Abstract
Whether cytokines can modulate the fate of primitive hematopoietic progenitor cells (HPCs) through successive in vitro cell divisions has not been established. Single human marrow CD34+CD38–/lo cells in the G0 phase of cell cycle were cultured under 7 different cytokine combinations, monitored for proliferation on days 3, 5, and 7, then assayed for long-term culture-initiating cell (LTC-IC) function on day 7. LTC-IC function was then retrospectively correlated with prior number of in vitro cell divisions to determine whether maintenance of LTC-IC function after in vitro cell division is dependent on cytokine exposure. In the presence of proliferation progression signals, initial cell division was independent of cytokine stimulation, suggesting that entry of primitive HPCs into the cell cycle is a stochastic property. However, kinetics of proliferation beyond day 3 and maintenance of LTC-IC function were sensitive to cytokine stimulation, such that LTC-IC underwent an initial long cell cycle, followed by more synchronized shorter cycles varying in length depending on the cytokine combination. Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) transplantation studies revealed analogous results to those obtained with LTC-ICs. These data suggest that although exit from quiescence and commitment to proliferation might be stochastic, kinetics of proliferation, and possibly fate of primitive HPCs, might be modulated by extrinsic factors.
Introduction
Self-renewal division of hematopoietic stem cells (HSCs) enables simultaneous sustained production of blood cells and maintenance of the stem cell pool. To support these functions, HSCs, under stochastic and deterministic regulatory mechanisms, balance differentiation and self-renewal to maintain steady-state hematopoiesis. Experimental evidence in human1,2 and animal systems3,4 clearly demonstrates that this balance is quickly broken in vitro where HSC proliferation is invariably associated with loss of self-renewal divisions. However, the extent of differentiation of primitive HSCs following a limited number of divisions in vitro is dependent on the nature of the proliferative stimulus5-7 with different growth factors influencing hematopoietic function either negatively,8 or positively.9,10 Distinct growth factor requirements for proliferation of different classes of primitive hematopoietic progenitor cells (HPCs), such as the long-term culture initiating cells (LTC-ICs) and extended LTC-ICs, have been identified.11 Maintenance and modest expansion of HSC activity in cultures supplemented with different cytokine combinations have been reported both in human12-15 and mouse systems.10 Although the association between number of cell divisions and maintenance of primitive HPC function has been previously investigated,16,17 comparison of in vitro and in vivo data has not been performed nor have these results been used to estimate the length of cell cycle of primitive HPCs in vitro. Recently, in vivo stem cell function of murine HSCs undergoing different types of cell division kinetics in vitro was examined, but the precise proliferative history of dividing cells was not recorded.18 Our laboratory19-21 and that of others9 examined the fate of human HPCs following a predetermined number of cell divisions in vitro. These studies concluded that after few divisions, the hematopoietic potential of primitive HPCs was compromised although other studies observed a slight increase in function among proliferating cells.22 However, these studies were not designed to examine the fate of individual primitive HPCs following successive cell divisions, although in some studies, the proliferation kinetics and growth onset23 or the proliferative history of individual clonogenic progenitors24,25 were assessed.
LTC-ICs are among the most primitive HPCs that can be assayed in culture.26 In an LTC-IC assay, the proliferative history, number of cell divisions, and the length of cell cycle of individual LTC-ICs cannot be evaluated. Single-cell studies of highly purified human fetal liver HPCs allowed for the assessment of the length of cell cycle but did not determine the functional properties of progeny cells.27 Other reports investigated the effects of positive and negative regulators of hematopoiesis on cell cycle kinetics of individual murine HPCs28 but only the high cloning efficiency of purified cells was examined leaving the relationship between cell cycle kinetics, length of cell cycle, and maintenance of clonogenic potential unanswered.
Mechanisms governing entry of HSCs into active phases of cell cycle and subsequent proliferation, differentiation or self-renewal, remain obscure. Although some investigators believe these functions are stochastic,29-32 others favor a more active role for extrinsic factors in stem cell regulation.6,33-35 Effects of extrinsic signaling have been examined by traditional recloning experiments,36-38 assessment of the bipotential of daughter cells,37,39 and more recently, by transplantation studies.40,41 However, it has been difficult to unequivocally endorse either model of HSC regulation due in part to the absence of assays that can measure the activity of a large number of individual primitive HPCs with a known proliferative history and to mathematically model the behavior of these cells.
Building on the rationale of our previous study,24 we designed an assay in which the fate of individual LTC-ICs could be determined following enumeration of the number of cell divisions accomplished after 7 days in culture. With this approach, we investigated the effects of extrinsic cytokine stimulation on the regulation of proliferation kinetics and maintenance of function of primitive HPCs and measured the length of cell cycle of LTC-ICs maintained under different cytokine combinations. We report here that exit from quiescence may be a stochastic property of primitive HPCs but that proliferation kinetics and maintenance of hematopoietic potential may be modulated by cytokines. Furthermore, these results indicate that the length of cell cycle of individual LTC-ICs is variable and may also be partially affected by extrinsic manipulations. Finally, in vivo studies in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice demonstrating maintenance of repopulating potential among cultured cells corroborated in vitro observations with LTC-IC function demonstrating the potential clinical translation of these results.
Materials and methods
BM collection and fractionation
Human bone marrow (BM) aspirates were collected from healthy adult volunteers after they provided informed consent according to guidelines of the Investigational Review Board of the Indiana University School of Medicine. Low-density BM cells were recovered by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density centrifugation. CD34+ cells were fractionated by magnetic-activated cell sorting (MACS) according to the manufacturer's directions (Miltenyi Biotec, Auburn, CA).
Cell staining and sorting
CD34+ cells were stained with allophycocyanin (APC)–conjugated CD34 monoclonal antibodies (Caltag Laboratories, Burlingame, CA) and phycoerythrin (PE)–conjugated CD38 (Becton Dickinson Immunocytometry Systems, San Jose, CA) and sorted to yield CD34+CD38–/lo cells. CD34+CD38–/lo cells were stained with Hoechst 33342 (Hst; Molecular Probes, Eugen, OR) and pyronin Y (PY; Polysciences, Warrington, PA) as previously described.42,43 Cells were sorted on a FACStar Plus (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) equipped with an argon laser providing 488 nm light and a krypton laser providing either 350 nm or 610 nm wavelength. Residual PE fluorescence on CD38–/lo cells was minimal and did not interfere with the PY signal. Cells with 2n DNA (determined by the Hst profile) and low PY fluorescence were selected as those in the G0 phase of cell cycle as previously demonstrated.24,43,44 Single CD34+CD38–/lo cells in G0 (G0CD34+CD38–/lo) were delivered into individual wells of round-bottomed 96-well plates using an automatic cell deposition unit (ACDU; BDIS). Plates were prepared with medium containing different cytokine combinations as described (see “Cell cultures”). Viability of sorted cells always exceeded 98%. Fluorescent beads were sorted individually into single wells of 96-well plates to verify the integrity of the ACDU. Single fluorescent beads were detected in more than 97% of the wells (6 plates analyzed) with less than 3% of the wells scored negative for the presence of any beads. None of the wells in this assay contained 2 or more beads (data not shown).
Cell cultures
Single G0CD34+CD38–/lo cells were sorted into individual wells of round-bottomed 96-well plates containing 100 μL medium supplemented with different cytokine combinations. The medium used in these experiments consisted of Iscove modified Dulbecco medium (IMDM) supplemented with 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Biowhittaker, Walkersville, MD), 10% fetal calf serum (FCS; Hyclone, Logan, UT), 10 μg/mL bovine serum albumin (BSA), 10 μg/mL bovine insulin, 200 μg/mL transferrin (all from StemCell Technologies, Vancouver, BC, Canada), 2 mM alanyl-glutamine, 1% (vol/vol) lipids cholesterol-rich, 1 mM sodium pyruvate (all from Sigma, St Louis, MO), 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10–5 M 2-mercaptoethanol. This medium (referred to hereafter as complete medium) was previously shown to maximize the number of cell divisions within 7 days and thereby the sensitivity of our assay without affecting cell survival or the relative differences among test cytokines to promote cellular proliferation.24
A total of 7 different cytokine combinations were used all of which contained a basic combination of stem cell factor (SCF), Flt-3 ligand (FL), and megakaryocyte growth and differentiation factor (MGDF). Tumor growth factor β (TGF-β) was included in one cytokine combination, and all combinations and cytokine concentrations are detailed in Table 1. For ease of data presentation, these cytokine combinations will be referred to as combinations 3 through 9 as indicated in Table 1. Either 1 or 2 plates (for a total of 96 or 192 individual wells) were prepared for every cytokine combination in every experiment. The number of cells contained in every well was determined on days 3, 5, and 7 by direct counting of the cells under an inverted microscope.
Cytokine combinations and concentrations used to initiate single cell cultures
. | . | Cytokine and cytokine concentration per mL culture medium . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Designation . | Combination . | SCF 100 ng . | FL 50 ng . | MGDF 50 ng . | IL-3 100 ng . | IL-6 100 ng . | Epo 3 U . | GM-CSF 20 ng . | TGF-β 10 ng . | |||||||
| 3 | SFM | X | X | X | ||||||||||||
| 4 | SFM3 | X | X | X | X | |||||||||||
| 5 | SFM36 | X | X | X | X | X | ||||||||||
| 6 | SFM36E | X | X | X | X | X | X | |||||||||
| 7 | SFM36EG | X | X | X | X | X | X | X | ||||||||
| 8 | SFM36G | X | X | X | X | X | X | |||||||||
| 9 | SFM36EGT | X | X | X | X | X | X | X | X | |||||||
. | . | Cytokine and cytokine concentration per mL culture medium . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Designation . | Combination . | SCF 100 ng . | FL 50 ng . | MGDF 50 ng . | IL-3 100 ng . | IL-6 100 ng . | Epo 3 U . | GM-CSF 20 ng . | TGF-β 10 ng . | |||||||
| 3 | SFM | X | X | X | ||||||||||||
| 4 | SFM3 | X | X | X | X | |||||||||||
| 5 | SFM36 | X | X | X | X | X | ||||||||||
| 6 | SFM36E | X | X | X | X | X | X | |||||||||
| 7 | SFM36EG | X | X | X | X | X | X | X | ||||||||
| 8 | SFM36G | X | X | X | X | X | X | |||||||||
| 9 | SFM36EGT | X | X | X | X | X | X | X | X | |||||||
Designations 3 through 9 are used throughout this report to correspond to the cytokine combinations shown in column 2.
Abbreviations: SCF, stem cell factor; FL, Flt-3 ligand; MGDF, megakaryocyte growth and development factor; IL-3, interleukin-3; IL-6, interleukin-6; Epo, erythropoietin; GM-CSF, granulocyte-macrophage colony-stimulating factor; TGF-β, tumor growth factor-β.
For bulk cultures, between 4.5 × 105 and 9.5 × 105 purified CD34+ cells (equivalent to the number of fresh cells transplanted into each mouse) were expanded in combinations 3 or 8 (Table 1) for 5 days. Cultures were replenished with fresh cytokines every 48 hours and cell density was maintained below 5 × 105/mL. On day 5, all the progeny of a single graft transplanted into one recipient on day 0 were used as a single graft (expansion equivalent).
Assessment of LTC-IC function
On day 7, selected wells (with small, round, translucent cells) from every plate were separated into 3 groups. Wells in the first group contained 1 to 4 (0, 1, or 2 divisions, assuming symmetrical division) cells. Wells in the second group contained between 5 and 8 cells (3 divisions), whereas group 3 consisted of wells with more than 8 cells. Cells in selected wells (up to 20 from each plate) were collected separately and transferred to a tube containing 1.5 × 104 irradiated (8000 cGy) M210B4 cells in 2 mL IMDM containing 10% FCS. After centrifugation, cells were resuspended in 200 μL myelocult medium (StemCell Technologies). The contents of each tube were transferred into individual wells of flat-bottomed 96-well plates and incubated at 37°C in a 100% humidified 5% CO2/5% O2/90% N2 atmosphere. At weekly intervals, all plates were fed by a half-medium change and at week 4 (5 weeks total), 120 μL was removed from each well and the remaining medium was mixed with 150 μL of a mixture consisting of 3 parts FCS and 4 parts 3.3% methylcellulose containing at final concentrations 5 × 10–5 M 2-mercaptoethanol, 100 ng/mL SCF, 10 ng/mL IL-3, 10 ng/mL IL-6, 5 ng/mL GM-CSF, and 2 U/mL Epo. Plates were incubated at 37°C in a 100% humidified 5% CO2 atmosphere. After 2 weeks, individual wells were scored for colony formation and those with one or more hematopoietic colonies were considered to have originated from a single LTC-IC. The proliferative history of clones in round-bottomed plates was retrospectively associated with LTC-IC activity.
Transplantation of test cells into NOD/SCID mice
NOD/LtSz-scid/scid (NOD/SCID) mice were bred and housed at Indiana University. Animal experiments were approved by the Animal Care Committee of the Indiana University School of Medicine. Mice were housed in microisolators and received autoclaved food and acidified water ad libitum. Nine- to 12-week-old NOD/SCID mice were sublethally irradiated with 3 Gy from a 137Cs source and received by intravenous injection as accessory cells, between 8 and 12 × 106 nonadherent CD34– adult human BM cells irradiated with 80 Gy. Two hours later, mice were given transplants of either fresh human CD34+ cells or their expansion equivalent (total number of cells generated in culture in 5 days from an equivalent number of fresh cells). Mice were killed 8 weeks later and BM cells were collected and analyzed for human chimerism as described before.43
Statistical analysis
Analysis of the frequency of clones scored as LTC-ICs and the effect of different culture conditions on the probability of maintaining LTC-IC activity were examined using logistic regression analysis. The outcome was presence or absence of LTC-IC activity in each clone. A total of 9 experiments were conducted using either 1 or 2 round-bottomed 96-well plates for each cytokine combination. From these plates, a total of 1776 clones were statistically analyzed for LTC-IC activity and retrospectively analyzed in regard to their individual proliferative history. The number of clones analyzed within each cytokine combination ranged from 100 to 428. To account for the correlation of observations within each experiment and plate, generalized estimating equation (GEE) methodology45 was applied to the logistic regression models. Multiple pair-wise comparisons between all cytokine combinations were adjusted with the Bonferroni procedure. The number of cells present in individual wells on day 5 ranged from 1 to 100 and on day 7 from 1 to 1000. However, on both days 5 and 7, the frequency of clones comprised of more than 8 cells was small. Therefore, observations where the number of cells was more than 8 were combined for analysis purposes. Thus the independent predictor used in the logistic regression model was number of cells present, which ranged from 1 to more than 8. The logistic regression model was run separately for each cytokine using either the number of cells present on day 5 or the number of cells present on day 7. A quadratic effect was also tested to determine if a curvilinear relationship exists between number of cells present and percent of cells with LTC-IC activity. For all statistical analyses (except where indicated), P < .05 was considered significant. An analysis of variance routine was applied to the log-transformed engraftment data to compare chimerism between groups of mice that had received transplants. Multiple comparisons were adjusted by the Tukey-Kramer method.
Results
A total of 4446 single cells in 9 experiments were initiated individually in round-bottomed 96-well plates and assessed for cell proliferation. However, only 1776 proliferating clones were assessed for LTC-IC potential. The G0 cell cycle status of human CD34+ cells displaying 2n DNA content (Hst staining) and low PY fluorescence has been previously documented.24,43,44 Accordingly, CD34+CD38–/lo cells fractionated based on their cell cycle status will be referred to as G0CD34+CD38–/lo cells.
Commitment of G0CD34+CD38–/lo cells to division
Whether culture conditions dictate the frequency at which G0CD34+CD38–/lo cells enter active phases of cell cycle and proceed to divide was investigated in 2 manners. First, the percentage of wells containing more than 2 cells on day 3 was calculated for all 7 cytokine combinations. Between 51.6% and 83.0% of individual wells in each group contained more than 2 cells on day 3 (Figure 1A).
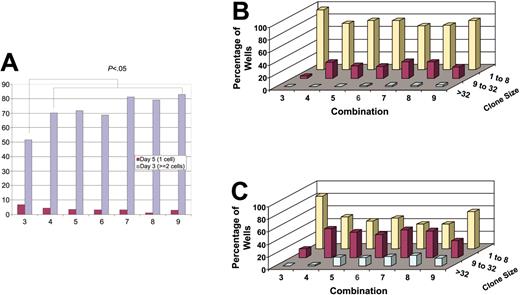
Proliferation kinetics of single G0CD34+CD38–/lo cells recorded on days 5 and 7. Single G0CD34+CD38–/lo cells were delivered into individual round-bottomed 96-well plates prepared with CM and 1 of the 7 cytokine cocktails depicted on the x-axis (Table 1 presents the details). Cell number in each well was counted on days 3 and 5 and the percentage of wells containing more than 2 cells on day 3 (red bars) or just one cell on day 5 (blue bars) were plotted in panel A. (B-C) Similarly, cell number in each well was counted on days 3, 5, and 7 and the percentage of wells (z-axis) containing clones composed of 1 to 8 cells (yellow bars), 9 to 32 cells (red bars), and more than 32 cells (green bars; y-axis) was calculated for each group on days 5 (B) and 7 (C). Between 288 and 984 clones were analyzed for each cytokine combination.
Proliferation kinetics of single G0CD34+CD38–/lo cells recorded on days 5 and 7. Single G0CD34+CD38–/lo cells were delivered into individual round-bottomed 96-well plates prepared with CM and 1 of the 7 cytokine cocktails depicted on the x-axis (Table 1 presents the details). Cell number in each well was counted on days 3 and 5 and the percentage of wells containing more than 2 cells on day 3 (red bars) or just one cell on day 5 (blue bars) were plotted in panel A. (B-C) Similarly, cell number in each well was counted on days 3, 5, and 7 and the percentage of wells (z-axis) containing clones composed of 1 to 8 cells (yellow bars), 9 to 32 cells (red bars), and more than 32 cells (green bars; y-axis) was calculated for each group on days 5 (B) and 7 (C). Between 288 and 984 clones were analyzed for each cytokine combination.
Comparison of the rates of cell division at day 3 showed that, adjusted for multiple comparisons, combination 3 was associated with lower rates of cell division compared to the remaining combinations. No other pair-wise comparison was statistically significant. These results demonstrate that in the presence of proliferation progression signals (combinations 4 through 9), commitment to division is a stochastic property of primitive HPCs. Second, between 1.6% and 7.1% of all wells analyzed under all 7 combinations contained only one cell on day 5. Comparison of these results indicated that on day 5, the frequency of cells that had not divided was not statistically different for any cytokine combination. These observations suggest that commitment to division may be a stochastic property of primitive HPCs, some of which require multiple or prolonged cytokine stimulation prior to their initial division.
Proliferation kinetics of single G0CD34+CD38–/lo cells in response to different cytokine combinations
Cell counts on days 3, 5, and 7 allowed for the examination of the rate at which G0CD34+CD38–/lo cells proliferate in response to different cytokine combinations and to estimate the minimum time required to execute the first division. As can be seen in Figure 1B-C, proliferation by day 5 or day 7 was divided into 3 groups depending on the number of cells detected. Clones in group 1 consisted of 1 to 8 cells (up to 3 divisions, assuming symmetrical divisions), group 2 included clones with 9 to 32 cells (4 and 5 divisions), whereas group 3 contained clones with more than 32 cells (> 5 divisions). Proliferation kinetics between day 0 and day 5 were similar in 6 of 7 culture conditions whereby approximately 75% of individual clones contained 8 or fewer cells (Figure 1B). In combination 3, however, 96.8% of wells analyzed on day 5 contained 8 or fewer cells. By day 7, between 37.1% and 46.3% of clones in all but 2 culture conditions had divided 4 times or more (Figure 1B). Conditions in which 60% or more of the clones remained with 8 cells or fewer were combination 3, probably due to the absence of proliferation progression signals7,42 and combination 9 (Figure 1B), which probably inhibited proliferation via TGF-β.46-48 These results suggest that although the decision to commence division may be a stochastic property of HPCs, cell division kinetics can be manipulated by extrinsic growth factors.
Frequency of LTC-ICs under different cytokine conditions
Whether different cytokine combinations altered the frequency of LTC-ICs among day 7 clones was examined (Figure 2). For all cytokine combinations except SFM3, the percentage of wells scored as LTC-IC at the end of the assay ranged from 27% to 42%. Under the influence of SFM3, 56% of the clones were scored as LTC-ICs. However, the overall treatment effect of all cytokine combinations was not significant (P > .05). Interestingly, pair-wise comparisons between treatments indicated that the outcome of LTC-IC readout under the effect of SFM3 was significantly different from other cytokine combinations (P for paired comparisons < .05). In addition, the difference between combinations 3 and 9 was also significant (P = .005). These differences suggest that whereas it is difficult to alter the commitment for initial cell division of HPCs, the frequency of cells initiating cell division under different culture conditions can be moderately modulated in vitro. No apparent differences were observed in types of colonies generated by LTC-ICs under different cytokine combinations.
Percentage of clones scored as LTC-ICs in each of the 7 cytokine combinations. Selected clones proliferating in round-bottomed 96-well plates seeded with individual G0CD34+CD38–/lo cells in the 7 cytokine combinations detailed in Table 1 were transferred to flat-bottomed 96-well plates on day 7 and assayed for LTC-IC function as described in “Materials and methods.” A total of 1776 clones were assayed for LTC-IC function and between 100 and 428 clones were examined for each cytokine combination. Data represent the percentage of clones in each group determined on day 49 to have originated from an LTC-IC.
Percentage of clones scored as LTC-ICs in each of the 7 cytokine combinations. Selected clones proliferating in round-bottomed 96-well plates seeded with individual G0CD34+CD38–/lo cells in the 7 cytokine combinations detailed in Table 1 were transferred to flat-bottomed 96-well plates on day 7 and assayed for LTC-IC function as described in “Materials and methods.” A total of 1776 clones were assayed for LTC-IC function and between 100 and 428 clones were examined for each cytokine combination. Data represent the percentage of clones in each group determined on day 49 to have originated from an LTC-IC.
Proliferation kinetics of single LTC-ICs in response to different cytokine combinations
By recording the proliferative history of each clone, the proliferation kinetics of LTC-ICs could be retrospectively examined (Figure 3). The rate of proliferation of individual LTC-ICs was different for every cytokine combination, similar to that observed for total G0CD34+CD38–/lo cells in Figure 1. Because the frequency of clones containing more than 8 cells on day 5 and day 7 was small, clones greater than 8 cells were combined for the analysis of LTC-IC function, resulting in compartmentalizing these clones into more restricted groups than those used for total G0CD34+CD38–/lo cells. The first contained 4 cells or fewer, depicting those that had remained dormant or divided up to 2 times only (Figure 3). The second contained between 5 and 8 cells (3 divisions), whereas the third group had more than 8 cells representing 4 or more divisions (Figure 3). By day 5, the vast majority of LTC-ICs maintained in combinations 3 or 4 (SFM or SFM3, respectively) remained as single cells or had divided once or twice only (Figure 3A). In contrast, all other conditions induced more than 50% of LTC-ICs to exceed 4 cells and 20% or more to proliferate beyond 8 cells. Interestingly, the inhibitory effect of TGF-β on proliferation of primitive HPCs could be gleaned through its ability to retain 60% more LTC-ICs in the 5- to 8-cell group compared to the same cytokine condition devoid of TGF-β (Figure 3A combinations 9 and 7, respectively). By day 7, the inability of combinations 3 and 4 to sustain multiple divisions of LTC-ICs was more evident because only one third of these clones divided beyond 8 cells, whereas more than 50% of clones in the remaining 5 conditions contained more than 8 cells (Figure 3B).
Relationship between cell proliferation and maintenance of LTC-IC function. Single G0CD34+CD38–/lo cells were delivered into individual round-bottomed 96-well plates prepared with complete medium and one of the 7 cytokine cocktails depicted on the x-axis (Table 1 presents details). On day 7, cells from 1776 individual wells were assayed for LTC-IC function as described in “Materials and methods.” Percentage of wells determined to have originated from an LTC-IC was plotted on the z-axis for all 7 cytokine combinations (x-axis) according to the size of the clone detected in each well (y-axis) on days 5 (A) and 7 (B). Cell numbers were grouped to represent 1 and 2 divisions (1-4 cells; yellow bars), 3 divisions (5-8 cells; red bars), and more than 3 divisions (> 8 cells; green bars). Between 100 and 428 clones were analyzed for each cytokine combination.
Relationship between cell proliferation and maintenance of LTC-IC function. Single G0CD34+CD38–/lo cells were delivered into individual round-bottomed 96-well plates prepared with complete medium and one of the 7 cytokine cocktails depicted on the x-axis (Table 1 presents details). On day 7, cells from 1776 individual wells were assayed for LTC-IC function as described in “Materials and methods.” Percentage of wells determined to have originated from an LTC-IC was plotted on the z-axis for all 7 cytokine combinations (x-axis) according to the size of the clone detected in each well (y-axis) on days 5 (A) and 7 (B). Cell numbers were grouped to represent 1 and 2 divisions (1-4 cells; yellow bars), 3 divisions (5-8 cells; red bars), and more than 3 divisions (> 8 cells; green bars). Between 100 and 428 clones were analyzed for each cytokine combination.
Relationship between number of cell divisions on day 5 and maintenance of LTC-IC activity
To investigate whether cytokine conditions modulate the fate of LTC-ICs through successive divisions, the proliferative histories of individual clones under each of the 7 conditions were used to predict the possibility of an LTC-IC–positive outcome using logistic regression. As can be seen in Figure 4, the actual probability of clones scored as LTC-ICs as a function of cell number per clone on day 5 (red data points) as well as the calculated prediction of detecting an LTC-IC (blue data points) varied from one cytokine combination to the other. Most striking was the quadratic or curvilinear effect observed with combination 4 (SFM3), indicating that the probability of detecting an LTC-IC under these conditions decreased significantly when individual clones exceeded 4 cells (P = .03). Under combinations 7, 8, and 9, a gradual increase in the probability of scoring an LTC-IC, albeit not statistically significant, was observed with increasing cell number per clone.
Maintenance of LTC-IC function through successive in vitro divisions under different cytokine conditions. Experimental (red data points) and predicted (blue plot) probability of detecting LTC-IC function among single sorted G0CD34+CD38–/lo cells was plotted for each of the 7 cytokine combinations segregating the data based on the number of cells contained in each well on day 5. Because few wells in all cases contained 9 or more cells, all data points in all the plots were pooled together and are reported here as 9 cells/well. Predicted values for each plot were calculated by regression analysis as indicated in “Materials and methods.” Experimental data points (red) are reported as mean ± SD. The number of cells in each well on day 5 was not a significant predictor of LTC-IC activity for all combinations (P > .05 for all) except combination 4 (SFM3; P = .033).
Maintenance of LTC-IC function through successive in vitro divisions under different cytokine conditions. Experimental (red data points) and predicted (blue plot) probability of detecting LTC-IC function among single sorted G0CD34+CD38–/lo cells was plotted for each of the 7 cytokine combinations segregating the data based on the number of cells contained in each well on day 5. Because few wells in all cases contained 9 or more cells, all data points in all the plots were pooled together and are reported here as 9 cells/well. Predicted values for each plot were calculated by regression analysis as indicated in “Materials and methods.” Experimental data points (red) are reported as mean ± SD. The number of cells in each well on day 5 was not a significant predictor of LTC-IC activity for all combinations (P > .05 for all) except combination 4 (SFM3; P = .033).
Logistic regression analysis of cell numbers in clones scored as LTC-IC on day 7 generated similar results with few notable differences. A significant curvilinear effect continued to be present for combination 4 (SFM3; P = .015). In addition, a significant curvilinear effect appeared for combination 5 (P = .029). Finally, the linear effect of cell number observed on day 5 for SFM36G persisted on day 7 but did not reach significance (P = .059). This trend suggested that under the influence of SFM36G, an increased probability for detecting LTC-IC function with increasing cell number may exist. These results document that the fate of individual LTC-ICs following successive cell divisions can be modulated by different cytokine combinations, some of which are detrimental (combinations 4 and 5 in Figure 4), whereas others are more conducive (combinations 8 and 9 in Figure 4) to maintenance of primitive HPC function.
Estimation of the length of cell cycle of LTC-ICs in vitro
Proliferation kinetics of individual LTC-ICs between day 0 and day 7 were used to estimate the length of cell cycle of LTC-ICs in vitro (Table 2). Data shown in Table 2 illustrate 4 important points. First, the estimated length of cell cycle varied considerably depending on the composition of the cytokine stimulation received by individual LTC-ICs. Second, on day 5 and day 7, the length of cell cycle was almost constant for each cytokine combination. However, at earlier observation points (day 3), longer cell cycle times were evident within every group suggesting a slow initial entry of these cells into active phases of cell cycle followed by a more rapid rate of turnover that brings cells under different conditions within a narrow range of cell cycle length. It is important to point out that at day 3, the average cell number in each well, under all conditions except one, was less than 3, demonstrating that, on average, fewer than 2 divisions had taken place (even if cell division of daughter cells was asymmetrical), thus emphasizing that any lag time in cell division was considered by these calculations as part of the actual length of the first cell cycle. Third, the highest rate of proliferation observed for combinations 7 and 8 on day 7 reduced the length of cell cycle to 33.6 hours and 34.9 hours, respectively, whereas a longer doubling time was maintained under combination 3 (estimated length of cell cycle was 62.0-69.2 hours). Fourth, presence of TGF-β in the culture medium produced a dramatic stabilization of the length of cell cycle at all times analyzed.
Estimated length of cell cycle of individual LTC-ICs
Culture conditions . | Cytokine combination . | Average no. cells*(estimated length of cell cycle, h†) . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | D 3 . | D 5 . | D 7 . | ||
| 3 | SFM | 1.74 (89.8) | 3.81 (62.0) | 5.35 (69.2) | ||
| 4 | SFM3 | 1.92 (76.6) | 5.31 (49.6) | 9.71 (51.1) | ||
| 5 | SFM36 | 2.13 (65.8) | 6.24 (45.3) | 13.46 (44.6) | ||
| 6 | SFM36E | 2.22 (62.4) | 6.17 (45.6) | 14.69 (43.2) | ||
| 7 | SFM36EG | 2.33 (58.8) | 9.18 (37.4) | 31.55 (33.6) | ||
| 8 | SFM36G | 2.79 (48.5) | 9.13 (37.5) | 27.7 (34.9) | ||
| 9 | SFM36EGT | 3.33 (41.3) | 7.46 (41.2) | 15.98 (41.9) | ||
Culture conditions . | Cytokine combination . | Average no. cells*(estimated length of cell cycle, h†) . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | D 3 . | D 5 . | D 7 . | ||
| 3 | SFM | 1.74 (89.8) | 3.81 (62.0) | 5.35 (69.2) | ||
| 4 | SFM3 | 1.92 (76.6) | 5.31 (49.6) | 9.71 (51.1) | ||
| 5 | SFM36 | 2.13 (65.8) | 6.24 (45.3) | 13.46 (44.6) | ||
| 6 | SFM36E | 2.22 (62.4) | 6.17 (45.6) | 14.69 (43.2) | ||
| 7 | SFM36EG | 2.33 (58.8) | 9.18 (37.4) | 31.55 (33.6) | ||
| 8 | SFM36G | 2.79 (48.5) | 9.13 (37.5) | 27.7 (34.9) | ||
| 9 | SFM36EGT | 3.33 (41.3) | 7.46 (41.2) | 15.98 (41.9) | ||
Average number of cells per LTC-IC clone detected on days 3, 5, and 7 was calculated by dividing the sum of cells in each clone counted on that day by the number of clones analyzed. Number of clones analyzed for each cytokine combination varied between 39 and 238. Total number of LTC-ICs analyzed was 699.
The length of cell cycle of individual LTC-IC was calculated by dividing the culture period in hours by log2 of the average number of cells calculated for every time point.
Transplantation of ex vivo expanded cells into NOD/SCID mice
To validate that maintenance of LTC-IC function correlates with preservation of an in vivo repopulating cell, CD34+ cells were expanded in combinations 3 or 8 for 5 days and the in vivo engraftment potential of expansion equivalent cells was compared to that of freshly isolated CD34+ cells. Cells cultured in combination 3 expanded 4.3-fold, whereas those in combination 8 expanded 8.1-fold reflecting the average size of clones maintained with these 2 combinations (Table 2). Analysis of chimerism data (Figure 5) using an analysis of variance routine revealed a significant difference in chimerism (at the 10% level) between the 3 groups (P = .08) and at the 5% level between combinations 3 and 8. As can be seen in Figure 5, cells expanded in combination 3 provided significantly less chimerism than fresh cells (7.2% ± 11.2% versus 19.1% ± 19.3%), whereas chimerism from cells expanded in combination 8 (23.2% ± 20.6%) was not significantly different from that observed with fresh cells but significantly different than chimerism observed with combination 3. Composition of the multilineage reconstitution in these mice was similar for all 3 groups (data not shown). These results suggest that observations made for LTC-ICs in vitro regarding maintenance of their number can be partially reproduced with in vivo repopulating cells as assessed by maintenance of the number or function of SCID repopulating cells.
Chimerism in NOD/SCID mice receiving fresh or expanded human BM CD34+ cells. Mice were conditioned as described in “Materials and methods” and were given injections through the tail vein of fresh (control) BM CD34+ cells or the expansion equivalent of equal numbers of cells expanded for 5 days in combinations 3 or 8. After 8 weeks, murine BM cells from individual mice were analyzed for the presence of human CD45+ cells. Data points represent the level of chimerism in individual mice and the horizontal bar for each set of points represents the mean. Results were pooled from 5 separate experiments and the total number of mice in each group is indicated in parentheses. Each mouse received between 4.5 × 105 and 9.5 × 105 fresh cells or the progeny of an equivalent number of cells. The difference between SFM and control groups and between SFM and SFM36G was significant at the 10% level.
Chimerism in NOD/SCID mice receiving fresh or expanded human BM CD34+ cells. Mice were conditioned as described in “Materials and methods” and were given injections through the tail vein of fresh (control) BM CD34+ cells or the expansion equivalent of equal numbers of cells expanded for 5 days in combinations 3 or 8. After 8 weeks, murine BM cells from individual mice were analyzed for the presence of human CD45+ cells. Data points represent the level of chimerism in individual mice and the horizontal bar for each set of points represents the mean. Results were pooled from 5 separate experiments and the total number of mice in each group is indicated in parentheses. Each mouse received between 4.5 × 105 and 9.5 × 105 fresh cells or the progeny of an equivalent number of cells. The difference between SFM and control groups and between SFM and SFM36G was significant at the 10% level.
Discussion
The experimental design of studies in this report allowed for the examination of whether exit of primitive HPCs from quiescence and execution of at least one cell division is a stochastic property of these cells or an event that may be regulated by exogenous stimuli. If commitment to cell division is deterministic, different percentages of G0CD34+CD38–/lo cells would have proliferated in response to different cytokine combinations within a specified period of time. Alternatively, if proliferation of G0CD34+CD38–/lo cells is a stochastic phenomenon, then regardless of the cytokine stimulus, a fixed percentage of cells will divide during a relatively short period of observation. Similar studies by Ramsfjell et al11 conducted with single CD34+CD38– BM cells demonstrated that addition of cytokines to SCF, FL, and MGDF did not alter the fraction of test cells recruited into proliferation when cell division was assessed 10 to 12 days later. Data from Ramsfjell et al,11 coupled with our present findings, suggest that initiation of cell division is a stochastic property of LTC-ICs. However, kinetics of proliferation subsequent to the first division was amenable to extrinsic cytokine modulation as is evident by changes in cell cycle length. These observations are in agreement with our previous findings42 that certain cytokines possess proliferation initiation characteristics, whereas others can act as proliferation progression signals.7 Recently, Ema and coworkers40 and Dykstra and colleagues41 reported controllable cell division of murine HSC in response to exogenous factors.
Whether the fate of primitive HPCs through successive in vitro cell divisions can be modulated by cytokine stimulation is not well established. We previously demonstrated21 that maintenance of primitive hematopoietic function among cultured CD34+ cells is gradually lost with successive in vitro cell divisions, most likely due to apoptosis. We repeated these experiments with single CD34+ cells residing in G0,24 only to observe similar findings, namely, that the hematopoietic function of dividing cells decreased with each division cycle. However, the proliferative history of single progenitors for retrospective analysis of the relationship between cell division and hematopoietic function was not recorded. When such an approach was taken in the present studies, it became evident that maintenance of LTC-IC function through cell proliferation was cytokine responsive. However, the ability to modulate preservation of LTC-IC function through cell division is rather limited and appears to be only moderately dependent on cytokine combinations used in culture. Although we could not perform direct measurements of self-renewal divisions, nor assess whether symmetrical or asymmetrical divisions dominated the proliferative behavior of these clones, it is certain that progeny of each LTC-IC clone containing 2 or more cells on day 7 resulted from at least one self-renewal division. Previous studies by Petzer and colleagues49 demonstrated that human LTC-ICs can proliferate in vitro without complete loss of their ability to be detected. Here we extended these studies and examined more closely the relationship between proliferative history and detection of LTC-IC function under multiple conditions.
Regulation of mitogenesis of HSCs and maintenance of their hematopoietic potential has been the subject of many studies trying to establish an association between both parameters or to confirm that these functions are dissociated. Our present studies provide evidence that these functions are not necessarily coupled and that extrinsic stimuli can alter the maintenance of function of HPCs proliferating in vitro. If LTC-IC activity is considered as a surrogate measure of stem cell function, our results appear to be in contradiction with those of Muller-Sieburg et al50 who concluded that self-renewal and differentiation decisions are more deterministic than stochastic. Other studies in which symmetry of initial cell divisions was examined concluded that extrinsic signals may alter lineage commitment and proliferation, but not symmetry of early divisions, which are most likely stochastic.16 Here, we reached similar conclusions regarding recruitment of primitive HPCs into cell cycle and subsequent proliferation kinetics and maintenance of LTC-IC function. Although these results suggest that it is possible to tailor culture conditions to promote a desired rate of proliferation and maintenance of function, early events in stem cell behavior including commitment to proliferation may not be affected by extrinsic conditions. Variability in the length of cell cycle under different conditions may reflect the susceptibility of LTC-ICs to extrinsic factors affecting both recruitment of cells into cell cycle and subsequent proliferation.
The almost horizontal plot of the predicted LTC-IC probability under combination 3 (Figure 4) suggests that SCF, FL, and MGDF (thrombopoietin [TPO]) are capable of sustaining self-renewal divisions. In similar in vivo studies49,51 and in others,9,13 the role of these 3 cytokines, alone or in different combinations, in supporting self-renewal was noted. The ability of IL-6 to partially restore loss of function observed with IL-3 (combination 5 in Figure 4) was not completely unexpected. That IL-3 abrogates the reconstituting potential of murine HSCs in irradiated8,52 and normal53 hosts is established. Conversely, IL-6 has been shown to promote self-renewal of HSCs,9 and in combination with IL-3, conserved the marrow repopulating potential of ex vivo manipulated cells.54,55 It is, therefore, possible that IL-6 reverses the quadratic effect observed for LTC-IC function in combination 4. However, this IL-6–mediated reversal was not long-lived because a significant quadratic effect was observed on day 7 for clones maintained in SFM36. It should be emphasized that Bryder and Jacobsen56 found IL-3 to support expansion of murine repopulating cells following up to 5 in vitro divisions in serum-free medium. Reasons that may explain differences between those results56 and ours are not apparent. Differences in the prediction of maintenance of LTC-IC function through successive divisions on day 5 and day 7 under the influence of combination 5 (SFM36) highlights the ability of this model to examine temporal effects of a given cytokine combination on the function of the same group of primitive HPCs. Such an advantage may be easily exploited for the design of retroviral-mediated gene transfer protocols. Effects observed for TGF-β are in line with many previous studies.57-59 Eaves and coworkers57 reported that proliferation of primitive HPCs could be prolonged when the effect of TGF-β was neutralized by specific antibodies. In our studies, TGF-β prolonged the cell cycle of LTC-ICs from 33.6 hours to 41.9 hours (Table 2, combinations 7 and 9, day 7) demonstrating the proliferation inhibitory effect of this modulator.
The length of cell cycle of HSC has been debated for a long time. Several in vivo murine studies established that stem cells cycle continuously, albeit at a very slow rate.60,61 Other studies on stem cell proliferation kinetics suggest that following a period of quiescence, HPCs divide in vitro in a synchronous manner and undergo cell cycle-related and reversible changes.62,63 We previously demonstrated that human HPCs cultured in vitro display a period of mitotic quiescence.19 A similar outcome was observed in the present studies whereby the first division took longer than subsequent divisions as reflected by the length of cell cycle calculated on days 3, 5, and 7. Interestingly, the length of cell cycle on day 5 and day 7 was similar within every cytokine combination suggesting synchrony.62,63 In response to combination 3, the length of cell cycle was very similar to the estimated 72-hour cycle of murine HSCs reported by Cheshire et al.61 Studies by Boezeman et al23 demonstrated that initiation of growth of single human BM primitive progenitor cells was delayed 2.6 to 3.1 days when cultured in the presence of SCF, IL-3, IL-6, granulocyte colony-stimulating factor (G-CSF), GM-CSF, and Epo. Under the influence of similar, but not identical cytokine combinations in our present studies (combination 6 or 7 in Table 2), the length of cell cycle on day 3 was 2.6 and 2.5 days, respectively (Table 2). These values verify that calculations made in our study for the length of cell cycle take into account any lag period observed with cultured cells by including this time into the length of the first cell cycle.
Transplantation studies of human BM CD34+ cells expanded in vitro with selected cytokine combinations demonstrated that observations made with LTC-ICs may be directly translated to long-term repopulating cells. The fact that combination 8 maintained LTC-IC function better than it supported long-term engrafting cells suggests that within G0CD34+CD38–/lo cells, LTC-IC and long-term reconstituting cells overlap but are not identical. However, our studies suggest that examination of LTC-IC function may be an informative preliminary in vitro assay that may prove useful in designing more complicated and costly in vivo studies.
Prepublished online as Blood First Edition Paper, December 21, 2004; DOI 10.1182/blood-2004-05-1773.
Supported by National Institutes of Health grant R01 HL55716 (E.F.S.) and in part by National Cancer Institute grant P30 CA082709. Herman B Wells Center for Pediatric Research is a Core Center of Excellence in Molecular Hematology (National Institute of Diabetes and Digestive and Kidney Disease P30 DK49218).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr Mervin Yoder for his critical review of this manuscript and the personnel of the Flow Cytometry Resource Facility for their professional assistance in sorting cells for these studies.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal