Abstract
We investigated the relevance of p53 deletions to the clinical outcome of patients with multiple myeloma (MM) treated with high-dose chemotherapy and autologous stem cell transplantation. Hemizygous p53 gene deletions were detected by fluorescence in situ hybridization in 10 of 105 (9.5%) patients studied. p53 deletions were associated with higher serum calcium (P = .0062) and creatinine (P = .013) levels, but there were no association with patient age, gender, β2-microglobulin, C-reactive protein, hemoglobin, albumin or bone lytic lesions, or immunoglobulin isotype. There were no associations of p53 deletions with 13q deletions or translocations t(11;14) or t(4;14). Patients with p53 deletions had significantly shorter progression-free (median, 7.9 versus 25.7 months, P = .0324) and overall survival (median, 14.7 versus 48.1 months, P = .0008) than patients without a p53 deletion. A multivariate analysis confirmed p53 deletion was an independent prognostic factor predicting shortened progression-free (P = .0009) or overall survival (P = .0002) in patients with MM after high-dose chemotherapy and autologous stem cell transplantation. (Blood. 2005;105:358-360)
Introduction
p53, a tumor suppressor gene, is implicated in the regulation of cell proliferation, differentiation, and apoptosis.1 Inactivation of p53 inactivation by mutation or allelic loss has been observed in various human neoplasms and associated with tumor progression.2,3 In multiple myeloma (MM), p53 mutations are rare and may represent late events in myeloma progression.4-6 The frequency of p53 deletions detected by fluorescence in situ hybridization (FISH) is reported to range from 9% to 34% of MMs.7-10 The p53 gene deletions are associated with poor survival in patients with MM treated with conventional chemotherapy regimens,7,8,10 but there is little information on the relevance of p53 deletions to survival of patients with MM treated with autologous stem cell transplantation (ASCT).
We recently reported that t(4;14) but not t(11;14) is an adverse prognostic factor in patients with MM undergoing ASCT.11 The current study extends our experience of cytoplasmic light-chain immunofluorescence combined with FISH (cIg-FISH) to investigate the frequency and prognostic significance of p53 deletions in a single institutional cohort of patients with MM treated with high-dose chemotherapy followed by autologous stem cell support.
Study design
Patients
Between January 1998 and December 2001, 128 consecutive patients were diagnosed and treated for MM with high-dose chemotherapy followed by ASCT at the Princess Margaret Hospital/University Health Network. Informed consent was provided by the patients in this study. The clinical and biologic features were previously reported.11 Karyotypic analysis was not routinely performed during this study period. All patients received 4 to 5 cycles of the VAD regimen (vincristine, Adriamycin [doxorubicin], and dexamethasone) followed by stem cell mobilization with cyclophosphamide 2.5 g/m2 and granulocyte colony-stimulating factor (G-CSF; 10 μg/kg) and 1 course of melphalan 200 mg/m2 immediately prior to ASCT. The median interval from diagnosis to transplantation was 9.1 months. The median follow-up from ASCT is 20 months.
Bone marrow aspirates
After institutional research review board approval was given, bone marrow samples from patients with MM were obtained at time of active disease. Mononuclear cells enriched by Ficoll-gradient centrifugation and cytospin slides were made and stored at -70°C. To improve the specificity for detection of myeloma cells, we combined interphase FISH with cytoplasmic light-chain immunofluorescence as previously described.11
FISH probes
To detect p53 deletions, a SpectrumRed-labeled DNA probe (LSI p53; Vysis, Downers Grove, IL) specific for the p53 locus on 17p13.1 was combined with a SpectrumGreen-labeled probe (CEP17, Vysis) for the chromosome 17 α-satellite-DNA centromere. The probes have been tested in normal cells and a normal pattern is considered to be a pair of red and green signals (2R2G). Loss of signal from the pair of probes (1R2G, 1R1G) indicates a p53 deletion. Patients were considered evaluable if at least 100 clonal plasma cells could be scored from each slide. Based on FISH studies of normal bone marrow mononuclear cells, the upper limit of normal plus 3 SDs was less than 10% for deletions of p53. We therefore used 10% as the background cut-off level for the probe sets. FISH studies for chromosome 13q14 deletions were described previously.12
Statistical analysis
Statistical evaluation used the Fisher exact, χ2, and the nonparametric Wilcoxon tests. Progression-free survival (PFS) and overall survival (OS) were calculated from the transplantation date by the Kaplan-Meier method. Differences between survival curves were analyzed by the log-rank test. A multivariate analysis of survival time was performed using the proportional hazards regression model of Cox to find adjusted impact of p53 deletions on PFS and OS.
Results and discussion
Correlation ofp53 deletions with laboratory and clinical features
A cIg-FISH analysis informative for p53 deletion was obtained in 105 of the 128 patients with MM. An interstitial p53 deletion identified by one red (p53) and 2 green (CEP17) signals was detected in 10 of 105 patients. The median percent of myeloma cells with an interstitial p53 deletion was 53% (range, 18%-95%). A FISH analysis informative for t(4;14) and t(11;14) was available for all 105 cases11 and identified only 2 cases (2%) with both a p53 deletion and an IgH translocation; one case involved t(4;14), the other case t(11;14). Of 93 cases informative for 13q status by FISH, 37 (40%) had 13 deletions and were not associated with p53 deletions. Patients with p53 deletions had significantly higher serum calcium (P = .0062) and creatinine (P = .013) levels, but there was no significant correlation between p53 status and age, gender, β2-microglobulin, C-reactive protein (CRP), albumin level, lytic bone lesions, or immunoglobulin isotype (Table 1).
Patient characteristics
. | . | p53 deletion . | . | . | |
|---|---|---|---|---|---|
| Characteristics . | Total, n = 105 . | Absent, n = 95 . | Present, n = 10 . | P . | |
| Sex, female/male | 42/62 | 40/54 | 2/8 | .17 | |
| Age, y (range) | 53 (31-71) | 54 (31-69) | 51 (40-71) | .84 | |
| β2-microglobulin level, mM (range) | 218.5 (118-4910) | 219 (118-4910) | 315 (201-430) | .67 | |
| CRP level, mg/L (range) | 3.5 (2.0-122.0) | 3.5 (2.5-122.0) | 2.0 (2.0-2.0) | .092 | |
| Calcium level, mM (range) | 2.4 (1.6-4.2) | 2.4 (1.6-4.2) | 2.6 (2.5-4.0) | .0062 | |
| Creatinine level, μM (range) | 88 (51-1759) | 88 (51-1025) | 140 (84-1759) | .013 | |
| Hemoglobulin level, g/dL (range) | 107 (52-192) | 107 (52-192) | 106 (82-137) | .84 | |
| Albumin level, g/L (range) | 39 (20-55) | 39 (20-55) | 35 (22-46) | .48 | |
| Time to ASCT, mo (range) | 9.0 (2.5-148.9) | 8.8 (2.5-148.9) | 10.1 (5.4-63.9) | .75 | |
| Chromosome abnormalities | |||||
| t(4:14), no. (%) | 12 (11.4) | 11 (11.6) | 1 (10.0) | .99 | |
| t(11:14), no. (%) | 15 (14.2) | 14 (14.7) | 1 (10.0) | .99 | |
| del(13q) (n = 93), no. (%) | 37 (39.8) | 31 (36.9) | 6 (66.7) | .15 | |
| Lytic lesions (n = 92), no. (%) | .14 | ||||
| 0 | 32 (34.8) | 31 (37.8) | 1 (10) | - | |
| 1-3 | 27 (29.3) | 24 (29.3) | 3 (30) | - | |
| More than 3 | 33 (35.8) | 27 (32.9) | 6 (60) | - | |
| Isotype (n = 101), no. (%) | .86 | ||||
| IgA | 25 (24.5) | 23 (25.0) | 2 (20.0) | - | |
| IgG | 56 (54.9) | 50 (54.4) | 6 (60.0) | - | |
| IgD | 3 (2.9) | 2 (2.0) | 1 (10.0) | - | |
| Light chains | 18 (17.7) | 17 (18.5) | 1 (10.0) | - | |
. | . | p53 deletion . | . | . | |
|---|---|---|---|---|---|
| Characteristics . | Total, n = 105 . | Absent, n = 95 . | Present, n = 10 . | P . | |
| Sex, female/male | 42/62 | 40/54 | 2/8 | .17 | |
| Age, y (range) | 53 (31-71) | 54 (31-69) | 51 (40-71) | .84 | |
| β2-microglobulin level, mM (range) | 218.5 (118-4910) | 219 (118-4910) | 315 (201-430) | .67 | |
| CRP level, mg/L (range) | 3.5 (2.0-122.0) | 3.5 (2.5-122.0) | 2.0 (2.0-2.0) | .092 | |
| Calcium level, mM (range) | 2.4 (1.6-4.2) | 2.4 (1.6-4.2) | 2.6 (2.5-4.0) | .0062 | |
| Creatinine level, μM (range) | 88 (51-1759) | 88 (51-1025) | 140 (84-1759) | .013 | |
| Hemoglobulin level, g/dL (range) | 107 (52-192) | 107 (52-192) | 106 (82-137) | .84 | |
| Albumin level, g/L (range) | 39 (20-55) | 39 (20-55) | 35 (22-46) | .48 | |
| Time to ASCT, mo (range) | 9.0 (2.5-148.9) | 8.8 (2.5-148.9) | 10.1 (5.4-63.9) | .75 | |
| Chromosome abnormalities | |||||
| t(4:14), no. (%) | 12 (11.4) | 11 (11.6) | 1 (10.0) | .99 | |
| t(11:14), no. (%) | 15 (14.2) | 14 (14.7) | 1 (10.0) | .99 | |
| del(13q) (n = 93), no. (%) | 37 (39.8) | 31 (36.9) | 6 (66.7) | .15 | |
| Lytic lesions (n = 92), no. (%) | .14 | ||||
| 0 | 32 (34.8) | 31 (37.8) | 1 (10) | - | |
| 1-3 | 27 (29.3) | 24 (29.3) | 3 (30) | - | |
| More than 3 | 33 (35.8) | 27 (32.9) | 6 (60) | - | |
| Isotype (n = 101), no. (%) | .86 | ||||
| IgA | 25 (24.5) | 23 (25.0) | 2 (20.0) | - | |
| IgG | 56 (54.9) | 50 (54.4) | 6 (60.0) | - | |
| IgD | 3 (2.9) | 2 (2.0) | 1 (10.0) | - | |
| Light chains | 18 (17.7) | 17 (18.5) | 1 (10.0) | - | |
- indicates not applicable (P values presented are from overall test).
Correlation ofp53 deletions and clinical outcome
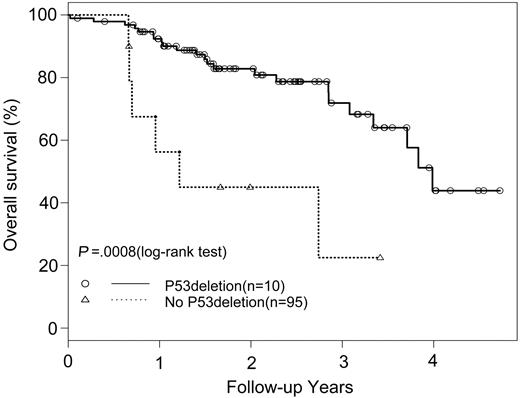
The median interval between diagnosis and ASCT was similar (10.1 versus 8.8 months) for patients with and without p53 deletions, respectively. The overall response rates were similar in patients with and without p53 deletions (67% versus 71%, respectively). However, patients with p53 deletions had significantly shorter PFS (median, 7.9 months) than patients without p53 deletions (median, 25.7 months, P = .0324). OS was also significantly shorter (median, 14.7 months) for patients with a p53 deletion than for patients without a deletion (median, 48.1 months, P = .0008; Figure 1). A univariate risk factor analysis including age, gender, β2-microglobulin, CRP, albumin, calcium, creatinine, lytic bone lesions, immunoglobulin isotype, t(4;14), t(11;14), and 13q status found that t(4;14) (P = .0012 for PFS, P = .0007 for OS) and 13q deletions (P = .0221 for PFS, P = .0565 for OS) were significant. A multivariate analysis confirmed p53 deletion as an independent risk factor for both PFS (P = .0009) and OS (P = .0002). Of note, one patient had coexistence of a p53 deletion and a t(11;14); his durations of PFS (6.1 months) and OS (8.5 months) were not significantly different from those of others with p53 deletions.
OS in patients with MM with and withoutp53 deletions who received autologous stem cell transplants.
OS in patients with MM with and withoutp53 deletions who received autologous stem cell transplants.
Increasing evidence indicates that high-dose chemotherapy followed by ASCT improves the clinical outcome of patients with MM, especially so for patients younger than age 65.13,14 It may therefore be clinically relevant to identify patients who could benefit from high-dose chemotherapy and ASCT from those who may not and are candidates for alternative treatment. Recently, adverse outcomes were reported in patients with MM with 13q deletions and t(4;14) undergoing ASCT.11,15,16 The current study identifies for the first time that patients with MM with p53 gene deletions treated with ASCT had adverse outcomes.
p53 deletions were detected by FISH in 9.5% of our patients, a frequency consistent with Avet-Loiseau et al9 and Fonseca et al,10 who found p53 deletions by FISH in 8.9% and 10.7% of their patients with MM, respectively. In contrast, Drach et al7 reported p53 deletions in 34% and 21.5%8 of their patients with MM. Because all authors used the same Vysis probe to detect p53 deletions, the variation in the p53 deletion frequency may be due to differences in FISH efficiency or to intrinsic differences in the different patient populations examined.
Drach et al7 and Fonseca et al10 reported p53 deletions were associated with an adverse outcome in patients with MM treated with conventional chemotherapy. Their studies raise the question as to whether high-dose chemotherapy and ASCT can overcome the negative influence of p53 deletions. However, our data indicate that this is not the case because these patients have significantly shorter PFS and OS compared with patients lacking the p53 deletions. Patients with p53 deletions are also more likely to have other features of aggressiveness such as hypercalcemia and elevated serum creatinine levels.
Because p53 deletions and t(4;14) are independent adverse prognostic factors and are found by FISH in 20% to 30% of patients with MM but are not easily detected by karyotypic study, FISH analysis of p53 and t(4;14) is warranted in patients with MM referred for high-dose chemotherapy and ASCT.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2004-04-1363.
Supported in part by grants from the Leukemia Research Fund of Canada (LRFC), National Cancer Institute of Canada (NCIC), Canadian Institute of Health Research (CIHR), and Multiple Myeloma Research Foundation (MMRF).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Bruce Patterson for reviewing the manuscript and Sara Samiee for her assistance with the myeloma database.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal