This study was launched to determine the prognostic significance of local tumor invasiveness (LTI) in 114 patients diagnosed with stage IE/IIE extranodal natural killer (NK)/T-cell lymphoma, nasal type (NTCL). LTI was defined as bony invasion or destruction or tumor invasion of the skin. Complete remission (CR), overall survival (OS), and disease-free survival (DFS) were compared between each group according to LTI, Ann Arbor stage, and International Prognostic Index (IPI). LTI was observed in 23 patients. Using multivariate analysis, factors associated with low probability of CR were the presence of LTI (P < .001), the presence of B symptoms (P = .003), and single-modality chemotherapy (P = .045). The presence of LTI (relative risk [RR] = 8.4, 95% confidence interval [CI] 3.9-17.9; P < .001) and high IPI score (RR = 2.8, 95% CI 1.2-6.8; P = .019) were also predictive of OS. The presence of LTI (RR = 7.3, 95% CI 3.2-16.5; P < .001) was an independently significant factor for reduced DFS. Ann Arbor staging system did not predict CR, OS, or DFS but IPI did have predictive power with regard to survival outcome. LTI is the most important prognostic factor in predicting low probability of CR and reduced OS and DFS in nasal stage IE/IIE NTCL.
Introduction
The Ann Arbor staging classification system was originally developed for Hodgkin lymphoma, but in the absence of a better alternative it has also been used for staging non-Hodgkin lymphomas (NHLs) for over 30 years.1 However, in some studies the Ann Arbor classification fails to identify the more aggressive prognostic subgroups of NHLs2,3 that spread to discontiguous lymph nodes and extranodal sites. For a more accurately systematized prediction of survival outcome, the International Prognostic Index (IPI) had been developed for aggressive B-cell lymphoma4 and has also been applied to T-cell lymphoma.5
The extranodal natural killer (NK)/T-cell lymphoma, nasal type (NTCL), is a distinct clinicopathologic entity that is very rare in Western populations but rather common among Asians, Mexicans, and South Americans of American Indian descent.6-8 A recent nationwide study of malignant lymphomas in Korea revealed that NTCL accounted for 8.7% to 10.5% of all NHLs and 74.1% of lymphomas arising in the nasal cavity and paranasal sinuses.9,10 Clinically, it often destroys the facial midline and spreads to or relapses at extranodal sites. Pathologically, it has a broad cytologic spectrum varying from pleomorphic mixed, small, medium, or large cells to predominantly large cells. The tumor cells are characteristically positive for CD56, CD2, cytoplasmic CD3 (CD3ϵ), and CD45R0 by immunophenotyping and positive for Epstein-Barr virus (EBV) by in situ hybridization.6,11,12 Patients who presented with nasal stage IIIE/IVE and extranasal NTCL exhibited more aggressive tumor behavior and poorer prognosis compared with patients of nasal stage IE/IIE NTCL13 . However, the Ann Arbor stage failed to predict survival differences between stage IE and stage IIE in the Korean multicenter study.13 It also posed clinical challenges in treatment selection due to its inability to predict the heterogeneous clinical behaviors of nasal stage IE/IIE NTCL, which included paranasal extension, bone destruction, and skin involvement.6,14-16 The extent of nasal lymphoma was considered as a prognostic factor in a few studies.17,18 Therefore, we aimed to compare the prognostic accuracies of a system based on local tumor invasiveness (LTI) with the existing Ann Arbor stage and the IPI, which has been shown to correlate with survival in recent studies in nasal stage IE/IIE NTCL.13,19
Patients, materials, and methods
Patients
We screened all 179 patients newly diagnosed with NTCL at Seoul National University Hospital (n = 134) and Korea Cancer Center Hospital (n = 45) in Seoul, Korea, between July 1991 and October 2003. Sixty-five cases were excluded in the analysis for the following reasons: 40 patients had extranasal NTCL, 16 patients had nasal stage IIIE/IVE NTCL, 1 patient was lost to follow-up, 1 patient had blastic NK cell lymphoma, and 7 patients received no treatment. A total of 114 patients with typical histologic features of NTCL,6 primary tumors localized to the upper aerodigestive tract, and Ann Arbor stage IE/IIE were included in a retrospective intent-to-treat analysis. All patients had undergone a staging work-up including panendoscopy of the upper aerodigestive tract, chest radiograph, computed tomography (CT)/magnetic resonance imaging (MRI) of the head and neck, CT of the abdomen and pelvis, and bone marrow examination. Contiguous disease extending to adjacent structures was staged as IE and lymph nodes that are 1.5 cm or greater were considered to be abnormal. Response to treatment was assessed according to the response criteria for NHLs.20 This study was approved by the institutional review board at the Seoul National University Hospital. Informed consent was provided according to the Declaration of Helsinki.
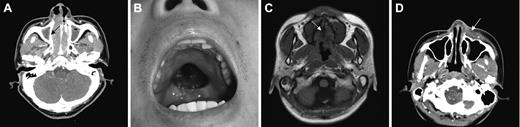
Local tumor invasiveness. (A) Thinning of right medial wall of maxillary bone (arrow) in CT of paranasal sinuses. (B) Palatal perforation on physical examination. (C) High signal intensity of hard palate is not delineated in T1-weighted MRI (arrow). (D) Skin infiltration by tumor (arrow) in CT of paranasal sinuses. Picture was taken with an Olympus Camedia C4000Z camera (Olympus, Tokyo, Japan). Adobe Photoshop 6.0 was used to process images (Adobe, San Jose, CA).
Local tumor invasiveness. (A) Thinning of right medial wall of maxillary bone (arrow) in CT of paranasal sinuses. (B) Palatal perforation on physical examination. (C) High signal intensity of hard palate is not delineated in T1-weighted MRI (arrow). (D) Skin infiltration by tumor (arrow) in CT of paranasal sinuses. Picture was taken with an Olympus Camedia C4000Z camera (Olympus, Tokyo, Japan). Adobe Photoshop 6.0 was used to process images (Adobe, San Jose, CA).
Local tumor invasiveness
LTI was defined as bony invasion or perforation or invasion of the skin. The involved bony structures included the anterior and medial walls of maxillary sinuses; the medial walls of the orbit; the anterior and inferior walls of ethmoidal sinuses; the skull base; and the inferior walls of frontal sinuses, hard palate, nasal bone, and nasal septal bones (perpendicular plate of ethmoid and vomer). We defined the extent of bone involvements based on CT and physical findings. Thinning or disorganized structure of bones due to the tumor was regarded as bony invasion (Figure 1A), and bone defect caused by the tumor was regarded as bony perforation (Figure 1B). Disruption of high signal intensity of bone marrow on T1-weighted MRI was also considered as bony invasion (Figure 1C). The infiltration of overlying skin around the tumor was regarded as skin invasion (Figure 1D). The CT/MRI findings of the head and neck were reviewed by radiologists (J.-H.K. and K.-H.C.) blinded to clinical outcomes.
Histology, immunophenotyping, and detection of EBV
All pathologic specimens were reviewed and reclassified based on strict morphologic criteria in adjunction with immunophenotypic analyses6 by a single pathologist (C.W.K.). Immunophenotypic procedures were performed on paraffin sections using a routine avidin-biotin–peroxidase complex method by using the following antibodies: CD3ϵ (DakoCytomation, Copenhagen, Denmark), CD20 (DakoCytomation), CD45R0 (DakoCytomation), and CD56 (Monosan, Uden, The Netherlands; DiNonA, Seoul, Korea). EBV RNA in situ hybridization (ISH) was performed using an ISH detection kit (Novocastra, Newcastle upon Tyne, United Kingdom).
Histologic findings showed angiocentricity (86% of patients), necrosis (98%), and pleomorphic infiltration (89%). Immunophenotypes were CD56+CD3ϵ+CD20– (92 patients), CD56–CD3ϵ+CD20– (12 patients), and CD56+CD3ϵ–CD20– (2 patients). Sixty-one (98%) of 62 patients expressed CD45R0. Forty-six (75%) of 61 patients harbored EBV RNA.
Treatments
Treatment modalities were given as follows: combination treatment of 3 to 6 cycles of chemotherapy with involved-field radiation therapy (56 patients), chemotherapy alone (45 patients), or radiation therapy alone (13 patients). Selection of treatment modality was at the discretion of the treating physicians. The chemotherapy regimens included CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone; 67 patients), COP-BLAM-V (cyclophosphamide, doxorubicin, vincristine, prednisolone, bleomycin, and procarbazine; 15 patients), ProMACE-CytaBOM (cytarabine, bleomycin, vincristine, methotrexate, leucovorin, prednisolone, doxorubicin, cyclophosphamide, and etoposide; 1 patient), EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin; 3 patients), and IMVP-16 (ifosfamide, methotrexate, etoposide, and prednisolone; 15 patients). The total radiation dose ranged from 25.2 Gy to 64.8 Gy (mean dose, 47.0 Gy).
Statistical analysis
The association between clinical factors and the probability of attaining complete remission (CR) was evaluated by the Pearson χ2 test. Overall survival (OS) was measured from the date of diagnosis to the date of death or the last follow-up visit. For patients in CR, disease-free survival (DFS) was calculated from the date of CR to the first evidence of relapse. OS and DFS curves were derived by the Kaplan and Meier method.21 Univariate analysis of OS or DFS was performed using the log-rank test. Factors independently associated with OS or DFS were identified by multivariate analysis using the Cox proportional hazards regression model.22 Two-sided P values of less than .05 were considered significant. All statistical analyses were performed using SPSS version 11.0 (SPSS, Chicago, IL).
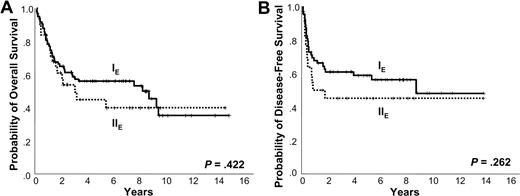
Kaplan-Meier plots of Ann Arbor stage. Kaplan-Meier plots of (A) overall survival and (B) disease-free survival according to Ann Arbor stage.
Kaplan-Meier plots of Ann Arbor stage. Kaplan-Meier plots of (A) overall survival and (B) disease-free survival according to Ann Arbor stage.
Results
Patients and treatment outcomes
The clinical characteristics of the 114 patients are summarized in Table 1. Median age of our sample was 47 years with a male-female ratio of 1.7:1. Median follow-up period for survivors was 78 months (range, 17-177 months). One third of the patients presented with systemic symptoms, most of whom had an ambulatory performance status (PS; Eastern Cooperative Oncology Group [ECOG] 0-1). Nearly three fourths of patients showed Ann Arbor stage IE, and elevated lactic dehydrogenase (LDH) level was observed in one third of the patients. One hundred (89%) of 112 patients were classified as having low IPI scores (0-1) and only 1 patient had bulky disease. Forty-six (82%) of 56 patients treated with combined modality achieved CR, but 16 (35%) of the 46 patients in CR eventually relapsed. CR was achieved in 28 (62%) of 45 patients receiving chemotherapy alone, of whom 17 (61%) subsequently relapsed. In the radiation alone group, 11 (85%) of 13 attained CR but 6 (55%) of 11 relapsed.
Characteristics of 114 nasal stage IE/IIE extranodal NK/T-cell lymphoma patients
Characteristic . | No. of patients (%) . |
|---|---|
| Age, n = 114 | |
| 60 y old or younger | 94 (82) |
| Older than 60 y | 20 (18) |
| Sex, n = 114 | |
| Male | 72 (63) |
| Female | 42 (37) |
| Primary sites of tumor, n = 114 | |
| Nasal cavity | 73 (64) |
| Nasopharynx | 21 (18) |
| Oral cavity/oropharynx | 15 (13) |
| Hypopharynx | 5 (4) |
| Systemic symptoms, n = 113 | |
| No | 78 (69) |
| Yes | 35 (31) |
| Performance status, n = 114 | |
| 0-1 | 108 (95) |
| 2 or higher | 6 (5) |
| Ann Arbor stage, n = 114 | |
| IE | 83 (73) |
| IIE | 31 (27) |
| LDH level, n = 109 | |
| Normal | 75 (69) |
| Elevated | 34 (31) |
| No. of extranodal sites, n = 114 | |
| 0-1 | 105 (92) |
| 2 or more | 9 (8) |
| International Prognostic Index rating, n = 112 | |
| 0-1 | 100 (89) |
| 2 or higher | 12 (11) |
| Immunophenotyping, n = 106 | |
| CD56+CD3ϵ+CD20- | 92 (87) |
| CD56-CD3ϵ+CD20- | 12 (11) |
| CD56+CD3ϵ-CD20- | 2 (2) |
Characteristic . | No. of patients (%) . |
|---|---|
| Age, n = 114 | |
| 60 y old or younger | 94 (82) |
| Older than 60 y | 20 (18) |
| Sex, n = 114 | |
| Male | 72 (63) |
| Female | 42 (37) |
| Primary sites of tumor, n = 114 | |
| Nasal cavity | 73 (64) |
| Nasopharynx | 21 (18) |
| Oral cavity/oropharynx | 15 (13) |
| Hypopharynx | 5 (4) |
| Systemic symptoms, n = 113 | |
| No | 78 (69) |
| Yes | 35 (31) |
| Performance status, n = 114 | |
| 0-1 | 108 (95) |
| 2 or higher | 6 (5) |
| Ann Arbor stage, n = 114 | |
| IE | 83 (73) |
| IIE | 31 (27) |
| LDH level, n = 109 | |
| Normal | 75 (69) |
| Elevated | 34 (31) |
| No. of extranodal sites, n = 114 | |
| 0-1 | 105 (92) |
| 2 or more | 9 (8) |
| International Prognostic Index rating, n = 112 | |
| 0-1 | 100 (89) |
| 2 or higher | 12 (11) |
| Immunophenotyping, n = 106 | |
| CD56+CD3ϵ+CD20- | 92 (87) |
| CD56-CD3ϵ+CD20- | 12 (11) |
| CD56+CD3ϵ-CD20- | 2 (2) |
Using univariate analysis, the factors associated with lower probability of achieving CR were the presence of LTI (relative risk [RR] = 15.0, 95% confidence interval [CI] 4.9-45.4; P < .001), ECOG PS of 2 or higher (RR = 7.5, 95% CI 1.3-43.9; P = .025), the presence of B symptoms (RR = 4.9, 95% CI 1.9-12.5; P = .001), and chemotherapy alone (RR = 3.3, 95% CI 1.3-8.1; P = .011). In a subsequent multivariate regression analysis, independently significant factors were the presence of LTI (RR = 16.0, 95% CI 4.2-61.5; P < .001), the presence of B symptoms (RR = 7.4, 95% CI 2.0-27.3; P = .003), and chemotherapy alone (RR = 3.6, 95% CI 1.0-12.9; P = .045). EBV RNA positivity did not adversely affect the attainment of CR (P = .125).
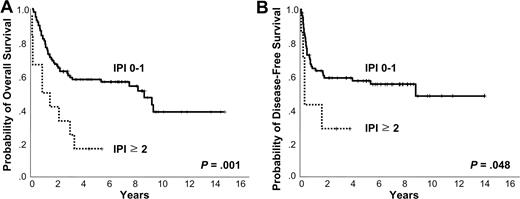
Kaplan-Meier plots of IPI. Kaplan-Meier plots of (A) overall survival and (B) disease-free survival according to International Prognostic Index.
Kaplan-Meier plots of IPI. Kaplan-Meier plots of (A) overall survival and (B) disease-free survival according to International Prognostic Index.
Survival analysis
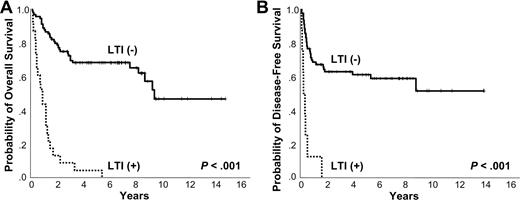
The 5-year OS and DFS were 53% and 55%, respectively. At the time of analysis, 56 patients were alive and 58 had died due to the lymphoma itself (n = 44), treatment-related complication (n = 8), and other comorbid disease (n = 6). Positive EBV RNA showed a trend of negative correlation with OS and DFS (5-year OS 46% vs 63%, P = .125; 3-year DFS 46% vs 69%, P = .099). Ann Arbor stage IE did not show better 5-year OS and DFS compared with Ann Arbor stage IIE (5-year OS 56% vs 44%, P = .422; 5-year DFS 59% vs 45%, P = .262; Figure 2A-B). However, the OS and DFS were superior in the low IPI score subgroup (0-1) compared with the high IPI score (≥ 2) group (5-year OS 58% vs 17%, P = .001; 3-year DFS 59% vs 29%, P = .048; Figure 3A-B). The presence of LTI reduced OS and DFS (5-year OS 4% vs 68%, P < .001; 1-year DFS 13% vs 68%, P < .001; Figure 4A-B). In terms of treatment modality, there were no significant differences in OS and DFS between the combined modality, radiation alone, and chemotherapy alone groups (5-year OS 56%, 69%, and 44%, respectively, P = .191; 5-year DFS 66%, 55%, and 39%, respectively, P = .087). In addition, chemotherapy regimens did not influence treatment outcome (data not shown).
Prediction of survival
The clinical factors associated with reduced OS in univariate analysis were the presence of LTI (RR = 8.5, 95% CI 4.7-15.2; P < .001), high IPI score (RR = 3.0, 95% CI 1.5-6.0; P = .002), number of extranodal sites (no. ENSs) of 2 or more (RR = 2.7, 95% CI 1.3-5.6; P = .006), and advanced age (> 60 years; RR = 1.8, 95% CI 1.0-3.4; P = .047; Table 2). Using multivariate analysis, independently significant factors were the presence of LTI (RR = 8.4, 95% CI 3.9-17.9; P < .001) and high IPI score (RR = 2.8, 95% CI 1.2-6.8; P = .019; Table 3). The presence of LTI and high IPI score remained independently significant in treatment modality–stratified multivariate analysis.
Kaplan-Meier plots of LTI. Kaplan-Meier plots of (A) overall survival and (B) disease-free survival according to local tumor invasiveness.
Kaplan-Meier plots of LTI. Kaplan-Meier plots of (A) overall survival and (B) disease-free survival according to local tumor invasiveness.
Clinical factors influencing overall survival in univariate analysis
Clinical factors . | RR . | 95% CI . | P . |
|---|---|---|---|
| Presence of LTI | 8.5 | 4.7-15.2 | < .001 |
| IPI score of 2 or higher | 3.0 | 1.5-6.0 | .002 |
| No. of extranodal sites of 2 or more | 2.7 | 1.3-5.6 | .006 |
| Age older than 60 years | 1.8 | 1.0-3.4 | .047 |
| ECOG PS of 2 or higher | 2.7 | 1.0-7.6 | .053 |
| Presence of B symptoms | 1.7 | 1.0-2.9 | .054 |
Clinical factors . | RR . | 95% CI . | P . |
|---|---|---|---|
| Presence of LTI | 8.5 | 4.7-15.2 | < .001 |
| IPI score of 2 or higher | 3.0 | 1.5-6.0 | .002 |
| No. of extranodal sites of 2 or more | 2.7 | 1.3-5.6 | .006 |
| Age older than 60 years | 1.8 | 1.0-3.4 | .047 |
| ECOG PS of 2 or higher | 2.7 | 1.0-7.6 | .053 |
| Presence of B symptoms | 1.7 | 1.0-2.9 | .054 |
Clinical factors influencing overall survival in multivariate analysis
Clinical factors . | RR . | 95% CI . | P . |
|---|---|---|---|
| Presence of LTI | 8.4 | 3.9-17.9 | < .001 |
| IPI score of 2 or higher | 2.8 | 1.2-6.8 | .019 |
Clinical factors . | RR . | 95% CI . | P . |
|---|---|---|---|
| Presence of LTI | 8.4 | 3.9-17.9 | < .001 |
| IPI score of 2 or higher | 2.8 | 1.2-6.8 | .019 |
In terms of DFS, the factors associated with reduced survival were the presence of LTI (RR = 7.0, 95% CI 3.1-16.0; P < .001), no. ENSs of 2 or more (RR = 2.9, 95% CI 1.0-8.2; P = .045), and elevated LDH level (RR = 1.9, 95% CI 1.0-3.7; P = .047) by univariate analysis. High IPI score showed trend of negatively affecting DFS (RR = 2.5, 95% CI 1.0-6.5; P = .057). The presence of LTI was an independently significant factor for reduced DFS (RR = 7.2, 95% CI 3.2-16.5; P < .001) using multivariate analysis. By treatment modality–stratified analysis, the presence of LTI and advanced age were significantly associated with reduced DFS. However, Ann Arbor stage did not affect OS and DFS in univariate analysis (P = .423 and .266, respectively).
Local tumor invasiveness
Twenty-three (21%) of 111 patients presented with LTI and characteristics are shown in Table 4. The median duration of presenting symptoms was 4 months (range, 1-13 months). The patterns of failure were as follows: local, 15 patients; regional, 2 patients; and systemic, 15 patients. The sites of systemic failure were gastrointestinal tract, lung, skin, bone marrow, liver, testis, and central nervous system (CNS). Eight (36%) of 22 evaluable patients attained CR but relapsed early (median DFS 3.0 months). All patients died of lymphoma (n = 20) or treatment-related complications (n = 3), and median OS was 10.6 months. Treatment modality significantly affected median OS (combined modality vs chemotherapy alone = 13.8 months vs 6.3 months, P = .037). According to treatment modality, the presence of LTI unchangeably reduced 2-year OS (combined modality 22% vs 82%, P < .001; and chemotherapy alone 7% vs 74%, P < .001; Table 5).
Characteristics of 23 patients with local tumor invasiveness
. | . | . | . | . | . | . | Outcomes . | . | |
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y/sex . | Sites of LTI . | Stage . | Tx . | Response to Tx . | Failure . | Cause of death . | OS (DFS), mo . | |
| 1 | 54/M | Palate | IEA | CTX | CR | L | DOD | 15 (7) | |
| 2 | 64/F | Skin | IEA | CTX | PD | L | DOD | 4 | |
| 3 | 52/M | Skull base | IEA | CTX | PD | L, S | DOD | 6 | |
| 4 | 31/M | Lip | IEB | CTX | PD | L | DOD | 27 | |
| 5 | 67/F | Palate | IEB | CTX | PD | L, S | DOD | 9 | |
| 6 | 36/F | Eyelid | IEB | CTX | PD | L | DOD | 5 | |
| 7 | 34/M | Eyelid | IEA | CTX | NE | S | TRM | 2 | |
| 8 | 43/M | Skin | IEA | CTX | PD | L, S | DOD | 8 | |
| 9 | 69/M | Palate | IEB | CTX | PD | L, S | DOD | 2 | |
| 10 | 55/M | Skin | IEB | CTX | PD | L, S | TRM | 1 | |
| 11 | 68/M | Ethmoid | IIEA | CTX | PD | L | DOD | 16 | |
| 12 | 46/F | Skin | IIEB | CTX | PR | L, S | DOD | 11 | |
| 13 | 42/M | Palate | IIEA | CTX | PD | L | DOD | 5 | |
| 14 | 41/M | Skull base | IEA | CM | CR | S | DOD | 40 (20) | |
| 15 | 61/M | Palate | IEA | CM | CR | S | DOD | 18 (4) | |
| 16 | 38/F | Palate | IEA | CM | CR | S | DOD | 14 (2) | |
| 17 | 18/M | Skin | IIEB | CM | PD | L | DOD | 6 | |
| 18 | 59/M | Lip | IIEA | CM | CR | S | DOD | 65 (5) | |
| 19 | 24/M | Palate | IIEA | CM | CR | R | DOD | 20 (3) | |
| 20 | 49/F | Maxilla | IIEB | CM | PD | S | TRM | 5 | |
| 21 | 52/F | Palate | IIEB | CM | CR | R, S | DOD | 11 (1) | |
| 22 | 44/F | Skull base | IIEB | CM | CR | L, S | DOD | 14 (2) | |
| 23 | 45/M | Palate | IIEA | CM | PD | L, S | DOD | 14 | |
. | . | . | . | . | . | . | Outcomes . | . | |
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y/sex . | Sites of LTI . | Stage . | Tx . | Response to Tx . | Failure . | Cause of death . | OS (DFS), mo . | |
| 1 | 54/M | Palate | IEA | CTX | CR | L | DOD | 15 (7) | |
| 2 | 64/F | Skin | IEA | CTX | PD | L | DOD | 4 | |
| 3 | 52/M | Skull base | IEA | CTX | PD | L, S | DOD | 6 | |
| 4 | 31/M | Lip | IEB | CTX | PD | L | DOD | 27 | |
| 5 | 67/F | Palate | IEB | CTX | PD | L, S | DOD | 9 | |
| 6 | 36/F | Eyelid | IEB | CTX | PD | L | DOD | 5 | |
| 7 | 34/M | Eyelid | IEA | CTX | NE | S | TRM | 2 | |
| 8 | 43/M | Skin | IEA | CTX | PD | L, S | DOD | 8 | |
| 9 | 69/M | Palate | IEB | CTX | PD | L, S | DOD | 2 | |
| 10 | 55/M | Skin | IEB | CTX | PD | L, S | TRM | 1 | |
| 11 | 68/M | Ethmoid | IIEA | CTX | PD | L | DOD | 16 | |
| 12 | 46/F | Skin | IIEB | CTX | PR | L, S | DOD | 11 | |
| 13 | 42/M | Palate | IIEA | CTX | PD | L | DOD | 5 | |
| 14 | 41/M | Skull base | IEA | CM | CR | S | DOD | 40 (20) | |
| 15 | 61/M | Palate | IEA | CM | CR | S | DOD | 18 (4) | |
| 16 | 38/F | Palate | IEA | CM | CR | S | DOD | 14 (2) | |
| 17 | 18/M | Skin | IIEB | CM | PD | L | DOD | 6 | |
| 18 | 59/M | Lip | IIEA | CM | CR | S | DOD | 65 (5) | |
| 19 | 24/M | Palate | IIEA | CM | CR | R | DOD | 20 (3) | |
| 20 | 49/F | Maxilla | IIEB | CM | PD | S | TRM | 5 | |
| 21 | 52/F | Palate | IIEB | CM | CR | R, S | DOD | 11 (1) | |
| 22 | 44/F | Skull base | IIEB | CM | CR | L, S | DOD | 14 (2) | |
| 23 | 45/M | Palate | IIEA | CM | PD | L, S | DOD | 14 | |
Tx indicates treatment modality; M, male; CTX, chemotherapy alone; L, local failure; DOD, died of disease; F, female; PD, progressive disease; S, systemic failure; NE, not evaluable; TRM, treatment-related mortality; PR, partial remission; CM, combined modality; and R, regional failure.
Comparison of overall survival according to local tumor invasiveness
. | 2-year OS, % . | . | . | |
|---|---|---|---|---|
| Treatment modality . | LTI- . | LTI+ . | P . | |
| Chemotherapy alone | 74 | 7 | < .001 | |
| Combined modality | 82 | 22 | < .001 | |
. | 2-year OS, % . | . | . | |
|---|---|---|---|---|
| Treatment modality . | LTI- . | LTI+ . | P . | |
| Chemotherapy alone | 74 | 7 | < .001 | |
| Combined modality | 82 | 22 | < .001 | |
LTI- indicates absence of LTI; LTI+, presence of LTI.
Discussion
The data presented here indicate that the presence of LTI provides the highest RR for reduced OS and DFS and low probability for CR in patients with nasal stage IE/IIE NTCL compared with other clinical factors. Although high IPI score was predictive of reduced OS, the IPI score itself did not predict CR and DFS in multivariate analysis. Ann Arbor stage was unable to dissect prognostic subgroups in our analysis.
Robbins et al17 previously reported that 5-year DFS was shortened in advanced T stages using the tumor-node-metastasis (TNM) staging system in stage IE/IIE lymphomas of nasal cavity and paranasal sinuses. Similarly, Logsdon et al18 reclassified stage IE into T stages according to the extent of the disease and showed that early T stages improved freedom from progression in patients treated with radiation therapy. Despite lack of immunophenotyping, the 2 studies used the TNM staging system and focused on the extent of the lymphoma, including bone destruction.18 The majority of studies to date showed the local invasive nature of nasal lymphomas extending to adjacent anatomic structures14,17,18,23,24 and destroying bone structures.6,16,25 Paranasal extension was a significant predictive factor of survival in several studies,17,18,26,27 whereas Cheung et al28 reported no prognostic significance of paranasal extension or the significance of bony invasion. Here, we investigated LTI that indicated a more advanced disease state than paranasal extension. With the presence of LTI, the majority of patients with systemic failure had the predilection sites of skin, gastrointestinal tract, liver, testis, and CNS, consistent with previous findings.29,30 Relative high frequency of systemic failure (65%) in patients with LTI resulted in reduced survival duration. Furthermore, systemic failure was not prevented by conventional treatments and led to death after the occurrence. Although combined modality of chemo and radiation therapies improved median OS in comparison with chemotherapy alone, problems of early relapse and mortality remained unresolved. In this study, LTI retained predictive capacity of OS and DFS in treatment modality–adjusted multivariate analyses. Nevertheless, the heterogeneity of treatment might weaken the prognostic significance of LTI.
Our study showed the adverse survival outcome of high IPI score, consistent with other previous reports.13,19 However, IPI lost the predictive capacity of DFS in multivariate analysis because only 2 IPI factors had univariate association with DFS. Nonetheless, in a nationwide survey of 326 Korean patients, IPI was a significant prognostic factor when patients with stage IIIE/IVE were included.13 Although most studies to date have found the Ann Arbor stage to be an independently significant prognostic factor predictive of survival,14,16,18,23,26,28,30 the staging system failed to predict OS, DFS, and probability of achieving CR in this study as well as in the previously reported multicenter collaborative study of 326 Korean patients.13 Such discrepancies may be a result of false-positive benign lymphoproliferative nodes associated with EBV in patients with Ann Arbor stage IIE.
In terms of treatment modality, it has been demonstrated that treatment with radiotherapy improved survival28 but addition of anthracycline-based regimens had no proven role.28,31 Early radiotherapy32 and additional booster radiotherapy16 were emphasized by a few investigators to reduce local failure. Two studies suggested the need for systemic chemotherapy in addition to radiation therapy to resolve the problem of frequent systemic failures in patients receiving radiation therapy alone.30,32 However, high expression of multidrug resistance protein 1 mRNA or its product, P glycoprotein, has led to resistance to chemotherapy and aggressive tumor behavior.33 In trying to tackle chemo-resistance, autologous stem cell transplantation was attempted, which if performed in the first CR showed a trend toward better OS compared with historic controls.34
In this study, chemotherapy alone deteriorated OS, DFS, and the probability of achieving CR in patients with nasal stage IE/IIE NTCL and decreased median OS in patients with LTI. Since no patients with LTI were treated with radiation therapy alone, we could cautiously conclude that combined modality is superior to chemotherapy alone for improving OS. However, the intrinsic problems of analysis that included heterogeneous chemotherapy regimens and treatment modalities remained unresolved. Therefore, it is not possible to draw meaningful conclusions on the optimal treatment modality and the role of radiation therapy in patients with LTI.
The presence of B symptoms was an independently significant factor for the low probability of achieving CR in our study, which was explored as a prognostic factor in previous studies.18,28,31 Rather, advanced age predicted reduced OS and DFS in univariate and treatment modality–adjusted multivariate analyses, respectively. However, the presence of B symptoms and advanced age should be further investigated as prognostic factors.
The status of EBV RNA failed to predict response and survival, but EBV RNA positivity had a tendency to reduce OS and DFS in our study; however, due to the limited number of EBV RNA tests, its significance is inconclusive on survival. Recently, Au et al35 showed that plasma EBV DNA at presentation correlated with stage and LDH level but did not correlate with IPI in 23 patients with NTCL. High-presentation EBV DNA was the most significant prognostic factor for reduced DFS and showed a trend of negatively affecting OS. Furthermore, there was evidence that cytotoxic molecules, such as perforin, granzyme B, and Fas ligand, produced tissue damage that was also induced by angiocentricity.36 Therefore, we should observe the correlation of LTI with plasma EBV DNA and also find cytotoxic molecules associated with LTI in the future.
In conclusion, this study demonstrated the importance of LTI as a prognostic factor in nasal stage IE/IIE NTCL. Ann Arbor stage dose not seem to predict survival and IPI lost predictive capacity of DFS in multivariate analysis. Consequently, LTI is the most important prognostic factor in nasal stage IE/IIE NTCL. Future efforts should be directed toward finding optimal treatment modalities including combined modality in managing patients with local tumor invasiveness.
Prepublished online as Blood First Edition Paper, August 18, 2005; DOI 10.1182/blood-2005-05-2056.
Supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (0412-CR01-0704-0001).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge the assistance of Sun Young Yum, MD, for revising the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal