Abstract
In this study, we investigated the in vitro and in vivo efficacy of patupilone (epothilone B, EPO906), a novel nontaxane microtubule stabilizing agent, in treatment of multiple myeloma (MM). Patupilone directly inhibited growth and survival of MM cells, including those resistant to conventional chemotherapies, such as the taxane paclitaxel. Patupilone induced G2M arrest of MM cells, with subsequent apoptosis. Interleukin-6 (IL-6) and insulin-like growth factor-1 (IGF-1), 2 known growth and survival factors for MM, did not protect MM.1S cells against patupilone-induced cell death. Proliferation of MM cells induced by adherence to bone marrow stromal cells (BMSCs) was also inhibited by patupilone and was paralleled by down-regulation of vascular endothelial growth factor (VEGF) secretion. Importantly, stimulation of cells from patients with MM, either with IL-6 or by adherence to BMSCs, enhanced the anti-proliferative and proapoptotic effects of patupilone. Moreover, patupilone was effective against MM cell lines that overexpress the MDR1/P-glycoprotein multidrug efflux pump. In addition, patupilone was effective in slowing tumor growth and prolonging median survival of mice that received orthotopical transplants with MM tumor cells. Taken together, these preclinical findings suggest that patupilone may be a safe and effective drug in the treatment of MM, providing the framework for clinical studies to improve patient outcome in MM. (Blood. 2005;105:350-357)
Introduction
Cytotoxic chemotherapy remains the standard therapy for multiple myeloma (MM). Typically, regimens include alkylating agents, corticosteroids, and anthracyclines. In eligible patients, high-dose chemotherapy with autologous stem cell transplantation can improve survival of patients with MM.1,2 However, none of these strategies are curative. Thalidomide and its immunomodulatory derivatives (IMiDs),3 the proteasome inhibitor bortezomib,4 and arsenic trioxide5-7 directly induce apoptosis or G1 growth arrest in drug-resistant MM cell lines and patient MM cells. Inhibitors of vascular endothelial growth factor (VEGF) signaling, such as PTK787 and GW654652 also block proliferation of drug-resistant MM cells.8,9 Importantly, these drugs target not only the MM cell, but also the bone marrow (BM) microenvironment, and overcome drug resistance both in vitro and in clinical studies. In addition, these drugs inhibit the transcription, secretion, and actions of cytokines such as interleukin 6 (IL-6), VEGF, and tumor necrosis factor α (TNFα), thereby blocking MM cell growth, adhesion, survival, drug resistance, and migration in the BM microenvironment.10
Patupilone (EPO906, epothilone B) belongs to a new class of natural product drugs with potential therapeutic benefits in multiple indications.11-15 Patupilone is one of several macrocyclic polyketides produced by myxobacterium Sorangium cellulosum12 that have biochemical properties similar to the taxane family of cytotoxic agents. Thus, like paclitaxel (Taxol) and docetaxel (Taxotere), patupilone promotes the formation of stable microtubule polymers from tubulin heterodimer subunits, competing with paclitaxel for binding to microtubules, suggesting that the binding sites for paclitaxel and patupilone on the β-tubulin subunit are overlapping.11,16 Patupilone is more potent than epothilone A and paclitaxel, both in terms of tubulin polymerization as well as cell growth inhibition.11,13-18 Interestingly, although the in vitro Ki for patupilone binding to microtubules is 0.71 μM,16 concentrations as low as 0.13 to 2.92 nM inhibit cancer cell proliferation by 50%,11,13-18 most likely because of high intracellular accumulation of patupilone.18 In contrast, resting human peripheral blood lymphocytes (HPBLs) were not affected by patupilone at concentrations as high as 1 μM. Patupilone has also been shown to produce significant responses in xenograft mouse models of human breast, epidermoid, thyroid, and ovarian carcinomas, as well as leukemias, including paclitaxel-resistant tumors.13,18-21
Although paclitaxel has been effective against various malignancies, several characteristics have limited its utility. Paclitaxel's poor aqueous solubility has necessitated the use of polyethoxylated castor oil (Cremophor) as a delivery vehicle, which can affect cardiac function and cause hypersensitivity responses.22 Epothilones in contrast, are more water soluble and, therefore, can be formulated in the absence of polyethoxylated castor oil.12-15 Furthermore, paclitaxel is a substrate for the MDR1/Pgp (P-glycoprotein) drug efflux pump overexpressed in various multidrug-resistant cancer cells.23,24 MDR1/Pgp expression has been detected in up to 41% of MM patient samples.25-27 In contrast to paclitaxel, epothilones in general are equally cytotoxic against paclitaxel-sensitive and paclitaxel-resistant human cancer cell lines, including those overexpressing MDR1/Pgp.11,13-16,28,29 In addition, although point mutations in the major expressed β-tubulin isoform result in a 24-fold increase in resistance to paclitaxel, these same mutations result in only a 1.3- to 4-fold cross-resistance to patupilone.28 To date, patupilone has been evaluated in 2 phase 1 studies, and several phase 2 clinical trials are ongoing.14,15 Whereas paclitaxel shows endotoxin-like activities associated with side effects such as myalgia, muscle weakness, and arthralgia, patupilone does not.30,31 The most common adverse side effects associated with patupilone treatment are diarrhea, fatigue, nausea, and sensory neuropathies.15
In this study, we demonstrate that patupilone can inhibit proliferation and induce apoptosis in MM cell lines and patient cells, including those resistant to conventional therapies, at concentrations that are minimally toxic to peripheral blood mononuclear cells (PBMCs) and bone marrow stromal cells (BMSCs). Patupilone-mediated MM cell cytotoxicity is associated with a G2M cell cycle arrest, which is followed by apoptosis induction. IL-6 or insulin-like growth factor-1 (IGF-1), known proliferation and survival factors for MM cells, do not protect against patupilone, but rather augment anti-MM activity. Importantly, patupilone inhibits BMSC adhesion-induced MM cell proliferation, which interestingly is paralleled by a reduction in secreted VEGF levels. In addition, patupilone prolongs survival of mice that received orthotopical transplants with human MM tumor cells, with minimal side effects. Taken together, these studies provide the framework for future clinical studies of patupilone to improve patient outcome in MM.
Materials and methods
MM-derived cell lines and patient cells
RPMI 8226 and U266 human MM cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Dexamethasone (Dex)-sensitive MM.1S and Dex-resistant MM.1R human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). RPMI 8226 human MM cell line resistant to doxorubicin (Dox40), melphalan (LR5), and mitoxantrone (MR20) were kindly provided by Dr William Dalton (Moffat Cancer Center, Tampa, FL). All human MM cell lines were cultured in RPMI-1640 media (Sigma Chemical, St Louis MO), containing 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine (L-glut; GIBCO, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin (P/S; GIBCO). Patient MM cells, purified from BM samples (as previously described) were 95% or more CD138+, CD45RA-. PBMCs were obtained from healthy donors by subjecting peripheral blood to standard Ficoll-Hypaque (Pharmacia-Amersham, Uppsala, Sweden) gradient separation. PBMCs were stimulated with 5 μg/mL phytohemagglutinin (PHA; Sigma) and 500 U/mL IL-2 (Sigma) for 48 hours, in the presence or absence of patupilone. Expression of MDR1 protein in cell lines was determined by flow cytometry using anti-CD243 (MDR1)-phycoerythrin (Immunotech, Miami, FL).
Bone marrow stromal cells (BMSCs)
BMSCs were prepared from BM aspirates of patents with MM as well as healthy donors as previously described.8 Cells were cultured in Iscoves modified Dulbecco media containing 20% FBS, 2 mM L-glutamine, and 100 μg/mL penicillin/streptomycin.
Reagents
Patupilone (EPO906, epothilone B) was provided by Novartis Pharma AG (Basel, Switzerland). Paclitaxel was supplied by Calbiochem (La Jolla, CA). The compounds were dissolved in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) and stored as 10 mM and 100 mM stock solutions, respectively, at -20°C until use. For all in vitro assays, the compound was diluted in culture medium to concentrations ranging from 0.001 to 10 000 nM. For in vivo mouse model experiments, patupilone was dissolved in PEG-300 (Fluka, St Gallen, Switzerland) as a stock solution and diluted to a 30% concentration with normal saline at the time of injection.
Proliferation and cell viability assays
MM cells were cultured in RPMI-1640 media containing 10% FBS and then plated into 96-well microtiter plates (Costar, Cambridge, MA), in the presence of drug or DMSO control. Proliferation was measured by incorporation of [3H]-deoxythymidine (dT; NEN Products, Boston, MA). Specifically, cells were pulsed with [3H]-dT (0.5 μCi/well [0.00185 MBq]) for the last 6 hours of 48-hour cultures, harvested onto glass filters with an automatic cell harvester (Cambridge Technology, Cambridge, MA), and counted using an LKB Betaplate scintillation counter (Wallac, Gaithersburg, MD). Measurement of cell viability was performed colorimetrically by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt (MTS) assay, using the CellTiter96 AQueous One Solution Reagent (Promega, Madison, WI). Cells were exposed to MTS for the last 2 hours of 48-hour cultures, and absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices, Sunnyvale, CA) at optical density (OD) of 490 nm.
Cell cycle and flow cytometry analysis
MM cells (1 × 106 cells) were cultured in the presence of patupilone or DMSO control for 24, 48, and 72 hours. Cells were then washed with phosphate-buffered saline (PBS), fixed with 70% ethanol, and treated with RNAse (Sigma). Cells were next stained with propidium iodide (PI; 5 μg/mL), and the cell cycle profile was determined using M software on an Epics flow cytometer (Coulter Immunology, Hialeah, FL).
Western blotting
Protein lysates from drug-treated and control MM cells were prepared using RIPA buffer in the presence of a protease inhibitor cocktail (Roche, Basel, Switzerland), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM sodium orthovanadate. Lysates were either analyzed directly on sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel or transferred onto Hybond C Super paper (Amersham, Arlington Heights, IL) and subsequently probed with a murine monoclonal antibody (MoAb) against bcl-2 (Santa Cruz, Santa Cruz, CA), bax (Santa Cruz), or poly(adenosine diphosphate-ribose) polymerase (PARP; Biomol, West Grove, PA); a rabbit polyclonal Ab against caspase 3 (Santa Cruz); or a goat polyclonal Ab against actin. Detection was using a horseradish peroxidase (HRP)-conjugated anti-murine or anti-goat Ab (both from Santa Cruz) and enhanced chemiluminescence (ECL) substrate solution (Amersham).
Proliferation of MM cells in an adhesion system
BMSCs (1 × 104 cells/well) were plated into 96-well microtiter plates and incubated at 37°C for 24 hours in Iscoves media (20% FBS). MM cells were then added to the BMSC-containing wells (5 × 104 cells/well), in the presence of drug or DMSO control. When MM.1S cells were used, both BMSCs and MM cells were starved for 12 hours in RPMI-1640 media containing 2% FBS. When patient MM cells were used, cocultures were performed in RPMI media containing 10% FBS. BMSCs and MM cells were also cultured separately as controls. After 48 hours, proliferation and cell viability were analyzed as described in “Proliferation and cell viability assays.” To ensure that all cells were collected for the proliferation assay, 10 × Trypsin (Sigma) was added to each well 10 minutes prior to harvesting.
Measurement of cytokine concentrations
Cytokine levels were measured in supernatants from the coculture system described in “Proliferation of MM cells in an adhesion system.” VEGF and IL-6 concentrations were measured by using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
Xenograft murine model
In a xenograft MM murine model32-37 mice were inoculated subcutaneously into the right flank with 3 × 107 MM cells in 100 μL RPMI-1640, together with 100 μL Matrigel basement membrane matrix (Becton Dickinson, Bedford, MA). On day +6 after injection, mice were assigned to 2 groups receiving patupilone or control treatment. Treatment with patupilone was given intravenously once weekly via tail vein at 2.5 mg/kg for 4 weeks, starting on day +6 or as a one-time 4 mg/kg dose on day +6. The control group received vehicle alone (30% PEG-300 in 0.9% sodium chloride) weekly. Caliper measurements of the longest perpendicular tumor diameters were performed twice per week to estimate tumor volume, using the following formula representing the 3-dimensional volume of an ellipse: 4/3 × (width/2)2 × (length/2). Animals were killed when their tumor reached 2 cm or when mice became moribund. Survival was evaluated from the first day of tumor injection until death.
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using Student t test. The minimal level of significance was a P value less than .05. Kaplan-Meier survival curves were generated, and log-rank analyses were used to calculate the mean overall mouse survival with 95% confidence intervals. Tumor volumes were analyzed using 1-way analysis of variance and Bonferroni post hoc tests.
Results
Effect of patupilone on patient myeloma cells and peripheral blood mononuclear cells
Patient MM cells were cultured for 24 hours in the presence of 10 nM patupilone or control vehicle and then compared under a light microscope (Figure 1A). Significant morphologic distortion was observed in treated patient cells compared with the untreated cells. Whereas untreated cells appeared as typical round to elliptical MM cells, treated cells were elongated, with abnormal cytoplasmic processes. Proliferation of myeloma cells from 3 patients with MM was next measured by [3H]-dT uptake after initial 48-hour cultures with IL-6 and patupilone. Fifty percent inhibition of proliferation was observed between 10 and 100 nM patupilone (P < .01) (Figure 1B, left). Patupilone also inhibited the growth of patient MM cells adherent to BMSCs (Figure 1B, right). In contrast, unactivated peripheral blood mononuclear cells from healthy donors were not affected by patupilone (Figure 1C). However, when PBMCs were stimulated using PHA and IL-2, a significant decrease in cell survival was observed with 100 nM patupilone (P = .01), consistent with the common understanding that cells need to proliferate to advance through mitosis, the cell cycle phase sensitive to microtubule-targeting drugs because of the essential role of microtubules in spindle formation and cell division.
Effect of patupilone on patient MM cells and peripheral blood mononuclear cells. (A) Patient cells treated for 24 hours in the presence of 10 nM patupilone were compared with untreated controls under a light microscope. MM.1S cells in RPMI media were examined under an Olympus BX60 inverted microscope (Olympus, Lake Success, NY) equipped with Hoffman objective lenses (X10/X20/X40; Diagnostic Instruments, Sterling Heights, MI) connected to a SPOT One Digital Camera (Diagnostic Instruments). Images were then processed using Adobe Photoshop software (Adobe, San Jose, CA) (B, left) Proliferation of tumor cells from 3 different patients with MM was measured by 3H-[dT] uptake 48 hours after initial exposure to IL-6 and patupilone. (Right) Patupilone inhibits growth of patient MM cells adherent to BMSCs. MM patient BMSCs were cultured with or without patient MM cells and in the presence of 0, 1, or 10 nM patupilone. Proliferation was measured at 48 hours. (C) Peripheral blood mononuclear cells (PBMCs; lower panel) from healthy donors either unstimulated (▴) or stimulated with PHA (▪), were treated with patupilone. Survival was determined using MTS assay. Error bars indicate ± standard deviation of triplicate experiments.
Effect of patupilone on patient MM cells and peripheral blood mononuclear cells. (A) Patient cells treated for 24 hours in the presence of 10 nM patupilone were compared with untreated controls under a light microscope. MM.1S cells in RPMI media were examined under an Olympus BX60 inverted microscope (Olympus, Lake Success, NY) equipped with Hoffman objective lenses (X10/X20/X40; Diagnostic Instruments, Sterling Heights, MI) connected to a SPOT One Digital Camera (Diagnostic Instruments). Images were then processed using Adobe Photoshop software (Adobe, San Jose, CA) (B, left) Proliferation of tumor cells from 3 different patients with MM was measured by 3H-[dT] uptake 48 hours after initial exposure to IL-6 and patupilone. (Right) Patupilone inhibits growth of patient MM cells adherent to BMSCs. MM patient BMSCs were cultured with or without patient MM cells and in the presence of 0, 1, or 10 nM patupilone. Proliferation was measured at 48 hours. (C) Peripheral blood mononuclear cells (PBMCs; lower panel) from healthy donors either unstimulated (▴) or stimulated with PHA (▪), were treated with patupilone. Survival was determined using MTS assay. Error bars indicate ± standard deviation of triplicate experiments.
Patupilone directly inhibits proliferation of MM cell lines
We next evaluated the direct effect of patupilone on proliferation and survival of MM cell lines. As shown in Figure 2A, patupilone in a dose-dependent manner decreased the survival of RPMI 8226, U266, Dex-sensitive MM.1S, Dex-resistant MM.1R cells; and doxorubicin-, melphalan-, and mitoxantrone-resistant RPMI 8226 Dox40, LR5, and MR20 cells, respectively (P < .01; Figure 2B). The IC50 for all MM cell lines after 72-hour incubation was between 1 and 10 nM. In contrast to patupilone, paclitaxel was not as effective in killing the tested MM cell lines. In particular, Dox-resistant RPMI cells, which overexpress the MDR1/Pgp efflux pump (as shown in Figure 2C), were resistant to paclitaxel even at high doses.
Patupilone reduces survival of MM cell lines either sensitive or resistant to conventional chemotherapy, including multidrug-resistant cells overexpressing MDR1. (A) Cell lines MM.1S (⋄), U266 (▵), and RPMI 8226 (□) were treated for 48 hours with 0, 1, 10, 100, 1000, or 10 000 nM patupilone. IC50 (inhibitory concentration 50%) was less than 10 nM for patupilone, as determined by MTS assay. (B) Patupilone reduces survival of multidrug-resistant cell lines, including cell lines that overexpress the MDR1/Pgp drug efflux pump. RPMI 8226-derived cell lines resistant to MR20, LR5, and Dox40 were treated for 48 hours with 0, 0.01, 0.1, 1, and 10 nM patupilone (left) or 0, 1, 10, 100, 1000, or 10 000 nM paclitaxel (right). Dox40 is shown by ♦; LR5, ▴; and MR20 ▪. Survival was determined by MTS assay. For patupilone, IC50 of Dox40 was less than 10 nM, whereas for paclitaxel, IC50 was not achieved even at 10 000 nM. The IC50s of LR5 and MR20 were 10 nM for patupilone and 100 nM for paclitaxel. (C) Flow cytometry demonstrates expression of MDR1 protein on RPMI 8226 as well as RPMI 8226-derived cell lines resistant to MR20, LR5, and Dox 40. Compared with parental RPMI 8226, almost a 2-log increase in MDR1 protein expression was observed in RPMI 8226-Dox40 cells. White histograms indicate isotype control; black histograms, anti-CD243 (MDR-1)-PE.
Patupilone reduces survival of MM cell lines either sensitive or resistant to conventional chemotherapy, including multidrug-resistant cells overexpressing MDR1. (A) Cell lines MM.1S (⋄), U266 (▵), and RPMI 8226 (□) were treated for 48 hours with 0, 1, 10, 100, 1000, or 10 000 nM patupilone. IC50 (inhibitory concentration 50%) was less than 10 nM for patupilone, as determined by MTS assay. (B) Patupilone reduces survival of multidrug-resistant cell lines, including cell lines that overexpress the MDR1/Pgp drug efflux pump. RPMI 8226-derived cell lines resistant to MR20, LR5, and Dox40 were treated for 48 hours with 0, 0.01, 0.1, 1, and 10 nM patupilone (left) or 0, 1, 10, 100, 1000, or 10 000 nM paclitaxel (right). Dox40 is shown by ♦; LR5, ▴; and MR20 ▪. Survival was determined by MTS assay. For patupilone, IC50 of Dox40 was less than 10 nM, whereas for paclitaxel, IC50 was not achieved even at 10 000 nM. The IC50s of LR5 and MR20 were 10 nM for patupilone and 100 nM for paclitaxel. (C) Flow cytometry demonstrates expression of MDR1 protein on RPMI 8226 as well as RPMI 8226-derived cell lines resistant to MR20, LR5, and Dox 40. Compared with parental RPMI 8226, almost a 2-log increase in MDR1 protein expression was observed in RPMI 8226-Dox40 cells. White histograms indicate isotype control; black histograms, anti-CD243 (MDR-1)-PE.
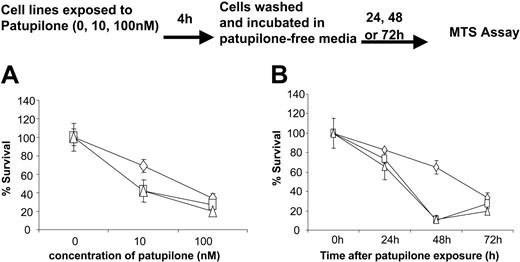
Effect of short-term exposure to patupilone
It has been reported that the in vitro sensitivity of colon carcinoma cells to patupilone was only minimally affected by drug exposure time, possibly because of intracellular accumulation and retention of the drug.13,18 To investigate whether MM cells are affected by short-term exposure to patupilone, MM cells were exposed to 10 nM or 100 nM patupilone for 4 hours and then incubated in patupilone-free medium for up to 72 hours. As can be seen in Figure 3A, patupilone retained activity even upon short-term drug exposure. The effect was most dramatic at 100 nM, but it was also seen at 10 nM (P < .01). Short-term exposure to 100 nM patupilone resulted in decreased tumor cell proliferation and viability as early as 24 hours but with more striking effects at 48 to 72 hours (Figure 3B).
Short-term exposure to patupilone suffices to inhibit survival of MM cell lines. MM.1S (⋄), U266 (□), and RPMI 8226 (▵) cells were exposed for 4 hours to 0, 10, or 100 nM patupilone, then incubated in culture media devoid of patupilone. (A) Cell survival was measured 24 hours after exposure to patupilone. (B) Cell survival was measured in cell lines exposed to 100 nM patupilone for 24, 48, or 72 hours.
Short-term exposure to patupilone suffices to inhibit survival of MM cell lines. MM.1S (⋄), U266 (□), and RPMI 8226 (▵) cells were exposed for 4 hours to 0, 10, or 100 nM patupilone, then incubated in culture media devoid of patupilone. (A) Cell survival was measured 24 hours after exposure to patupilone. (B) Cell survival was measured in cell lines exposed to 100 nM patupilone for 24, 48, or 72 hours.
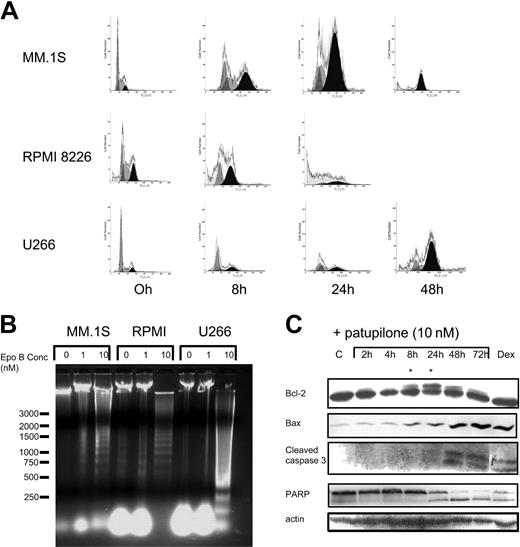
Effects of patupilone on cell cycle and apoptotic signaling
We next examined the cell cycle profile of drug-treated MM cell lines using propidium iodide staining. As can be seen in Figure 4A, addition of 10 nM patupilone induced a shift of cells from G1 to G2M within 24 hours, with a subsequent increase in sub-G1 cells. By 48 hours, remaining viable cells were in G2M phase (Figure 4A). DNA laddering appeared within 48 hours of incubation with 10 nM patupilone (Figure 4B). Previous studies have shown that treatment with patupilone modulates expression of apoptosis-regulating proteins. We, therefore, next examined the protein levels of the antiapoptotic and proapoptotic proteins bcl-2 and bax, respectively, in patupilone-treated MM.1S cells. Incubation of MM.1S cells with 10 nM patupilone for 8 to 24 hours was associated with increased expression of bax as well as decreased expression of bcl-2 and appearance of a bcl-2 form with reduced electrophoretic mobility (Figure 4C). This modification of bcl-2 indicative of posttranslational modification (most likely a mitosis-associated phosphorylation event) has also been observed in MM cells treated with paclitaxel and was associated with drug sensitivity.38 Cleavage of caspase 3 and PARP was observed by 24 hours, consistent with apoptosis.
Patupilone induces G2M arrest of MM cells, followed by apoptosis. (A) MM.1S, RPMI 8226, and U266 cells were incubated in culture medium containing 10 nM patupilone for 0, 8, and 24 hours; cell cycle profile was obtained as described in “Materials and methods.” By 48 hours, remaining viable cells were in G2M phase. Gray histograms indicate G1/S phase; black histograms, G2M phase; and white outline, all phases. (B) Cell lines were exposed to 0, 1, or 10 nM patupilone. DNA laddering, indicative of apoptosis, was observed after 24-hour exposure of MM.1S, RPMI 8226, and U266. (C) Western blot analysis of protein lysates obtained from MM.1S cells treated for 0, 2, 4, 8, 24, 48, and 72 hours with 10 nM patupilone were probed with antibodies to bcl-2, bax, caspase 3, and PARP. Treatment for 8 and 24 hours led to emergence of an extra bcl-2 band (*), consistent with phosphorylated bcl-2. Cleaved forms of caspase 3 and PARP, indicative of caspase activation and apoptosis, respectively, appeared by 24 hours. Dexamethasone-treated cells served as a control for apoptosis, and actin served as a protein loading control.
Patupilone induces G2M arrest of MM cells, followed by apoptosis. (A) MM.1S, RPMI 8226, and U266 cells were incubated in culture medium containing 10 nM patupilone for 0, 8, and 24 hours; cell cycle profile was obtained as described in “Materials and methods.” By 48 hours, remaining viable cells were in G2M phase. Gray histograms indicate G1/S phase; black histograms, G2M phase; and white outline, all phases. (B) Cell lines were exposed to 0, 1, or 10 nM patupilone. DNA laddering, indicative of apoptosis, was observed after 24-hour exposure of MM.1S, RPMI 8226, and U266. (C) Western blot analysis of protein lysates obtained from MM.1S cells treated for 0, 2, 4, 8, 24, 48, and 72 hours with 10 nM patupilone were probed with antibodies to bcl-2, bax, caspase 3, and PARP. Treatment for 8 and 24 hours led to emergence of an extra bcl-2 band (*), consistent with phosphorylated bcl-2. Cleaved forms of caspase 3 and PARP, indicative of caspase activation and apoptosis, respectively, appeared by 24 hours. Dexamethasone-treated cells served as a control for apoptosis, and actin served as a protein loading control.
Effect of patupilone on survival of MM.1S cells in the presence of dexamethasone, IL-6, and IGF-1
Because Dex is a principal treatment for MM, we next determined whether patupilone added to the in vitro effect of Dex on MM cells. As shown in Figure 5, both patupilone and Dex block proliferation of MM.1S cells, and their growth inhibitory effects are additive. Interleukin-6 and IGF-1 are major growth and survival factors for MM cells, which protect against Dex-induced apoptosis. Because patupilone is cytotoxic to proliferating cells, we next examined whether IL-6 or IGF-1 could enhance, rather than protect against, patupilone. MM.1S cells were incubated in the presence or absence of IL-6 (100 ng/mL) or IGF-1 (200 ng/mL) with various concentrations of patupilone. Proliferation and survival were then assessed after 24 hours. As expected, the addition of IL-6 and IGF-1 increased proliferation of MM.1S cells (not shown), and 10 nM patupilone resulted in a consistent decrease in the proliferation rate of the cells, in the presence or absence of IL-6 or IGF-1. However, coincubation of patupilone with IL-6 or IGF-1 resulted in enhanced reduction in the fraction of proliferating MM.1S cells, as determined by thymidine uptake (Figure 6A), with significantly decreased survival by MTS assay seen at 24 hours in IL-6- and IGF-1-treated groups (Figure 6B). There was not a significant difference in patupilone-mediated killing between IL-6- or IGF-1-treated cells.
Effects of dexamethasone in combination with patupilone on MM cell proliferation and survival. MM.1S cells were incubated in the presence of dexamethasone (0, 0.01, 0.1, and 1 μM) and patupilone (0, 0.01, 0.1, 1, and 10 nM) for 48 hours. Proliferation was measured by 3H-[dT] uptake (A), and survival was measured by MTS assay (B) relative to untreated control cells. Patupilone appeared to be responsible for most of the response at high patupilone concentration. Error bars indicate ± standard deviation of triplicate experiments.
Effects of dexamethasone in combination with patupilone on MM cell proliferation and survival. MM.1S cells were incubated in the presence of dexamethasone (0, 0.01, 0.1, and 1 μM) and patupilone (0, 0.01, 0.1, 1, and 10 nM) for 48 hours. Proliferation was measured by 3H-[dT] uptake (A), and survival was measured by MTS assay (B) relative to untreated control cells. Patupilone appeared to be responsible for most of the response at high patupilone concentration. Error bars indicate ± standard deviation of triplicate experiments.
IL-6 and IGF-1 do not protect against patupilone. IL-6 and IGF-1 are growth and survival factors for MM cells. Both inhibit dexamethasone-induced apoptosis. MM.1S cells were incubated for 24 hours in the absence (⋄) or presence of IL-6 (100 ng/mL, ▵), IGF-1 (200 ng/mL, □), or patupilone 0, 0.1, 1, or 10 nM. Proliferation was measured by 3H-[dT] uptake (A), and survival was measured by MTS assay (B). Both assays were measured relative to internal controls without patupilone, in the absence or presence of IL-6 or IGF-1. Error bars indicate ± standard deviation of triplicate experiments.
IL-6 and IGF-1 do not protect against patupilone. IL-6 and IGF-1 are growth and survival factors for MM cells. Both inhibit dexamethasone-induced apoptosis. MM.1S cells were incubated for 24 hours in the absence (⋄) or presence of IL-6 (100 ng/mL, ▵), IGF-1 (200 ng/mL, □), or patupilone 0, 0.1, 1, or 10 nM. Proliferation was measured by 3H-[dT] uptake (A), and survival was measured by MTS assay (B). Both assays were measured relative to internal controls without patupilone, in the absence or presence of IL-6 or IGF-1. Error bars indicate ± standard deviation of triplicate experiments.
Effect of patupilone on the proliferation of MM.1S cells cultured with BMSCs
We next investigated the effect of patupilone on MM cell growth in the bone marrow microenvironment. As shown in Figure 7A, coculture of MM.1S cells with BMSCs significantly increased the proliferation rate of the tumor cells. Importantly, patupilone (10 nM) significantly decreased the proliferation of MM.1S cells adherent to BMSCs. These results are consistent with the results observed using patient MM cells, as described (Figure 1B). Our recent studies have established the importance of MM cell adhesion to BMSCs in promoting tumor growth and survival, through both direct physical interaction and via cytokines. Specifically, adherence of MM cells to BMSCs triggers IL-6 and VEGF secretion by BMSCs. As seen in Figure 7B, patupilone inhibited tumor cell adhesion-induced up-regulation of VEGF secretion. IL-6 secretion was also induced in cultures of MM cells and BMSCs (Figure 7C); however, 10 nM patupilone did not abrogate this increase, despite decreasing adhesion-induced proliferation.
Patupilone inhibits proliferation of MM cells adherent to BMSCs. MM patient BMSCs were cultured with or without MM.1S cells in the presence of 0, 1, or 10 nM patupilone. Subsequent proliferation at 48 hours was measured by 3H-[dT] uptake (A). Supernatants from the coculture of BMSCs with MM cells were analyzed by ELISA for VEGF (B) and IL-6 (C), 2 important factors for MM cell migration and proliferation.
Patupilone inhibits proliferation of MM cells adherent to BMSCs. MM patient BMSCs were cultured with or without MM.1S cells in the presence of 0, 1, or 10 nM patupilone. Subsequent proliferation at 48 hours was measured by 3H-[dT] uptake (A). Supernatants from the coculture of BMSCs with MM cells were analyzed by ELISA for VEGF (B) and IL-6 (C), 2 important factors for MM cell migration and proliferation.
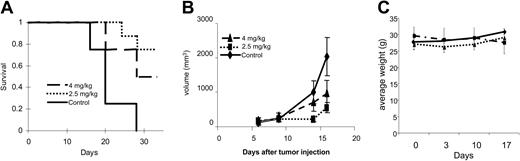
Patupilone prolongs survival in vivo in a plasmacytoma mouse model
We next tested safety and efficacy of patupilone treatment in vivo in a plasmacytoma mouse model.32-37 Twenty-four beige-nude-xid mice were injected subcutaneously with RPMI 8226 cells in their flanks; 6 days after injection, they were randomly separated into 3 groups: 1 group was injected intravenously with a 1-time dose of patupilone at 4 mg/kg on day +6 following tumor implantation; a second group received 4 weekly intravenous doses of patupilone at 2.5 mg/kg on days 6, 13, 20, and 27; and a third group injected with vehicle (30% PEG-300) served as a control. Tumor measurements were performed twice per week, and mice were killed when the longest tumor dimension reached 2 cm. Survival was assessed using Kaplan-Meier curves. As shown in Figure 8A, the mean overall survival (OS) was 21 days (95% confidence interval [CI], 18-24 days) in the control cohort versus 26 days (95% CI, 23-29 days) and 28 days (95% CI, 26-29 days) in groups treated with patupilone 2.5 and 4 mg/kg, respectively. There was no statistically significant difference in mean OS of mice treated with 4 mg/kg versus 2.5 mg/kg (P = .24). In contrast, a statistically significant prolongation in mean OS compared with control mice was observed in animals treated with 2.5 mg/kg (P = .011) and 4 mg/kg (P = .001) patupilone. In the experiments assessing OS, all control mice were killed by day +27 after tumor injection, whereas 75% of mice injected with 4 mg/kg 1-time dose and 50% of mice injected with 2.5 mg/kg weekly dose remained alive at that time point.
Patupilone extends survival and inhibits tumor growth in an MM xenograft mouse model. RPMI 8226 cells were injected into the flanks of 24 beige-xid nude mice. Six days after tumor cell injection, mice were randomly separated into 1 of 3 treatment groups: control vehicle only (long dashed line), 1 dose of patupilone at 4 mg/kg intravenously (long and short dashed line), or 4 weekly doses of patupilone at 2.5 mg/kg intravenously (short dashed line). Tumor measurements were performed twice weekly, and tumor volumes were calculated. When tumors reached 2 cm in the longest diameter, mice were killed. (A) Kaplan-Meier survival curves of the mice in each group were plotted relative to the number of days after tumor injection. (B) Mean tumor volumes for mice in each group were calculated until day 16, the day when the first mice were killed. Control (♦), patupilone at 4 mg/kg intravenously (▴), or 4 weekly doses of patupilone at 2.5 mg/kg intravenously (▪). (C) Mice were weighed once per week, and average weight was calculated.
Patupilone extends survival and inhibits tumor growth in an MM xenograft mouse model. RPMI 8226 cells were injected into the flanks of 24 beige-xid nude mice. Six days after tumor cell injection, mice were randomly separated into 1 of 3 treatment groups: control vehicle only (long dashed line), 1 dose of patupilone at 4 mg/kg intravenously (long and short dashed line), or 4 weekly doses of patupilone at 2.5 mg/kg intravenously (short dashed line). Tumor measurements were performed twice weekly, and tumor volumes were calculated. When tumors reached 2 cm in the longest diameter, mice were killed. (A) Kaplan-Meier survival curves of the mice in each group were plotted relative to the number of days after tumor injection. (B) Mean tumor volumes for mice in each group were calculated until day 16, the day when the first mice were killed. Control (♦), patupilone at 4 mg/kg intravenously (▴), or 4 weekly doses of patupilone at 2.5 mg/kg intravenously (▪). (C) Mice were weighed once per week, and average weight was calculated.
As can be seen in Figure 8B, tumor growth was significantly delayed in both patupilone-treated groups versus controls. Comparisons of tumor volumes on day 16 following tumor implantation showed statistically significant differences across treatment groups (P < .001, 1-way analysis of variance), and Bonferroni post hoc tests revealed significantly lower tumor volumes in the patupilone 4 mg/kg group versus the control group (P < .001), as well as in the patupilone 2.5 mg/kg group versus the control group (P = .001). In contrast, there was no significant difference between the 2 patupilone treatment groups (P = .328). Two mice in the weekly treatment group died suddenly (on day +34 and day +38), with significant weight loss prior to death. None of the mice in the 4 mg/kg 1-time treatment group died unexpectedly.
To look for specific toxicities related to patupilone, we monitored body weight development (Figure 8C) and performed complete blood counts for all mice on day 17 following tumor inoculation (Table 1). Mice in the 2.5 mg/kg weekly treatment group, but not the 4 mg/kg single administration cohort, showed significant weight loss by day +17. There were no appreciable differences in white blood cell count, hemoglobin concentration, platelet count, or absolute neutrophil counts among the 3 groups. In addition, there were no significant differences in the gross histology of liver, gut, kidney, or bone marrow in treated versus control groups (data not shown).
Patupilone's effect on survival and tumor growth in an MM xenograft mouse model
. | No. . | White blood cell count, × 109/L . | Hematocrit . | Platelet count × 109/L . | Absolute neutrophil count, × 109/L . |
|---|---|---|---|---|---|
| Control | 8 | 5.1 | 36.2 | 1080 | 2.7 |
| Patupilone, 2.5 mg/kg per wk, 4 doses | 8 | 5.5 | 38.3 | 1023 | 3.8 |
| Patupilone, 4 mg/kg, 1 dose | 8 | 6.3 | 37.4 | 1282 | 2.3 |
. | No. . | White blood cell count, × 109/L . | Hematocrit . | Platelet count × 109/L . | Absolute neutrophil count, × 109/L . |
|---|---|---|---|---|---|
| Control | 8 | 5.1 | 36.2 | 1080 | 2.7 |
| Patupilone, 2.5 mg/kg per wk, 4 doses | 8 | 5.5 | 38.3 | 1023 | 3.8 |
| Patupilone, 4 mg/kg, 1 dose | 8 | 6.3 | 37.4 | 1282 | 2.3 |
Blood counts were measured when mice were killed. No significant difference was observed between treated mice and controls.
Discussion
Epothilones and paclitaxel, despite having unrelated chemical structures, promote tubulin polymerization and suppress microtubule depolymerization, thereby interfering with microtubule dynamics, a microtubule function of particular importance during mitosis.39-41 The use of the microtubule-stabilizing drugs paclitaxel and docetaxel has been increasing in recent years, due in part to their efficacy in solid tumors. However, the use of paclitaxel and docetaxel in the treatment of MM has had limited success. In 1998, the Eastern Cooperative Oncology Group (ECOG) reported that initial therapy with paclitaxel administered at a dose of 250 mg/m2 by continuous intravenous infusion for 24 hours every 21 days for 4 cycles, resulted in 4 (29%) objective responses in 14 patients with newly diagnosed MM; however, there were no complete responses and 3 lethal toxicities were observed.42 Although the median survival of all eligible patients was 2.8 years, comparable with median survival observed after melphalan and prednisone treatment, the toxicity of paclitaxel as used in this study was excessive. Clinical activity in MM was nevertheless demonstrated, a concept supported by the use of paclitaxel in the combination d-TEC (dexamethasone, paclitaxel, etoposide, cyclophosphamide), which was successfully used in MM for stem cell mobilization.43 In 2003, ECOG reported a clinical study of docetaxel given at 75 mg/m2 intravenously over 1 hour every 3 weeks in patients with relapsing or refractory MM, who had received no more than 2 prior combination chemotherapy regimens. Eighty percent of patients developed grade 3 to 4 granulocytopenia, 23% experienced grade 3 to 4 thrombocytopenia, and the median survival was 9.9 months.44
The limited efficacy of paclitaxel in MM may partially reflect the fact that it serves as a substrate for the MDR1/Pgp drug efflux pump. Although patients with MM at presentation have a low percentage of plasma cells that express MDR1, this percentage increases to 50% of patients after treatment with chemotherapy.25-27 These findings suggest that microtubule-stabilizing agents could be an effective treatment for MM if MDR1/Pgp-mediated multidrug resistance can be overcome. In this study, we demonstrate potent cytotoxic activity of the novel, non-taxane microtubule-stabilizing agent patupilone, against MM cells. Myeloma cell lines with a high proliferative rate were most sensitive to patupilone. Importantly, in agreement with similar observations reported for other preclinical cancer models, we show that patupilone is effective against paclitaxel-resistant MM cells that over-express the MDR1/Pgp drug efflux pump. Moreover, the retention of efficacy we observed even following short drug-exposure times may be related to the ability of patupilone to achieve and retain high intracellular concentrations18 that might be due to the higher affinity of patupilone for microtubules compared with paclitaxel.16
At nanomolar concentrations of patupilone we observed morphologic changes and inhibition of proliferation in MM cells, even in tumor cells bound to BMSCs. IL-6 secretion induced by binding of MM cells to BMSCs remained high in the coculture system in the presence of patupilone, and, in fact, IL-6-treated MM cells may be even more sensitive to patupilone. In contrast, patupilone led to a reduction in the levels of secreted VEGF in the coculture system. It is currently unclear whether this is an epigenetic phenomenon or due to a decrease in MM cells.
There was no toxicity against normal PBMCs, but some toxicity was observed against PHA-stimulated PBMCs, further supporting the view that patupilone is selectively cytotoxic against proliferating cells. Within 8 hours of exposure, G2M arrest was observed, accompanied by up-regulation of bax and the appearance of a mobility-shifted form of bcl-2 indicative of posttranslational modification, most likely phosphorylation. These events were followed by cleavage of caspase 3 and PARP, signs of engagement of the apoptotic execution machinery. Importantly, patupilone was well tolerated and very effective in a murine MM model, evidenced by significant inhibition of MM tumor growth in mice treated with either 1 dose of patupilone at 4 mg/kg intravenously, or 4 weekly doses of patupilone at 2.5 mg/kg intravenously. These data suggest that either a weekly treatment or less frequent treatment with higher doses of patupilone may be effective in MM.
In summary, we have shown that patupilone acts both directly on MM cells as well as in the BM milieu to inhibit MM cell growth and survival, overcoming cancer cell-intrinsic as well as cell-interaction mediated mechanisms of drug resistance. These observations, coupled with its lack of major toxicity in preclinical mouse models, provide the framework for clinical studies with patupilone directed at improving patient outcome in MM.
Prepublished online as Blood First Edition Paper, September 14, 2004; DOI 10.1182/blood-2004-06-2499.
Supported by grants from the National Institutes of Health (grants RO-1 50947 and PO-1 78378), as well as the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
One of the authors (M.W.) is employed by a company (Novartis Pharma AG) whose compound patupilone was studied in the present work.
B.L. and L.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Effect of patupilone on patient MM cells and peripheral blood mononuclear cells. (A) Patient cells treated for 24 hours in the presence of 10 nM patupilone were compared with untreated controls under a light microscope. MM.1S cells in RPMI media were examined under an Olympus BX60 inverted microscope (Olympus, Lake Success, NY) equipped with Hoffman objective lenses (X10/X20/X40; Diagnostic Instruments, Sterling Heights, MI) connected to a SPOT One Digital Camera (Diagnostic Instruments). Images were then processed using Adobe Photoshop software (Adobe, San Jose, CA) (B, left) Proliferation of tumor cells from 3 different patients with MM was measured by 3H-[dT] uptake 48 hours after initial exposure to IL-6 and patupilone. (Right) Patupilone inhibits growth of patient MM cells adherent to BMSCs. MM patient BMSCs were cultured with or without patient MM cells and in the presence of 0, 1, or 10 nM patupilone. Proliferation was measured at 48 hours. (C) Peripheral blood mononuclear cells (PBMCs; lower panel) from healthy donors either unstimulated (▴) or stimulated with PHA (▪), were treated with patupilone. Survival was determined using MTS assay. Error bars indicate ± standard deviation of triplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2499/6/m_zh80010571480001.jpeg?Expires=1767900297&Signature=CHQxabWie59Lb1MyO2KeRsbgKcq6R9710~YNA22i0unuAwCDD2eU-0Xp~H~xI~k7lfTQhTV~vH0sXgC~~f4Ia45ibYxN05TEH2NOE2q5PvfCl1Hw0TCqQueHL-TyYlYDSI7kLse2TE9MZZF6DmXJzKJJwjt1f43ourzf~gDsCZWl~XRR9jXY6DxMSRWFBrePwitpJD64Q~vzYzpcpe4HzTOCac9ElX9zHJP2bl7M-DrWegP9606nE6-drHMo9IR6lpW0RdevWNxXPfT~TYy9ptWOa~5ERLkbfkg7JqSmoHRUa-k0dw6FsYqZ9vkOUe9JA~DEY-7ATwkl3YvrmqW6MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Effects of dexamethasone in combination with patupilone on MM cell proliferation and survival. MM.1S cells were incubated in the presence of dexamethasone (0, 0.01, 0.1, and 1 μM) and patupilone (0, 0.01, 0.1, 1, and 10 nM) for 48 hours. Proliferation was measured by 3H-[dT] uptake (A), and survival was measured by MTS assay (B) relative to untreated control cells. Patupilone appeared to be responsible for most of the response at high patupilone concentration. Error bars indicate ± standard deviation of triplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2499/6/m_zh80010571480005.jpeg?Expires=1767900297&Signature=xWR7MuiD2ku5LvwkPKYbz0CyjOx23-5ityJEULtF3tMZqRcAnc3a5XQm263JGAiLhXcGQ2FxyMQPQ1DleItsP4-6XHeZuCN3oTdX8mjzECtiU3zb9rYhFLAGOvR9DlyMl~A8cagcpjrjzDySzwZ77obKHOInsxcfb178ZBgK3xlLs8pRJ5ww14nZM8vRLx-iD5txfULY00KgXvtm8rRmI61HpyFbb3wOsQeFkIqJQRK-nBytktVmcF2JVnC55rZn44o0cY4KDvX7ljop0-7R3Et0atNsfh1R-dins10dXLyB8wEEzJar5qa53lI5r0SL8eeljVO1CDmOTsRYaVLgNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. IL-6 and IGF-1 do not protect against patupilone. IL-6 and IGF-1 are growth and survival factors for MM cells. Both inhibit dexamethasone-induced apoptosis. MM.1S cells were incubated for 24 hours in the absence (⋄) or presence of IL-6 (100 ng/mL, ▵), IGF-1 (200 ng/mL, □), or patupilone 0, 0.1, 1, or 10 nM. Proliferation was measured by 3H-[dT] uptake (A), and survival was measured by MTS assay (B). Both assays were measured relative to internal controls without patupilone, in the absence or presence of IL-6 or IGF-1. Error bars indicate ± standard deviation of triplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2499/6/m_zh80010571480006.jpeg?Expires=1767900297&Signature=fJRM04bC-ds9kqA6CNIcVbkpbqIomxcGGogoEd9dyeYre-4Q2jwQ72GGxtB3dyZuSQ8i2m~mVWtH9VaR75RMrpHmZQtU0bDzK71lCcc7qu2oeG2i7lQOG1YNsng-a02qt-pHDJDDnBJVSa12MJ3JMzQ6Izuiq7FcH-5FUtU2yPc5AgtMGEjqKDw8hYwRduaZfb~8v3OzgQ2rVCpheotBhCWXZJFWwHQL-LW5yg5rP44JWST7tk9XANoE1pshS759W3B250Z01RlIRX5TtlJ0M7wpYSxtJkW6C-4kmCjc4tImmj2kEivLH37QO3x5L~2psTXqCWHYIa0Jb3LWmvWlMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Patupilone inhibits proliferation of MM cells adherent to BMSCs. MM patient BMSCs were cultured with or without MM.1S cells in the presence of 0, 1, or 10 nM patupilone. Subsequent proliferation at 48 hours was measured by 3H-[dT] uptake (A). Supernatants from the coculture of BMSCs with MM cells were analyzed by ELISA for VEGF (B) and IL-6 (C), 2 important factors for MM cell migration and proliferation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2499/6/m_zh80010571480007.jpeg?Expires=1767900297&Signature=FDN0qrjNHBnF26xivDwrv3DQ68HXZiKAUNtM6YUfUWV4VXa1KLfuh7ba9pWtltbl1q0mYzH6R1tC6JfbItxMATxTm1OMUJ34CcHiwGSudFAc3Tr52o5-RImQTpcYt72TjOJ~lw6Spj-aGW1UNRtSX3Ux~NrfiFsfCM7CO~XuyQfSsdza4o74u7q7dtluAVLluWXjY6yuav2mEiQ9rNUk~2Fxg09Pr1D3kXawGQvUtj5Io02lULuBTgbM6JU3vlN7baqTyANKEs7rG8PqUsUuPDay1ECxe7EORSJeHx4y6vFPP0~dj6Zk93kQsywEo6qrBBzj0n~vNpgaBauLIVyEaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal