Bortezomib, a proteasome inhibitor with efficacy in multiple myeloma, is associated with thrombocytopenia, the cause and kinetics of which are different from those of standard cytotoxic agents. We assessed the frequency, kinetics, and mechanism of thrombocytopenia following treatment with bortezomib 1.3 mg/m2 in 228 patients with relapsed and/or refractory myeloma in 2 phase 2 trials. The mean platelet count decreased by approximately 60% during treatment but recovered rapidly between treatments in a cyclic fashion. Among responders, the pretreatment platelet count increased significantly during subsequent cycles of therapy. The mean percent reduction in platelets was independent of baseline platelet count, M-protein concentration, and marrow plasmacytosis. Plasma thrombopoietin levels inversely correlated with platelet count. Murine studies demonstrated a reduction in peripheral platelet count following a single bortezomib dose without negative effects on megakaryocytic cellularity, ploidy, or morphology. These data suggest that bortezomib-induced thrombocytopenia is due to a reversible effect on megakaryocytic function rather than a direct cytotoxic effect on megakaryocytes or their progenitors. The exact mechanism underlying bortezomib-induced thrombocytopenia remains unknown but it is unlikely to be related to marrow injury or decreased thrombopoietin production.
Introduction
Thrombocytopenia is a frequent complication of chemotherapy that may result in bleeding as well as reductions in the delivered dose of chemotherapy. The development of thrombocytopenia in patients with cancer is often attributable to the cytotoxic effects of cell cycle–active agents on megakaryocytes.1 Thrombopoietin (TPO), a megakaryocyte growth and differentiation factor, is made constitutively by the liver, kidney, and bone marrow stroma.2 Circulating TPO is cleared via binding to circulating platelets and bone marrow megakaryocytes.3,4 Marrow megakaryopoiesis is then regulated by the circulating platelet count as well as by marrow megakaryocyte content. When circulating platelet counts or megakaryocyte content of the marrow decreases as a result of cytotoxic injury, peripheral TPO levels increase due to decreased TPO clearance.1,5
Reciprocal modulations of platelet counts and TPO are commonly observed in patients who receive myelosuppressive chemotherapy.6-10 Platelet budding from megakaryocytes is a tightly regulated process that in part depends on activity of nuclear factor κB (NF-κB).11 The kinetics of megakaryopoiesis are such that megakaryocyte differentiation often requires 10 days following cytotoxic drug treatment, and prolonged periods of thrombocytopenia are commonly seen following cytotoxic chemotherapy as a result of marrow injury. Because specific and effective megakaryocyte growth factors are not commercially available, this delayed recovery of peripheral platelet counts can limit dose-intense delivery of chemotherapy.
Bortezomib is a potent and reversible proteasome inhibitor that has been extensively studied in preclinical and clinical studies.12 Two phase 2 clinical trials, Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition Therapy (SUMMIT) and Clinical Response and Efficacy Study of Bortezomib in the Treatment of Relapsing Multiple Myeloma (CREST), established the activity of bortezomib in the treatment of relapsed and/or refractory multiple myeloma. The SUMMIT trial evaluated the use of bortezomib at a dose of 1.3 mg/m2 in patients with relapsed, refractory multiple myeloma, with an overall response rate of 35% for bortezomib alone13 by the European Group for Blood and Marrow Transplantation (EBMT) criteria.14 Patients who had relapsed after front-line therapy were treated with bortezomib at a dose of 1.0 or 1.3 mg/m2 in the CREST trial and demonstrated overall response rates of 33% and 50%, respectively, to bortezomib alone.15
The most common grade 3 adverse event reported during SUMMIT and CREST was a cyclic thrombocytopenia, which was reported in 28% of patients in SUMMIT and 24% of patients in CREST.13,15 The specific mechanism by which bortezomib causes thrombocytopenia is not known. However, unlike that of many other antineoplastic agents,16,17 preclinical evidence suggested that bortezomib did not adversely affect stem cell function.18 Proteasome inhibition with bortezomib prevents the activation of NF-κB,19 which may potentially prevent platelet budding from megakaryocytes during treatment.
We conducted an analysis of the relationship of blood platelet count to bortezomib treatment to characterize the severity and predictability of thrombocytopenia in patients with relapsed or refractory multiple myeloma treated in SUMMIT and CREST. We also evaluated circulating TPO levels among patients receiving bortezomib based on the observation that bone marrow stromal cells produce thrombopoietin and that stromal-myeloma cell interactions are inhibited by bortezomib. We initially hypothesized that the thrombocytopenia seen with bortezomib therapy may be related to a reduction in bone marrow stromal TPO production and sought to test this hypothesis. Pretreatment characteristics were evaluated to determine potential risk factors for the development of grade 3/4 thrombocytopenia, and the relationship between platelet and thrombopoietin levels during treatment was also examined. In a complementary preclinical laboratory experiment we evaluated peripheral platelet count, ploidy, and megakaryocyte content in the marrow of mice treated with a single dose of bortezomib.
Patients, materials, and methods
Eligibility criteria from SUMMIT and CREST have been reported previously.13,15 In brief, patients were 18 years of age or older with measurable disease and relapsed or refractory disease following front-line chemotherapy (CREST) or relapsed and refractory disease after at least 2 prior regimens (SUMMIT). Patients were required to have a Karnofsky performance status (KPS) of at least 60% and a platelet count of 50 × 109/L or greater; if they had extensive bone marrow involvement, the threshold was lowered to 30 × 109/L or greater. Patients were allowed to have unlimited transfusion support and could undergo transfusion at study entry to meet this platelet count. All patients provided written, informed consent before study entry; studies were conducted in accordance with the Declaration of Helsinki, and approval was obtained from the local institutional review board (IRB) of each study center.
Treatment with bortezomib 1.3 mg/m2 was administered as an intravenous push over 3 to 5 seconds twice weekly (days 1, 4, 8, and 11) for the first 2 weeks of each 3-week treatment cycle for up to 8 cycles. Treatment was to be withheld in patients who developed grade 3 or higher nonhematologic or grade 4 hematologic toxicities and resumed at a 25% dose reduction (1.3 to 1.0 mg/m2 and 1.0 to 0.7 mg/m2) after resolution to grade 1 or better. Oral dexamethasone 20 mg was administered on the day of treatment with bortezomib and on the day after treatment in patients who had progressive disease after 2 or stable disease after the first 4 treatment cycles.
Baseline evaluations included platelet counts, serum myeloma protein concentrations, the percentage of bone marrow infiltration by plasma cells, and the number of prior treatment regimens. Platelet counts were also monitored before the administration of bortezomib on days 1, 4, 8, and 11 of each treatment cycle. EBMT criteria14 were used by an independent review committee to evaluate responses after cycles 2, 4, 6, and 8.
The incidence and severity of thrombocytopenia were monitored by adverse event reporting and laboratory evaluation of plasma samples. The Common Toxicity Criteria version 2.0 of the National Cancer Institute was used to grade thrombocytopenia: grade 1, 75.0 × 109/L to less than the lower limit of normal; grade 2, 50 × 109/L or greater to less than 75 × 109/L; grade 3, 10 × 109/L or greater to less than 50 × 109/L; grade 4, less than 10 × 109/L.20
TPO concentrations were measured in a subset of patients with refractory multiple myeloma who received bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of the first 8 cycles followed by once-weekly treatment for the first 4 of 5 weeks (6 patients received twice-weekly therapy, and 1 patient received once-weekly therapy) as part of a separate IRB-approved study, Assessment of Proteasome Inhibition for Extending Remissions (APEX); these patients also signed written, informed consent.21 Platelet counts were measured on the day of bortezomib administration. Plasma samples were obtained during treatment with bortezomib and were frozen for later analysis of TPO levels using an enzyme-linked immunosorbent assay (ELISA; R & D Systems Kit, Minneapolis, MN). TPO levels were also measured in patients receiving maintenance therapy with bortezomib on a weekly schedule protocol. Murine plasma and serum TPO levels were collected and analyzed using an ELISA assay kit (R & D Systems). TPO concentrations were 3 to 4.7 times higher in serum compared with paired plasma samples. For some time points serum TPO concentrations were converted to equivalent plasma values.
Murine experiments
Male BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used for these experiments at 16 weeks of age. Mice were housed in microisolator cages in the Emory University Animal Care Facility, with acidified water and standard chow available at all times. Animal handling and experimental procedures were approved by the Emory University Institutional Animal Care and Use Committee. Bortezomib was dissolved at a concentration of 100 mM (38.424 mg/kg) in dimethyl sulfoxide (DMSO) and stored at –20°C. This stock was diluted to 0.25 mg/mL bortezomib in sterile 0.9% sodium chloride, and experimental mice received 1.0 to 2.5 mg/kg bortezomib via tail-vein injection. Control mice received only DMSO. In another series of experiments, 2 separate groups of mice (n = 12 DMSO, n = 21 bortezomib treated) were treated with 1.5 mg/kg bortezomib or DMSO on a standard day 1, 4, 8, and 11 schedule (per human dosing scheme). Blood samples were collected via the retro-orbital sinus using 20 μL ethylenediaminetetraacetic acid (EDTA)–coated microcapillary tubes (Drew Scientific, Oxford, CT) from mice anesthetized with isoflurane inhalation. Platelet counts were obtained using an automated hematology analyzer (Beckman Coulter AcT Diff with veterinary software; Beckman Coulter, Fullerton, CA). Bone marrow megakaryocyte content and morphology from bortezomib- and control-treated mice were determined from femora that were fixed in 10% buffered formalin, decalcified, and embedded in paraffin. Coded histologic sections were stained with hematoxylin and eosin, and mounted with cytoseal medium (Richard Allen Scientific, Kalamazoo, MI) for standard light microscopy using an Eclipse E600 microscope (Nikon, Melville, NY) with Plan Fluor 20 × 0.50 numeric aperture (NA) and 60 × 0.85 NA objective lenses. Images were acquired using a Spot Insight QE camera (Diagnostic Instruments, Sterling Heights, MI) and processed using Photoshop CS version 8.0 software (Adobe Systems, San Jose, CA). The number of megakaryocytes per × 200 field was determined by a trained pathologist (D.L.J.) without knowledge of treatment conditions. Megakaryocyte ploidy was determined from bone marrow suspensions prepared from flushing the intramedullary cavity of a femur and by counting the number of nucleated cells using the Beckman CoulterAcT Diff and labeling cell-surface antigens and DNA content with fluorescent dyes, using a method modified from Schmid et al.22 Briefly, cells were stained with fluorescein isothiocyanate (FITC)–conjugated antibodies against CD41 and CD61 and a phycoerythrin-conjugated antibody against Gr-1 (BD Biosciences Pharmingen, San Diego, CA), fixed with 0.25% paraformaldehyde, and permeabilized using 0.05% Tween 20 prior to staining the DNA with 25 μg/mL 7-aminoactinomycin D (7-AAD). Multiparametric flow cytometry was performed using a FACSAria instrument and FACSDIVA software (BD Biosciences, San Jose, CA), and digital list mode files were analyzed for determinations of megakaryocyte content and ploidy distribution using FlowJo software (Tree Star, Ashland, OR). Cells that did not stain with 7-AAD were excluded from analysis. Two list mode files were acquired for each sample: an ungated data file containing approximately 5 × 105 events for determination of the megakaryocyte fraction and a file excluding low FITC fluorescence and containing approximately 1 × 106 events for determination of megakaryocyte ploidy distribution. Megakaryocytes were defined by positive staining for CD41/61 and negative staining for Gr-1. The level of staining for the 2N and 4N populations was identified by gating on monocytes. The 7-AAD fluorescence distribution of gated megakaryocytes identified the 2N, 4N, 8N, 16N, 32N, and 64N populations (modified from Corash et al23 ). The absolute number of CD41/61+Gr-1– megakaryocytes in each ploidy category (2N, 4N, 8N, 16N, 32N, and 64N) was determined by multiplying the total cell number (1 femur plus 2 tibiae) by the fraction of bone marrow cells represented in each category.
Statistics
All statistical summary analyses, including standard deviations (SDs), were performed using SAS version 8.2 (SAS Institute, Cary, NC). Multivariate analyses were performed using logistic regression to determine whether there was an association between baseline platelet counts, baseline M-protein concentration, number of prior regimens (lines of therapy), age, or the baseline percentage of bone marrow plasmacytosis (BMPC) on the development of grade 3 or 4 thrombocytopenia.
Results
Patient characteristics
Demographics and baseline characteristics are shown in Table 1 for all patients in SUMMIT and CREST who received bortezomib 1.3 mg/m2 (n = 228).
Patient demographics and baseline characteristics
. | Data . |
|---|---|
| No. patients studied | 228 |
| Median age, y (range) | 59 (30-84) |
| Male/female, % | 57/43 |
| Ethnicity, % | |
| European American | 81 |
| African American | 11 |
| Other | 9 |
| Myeloma type, % | |
| IgG | 61 |
| IgA | 24 |
| Other | 15 |
| Median no. of previous regimens (range) | 5 (1-15) |
| Prior therapies, % | |
| Any corticosteroid | 99.6 |
| Any alkylating agent | 89 |
| Any anthracycline | 79 |
| Prior thalidomide | 77 |
| Stem cell transplantation or other high-dose therapy | 61 |
| KPS | |
| No. patients | 222 |
| 70% or less, % of patients | 20 |
| 80%, % of patients | 37 |
| 90% or greater, % of patients | 43 |
| Elevated β2-microglobin level | |
| No. patients | 210 |
| 4 mg/L or greater, % | 44 |
| 6 mg/L or greater, % | 28 |
| Bone marrow cytogenetics | |
| No. patients | 195 |
| Any abnormalities, % | 37 |
| Chromosome 13 deletion, % | 15 |
| Hemoglobin level less than 100 g/L, % | 40 |
| Mean platelet count, × 109/L (median) | 167.2 (166.5) |
| % of patients given platelet transfusions | 14 |
. | Data . |
|---|---|
| No. patients studied | 228 |
| Median age, y (range) | 59 (30-84) |
| Male/female, % | 57/43 |
| Ethnicity, % | |
| European American | 81 |
| African American | 11 |
| Other | 9 |
| Myeloma type, % | |
| IgG | 61 |
| IgA | 24 |
| Other | 15 |
| Median no. of previous regimens (range) | 5 (1-15) |
| Prior therapies, % | |
| Any corticosteroid | 99.6 |
| Any alkylating agent | 89 |
| Any anthracycline | 79 |
| Prior thalidomide | 77 |
| Stem cell transplantation or other high-dose therapy | 61 |
| KPS | |
| No. patients | 222 |
| 70% or less, % of patients | 20 |
| 80%, % of patients | 37 |
| 90% or greater, % of patients | 43 |
| Elevated β2-microglobin level | |
| No. patients | 210 |
| 4 mg/L or greater, % | 44 |
| 6 mg/L or greater, % | 28 |
| Bone marrow cytogenetics | |
| No. patients | 195 |
| Any abnormalities, % | 37 |
| Chromosome 13 deletion, % | 15 |
| Hemoglobin level less than 100 g/L, % | 40 |
| Mean platelet count, × 109/L (median) | 167.2 (166.5) |
| % of patients given platelet transfusions | 14 |
To convert β2-microglobulin level from milligrams per liter to nanomoles per liter, multiply milligrams per liter by 85.
IgG indicates immunoglobulin G.
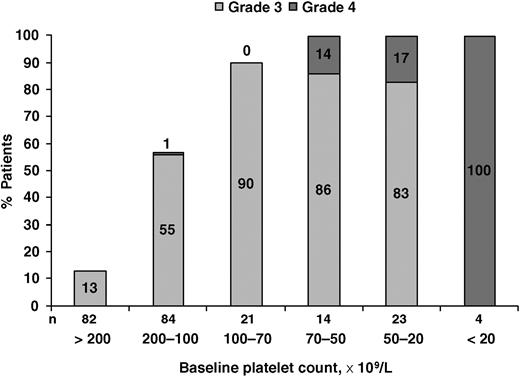
Incidence of grade 3/4 thrombocytopenia according to baseline platelet counts.
Incidence of grade 3/4 thrombocytopenia according to baseline platelet counts.
Thrombocytopenia: frequency, severity, and risk factors
The mean baseline platelet count was 165 × 109/L (SD 91.3), and the mean cycle 1 day 11 platelet count was 107 × 109/L (SD 65.7). The incidence of all grades of treatment-emergent thrombocytopenia with bortezomib 1.3 mg/m2 was 42% (96 of 228 patients), as measured by adverse event reporting. The incidence of grade 3/4 thrombocytopenia was 30% (68 of 228 patients) and it was inversely related to the baseline platelet count (Figure 1). Grade 4 thrombocytopenia (< 10 × 109/L) occurred in 24% of patients with baseline platelet counts less than 70 × 109/L, whereas its incidence was low (≤ 1%) in patients with higher baseline counts.
Mean platelet concentrations in all patients on days 1, 4, 8, and 11 of each treatment cycle followed a biphasic and cyclic pattern, decreasing during the portion of the cycle when bortezomib was administered and recovering to baseline during the rest periods of each cycle (Figure 2). Among responding patients, the day 1 mean platelet count increased between cycles 1 and 8 from 178.8 (SD 97.7) to 200.1 (SD 102.8) (P < .001).
Significant predictors of grade 3/4 thrombocytopenia by multivariate analysis included low baseline platelet count (odds ratio 0.974; P < .001), high baseline M-protein concentration (odds ratio 0.978; P = .032), and number of prior regimens (odds ratio 1.259; P = .007).
Patients with less bone marrow plasma cell infiltration (ie, < 50% BMPC) had significantly higher baseline pretreatment platelet counts (P < .001) and nadir platelet counts (P < .001) and greater absolute platelet changes (absolute platelet change = baseline platelet count – nadir platelet count) from baseline in platelet counts over the course of the study (P < .001) than did patients with greater than 50% BMPC (Figure 3). Similarly, patients with lower baseline M-protein levels (< 31 g/L) began therapy with a higher platelet count and had a higher platelet nadir than did patients with M-protein concentrations of greater than 31 g/L (Table 2).
Association of baseline bone marrow plasmacytosis (BMPC) and M-protein with mean platelet parameters
. | 50% or greater BMPC . | Less than 50% BMPC . | M-protein concentration greater than 31 g/L . | M-protein concentration less than 31 g/L . |
|---|---|---|---|---|
| Baseline platelet count, ×109/L | 135.57 | 198.92* | 139.94 | 188.45* |
| Platelet nadir, ×109/L | 48.2 | 80.34* | 54.92 | 70.47‡ |
| Platelet change, ×109/L | -87.37 | -119.88* | -85.82 | -117.98§ |
| Platelet change, % | 63.7 | 58.3† | 59.1 | 63.3∥ |
| Baseline BMPC, % | — | — | 66.32 | 38.61* |
| Baseline M-protein concentration | 39.78 | 23.96* | — | — |
. | 50% or greater BMPC . | Less than 50% BMPC . | M-protein concentration greater than 31 g/L . | M-protein concentration less than 31 g/L . |
|---|---|---|---|---|
| Baseline platelet count, ×109/L | 135.57 | 198.92* | 139.94 | 188.45* |
| Platelet nadir, ×109/L | 48.2 | 80.34* | 54.92 | 70.47‡ |
| Platelet change, ×109/L | -87.37 | -119.88* | -85.82 | -117.98§ |
| Platelet change, % | 63.7 | 58.3† | 59.1 | 63.3∥ |
| Baseline BMPC, % | — | — | 66.32 | 38.61* |
| Baseline M-protein concentration | 39.78 | 23.96* | — | — |
— indicates not applicable.
P < .001.
P = .06.
P = .03.
P = .001.
P = .18
Further analysis of therapy-related thrombocytopenia demonstrated that the mean percent reduction in peripheral platelet count (percent reduction of platelet count = [baseline platelet count – nadir platelet count]/[baseline platelet count] × 100) was similar across several different treatment groups. All patients experienced an approximately 60% reduction in platelet counts from start to nadir, regardless of their baseline platelet count, serum M-protein concentration, or percentage of BMPC (Table 2). The lowest measured mean platelet nadir among all patients (n = 228) and in nonresponders (n = 156) occurred on day 4 of cycle 4. Among patients with a complete or partial response to bortezomib therapy (n = 63), the lowest platelet nadir occurred earlier, on day 8 of cycle 1. Thus, mean platelet nadirs occurred early, in cycle 1, among patients who responded, and mean platelet counts were higher in subsequent cycles.
Mean platelet count with standard error during each treatment cycle.
Platelet recovery. (A) Platelet recovery over time in patients with less than 50% plasma cell infiltration in the bone marrow (n = 103). (B) Platelet recovery over time in patients with 50% or greater plasma cell infiltration in the bone marrow (n = 116).
Platelet recovery. (A) Platelet recovery over time in patients with less than 50% plasma cell infiltration in the bone marrow (n = 103). (B) Platelet recovery over time in patients with 50% or greater plasma cell infiltration in the bone marrow (n = 116).
Although there was a tendency toward a lower incidence of grade 3/4 thrombocytopenia in complete or partial responders (41%) compared with nonresponders (56%), the difference did not reach significance (P = .054). Importantly, overall response rates were similar between patients with baseline platelet counts less than 75 × 109/L and in those with baseline platelet counts of 75 × 109/L or greater (Table 3). Although no patients with a baseline platelet count less than 50 × 109/L were enrolled into CREST, 22 patients with a baseline platelet count less than 50 × 109/L were enrolled into SUMMIT, and stable disease or better was observed in 9 of these patients (41%).
Treatment response in evaluable patients according to baseline platelet counts
. | SUMMIT . | . | CREST* . | |
|---|---|---|---|---|
| . | BPC less than 75 × 109/L . | BPC 75 × 109/L or greater . | BPC 75 × 109/L or greater . | |
| No. patients | 35 | 157 | 26 | |
| CR, no. (%) | 3 (9) | 15 (10) | 1 (4) | |
| PR, no. (%) | 8 (23) | 26 (17) | 9 (35) | |
| MR, no. (%) | 2 (6) | 12 (8) | 3 (12) | |
| Overall response (CR, PR, MR), no. (%) | 13 (37) | 53 (34) | 13 (50) | |
. | SUMMIT . | . | CREST* . | |
|---|---|---|---|---|
| . | BPC less than 75 × 109/L . | BPC 75 × 109/L or greater . | BPC 75 × 109/L or greater . | |
| No. patients | 35 | 157 | 26 | |
| CR, no. (%) | 3 (9) | 15 (10) | 1 (4) | |
| PR, no. (%) | 8 (23) | 26 (17) | 9 (35) | |
| MR, no. (%) | 2 (6) | 12 (8) | 3 (12) | |
| Overall response (CR, PR, MR), no. (%) | 13 (37) | 53 (34) | 13 (50) | |
BPC indicates baseline platelet count; CR, complete response; PR, partial response; and MR, minor response.
No patient in CREST receiving bortezomib 1.3 mg/m2 had a baseline platelet count less than 75 × 109/L.
Clinical sequelae and platelet support
No bleeding that required hospitalization was reported in this population. Bleeding associated with thrombocytopenia was reported in 2 patients. Of these, one patient developed gastrointestinal bleeding secondary to grade 3 thrombocytopenia. The other patient experienced grade 1 epistaxis and gingival and hemorrhoidal bleeding during an episode of grade 4 thrombocytopenia (platelet count, 6 × 109/L).
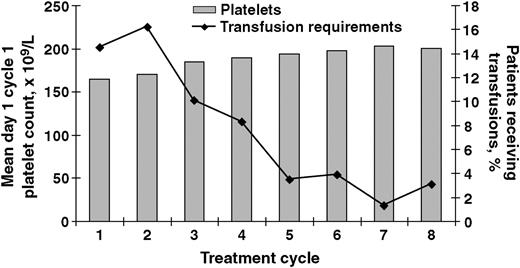
During cycles 1 and 2, platelet transfusions were received by 14.2% and 16.5% of patients, respectively. As expected, median baseline platelet counts were lower in patients who required transfusions relative to those who did not require transfusions (66 × 109/L vs 182 × 109/L). With increasing cycles of treatment, the percentage of patients requiring transfusions decreased, whereas mean platelet counts increased (Figure 4). The percentage of patients requiring platelet transfusions and erythropoietin administration was highest during cycles 1 and 2 (Figure 5). Among the 73 patients who received platelet transfusions, 13 patients (18%) ultimately achieved a complete or partial response; a meaningful analysis of platelet levels over time among these responders was precluded due to the small sample size.
Thirty-seven (16%) of 228 patients had at least one dose of bortezomib held because of thrombocytopenia. Nine patients had at least one dose reduction, and 9 patients discontinued because of thrombocytopenia. These interventions were made early in the phase 2 experience, before the kinetics of platelet recovery were better understood.
Thrombopoietin levels
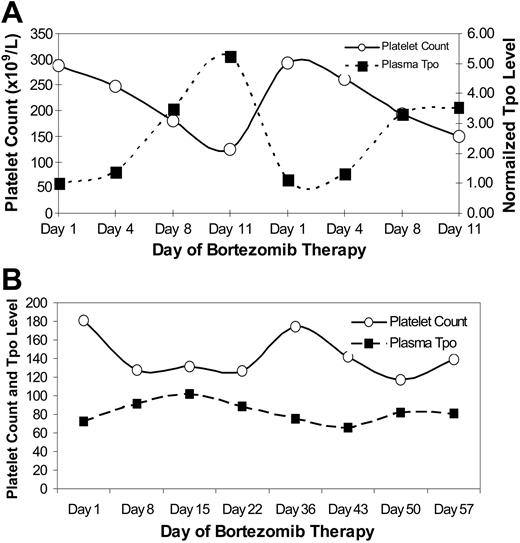
There was an inverse relationship between TPO and platelet levels during treatment with bortezomib twice weekly for the first 2 weeks of every 3-week cycle (n = 5; Figure 6A). Treatment with bortezomib once weekly for the first 4 weeks of a 5-week schedule was associated with a relatively stable platelet count that fluctuated little (n = 1; Figure 6B). Similarly, TPO levels were generally stable during treatment with the less intensive weekly regimen, whereas the thrombopoietin level increased in response to a more significant reduction in the peripheral platelet count among the more intensely treated patients.
Mean day 1 cycle 1 platelet counts and the percentage of patients treated with bortezomib 1.3 mg/m2 over time.
Mean day 1 cycle 1 platelet counts and the percentage of patients treated with bortezomib 1.3 mg/m2 over time.
Platelet transfusions and erythropoietin. (A) Administration of platelet transfusions or erythropoietin (EPO) in all patients (n = 228) and (B) in responders with complete, partial, or minimal response (n = 80).
Platelet transfusions and erythropoietin. (A) Administration of platelet transfusions or erythropoietin (EPO) in all patients (n = 228) and (B) in responders with complete, partial, or minimal response (n = 80).
TPO and platelet counts. (A) Relationship of plasma thrombopoietin levels and peripheral platelet counts (× 109/L) during treatment with bortezomib twice weekly every 3 weeks for 8 cycles. The thrombopoietin level was measured in pg/mL and normalized to the day-1 level in order to combine the results from 5 patients. The platelet count represents the median platelet count for the same 5 patients in whom TPO was measured. (B) Similar data from a single patient receiving weekly bortezomib demonstrating less variance in the platelet count (× 109/L) and plasma TPO level (pg/mL).
TPO and platelet counts. (A) Relationship of plasma thrombopoietin levels and peripheral platelet counts (× 109/L) during treatment with bortezomib twice weekly every 3 weeks for 8 cycles. The thrombopoietin level was measured in pg/mL and normalized to the day-1 level in order to combine the results from 5 patients. The platelet count represents the median platelet count for the same 5 patients in whom TPO was measured. (B) Similar data from a single patient receiving weekly bortezomib demonstrating less variance in the platelet count (× 109/L) and plasma TPO level (pg/mL).
Bortezomib-treated mice, platelet counts, and megakaryocytes. (A) Platelet counts from BALB/c mice after intravenous administration of bortezomib 2.5 mg/kg. (B) Absolute numbers of megakaryocytes (1 femur, 2 tibiae) in different ploidy subsets from mice killed 4 or 15 days after receiving bortezomib 2.5 mg/kg. Untreated mice served as baseline controls and there were 4 to 5 mice per group. Error bars represent standard deviation. (C-E) Bone marrow sections showing megakaryocyte cells. (C) Untreated control. (D) Four days after bortezomib 2.5 mg/kg. (E) Fifteen days after bortezomib 2.5 mg/kg. Original magnifications, × 200; inset, × 600.
Bortezomib-treated mice, platelet counts, and megakaryocytes. (A) Platelet counts from BALB/c mice after intravenous administration of bortezomib 2.5 mg/kg. (B) Absolute numbers of megakaryocytes (1 femur, 2 tibiae) in different ploidy subsets from mice killed 4 or 15 days after receiving bortezomib 2.5 mg/kg. Untreated mice served as baseline controls and there were 4 to 5 mice per group. Error bars represent standard deviation. (C-E) Bone marrow sections showing megakaryocyte cells. (C) Untreated control. (D) Four days after bortezomib 2.5 mg/kg. (E) Fifteen days after bortezomib 2.5 mg/kg. Original magnifications, × 200; inset, × 600.
Effects of bortezomib on murine platelet count and megakaryopoiesis
To study the effects of a single dose of bortezomib on thrombopoiesis, mice received single intravenous doses of 0.8 to 2.5 mg/kg bortezomib, and blood samples were collected 1, 2, 3, 4, 6, 8, and 14 days later. A dose-dependent thrombocytopenia was observed, with significant decreases in blood platelet counts seen among recipients of greater than 1.5 mg/kg bortezomib. Lower doses of bortezomib were associated with a reduced effect on peripheral platelet counts (data not shown). Mice that received bortezomib 2.5 mg/kg had a significant reduction in blood platelet counts 2 to 4 days after treatment, with a return to normal platelet counts 6 to 14 days after treatment (Figure 7A). Control mice that received DMSO did not have significant variation in platelet counts over the 2-week time period. To investigate whether bortezomib has a direct cytopathic effect on megakaryocytes that results in the observed peripheral thrombocytopenia associated with bortezomib therapy, additional mice were treated with bortezomib 2.5 mg/kg and killed after 4 days (lowest platelet counts) or 15 days (after recovery of platelet counts) to evaluate bone marrow megakaryocyte content, ploidy, and morphology. Megakaryocyte counts were similar between control and bortezomib-treated mice 4 days after treatment; however, representation of 32N and 64N megakaryocytes was significantly increased in bortezomib-treated mice at this time point (P = .047 and P = .010, respectively; Figure 7B). Fifteen days after bortezomib treatment, numbers of 4N, 8N, 16N, and 32N megakaryocytes were significantly higher in bortezomib-treated mice compared with control mice (P = .028, P < .001, P = .003, and P = .015, respectively). Megakaryocytes were morphologically and numerically similar on hematoxylin and eosin–stained sections across all conditions and contained approximately 25 to 30 megakaryocytes per × 200 field (Figures 7C-E).
In additional experiments examining potential effects of bortezomib on TPO levels, mice were administered bortezomib (1.5 mg/kg) on days 1, 4, 8, and 11 to mimic the dosing schedule used in patients. Platelet counts and thrombopoietin levels were tracked over a 20-day period. Platelet counts dropped after 2 bortezomib doses, but the kinetics of platelet recovery were faster than in humans, resulting in a bi-modal curve with platelet nadirs at days 6 and 13 (Figure 8). As in humans, plasma thrombopoietin levels were inversely related to platelet counts (Figure 8). The data show that TPO synthesis is not directly affected by bortezomib and that circulating TPO levels remain dependent on platelet concentration. Although repeated dosing with bortezomib 1.5 mg/kg was at the limit of toxicity—mice lost an average of 20% body weight by day 11 and many had signs of motor dysfunction—these effects were reversible when dosing was stopped. This dosing scheme also resulted in decreased bone marrow cellularity, determined by flushing marrow from femora and tibiae and determining total cell counts as well as by objective pathologic review of fixed marrow sections, but overall megakaryocyte numbers were not significantly affected as analyzed by flow cytometry and histologic staining (data not shown).
Relationship of average plasma thrombopoietin levels (pg/mL) and peripheral platelet counts (× 106/mL) in BALB/c mice during multidose treatment with bortezomib on days 1, 4, 8, 11 (arrows). Four to 6 mice per time point. Error bars represent the standard error of the mean.
Relationship of average plasma thrombopoietin levels (pg/mL) and peripheral platelet counts (× 106/mL) in BALB/c mice during multidose treatment with bortezomib on days 1, 4, 8, 11 (arrows). Four to 6 mice per time point. Error bars represent the standard error of the mean.
Discussion
Thrombocytopenia with or without concomitant leukopenia can represent a major limitation in the delivery of effective anticancer therapy. When isolated thrombocytopenia is present at the time the next dose or cycle of therapy is due, doses are often held or attenuated to preserve marrow function and lower the risk of long-term thrombocytopenia. Our analysis defines the pattern of thrombocytopenia seen with the proteasome inhibitor bortezomib and highlights the fact that the kinetics and mechanism of thrombocytopenia are different from those seen with standard cytotoxic therapy. These differences include a shorter recovery time than that seen with cytotoxic marrow injury, the absence of a lethal cytotoxic effect of bortezomib on marrow megakaryocytes, and, based on emerging clinical data, the absence of cumulative or persistent thrombocytopenia. In contrast with other models of chemotherapy-induced thrombocytopenia, platelet counts actually increased, despite repeated dosing among responders.
Thrombocytopenia seen with bortezomib therapy was transient, predictably decreasing during the first 10 days of each cycle and recovering toward baseline during the 10-day rest period between cycles. Grade 3/4 thrombocytopenia was identified in 30% of patients (68 of 228) who were treated with bortezomib 1.3 mg/m2, comparable to the 29% incidence of grade 3/4 thrombocytopenia associated with the 1.0 mg/m2 dose administered to 28 patients in the CREST trial.15
Although all patients experienced some reduction in the peripheral platelet count, this analysis defined predictors for the development of grade 3/4 thrombocytopenia. A mean reduction in platelet counts from baseline to nadir of approximately 60% was observed regardless of baseline BMPC, M-protein concentrations, or platelet count. This relatively constant reduction suggests that the magnitude of the effect on platelets and megakaryocytes is fixed and that the degree of thrombocytopenia is dependent upon the initial starting platelet count. Grade 3/4 thrombocytopenia was more frequently observed in patients with low platelet counts at baseline. Rates of response to bortezomib were equivalent regardless of the baseline platelet count.
There was a consistent cyclic pattern of platelet count decrease and recovery over the 8 cycles, with no evidence of cumulative toxicity. These observations are consistent with the pharmacodynamics of bortezomib, in which proteasome inhibition is maximal within 1 hour after dosing with return to baseline levels of proteasome activity within the next 72 hours.24 The clinical observation of progressively higher nadir platelet counts among bortezomib-treated patients in the absence of transfusion support may represent an early predictor of response.
It is important to note that platelet counts were not routinely measured between days 12 and 22, that is, after day 11 of each cycle per protocol; hence, it is not known when the true nadir occurred in each cycle. Based on the pattern of thrombocytopenia and platelet kinetics observed after each dose, the nadir most likely occurred at approximately day 14. From the standpoint of clinical management, patients with a platelet count less than 30 × 109/L on day 11 of each cycle of bortezomib therapy should have an additional platelet count done between days 14 and 15 to ensure that the count has not dropped to less than 20 × 109/L. There were no reports of serious bleeding complications from thrombocytopenia, and platelet transfusions were required in less than 15% of all patients in these studies. This observation suggests that patients experiencing thrombocytopenia should be able to continue treatment with bortezomib without dose interruptions as long as platelet transfusions are available for support.
Recovery of the platelet count was rapid, suggesting that megakaryocyte cytotoxicity was not the cause. The predicted time for recovery of platelet counts from nadir to baseline values would seem to be fewer than 7 days, based on a maximum nadir around day 15 and restoration of baseline values by the start of the next cycle (day 22). This clinical pattern of thrombocytopenia is not typically observed with other cytotoxic agents. With other agents, nadirs are apparent from 7 to 14 days after chemotherapy begins and they typically require 3 to 4 weeks for resolution.25 Thrombocytopenia may be even more prolonged if significant injury to hematopoietic stem cells occurs following high doses of certain cytotoxic agents. Drugs that are more likely to damage marrow stem cells include alkylating agents such as melphalan and nonspecific agents such as carboplatin that affect both cycling and noncycling cells.16,17 In contrast, cell cycle–specific agents, such as the antimicrotubule agents paclitaxel and docetaxel, are most toxic to cells in a specific phase of the cell cycle and therefore tend to cause less severe myelosuppression, but thrombocytopenia can still occur. With bortezomib, thrombocytopenia resolved during the 10-day rest period, suggesting that the mechanism resulting in thrombocytopenia is different than that seen with other cytotoxic agents and that no significant megakaryocytic or stem cell injury had occurred.
Our initial hypothesis was that bone marrow stromal production of TPO may be inhibited by bortezomib. This was initially tested among patients receiving a standard bortezomib dose and schedule, and an inverse relationship was noted between circulating platelet counts and plasma TPO levels. Review of historic data addressing the relationship between plasma TPO and platelet counts clearly supports the inverse relationship between the two, but the degree of elevation of TPO varies significantly. In a paper from Lin et al,26 the height of the rise in plasma TPO was proportional to the degree of thrombocytopenia, and in a group of patients maintaining a platelet count of greater than 100 × 109/L, the plasma TPO level was 266 ± 231 pg/mL (not statistically different from healthy individuals). A second paper, from Engel et al,7 measured the relationship between plasma TPO and platelets among a group of patients receiving different intensity chemotherapy for Hodgkin disease and found that the plasma TPO level ranged between 50 and 400, a value similar to the range we observed when the platelet count was above 100 × 109/L. Thus, the degree of rise in TPO was inversely related to the platelet count and did appear to be proportional to the effect observed by others with similar degrees of thrombocytopenia. An inverse relationship between TPO and platelet counts was also observed in the murine repeated dosing experiments, further supporting the conclusion that bortezomib itself does not suppress TPO synthesis (Figure 8). Bortezomib-induced inhibition of NF-κB is hypothesized to prevent platelet budding, with return of proteasome function within 72 hours after dosing.24 The observation of morphologically normal megakaryocytes in the bone marrow at the time of peripheral thrombocytopenia, with a subsequent prompt recovery of blood platelet counts, suggests either transient inhibition of thrombopoiesis with intact megakaryocytes or a transient increase in platelet clearance from the peripheral circulation. The absence of clinical features associated with marked enhanced platelet consumption, such as disseminated intravascular coagulation, and the predicted increase in peripheral blood platelet counts following transfusion argue against the hypothesis that bortezomib-induced thrombocytopenia is due to increased platelet consumption in the peripheral circulation. Preservation of megakaryocyte progenitors during treatment with bortezomib is corroborated by a preclinical study that reported no adverse effects on stem cells from mice following treatment with bortezomib: lethally irradiated mice who received these cells had recovery of hematopoietic stem cell function comparable to that of controls.18 The increase in megakaryocyte ploidy seen in the treated group compared with the control animals suggests that although the transient defect may be in megakaryocyte differentiation (or budding), the standard compensatory mechanism remains intact given the degree of peripheral thrombocytopenia. Thus, while there is no effect on marrow megakaryocyte content during therapy, the reduction in the circulating platelet count induces an increase in circulating thrombopoietin, thereby increasing the megakaryocyte ploidy in the marrow compared with that of untreated controls.
From a clinical perspective, treatment with bortezomib resulted in a transient predictable reduction in platelet counts and a low incidence of platelet support (and bleeding); thus, continued bortezomib therapy could be safely administered to thrombocytopenic patients. While the data presented in this paper describe the experience with thrombocytopenia in the SUMMIT and CREST trials, results from a large randomized phase 3 clinical trial of bortezomib compared with high-dose dexamethasone in relapsed myeloma demonstrated the clinical benefit of bortezomib and confirmed the cyclic and recoverable nature of the thrombocytopenia after treatment with bortezomib alone.21 Thus, the mechanisms of platelet reduction associated with bortezomib therapy may be unique compared with those of conventional chemotherapies. Based on the murine studies, bortezomib-induced thrombocytopenia is not a result of a lethal effect on bone marrow but is rather more likely a transient effect on megakaryocyte function, possibly related to platelet budding. The absolute decrease in platelet count of approximately 60% regardless of baseline parameters suggests that the mechanism by which bortezomib induces thrombocytopenia is saturable and specific, and its transient nature suggests that it is fully and rapidly reversible. To maximize the likelihood of benefit from bortezomib therapy, patients with multiple myeloma and thrombocytopenia should be supported with platelet transfusions as clinically indicated rather than having treatment reduced or delayed because of thrombocytopenia.
Appendix
SUMMIT/CREST investigators: Gordon Srkalovic, Cleveland Clinic Foundation, Cleveland, OH; Melissa Alsina, H. Lee Moffitt Cancer Center, Tampa, FL; Raymond Alexanian, M. D. Anderson Cancer Center, Houston, TX; David Siegel, Carol G. Simon Cancer Center, Morristown, NJ; Robert Z. Orlowski, University of North Carolina, Chapel Hill; David Kuter, Massachusetts General Hospital, Boston, MA; Steven Limentani, Charlotte Medical Clinic, Charlotte, NC; Bart Barlogie, University of Arkansas for Medical Sciences, Little Rock; Ruben Niesvizky, New York–Presbyterian Hospital/Weill Medical College, Cornell University, New York, NY; David Irwin, Alta Bates Comprehensive Cancer Center, Berkeley, CA; and Michael Schuster, New York–Presbyterian Hospital/Weill Medical College, Cornell University, New York, NY.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2005-03-1173.
A complete list of the members of SUMMIT/CREST appears in “Appendix.”
Supported by the Lymphoma Research Foundation (S.L.) and the National Institutes of Health (grant DK60647) (D.L.J.).
S.L. is a consultant for and on the Speakers' Bureau for Millennium Pharmaceuticals, Inc. P.G.R. is on the Speakers' Bureau and Advisory Board for Millennium Pharmaceuticals, Inc. S.J. is a consultant for and on the Speakers' Bureau for Millennium Pharmaceuticals, Inc. B.B. receives research support from Millennium Pharmaceuticals, Inc, and serves on the company's scientific advisory board. J.R.B. receives honoraria from the Speakers' Bureau, grants, and consultant fees from Millennium Pharmaceuticals, Inc, and is on the Advisory Board of Millennium Pharmaceuticals, Inc. D.P.S. and D.-L.W.E. are full-time employees of and own stock in Millennium Pharmaceuticals, Inc. J.A. owns stock and stock options in Millennium Pharmaceuticals, Inc. H.X. is a contractor-biostatistician working with Millennium Pharmaceuticals, Inc. K.C.A. receives grant support from Millennium Pharmaceuticals, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal