. The tyrosyl-phosphorylated proteins pulled down by anti-FLAG (PTPROt) were detected by immunoblotting with the antiphosphotyrosine antibody 4G10 (top panel). The blot was then stripped and reprobed with anti-SYK (middle panel) and anti-FLAG (bottom panel). (B) PTPROt expression in lymphoblastoid B cells and tet-inducible PTPROt clones. Lanes 1, 2, and and 4 show total cell lysates from PTPROt WT cells without or with Dox induction (lanes 1 and 2, respectively) and CRL-8062 lymphoblastoid cells (lane 4). Lane 3 shows control anti-FLAG (PTPROt) immunoprecipitate from Dox-induced PTPROt WT cells. Samples were size-fractionated, blotted, and analyzed with anti PTPROt (top panel) or β-actin antibody (bottom panel). (C) Coimmunoprecipitation of SYK and PTPROt in lymphoblastoid cells. CRL-8062 cells were serum starved and subsequently stimulated with anti-IgG. Thereafter, cells were lysed and size-fractionated directly (lane 1) or following immunoprecipitation with anti-SYK antibody (lane 2). Anti-FLAG (PTPROt) immunoprecipitate from Dox-induced PTPROt-WT cells was analyzed simultaneously to serve as a positive control (lane 3). After size fractionation, the samples were immunoblotted and analyzed with PTPROt antiserum (top panel). Thereafter, the blot was stripped and reprobed with anti-SYK (bottom panel).
PTPROt overexpression inhibits SYK tyrosyl phosphorylation
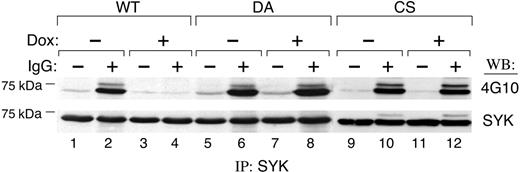
We next used the Dox-inducible PTPROt cell lines to define the relationship between BCR cross-linking, SYK tyrosyl phosphorylation, and expression of WT or mutant PTPROt. If SYK is a PTPROt substrate, we postulated that overexpression of WT PTPROt would inhibit BCR-triggered SYK tyrosyl phosphorylation. For this reason, tet-inducible PTPROt lymphoma cells were treated with Dox and, subsequently, anti–human Ig. Thereafter, SYK was immunoprecipitated and immunoblotted with the antiphosphotyrosine antibody 4G10. The tyrosyl phosphorylation of SYK was significantly inhibited by PTPROt-WT expression (Figure 3; compare lanes 2 and 4). Similar results were obtained with additional independent PTPROt-WT clones (data not shown). In contrast, Dox-induced expression of DA- or CS-mutant PTPROt had little effect on SYK tyrosyl phosphorylation (Figure 3, lanes 8 and 12), confirming the specificity of the PTPROt-WT effect. Taken together, these data demonstrate that PTPROt modulates BCR-triggered SYK tyrosyl phosphorylation in vivo.
PTPROt inhibits SYK tyrosyl phosphorylation. Tet-inducible FLAG-tagged WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, and stimulated thereafter with goat anti–human IgG (10 μg/mL) for 8 minutes or left untreated. Cells were lysed and immunoprecipitations were performed with anti-SYK antibody. The phosphorylation of SYK was detected by Western blot with the antiphosphotyrosine antibody 4G10 (top panel). The blot was then stripped and reprobed with anti-SYK (bottom panel).
PTPROt inhibits SYK tyrosyl phosphorylation. Tet-inducible FLAG-tagged WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, and stimulated thereafter with goat anti–human IgG (10 μg/mL) for 8 minutes or left untreated. Cells were lysed and immunoprecipitations were performed with anti-SYK antibody. The phosphorylation of SYK was detected by Western blot with the antiphosphotyrosine antibody 4G10 (top panel). The blot was then stripped and reprobed with anti-SYK (bottom panel).
PTPROt overexpression inhibits MAPK /ERK signaling and cell proliferation and induces cell apoptosis
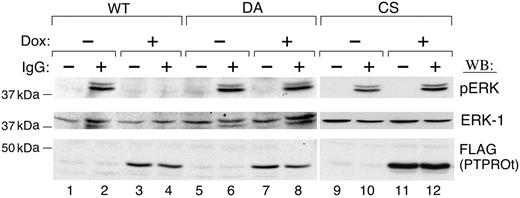
We next asked whether the inhibition of SYK tyrosyl phosphorylation by PTPROt altered the phosphorylation of downstream SYK targets and associated cellular proliferation. Since the MAPK/ERK signaling pathway is one of the most important downstream signaling pathways regulated by SYK,24 we examined the phosphorylation of ERK1/2 in Dox-induced PTPROt-WT and mutant transfectants, probing immunoblotted cell lysates with a phospho-ERK1/2 antibody. ERK1/2 was strongly phosphorylated after BCR cross-linking (Figure 4, lane 2), and ERK1/2 phosphorylation was significantly inhibited by PTPROt-WT overexpression (Figure 4; compare lanes 2 and 4). In contrast, overexpression of PTPROt-CS or -DA mutants had little effect on ERK1/2 phosphorylation (Figure 4, lanes 8 and 12).
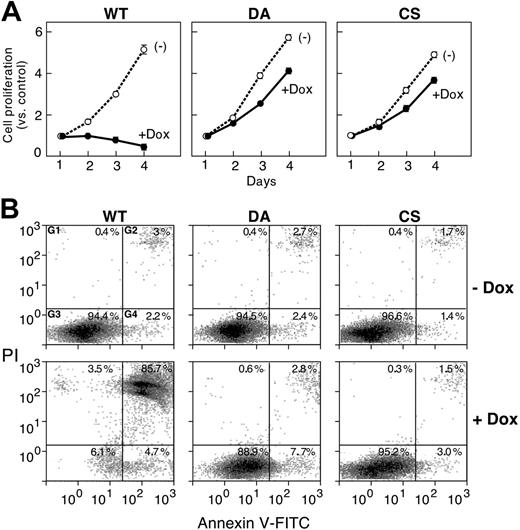
Given the role of the MAPK/ERK signaling pathway in regulating cellular proliferation,25 we next examined the effect of PTPROt overexpression on cell growth using an MTT assay. Cellular proliferation was completely inhibited when PTPROt-WT transfectants were induced with Dox, although these cells grew normally in the absence of Dox (and PTPROt-WT expression) (Figure 5A, left panel). In marked contrast, cellular proliferation was not significantly altered by the induction of either PTPROt mutant (DA, CS) (Figure 5A, middle and left panels).
In companion experiments, we also assessed the effect of PTPROt overexpression on cellular apoptosis with annexin V–FITC/PI staining. Dox-induced PTPROt-WT dramatically increased the apoptotic-cell fraction, whereas neither PTPROt mutant (DA or CS) had this effect (Figure 5B; compare left vs middle/right panels).
PTPROt overexpression inhibits SHC and BLNK tyrosyl phosphorylation
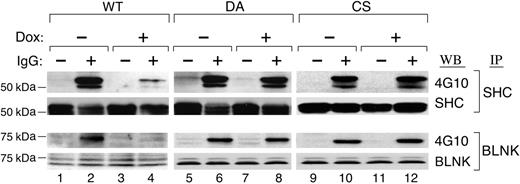
Two important adaptor proteins, SHC and BLNK, are direct targets of SYK that undergo tyrosyl phosphorylation following BCR engagement.26 For this reason, we postulated that PTPROt-associated inhibition of SYK would decrease SHC and BLNK tyrosyl phosphorylation. To test this assumption, SHC and BLNK were immunoprecipitated from Dox-induced PTPROt-WT and mutant transfectants, immunoblotted, and analyzed with 4G10 (Figure 6). The tyrosyl phosphorylation of SHC and BLNK was dramatically decreased following overexpression of PTPROt-WT (Figure 6, lane 4), whereas DA and CS mutant overexpression did not alter SHC or BLNK phosphorylation (Figure 6, lanes 8 and 12).
PTPROt inhibits ERK phosphorylation. Tet-inducible FLAG-tagged WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, and stimulated with goat anti–human IgG (10 μg/mL) for 8 minutes or left untreated. Thereafter, total cell lysates were size fractionated, blotted, and analyzed with anti–phospho-ERK1/2 antibody (top panel). The blot was then stripped and reprobed with anti-ERK1 (middle panel) and anti-FLAG antibodies (bottom panel).
PTPROt inhibits ERK phosphorylation. Tet-inducible FLAG-tagged WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, and stimulated with goat anti–human IgG (10 μg/mL) for 8 minutes or left untreated. Thereafter, total cell lysates were size fractionated, blotted, and analyzed with anti–phospho-ERK1/2 antibody (top panel). The blot was then stripped and reprobed with anti-ERK1 (middle panel) and anti-FLAG antibodies (bottom panel).
PTPROt overexpression inhibits cellular proliferation and induces apoptosis. (A) Cellular proliferation of tet-inducible PTPROt clones. Tet-inducible FLAG-tagged WT, CS, or DA PTPROt clones were cultured with or without Dox for 1 to 4 days (x-axis) and analyzed in MTT proliferation assays. Proliferation on days 2 to 4 is represented as fold increases compared with the initial day-1 measurement (y-axis). (B) Apoptosis of tet-inducible PTPROt clones. Tet-inducible FLAG-tagged WT, CS, or DA PTPROt clones were cultured with or without Dox for 4 days and analyzed thereafter with annexin V–FITC/PI staining. (x- and y-axes, respectively). The percentages of cells staining with PI alone (G1), annexin V and PI (G2), annexin V alone (G4), or neither reagent (G3) are indicated.
PTPROt overexpression inhibits cellular proliferation and induces apoptosis. (A) Cellular proliferation of tet-inducible PTPROt clones. Tet-inducible FLAG-tagged WT, CS, or DA PTPROt clones were cultured with or without Dox for 1 to 4 days (x-axis) and analyzed in MTT proliferation assays. Proliferation on days 2 to 4 is represented as fold increases compared with the initial day-1 measurement (y-axis). (B) Apoptosis of tet-inducible PTPROt clones. Tet-inducible FLAG-tagged WT, CS, or DA PTPROt clones were cultured with or without Dox for 4 days and analyzed thereafter with annexin V–FITC/PI staining. (x- and y-axes, respectively). The percentages of cells staining with PI alone (G1), annexin V and PI (G2), annexin V alone (G4), or neither reagent (G3) are indicated.
PTPROt overexpression decreases Ca2+ levels
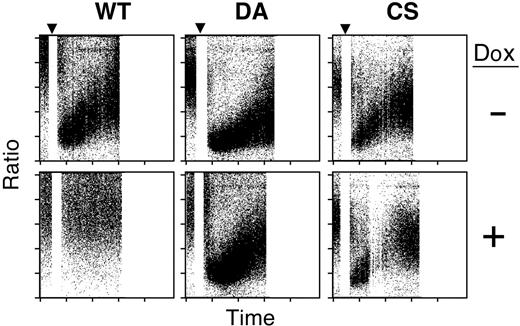
Since SYK and BLNK are essential for phospholipase C (PLC)–γ2–induced Ca2+ influx and release from internal stores,27,28 we reasoned that PTPROt might decrease Ca2+ levels after BCR cross-linking. To assess this possibility, PTPROt-WT and mutant transfectants were loaded with Indo-1 am (10 μg/mL), and Ca2+ levels were measured by dual-laser flow cytometric assay. In this assay, a decrease in measured indo-1 am fluorescence correlates with a rise in intracellular Ca2+.20 As a first step, we measured Ca2+ levels in the Dox-inducible transfectants in the absence of Dox (Figure 7, top panels). Prior to BCR engagement, most cells had low baseline Ca2+ levels. Following BCR cross-linking, intracellular Ca2+ levels markedly increased in all 3 cell lines (Figure 7, top panels).
PTPROt overexpression inhibits SHC and BLNK tyrosyl phosphorylation. Tet-inducible FLAG-tagged WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, and stimulated with goat anti–human IgG for 8 minutes or left untreated. Thereafter, cells were lysed and immunoprecipitated with anti-SHC or anti-BLNK antibody. SHC immunoprecipitates were size fractionated, blotted, and analyzed with the phosphotyrosine antibody 4G10 (top panel). The blot was subsequently stripped and blotted with an anti-SHC antibody (bottom panel). BLNK immunoprecipitates were size fractionated, blotted, and analyzed with the phosphotyrosine antibody 4G10 (top panel). The blot was subsequently stripped and blotted with an anti-BLNK antibody (bottom panel).
PTPROt overexpression inhibits SHC and BLNK tyrosyl phosphorylation. Tet-inducible FLAG-tagged WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, and stimulated with goat anti–human IgG for 8 minutes or left untreated. Thereafter, cells were lysed and immunoprecipitated with anti-SHC or anti-BLNK antibody. SHC immunoprecipitates were size fractionated, blotted, and analyzed with the phosphotyrosine antibody 4G10 (top panel). The blot was subsequently stripped and blotted with an anti-SHC antibody (bottom panel). BLNK immunoprecipitates were size fractionated, blotted, and analyzed with the phosphotyrosine antibody 4G10 (top panel). The blot was subsequently stripped and blotted with an anti-BLNK antibody (bottom panel).
PTPROt overexpression decreases Ca2+ levels. Tet-inducible WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, loaded with Indo-1 am, and analyzed for Ca2+ influx. Baseline values are represented on the left. At 30 seconds, cells were stimulated with goat anti–human IgG (▾). Subsequent Ca2+ flux is indicated on the right. The fluorescence ratio (530 nm/408 nm) is shown on the y-axis in relation to time on the x-axis. Note that a decrease in indo-1 am fluorescence correlates with an increase in Ca2+ levels. Each point represents the ratio for a single cell. The density of shading is proportional to cell number.
PTPROt overexpression decreases Ca2+ levels. Tet-inducible WT or mutant (DA and CS) PTPROt clones were cultured with or without Dox, serum starved, loaded with Indo-1 am, and analyzed for Ca2+ influx. Baseline values are represented on the left. At 30 seconds, cells were stimulated with goat anti–human IgG (▾). Subsequent Ca2+ flux is indicated on the right. The fluorescence ratio (530 nm/408 nm) is shown on the y-axis in relation to time on the x-axis. Note that a decrease in indo-1 am fluorescence correlates with an increase in Ca2+ levels. Each point represents the ratio for a single cell. The density of shading is proportional to cell number.
We then examined the effect of PTPROt-WT expression on intracellular Ca2+ levels. As expected, Dox-induced PTPROt-WT expression significantly decreased Ca2+ influx (Figure 7; compare top and bottom panels). In marked contrast, there was strong Ca2+ influx after BCR cross-linking in Dox-induced PTPROt-CS or -DA cells, demonstrating that the inactive phosphatase had little effect on intracellular Ca2+ levels (Figure 7, bottom right panels).
Discussion
PTPROt is a tissue-specific PTP that is expressed and developmentally regulated in B lymphocytes.7 In previous studies, we found that PTPROt overexpression markedly increased G0/G1 arrest, providing the first evidence that this PTP regulated B-cell growth.7 Herein, we identify the protein tyrosine kinase, SYK, as a major PTPROt substrate. The overexpression of PTPROt inhibited BCR-triggered SYK tyrosyl phosphorylation, activation of associated adaptor proteins, and downstream signaling events including calcium mobilization, MAPK/ERK activation, and cellular proliferation.
Recent studies highlight the critical role of PTPs in regulating SYK activation, proximal BCR signaling, and signal amplification.3,5 In these model systems, the reversible oxidation and inactivation of PTPs increased SYK phosphorylation and both the strength and duration of BCR signals.2,5,6 PTK signaling pathways and regulatory PTPs are often synergistic and sometimes redundant. For example, an additional PTP, SHP-1, limits proximal BCR signaling and SYK activation indirectly, by dephosphorylating the BCR phosphorylated ITAM.5 Our current studies identify a direct regulatory effect of an additional PTP, PTPROt, on SYK-mediated downstream signaling.
Of interest, the overexpression of PTPROt also inhibits lymphoma-cell proliferation and induces apoptosis in the absence of BCR cross-linking, suggesting that PTPROt modulates tonic BCR signaling. Although the nature of tonic BCR signaling is not well understood, recent studies indicate that BCRs transmit low-level survival signals in the absence of receptor engagement.29-32 Additional studies highlight the likely role of PTPs in tonic BCR signaling, demonstrating that BCR-proximal PTKs can be activated by pervanadate/H2O2 without BCR cross-linking.5,30,31,33 Since tonic BCR signaling involves the transient oxidation of BCR-proximal PTPs,30 PTPROt may play an important role in this process.
In earlier studies, we found that PTPROt was developmentally regulated in normal B cells: highly expressed by naive B cells; markedly reduced in antigen-stimulated, actively proliferating GC B cells; and again up-regulated in memory B cells.7 The functional consequences of PTPROt overexpression—decreased SYK phosphorylation, BCR signaling, and cellular proliferation—highlight its likely role in quiescent naive and memory B cells. These observations are of additional interest as SYK is required for B-cell maturation, follicle entry, and recirculation.34-37
In addition to its role in B-lymphoid cells, PTPROt is also expressed by phagocytic cells, including macrophages and osteoclasts.38,39 In leukocytes, SYK regulates immunoreceptor-related pathways and additional integrin-signaling cascades,40 raising the possibility that PTPROt modulation of SYK may have an important role in these additional cell types.
Given the functional effects of PTPROt overexpression and the recent suggestions that PTPRO (full length) may function as a tumor suppressor,13-16 it will be important to elucidate PTPROt regulatory mechanisms. We previously found that the 5′ untranslated region of PTPROT serves as an intron that is spliced out of the larger PTPRO transcript.7 The alternative PTPROT (PTP-oc) promoter, which was recently characterized, exhibits tissue specificity and contains flanking potential repressor elements.12 Unlike the PTPRO promoter, the PTPROT 5′ regulatory region does not include a CpG island12 (P.J., unpublished observations, November 2005), suggesting that PTPROT is less likely to be regulated by tumor-specific methylation. Since PTPROt modulates SYK phosphorylation and proximal BCR activity and likely limits tonic BCR-survival signals, the regulation of this phosphatase in normal and malignant B cells will be of particular interest.
Prepublished online as Blood First Edition Paper, August 3, 2006; DOI 10.1182/blood-2006-03-013821.
The authors declare no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Ricardo C. T. Aguiar's current address: Department of Medicine-Hematology, San Antonio Cancer Institute, University of Texas Health Science Center, San Antonio, TX 78229.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal