Abstract
An acquired somatic mutation, Jak2V617F, was recently discovered in most patients with polycythemia vera (PV), chronic idiopathic myelofibrosis (CIMF), and essential thrombocythemia (ET). To investigate the role of this mutation in vivo, we transplanted bone marrow (BM) transduced with a retrovirus expressing either Jak2 wild-type (wt) or Jak2V617F into lethally irradiated syngeneic recipient mice. Expression of Jak2V617F, but not Jak2wt, resulted in clinicopathologic features that closely resembled PV in humans. These included striking elevation in hemoglobin level/hematocrit, leukocytosis, megakaryocyte hyperplasia, extramedullary hematopoiesis resulting in splenomegaly, and reticulin fibrosis in the bone marrow. Histopathologic and flow cytometric analyses showed an increase in maturing myeloid lineage progenitors, although megakaryocytes showed decreased polyploidization and staining for acetylcholinesterase. In vitro analysis of primary cells showed constitutive activation of Stat5 and cytokine-independent growth of erythroid colony-forming unit (CFU-E) and erythropoietin hypersensitivity, and Southern blot analysis for retroviral integration indicated that the disease was oligoclonal. Furthermore, we observed strain-specific differences in phenotype, with Balb/c mice demonstrating markedly elevated leukocyte counts, splenomegaly, and reticulin fibrosis compared with C57Bl/6 mice. We conclude that Jak2V617F expression in bone marrow progenitors results in a PV-like syndrome with myelofibrosis and that there are strain-specific modifiers that may in part explain phenotypic pleiotropy of Jak2V617F-associated myeloproliferative disease in humans.
Introduction
A G>T point mutation resulting in the substitution of phenylalanine for valine at position 617 (V617F) in Janus kinase 2 (Jak2) is present in most patients with polycythemia vera (PV), chronic idiopathic myelofibrosis (CIMF), and essential thrombocythemia (ET). The most recent estimates using sensitive detection methods indicate that JAK2V617F is present in approximately 100% of patients with PV, approximately 60% to 70% of patients with ET, and approximately 50% of patients with CIMF, respectively.1,6 X-inactivation–based analysis of women with myeloproliferative disease (MPD) and karyotypic analysis have demonstrated that these MPDs are clonal stem cell disorders characterized by acquired somatic mutation in hematopoietic progenitors.7,12 Clinical features of PV include polycythemia in the absence of any cause of secondary erythrocytosis and splenomegaly caused by extramedullary hematopoiesis. Most patients with PV also have varying degrees of leukocytosis and thrombocytosis and variable bone marrow fibrosis that may progress with time. One diagnostic hallmark is erythropoietin hypersensitivity of erythroid progenitors in vitro,13,15 including growth in the absence of erythropoietin. Complications of PV may include arterial and venous thrombosis, development of myelofibrosis, and a relatively low rate of progression to acute myeloid leukemia (AML).
JAK2, and the other Janus kinase family members—JAK1, JAK3, and TYK2—interact with type I and II cytokine receptors and are key effectors of cytokine receptor signaling.16,17 We and others have previously reported that Jak2V617F is able to confer factor-independent growth of Ba/F31,2 or factor-dependent cell Paterson (FDCP) cells1,2 that are stably transduced with the erythropoietin receptor (EpoR) and that both Jak2V617F and its downstream effector Stat5 are constitutively activated in these cells, as assessed by tyrosine phosphorylation. There are several important questions about the role of JAK2V617F in the pathophysiology of MPD, including the phenotypic pleiotropy in humans who have the same mutant allele of JAK2. We have evaluated the effect of expressing Jak2V617F in vivo in a murine bone marrow transplant model, and we report that Jak2V617F induces many, but not all, of the features of PV, including polycythemia, leukocytosis, extramedullary hematopoiesis resulting in splenomegaly, and variable reticulin fibrosis. Although we observed megakaryocyte hyperplasia in the bone marrow and spleen, peripheral blood platelet counts were normal. With the caveat that this is a murine model system, these data suggest that Jak2V617F is responsible for many, but not all, the clinicopathologic features of these MPDs and that second mutations may be required for the genesis of thrombocytosis. In addition, there were strain-specific differences in the degree of leukocytosis, splenomegaly, and reticulin fibrosis. Balb/c mice that underwent transduction with Jak2V617F had significantly higher degrees of leukocytosis, a corresponding increase in the degree of splenomegaly, and an increase in bone marrow fibrosis compared with C57Bl/6 mice. These data also suggest that host modifiers may account for phenotypic pleiotropy in murine models of disease and in humans with JAK2V617F-associated myeloproliferative disease.
Materials and methods
Expression vectors and viral supernatants
The murine Jak2 cDNA was cloned into the retroviral vector MSCV-IRES EGFP. The V617F mutation in Jak2 was generated using site-directed mutagenesis (Quikchange-XL; Stratagene, La Jolla, CA) and was confirmed by full-length DNA sequencing. We cultured 293T cells in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). Transient cotransfection of 293T cells and generation of retroviral supernatant were performed using FuGENE (Roche, Nutley, NJ) according to the manufacturer's protocol. Briefly, equal amounts of expression vectors and packaging plasmid (Ecopak, Auckland, New Zealand) were combined, preincubated for 15 minutes at room temperature with FuGENE (Roche), and added with fresh DMEM with 10% FCS to 293T cells. Viral supernatant was harvested after 48 hours and was used to transduce whole BM or Ba/F3 cells to assess viral titer.
Bone marrow transplantation and analysis of mice
Murine BM transplantation experiments were performed as previously described.18,19 Briefly, the viral titer of MSCV-EGFP retroviral supernatants was determined by transducing Ba/F3 cells with supernatant (plus polybrene, 10 μg/mL) and analyzed for the percentage of green fluorescent protein–positive (GFP+) cells by flow cytometry 2 days after transduction. C57Bl/6 or Balb/c donor mice (Taconic, Germantown, NY) were treated for 5 days with 5-fluorouracil (150 mg/kg, intraperitoneal injection). Bone marrow cells from donor mice were harvested by flushing femurs and tibias and were cultured for 24 hours in transplant medium (RPMI + 10% FBS + 6 ng/mL IL-3, 10 ng/mL IL-6, and 10 ng/mL stem cell factor). Cells were treated by spin infection with retroviral supernatants (1 mL supernatant per 4 × 106 cells, plus polybrene, plus HEPES 30 μL/4 mL) and centrifuged at 1800 g for 90 minutes at 24 hours before and on the day of transplantation. Whole BM cells (1.0 × 106) were resuspended in Hanks balanced salt solution and were injected into lateral tail veins of lethally irradiated (2 × 5.5 Gy [550 rads]) C57Bl/6 or (2 × 4.5 Gy [450 rads]) Balb/c recipient mice. Mice were maintained on acidified water.
Analysis of disease in mice
Balb/c or C57Bl/6 mice were killed at the times indicated based on an Institutional Animal Care and Use Committee (IACUC)–approved protocol that included assessment of morbidity by more than 10% loss of weight, scruffy appearance, lethargy, or splenomegaly extending across the midline. Peripheral blood was collected from the retroorbital cavity using EDTA glass capillary tubes and analyzed by automated complete and differential blood cell counts and blood smears (stained with Wright-Giemsa). Normal ranges for complete blood counts from peripheral blood were obtained from the Jackson Laboratory (Bar Harbor, ME) homepage (http://aretha.jax.org/pub-cgi/phenome/mpdcgi?rtn=docs/home). For the Balb/c strain (n = 35), the white blood cell (WBC) count ranged from 3.56 to 6.6 × 109/L and consisted of 74% to 80% lymphocytes, 14.8% to 20.2% granulocytes, and 1.2% to 1.97% monocytes; the red blood cell (RBC) count ranged from 8.28 to 9.45 × 1012/L; the hematocrit (HCT) was between 0.387 and 0.445; and the platelet count ranged from 1132 to 1449 × 109/L. For the C57Bl/6 mice (n = 76), the WBC count ranged from 6.32 to 8.15 × 109/L and consisted of 75.5% to 86.2% lymphocytes, 9.14% to 15.3% neutrophils, and 1.37% to 1.81% monocytes; the RBC count ranged from 9.11 to 9.63 × 1012/L; the HCT ranged from 0.405 to 0.445; and the platelet count ranged from 918 to 1186 × 109/L.
Single-cell suspensions of spleen were prepared by pressing tissue through a cell strainer, and single-cell suspensions of BM were prepared by flushing bones, followed by RBC lysis. Cells were frozen in 90% FBS and 10% DMSO. For histopathologic examination, tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin or to assess for fibrosis stained with reticulin. Images of histologic slides were obtained on a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) equipped with a SPOT RT color digital camera (model 2.1.1; Diagnostic Instruments, Sterling Heights, MI). The microscope was equipped with a 10 ×/22 NA ocular lens. Low-power images (100 ×) were obtained with a 10 ×/0.25 objective lens. High-power images (600 ×) were obtained with a 60 ×/1.4 objective lens with oil (Trak 300; Richard Allan Scientific, Kalamazoo, MI). Images were analyzed in Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
For flow cytometry, cells were washed in PBS + 1% bovine serum albumin (BSA), blocked with Fc-block (BD PharMingen, San Diego, CA) for 10 minutes on ice, and stained with monoclonal antibodies in PBS + 1% FCS for 30 minutes on ice. Antibodies used were allophycocyanin (APC)–conjugated ter119, Gr-1, and B220 and phycoerythrin (PE)–conjugated CD41, Mac1, CD3, and CD71 rat antimouse antibodies (BD PharMingen). After washing, cells were resuspended in PBS + 1% FCS containing 0.5 μg/mL 7-amino-actinomycin D (7-AAD; BD PharMingen) to allow discrimination of nonviable cells. Flow cytometry was performed on a FACSCalibur cytometer (BD Biosciences, San Jose, CA). At least 10 000 events were acquired, and data were analyzed using CellQuest software (BD Biosciences). Results are presented as dot plots of viable cells selected on the basis of scatter and 7-AAD staining. For megakaryocytic analysis, freshly derived BM cells from 2 femurs and 2 tibias for Jak2 or for Jak2V617F were cultured at 2 different time points in DMEM + 10% fetal calf serum with 10 ng/mL recombinant murine thrombopoietin (rmTPO; Sigma-Aldrich, St Louis, MO) and 50 ng/mL recombinant human interleukin-11 (rhIL-11; StemCell Technologies, Vancouver, BC, Canada) for 4 days before flow cytometric analysis of DNA content. BM megakaryocytes were stained with rat anti–mouse CD41 (BD PharMingen) and anti–rat APC (BD PharMingen), followed by 50 μg/mL propidium iodide in 0.1% sodium citrate buffer and then incubated with 50 μg/mL RNase (Qiagen, Valencia, CA), as previously described.20,21
Southern blot analysis for proviral insertion and clonality
Genomic DNA was prepared from single-cell suspensions of spleens of Jak2 and Jak2V617F animals that underwent transplantation and one untreated animal using DNA lysis buffer (Puregene cell lysis solution; Gentra, Minneapolis, MN). Ten micrograms of genomic DNA was digested with NcoI, which cuts once within the proviral sequence and once within the integrated locus, and was subjected to electrophoresis and hybridization according to standard protocols. The DNA was hybridized with an EGFP probe (NcoI and SalI digest of MSCV-IRES-EGFP). Miracle Hyb (Stratagene) was used for hybridization according to the manufacturer's recommendations.
Erythropoietin ELISA
For quantitative determination of mouse EPO concentrations, the mouse EPO Immunoassay (Quantikine, Minneapolis, MN) was used. Briefly, mouse serum was collected, centrifuged for 20 minutes at 2000g, and processed according to the manufacturer's protocol. Optical density was determined on a microplate reader at 450 nm.
Protein lysates and Western blot analysis
Ba/F3 cells retrovirally transfected with Jak2, Jak2V617F, or MIG were double sorted for GFP expression within 48 hours and starved for 4 hours in RPMI medium containing 1% BSA at 37°C. Cells were lysed in lysis buffer (Tris HCl 20 mM, 1% Triton X-100, NaCl 150 mM, EDTA 5 mM, 200 mM Na3VO4, 200 mM phenylarsine oxide and protease arrest [Genotec, St Louis, MO]), and 1 g protein was immunoprecipitated with either anti-Stat5 or anti-Jak2 antibody at 4°C overnight. Before electrophoresis on 3% to 8% Tris acetate sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Invitrogen, Carlsbad, CA), all the protein lysate was combined with reducing SDS sample buffer (Invitrogen) and was transferred to nitrocellulose membranes and blotted with the indicated antibodies: anti–phospho-STAT5 (1:500; Cell Signaling, Beverly, MA), anti–phospho-JAK2 (1:500; Cell Signaling), anti-JAK2 (1:1000, polyclonal antibody raised against the JH1 domain of JAK2), anti-Stat5 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), and peroxidase-conjugated anti–rabbit immunoglobulin (1:5000; Amersham Biosciences, Piscataway, NJ).
Flow cytometric staining of intracellular phosphorylated Stat5
After transplantation, freshly isolated spleens and BM from Jak2 and V617F Jak2 animals were cultured for 4 hours in RPMI + 1% BSA at 37°C. Staining was performed as previously described.22 Cells were incubated with anti–human phospho-STAT5 (Cell Signaling). After a final wash, the samples were analyzed by flow cytometry, and probability was calculated with the 2-paired Student t test.
Colony assays
To detect CFU-E colonies, splenocytes from Jak2wt and Jak2V617F mice were plated in semisolid methylcellulose according to the manufacturer's protocol. To test EPO sensitivity of CFU-Es, 1 × 106 cells were plated in duplicate in methylcellulose (M3234; StemCell Technologies) containing varying concentrations (0 U, 0.01 U, 0.1 U) of EPO. Benzidine-positive clusters (benzidine provided by Sigma) were counted after 2 days in culture. To detect erythroid burst-forming unit (BFU-E) colonies, 5 × 105 BM and 2 × 105 spleen cells from Jak2 mice were plated in duplicate in methylcellulose (M3434: StemCell Technologies) containing 3 U/mL EPO, 10 ng/mL recombinant murine interleukin-3 (rmIL-3), 10 ng/mL rmIL-6, and 50 ng/mL recombinant murine stem cell factor according to the manufacturer's protocol. Only hemoglobin-expressing clones were counted as BFU-E colonies on day 8 in culture. Megakaryocyte-plating assays were performed in collagen-containing medium according to the manufacturer's protocol (MegaCult-C; StemCell Technologies). Slides were fixed and stained for 6 hours with acetylcholine iodide (Sigma), according to the MegaCult-C protocol, and were counterstained with Harris hematoxylin solution (Sigma).
Splenomegaly and reticulin fibrosis in Jak2V617F mice after BM transplantation. (A) Bar graphs indicate an 11-fold increase in spleen weight in Jak2V617F Balb/c compared with Jak2wt Balb/c animals and an approximately 4-fold increase in spleen weight in Jak2V617F C57Bl/6 compared with Jak2wt C57Bl/6 mice. (B) Moderate to marked diffusely increased reticulin fibrosis in BM of Balb/c mice that had received transplants of Jak2V617F 4.5 weeks after transplantation, which was absent in Jak2wt BM (C).
Splenomegaly and reticulin fibrosis in Jak2V617F mice after BM transplantation. (A) Bar graphs indicate an 11-fold increase in spleen weight in Jak2V617F Balb/c compared with Jak2wt Balb/c animals and an approximately 4-fold increase in spleen weight in Jak2V617F C57Bl/6 compared with Jak2wt C57Bl/6 mice. (B) Moderate to marked diffusely increased reticulin fibrosis in BM of Balb/c mice that had received transplants of Jak2V617F 4.5 weeks after transplantation, which was absent in Jak2wt BM (C).
Results
Jak2V617F expression in BM cells confers a phenotype similar to human PV in mice
Freshly harvested BM was transduced with MSCV-IRES-EGFP vectors containing Jak2wt, Jak2V617F, or no insert, respectively, and was transplanted into lethally irradiated Balb/c or C57Bl/6 recipient mice. Four weeks after transplantation, Jak2V617F-transduced animals had developed elevated HCTs (mean .74, n = 22) with no apparent strain-specific differences (P = .49), whereas both strains of mice that received Jak2wt-transduced BM had normal HCTs (Table 1). Jak2V617F-transduced mice had elevated HCTs for as long as 20 weeks (Figure S3). At the time of humane killing, Jak2V617F Balb/c mice (n = 16) had marked splenomegaly and higher average spleen weights (1.06 g) than Jak2wt-transduced mice (n = 10), whose average spleen weight was 0.1 g (Figure 1A). In addition, Balb/c and C57Bl/6 mice that received BM transduced Jak2V617F had leukocytosis and splenomegaly, in contrast to mice that that received BM transduced with Jak2wt, but the degree of leukocytosis was markedly higher in the Balb/c mice (mean, 112 × 109/L; n = 22) than in the C57Bl/6 mice (mean, 20 × 109/L; n = 18). WBC counts were abnormally elevated in only 3 of 18 animals (Table 1).
Differential blood counts in Balb/c or C57B1/6 mice expressing Jak2V617F or Jak2wt 50 days after transplantation
. | Jak2V617F . | Jak2wt . |
|---|---|---|
| Balb/c | ||
| No. mice | 22 | 10 |
| WBC count × 109/L | 112 ± 43 | 6.5 ± 2.6 |
| HCT | .737 ± .065 | .497 ± .043 |
| Platelet count × 109/L | 591 ± 150 | 263 ± 144 |
| C57B1/6 | ||
| No. mice | 18 | 10 |
| WBC count × 109/L | 20 ± 22 | 10.7 ± 3.3 |
| HCT | .725 ± .038 | .449 ± .025 |
| Platelet count × 109/L | 524 ± 94 | 576 ± 88 |
. | Jak2V617F . | Jak2wt . |
|---|---|---|
| Balb/c | ||
| No. mice | 22 | 10 |
| WBC count × 109/L | 112 ± 43 | 6.5 ± 2.6 |
| HCT | .737 ± .065 | .497 ± .043 |
| Platelet count × 109/L | 591 ± 150 | 263 ± 144 |
| C57B1/6 | ||
| No. mice | 18 | 10 |
| WBC count × 109/L | 20 ± 22 | 10.7 ± 3.3 |
| HCT | .725 ± .038 | .449 ± .025 |
| Platelet count × 109/L | 524 ± 94 | 576 ± 88 |
After transplantation, we performed detailed histopathologic analysis of the animals in both mouse strains. Peripheral blood (PB) smears from Jak2V617F Balb/c mice showed reticulocytosis and marked leukocytosis, predominantly composed of maturing myeloid elements with rare immature forms (Figure 2A), compared with normal blood smears in Jak2wt mice (Figure 2B). There was an increase in platelet size accompanied by a significant increase in the mean platelet volume (MPV) value (P < .01) in Jak2V617F Balb/c (data not shown).
Histopathologic analysis of BM from Jak2V617F mice showed a prominent population of maturing myeloid cells with mildly to moderately increased numbers of megakaryocytes, including large, atypical forms occurring in occasional clusters and showing emperipolesis of neutrophils in megakaryocyte cytoplasm (Figure 2C). In contrast, Jak2wt-transduced BM sections showed preserved marrow architecture with normal ratios of myeloid to erythroid elements and unremarkable megakaryocytes (Figure 2D). Spleen sections from Jak2V617F Balb/c mice exhibited complete effacement of normal splenic architecture and expansion of red pulp by an atypical population of maturing myeloid forms, erythroid elements, and clusters of dysplastic megakaryocytes (Figure 2E,G) compared with Jak2wt spleens, in which was found preserved normal splenic architecture with unperturbed red and white pulp (Figure 2F,H). Liver sections of Jak2V617F mice demonstrated extensive amounts of extramedullary hematopoiesis with clusters of maturing myeloid cells, occasional dysplastic megakaryocytes, and erythroid elements, in contrast to normal liver histopathology in Jak2wt animals (Figure 2I-L). We also observed moderately to markedly increased diffuse reticulin fibrosis in the BM of Jak2V617F Balb/c mice, but there was no fibrosis in the C57Bl/6 background or in animals of either background that received BM transduced with Jak2wt (Figure 1B-C).
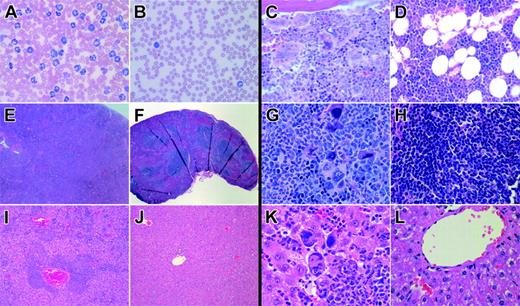
Histopathology of Jak2V617F and Jak2wt BM transplant models. Histology of Jak2V617F- and Jak2wt-transduced Balb/c mice showing images of PB (A-B) and pathology in representative sections of BM (C-D), spleen (E-H), and liver (I-L). PB smear (B, 600 ×; Wright-Giemsa stain) of a representative Jak2wt animal displays normal white blood cell counts with an unremarkable differential. In contrast, PB smear (A, 600 ×; Wright-Giemsa stain) of a representative Jak2V617F mutant animal reveals marked leukocytosis consisting predominantly of maturing myeloid elements. Bone marrow images from Jak2wt animals display preserved marrow architecture with normal ratios of myeloid/erythroid elements and unremarkable megakaryocytes (D, 600 ×, H&E stain). In comparison, BM sections from Jak2V617F mutant animals demonstrate marrow elements composed of a prominent population of maturing myeloid cells with mildly to moderately increased numbers of megakaryocytes, including large, atypical forms occurring in occasional clusters (C; 600 ×, H&E stain) and showing emperipolesis of neutrophils in megakaryocyte cytoplasm. Spleen sections from Jak2V617F mice display complete effacement of normal splenic architecture (E; 40 ×, H&E stain) with a marked expansion of red pulp predominantly composed of maturing myeloid elements, frequent atypical megakaryocytes in clusters, and occasional erythroid elements (G; 600 ×, H&E stain) compared with unremarkable Jak2wt spleens (F, H; 40 ×, 600 ×, H&E stain). Liver images from Jak2V617F mice illustrate evidence of extensive extramedullary hematopoiesis in a perivascular and sinusoidal distribution (I; 100 ×, H&E stain) composed of frequent large, atypical megakaryocytes, maturing myeloid cells, and erythroid forms (K; 600 ×, H&E stain) notably absent in livers from Jak2wt animals (J, L; 100 ×, 600 ×, H&E stain).
Histopathology of Jak2V617F and Jak2wt BM transplant models. Histology of Jak2V617F- and Jak2wt-transduced Balb/c mice showing images of PB (A-B) and pathology in representative sections of BM (C-D), spleen (E-H), and liver (I-L). PB smear (B, 600 ×; Wright-Giemsa stain) of a representative Jak2wt animal displays normal white blood cell counts with an unremarkable differential. In contrast, PB smear (A, 600 ×; Wright-Giemsa stain) of a representative Jak2V617F mutant animal reveals marked leukocytosis consisting predominantly of maturing myeloid elements. Bone marrow images from Jak2wt animals display preserved marrow architecture with normal ratios of myeloid/erythroid elements and unremarkable megakaryocytes (D, 600 ×, H&E stain). In comparison, BM sections from Jak2V617F mutant animals demonstrate marrow elements composed of a prominent population of maturing myeloid cells with mildly to moderately increased numbers of megakaryocytes, including large, atypical forms occurring in occasional clusters (C; 600 ×, H&E stain) and showing emperipolesis of neutrophils in megakaryocyte cytoplasm. Spleen sections from Jak2V617F mice display complete effacement of normal splenic architecture (E; 40 ×, H&E stain) with a marked expansion of red pulp predominantly composed of maturing myeloid elements, frequent atypical megakaryocytes in clusters, and occasional erythroid elements (G; 600 ×, H&E stain) compared with unremarkable Jak2wt spleens (F, H; 40 ×, 600 ×, H&E stain). Liver images from Jak2V617F mice illustrate evidence of extensive extramedullary hematopoiesis in a perivascular and sinusoidal distribution (I; 100 ×, H&E stain) composed of frequent large, atypical megakaryocytes, maturing myeloid cells, and erythroid forms (K; 600 ×, H&E stain) notably absent in livers from Jak2wt animals (J, L; 100 ×, 600 ×, H&E stain).
Consistent with these histopathologic findings, flow cytometric analysis of Balb/c Jak2V617F mice showed an approximately 10-fold increase in the ter119+ and the CD71+/ter119+ populations in the spleen compared with Jak2wt animals (Figure 3, upper left). In addition, there was an increase in the Gr1+/Mac1+ population in BM (approximately 3-fold) or spleen (approximately 10-fold) of Jak2V617F-compared with Jak2wt-expressing mice (Figure 3, upper right). B220+ B cells were proportionately decreased in the BM and spleens of Jak2V617F-compared with the Jak2wt-expressing Balb/c mice.
In contrast to what was observed in Balb/c mice, examination of PB smears from C57Bl/6 mice after transplantation with Jak2V617F or Jak2wt revealed only modest leukocytosis in 3 Jak2V617F animals (Figure S1A-B, available on the Blood website; see the Supplemental Figures link at the top of the online article). Like Balb/c mutant mice, Jak2V617F C57Bl/6 animals showed increased reticulocytosis and enlarged platelets, accompanied by a significant increase in the mean platelet volume (MPV) (P < .01) compared with Jak2wt mice. The BM of Jak2V617F C57Bl/6 mice also displayed a prominent population of maturing myeloid cells with increased numbers of megakaryocytes, including large atypical forms occurring in occasional clusters. Emperipolesis of granulocytes in the cytoplasm of megakaryocytes was commonly noted in Jak2V617F animals but rarely occurred in Jak2wt mice with normal megakaryocyte morphology after BM transplantation (Figure S1C-D). Splenomegaly was less pronounced in Jak2V617F C57Bl/6 animals than in Balb/c mutant mice and was mainly attributable to a significant expansion of the red pulp by a predominant population of erythroid elements (Figure S1E) and occasional admixed myeloid forms and megakaryocytes (Figure S1G). Similarly, we found evidence of focal extramedullary hematopoiesis with occasional megakaryocytes and focal clusters of granulocytes in the liver (Figure S1I-L and data not shown) that were absent in Jak2wt animals. Flow cytometric analysis revealed an approximately 5-fold increase in ter119+/CD71+ and ter119+ populations in spleens of Jak2V617F C57Bl/6 mice compared with Jak2wt mice (Figure S2).
Flow cytometric analysis of BM and spleen after transplantation with BM transduced with either Jak2V617F or Jak2wt in Balb/c mice. Dot plots demonstrate an approximately 10-fold increase in the ter119+ and the CD71+/ter119+ population in the spleens of Jak2V617F compared with Jak2wt animals. Most CD71+/ter119+ cells represented a more immature erythroid population at the level of BFU-Es. Representative plots from 1 of 12 independent experiments for the mutant mice and 1 of 10 experiments for the Jak2wt mice are shown. In the BM of Jak2V617F mice, a prominent population of mature Gr1+/Mac1+ myeloid cells was detectable that had increased approximately 3-fold compared with the BM of Jak2wt mice and approximately 10-fold in spleens of Jak2V617F compared with spleens of Jak2wt mice. B220+ B cells were proportionately decreased in the BM and spleens of Jak2V617F mice compared with Jak2wt mice.
Flow cytometric analysis of BM and spleen after transplantation with BM transduced with either Jak2V617F or Jak2wt in Balb/c mice. Dot plots demonstrate an approximately 10-fold increase in the ter119+ and the CD71+/ter119+ population in the spleens of Jak2V617F compared with Jak2wt animals. Most CD71+/ter119+ cells represented a more immature erythroid population at the level of BFU-Es. Representative plots from 1 of 12 independent experiments for the mutant mice and 1 of 10 experiments for the Jak2wt mice are shown. In the BM of Jak2V617F mice, a prominent population of mature Gr1+/Mac1+ myeloid cells was detectable that had increased approximately 3-fold compared with the BM of Jak2wt mice and approximately 10-fold in spleens of Jak2V617F compared with spleens of Jak2wt mice. B220+ B cells were proportionately decreased in the BM and spleens of Jak2V617F mice compared with Jak2wt mice.
Increase in survival in Jak2V617F C57Bl/6 compared with Jak2V617F Balb/c mice. (A) Kaplan-Meier survival plot showing death of all Jak2V617F-expressing Balb/c mice between 50 and 95 days marked by the red line (n = 22), of 3 C57Bl/6 mice between 65 and 75 days marked by the blue line (3 of 18), and normal life expectancy for Jak2wt Balb/c and C57Bl/6 mice marked by the green line (n = 10 for each group). (B) Southern blot analysis demonstrating oligoclonal retroviral integration in spleen cells of 5 Jak2V617F mice after transplantation, which was polyclonal in Jak2wt mice and absent in the spleen of an untransduced wild-type animal. (C) Inverse correlation between high endogenous HCTs of 0.65 to 0.75 and low serum EPO levels of 10 pg/mL in 4 Balb/c mice and 1 C57Bl/6 mouse expressing Jak2V617F and normal serum EPO levels of 150 and 250 pg/mL in 1 Balb/c mouse and 1 C57Bl/6 mouse expressing Jak2wt with normal HCTs and appropriately high serum EPO levels in 5-fluorouracil–treated anemic animals.
Increase in survival in Jak2V617F C57Bl/6 compared with Jak2V617F Balb/c mice. (A) Kaplan-Meier survival plot showing death of all Jak2V617F-expressing Balb/c mice between 50 and 95 days marked by the red line (n = 22), of 3 C57Bl/6 mice between 65 and 75 days marked by the blue line (3 of 18), and normal life expectancy for Jak2wt Balb/c and C57Bl/6 mice marked by the green line (n = 10 for each group). (B) Southern blot analysis demonstrating oligoclonal retroviral integration in spleen cells of 5 Jak2V617F mice after transplantation, which was polyclonal in Jak2wt mice and absent in the spleen of an untransduced wild-type animal. (C) Inverse correlation between high endogenous HCTs of 0.65 to 0.75 and low serum EPO levels of 10 pg/mL in 4 Balb/c mice and 1 C57Bl/6 mouse expressing Jak2V617F and normal serum EPO levels of 150 and 250 pg/mL in 1 Balb/c mouse and 1 C57Bl/6 mouse expressing Jak2wt with normal HCTs and appropriately high serum EPO levels in 5-fluorouracil–treated anemic animals.
In accordance with the generally more aggressive clinical phenotype observed in Balb/c mice, all animals were killed because of associated morbidities within 50 to 95 days of transplantation (Figure S3). In contrast, only 3 of 18 C57Bl/6 mice developed end-stage disease mandating humane killing during an observation period of up to 32 weeks (Figure 4A).
Clonality, serum EPO levels, and transplantability
Southern blot analysis demonstrated oligoclonal retroviral integration in cells derived from single-cell suspensions in the spleens of the Jak2V617F BM transplant model (Figure 4B). Because erythrocytosis is an unusual phenotype in murine bone marrow transplant models of hematopoietic malignancies, we also assessed serum EPO levels to test the unlikely possibility that there were secondary causes of erythrocytosis. After transplantation, Jak2V617F mice of both strains with high HCTs (.65-.75) had serum EPO levels in the lowest detectable range (approximately 10 pg/mL), in contrast to Jak2wt mice with normal HCTs and normal serum EPO levels. In control experiments, anemic animals assayed after treatment with 5-fluorouracil showed appropriately high serum EPO levels (Figure 4C).
Jak2V617F and Stat5 are constitutively activated in vivo. (A) Immunoprecipitation and Western blot analysis in GFP-sorted, Jak2V617F, Jak2wt, and empty vector transfected Ba/F3 cells showing constitutively active Jak2 and Stat5 after 4 hours of starvation in 1% bovine serum albumin in Jak2V617F stable Ba/F3 cells. (B) Phospho-flow analysis showing significant increases in phosphorylated Stat5 in the BM and spleens of Jak2V617F mice compared with Jak2wt mice, as indicated by the significant shift to the left (P < .001).
Jak2V617F and Stat5 are constitutively activated in vivo. (A) Immunoprecipitation and Western blot analysis in GFP-sorted, Jak2V617F, Jak2wt, and empty vector transfected Ba/F3 cells showing constitutively active Jak2 and Stat5 after 4 hours of starvation in 1% bovine serum albumin in Jak2V617F stable Ba/F3 cells. (B) Phospho-flow analysis showing significant increases in phosphorylated Stat5 in the BM and spleens of Jak2V617F mice compared with Jak2wt mice, as indicated by the significant shift to the left (P < .001).
The disease phenotype was not transplantable in C57Bl/6 mice under conditions using 1 × 106 diseased whole BM cells injected into sublethally irradiated (1 × 5.5 Gy [1 × 550 rad]) hosts (n = 5). Increased doses of 4 × 106 whole BM cells (n = 2) or splenocytes (n = 3) of diseased animals transplanted into lethally irradiated recipients with the congenic marker Ly5.1 also failed to confer elevated HCT levels in recipient animals.
Jak2V617F and Stat5 are constitutively activated in vivo, as assessed by tyrosine phosphorylation
In control experiments, tyrosine phosphorylation of Jak2 and Stat5 was assayed by transfecting Ba/F3 cells with MSCV-IRES-EGFP vectors containing Jak2V617F, Jak2wt, or no insert. FACS-sorted GFP-expressing cells were immunoprecipitated with anti-Jak2 or anti-Stat5 and blotted with anti–phospho-Jak2 or anti–phospho-Stat5, respectively. As previously reported, there was constitutive activation of Jak2 and Stat5 in Jak2V617F-expressing Ba/F3 cells, as assessed by tyrosine phosphorylation, that was not detectable in Jak2wt or empty vector (Figure 5A). We next tested for increased Stat5 activation as assessed by phospho-flow analysis of primary hematopoietic cells derived from Jak2V617F or Jak2wt animals. There was a significant increase in phosphorylated Stat5 in BM and spleens of Jak2V617F animals in comparison with Jak2wt animals. These data indicate that Jak2V617F expression in vivo also results in the activation of Stat5 (Figure 5B).
Jak2V617F increases the number of erythroid colonies (BFU-E and CFU-E) and total numbers of colonies in a BM transplant model. (A) CFU assays in methylcellulose show an approximately 2-fold increase in the total number of colonies in BM (P < .01) and an approximately 5-fold increase in the spleens of Jak2V617F-compared with Jak2wt-expressing mice (P < .01). Distributions of all types of colonies were comparable with the exception of Jak2wt spleens, in which no GEMMs were detectable (n = 5 Jak2V617F BM; n = 4 Jak2wt BM; n = 8 Jak2V617F spleen; n = 4 Jak2wt spleen). (B) Total numbers of BFU-Es were increased approximately 4-fold in Jak2V617F BM compared with Jak2wt BM mice (P < .01; n = 5 Jak2V617F BM; n = 4 Jak2wt BM, in duplicate) and approximately 9-fold increased in spleens of Jak2V617F compared with Jak2wt mice (P < .01; n = 8 Jak2V617F BM; n = 4 Jak2wt spleens, in duplicate). (C) Growth of CFU-Es in the absence of cytokines and hypersensitivity of CFU-E colonies to EPO in Jak2V617F spleens (P < .01) but not in Jak2wt spleens.
Jak2V617F increases the number of erythroid colonies (BFU-E and CFU-E) and total numbers of colonies in a BM transplant model. (A) CFU assays in methylcellulose show an approximately 2-fold increase in the total number of colonies in BM (P < .01) and an approximately 5-fold increase in the spleens of Jak2V617F-compared with Jak2wt-expressing mice (P < .01). Distributions of all types of colonies were comparable with the exception of Jak2wt spleens, in which no GEMMs were detectable (n = 5 Jak2V617F BM; n = 4 Jak2wt BM; n = 8 Jak2V617F spleen; n = 4 Jak2wt spleen). (B) Total numbers of BFU-Es were increased approximately 4-fold in Jak2V617F BM compared with Jak2wt BM mice (P < .01; n = 5 Jak2V617F BM; n = 4 Jak2wt BM, in duplicate) and approximately 9-fold increased in spleens of Jak2V617F compared with Jak2wt mice (P < .01; n = 8 Jak2V617F BM; n = 4 Jak2wt spleens, in duplicate). (C) Growth of CFU-Es in the absence of cytokines and hypersensitivity of CFU-E colonies to EPO in Jak2V617F spleens (P < .01) but not in Jak2wt spleens.
Jak2V617F enhances growth and proliferation of BM and spleen cells and confers EPO-independent growth to erythroid colonies
Total hematopoietic colony numbers derived from BM or spleen on day 8 after plating in methylcellulose with growth factors were increased in Jak2V617F animals compared with Jak2wt (P < .01 BM; P < .01 spleen) (Figure 6A). All types of hematopoietic colonies were present at normal percentages, with the exception of spleen cells of Jak2wt in which no CFU–granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM) colonies were detected (Figure 6A). There was also a significant increase in total numbers of BFU-Es in Jak2V617F-expressing BM and spleen cells compared with Jak2wt BM and spleen cells (BM, P < .01; spleen, P < .01) (Figure 6B). Fresh spleen cells of Jak2V617F animals were plated in methylcellulose in the absence of cytokines or with 0.01 U or 0.1 U EPO at a density of 3.2 × 105 cells/mL. CFU-E growth was detected in the absence of cytokines in Jak2V617F but not in Jak2wt spleens. In addition, there was evidence of hypersensitivity of CFU-E to EPO in colonies derived from Jak2V617F spleens (Figure 6C).
Maturation defect in megakaryocytes derived from Jak2V617F animals
Flow cytometric analysis of cultured megakaryocytes revealed a decrease in polyploid megakaryocytes in BM from Jak2V617F mice compared with BM megakaryocytes derived from Jak2wt BM (Figure 7A, red overlay). In addition, there was a decrease in the total number of acetylcholine-positive megakaryocyte colonies in BM derived from Jak2V617F compared with Jak2wt BM (Figure 7B). Together these data indicate that although BM from Jak2V617F showed megakaryocyte hyperplasia, there was a defect in the maturation of these megakaryocytes.
Discussion
JAK2V617F has recently been identified by several groups as an acquired somatic mutation present in most MPD patients with PV, CIMF, or ET.1,4,6 The mutant allele has certain properties of other constitutively activated tyrosine kinases associated with hematopoietic malignancies, including enhanced tyrosine autophosphorylation in hematopoietic and nonhematopoietic cell contexts. In addition, JAK2V617F confers factor-independent growth to hematopoietic cell lines expressing the EpoR and hypersensitivity to stimulation with EPO. JAK2V617F had also been reported to induce polycythemia in a murine BM transplant model of disease, though detailed phenotypic analysis was not provided.1 We were interested in understanding the basis for the apparent phenotypic pleiotropy of a single disease allele that segregates with 3 clinically distinct, though related, myeloproliferative diseases. In addition, it is a puzzle that only a single mutant-activating allele has been identified in these diseases, in contrast to, for example, FLT3 mutations in acute myeloid leukemia, encompassing a broad spectrum of activating mutations that have been identified in the juxtamembrane domain and the activation loop of FLT3. The data generated from these analyses provide some insight into these questions.
We expressed Jak2V617F and control Jak2wt cDNA in the murine hematopoietic system through ex vivo transduction and BM transplantation. The mice developed striking polycythemia after a short latency with a high degree of penetrance. We did not observe polycythemia in a spectrum of BM transplant experiments using constitutively activated tyrosine kinases associated with myeloid malignancies—including breakpoint cluster region–Abelson murine leukemia (BCR-ABL) virus, TEL-PDGFRB, TEL-ABL, FIP1L1-PDGFRA, and ZNF198-FGFR1, among others—in humans, suggesting a specific role for the JAK2V617F allele in the induction of polycythemia in this murine model and in humans. The polycythemia in the mouse model was stable up to at least 20 weeks and was associated with other key features of PV, including leukocytosis, splenomegaly caused by extramedullary hematopoiesis, cytokine-independent colony formation in vitro, and low serum erythropoietin levels.14,23 These data indicate that Jak2V617F is able to confer a disease phenotype in mice with clinicopathologic features reminiscent of PV. In addition, data indicate that Stat5 is activated in vivo in hematopoietic progenitors, as has previously been reported in vitro.1,2
Loss of polyploid megakaryocytes in Jak2V617F animals. (A, top) Unremarkable 2n, 4n, 8n, 16n, 32n, and 64n peaks in megakaryocytes of Jak2wt in gray. (Middle) Normal 2n and 4n peaks in Jak2V617F but loss or decrease of 8n to 64n peaks in the mutant in red. (Bottom) Overlay. Megakaryocytes were distinguished by size and positive staining for CD41, and polyploidy was distinguished by propidium iodide staining. (B) Megacult assay showing an approximately 2-fold decrease in the BM of Jak2V617F-expressing animals (P < .01).
Loss of polyploid megakaryocytes in Jak2V617F animals. (A, top) Unremarkable 2n, 4n, 8n, 16n, 32n, and 64n peaks in megakaryocytes of Jak2wt in gray. (Middle) Normal 2n and 4n peaks in Jak2V617F but loss or decrease of 8n to 64n peaks in the mutant in red. (Bottom) Overlay. Megakaryocytes were distinguished by size and positive staining for CD41, and polyploidy was distinguished by propidium iodide staining. (B) Megacult assay showing an approximately 2-fold decrease in the BM of Jak2V617F-expressing animals (P < .01).
In addition, we observed several important strain-specific differences in phenotype. First, reticulin fibrosis, a concomitant of disease in some patients with PV, was observed in the BM of Balb/c but not of C57Bl/6 mice. Although larger numbers of mice are required to fully annotate this observation, the data are consistent with strain-specific modifiers of the fibrosis phenotype. In addition, we observed significantly increased leukocytosis in the Balb/c background compared with C57Bl/6, again suggesting the influence of host modifiers. We and others have observed similar strain-specific differences in phenotype between Balb/c and C57Bl/6 mice transduced with constitutively activated tyrosine kinase alleles, such as BCR-ABL and FLT3-ITD (D.G.G., unpublished observations, June 2002). C57Bl/6 in general appears to attenuate phenotype. It will be important to monitor the effect of the Jak2V617F allele in the C57Bl/6 background in the event that during long-term follow-up additional events result in other attributes of MPD, such as thrombocytosis or progressive fibrosis.
Although there was marked megakaryocyte hyperplasia in mice of both backgrounds, megakaryocyte differentiation seemed to be impaired and animals had normal platelet counts. In contrast, most humans with PV have some degree of thrombocytosis. These data may be consistent with a requirement for additional mutations that potentiate platelet production, though other explanations are possible. Preliminary studies indicate a highly variable degree of penetrance of PV-like disease in 6 additional mouse inbred strains examined, suggesting that it may be possible to identify genetic modifiers using conventional mapping strategies in mice (G.W. and D.G.G., unpublished observations, July 2005). Data that support a requirement for additional mutations in some contexts include the observation of mitotic recombination that generates 2 copies of the JAKV617F allele in some patients with PV, ET, or CIMF.2 The phenotypic consequences, if any, of a heterozygous compared with a homozygous JAK2V617F allele in humans are unknown, but at a minimum the genetic data indicate a selection for clones that have acquired 2 mutant alleles through mitotic recombination. To accurately assess the role of a heterozygous compared with a homozygous Jak2V617F mutation, it will probably be necessary to generate conditional knock-in alleles. As further evidence of a requirement for second mutations, certain pedigrees have a heritable proclivity to develop PV that maps to a genomic locus other than the JAK2 locus, despite the fact that JAK2V617F is acquired with disease progression. This observation suggests a second disease allele in the germline that complements the activity of the JAK2V617F allele.24 Data also indicate that a percentage of JAK2V617F+ patients have secondarily acquired cytogenetic aberrations, such as 20q–. Finally, we have recently observed that in some female patients with clonally derived granulocytes, JAK2V617F is present in only a fraction of cells by quantitative genomic PCR, suggesting that a JAK2V617F mutation may be a secondary event in this instance (R.L.L. and D.G.G., unpublished observations, July 2005).
These findings do not address the basis for selecting a single activating allele of JAK2. It will be important to test other activating alleles of JAK2 to determine whether only JAK2V617F can cause PV. However, we have recently observed that although JAK2 normally signals from the engagement of a broad spectrum of type I and type II cytokine receptors, JAK2V617F is most efficient in transforming hematopoietic cells when expressed from type I cytokine receptors that lack a common chain (EpoR, Mpl, G-CSR, or prolactin receptor).25 These data suggest that qualitatively altered cytokine signaling in the mutant allele specifies phenotype. Further experiments in this murine BM transplantation assay in appropriate backgrounds (such as G-CSFR–deficient animals) should help to clarify this question. Finally, this murine BM transplantation model provides a robust and reproducible in vivo assay system to assess the phenotypic consequences and therapeutic efficacy of JAK2-selective inhibitors.
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-12-4824.
Supported in part by grants from the National Institutes of Health (CA66996, DK50654, CA04002), the Leukemia and Lymphoma Society of America, and the Howard Hughes Medical Institute.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marius Wernig for insightful discussions and contributions to this work and Elizabeth McDowell, Dana Cullen, Sandra Moore, Allison Coburn, and Maricel Gozo for their outstanding technical assistance.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal