Abstract
Graft versus host disease (GVHD) is triggered by host antigen-presenting cells (APCs) that activate donor T cells to proliferate and differentiate, but which APC-activated donor T-cell subsets mediate GVHD versus beneficial antitumor effects is not known. Using a CD8+ T cell–dependent mouse model of human GVHD, we found that host dendritic cell (DC)–induced CD44hiCD8+ effector/memory T cells were functionally defective in inducing GVHD, whereas CD44loCD8+ naive phenotype T cells were extremely potent GVHD inducers. Depletion of CD44loCD8+ T cells from host DC-stimulated T cells before transplantation prevented GVHD without impairing their antitumor activity in vivo. Compared with CD44loCD8+ T cells, CD44hiCD8+ T cells expressed high levels of Fas and were efficiently deleted in vivo following transplantation. These results suggest that ex vivo allogeneic DC stimulation of donor CD8+ T cells may be useful for the prevention of GVHD and for optimizing antitumor therapies in vivo.
Introduction
Graft-versus-host disease (GVHD) remains the major toxicity of allogeneic bone marrow transplantation (allo-BMT). Even with pharmacologic prophylaxis and treatment, 10% to 50% of patients who receive major histocompatibility complex (MHC)–identical donor transplants die from GVHD-related complications.1-3 Highefficiency T-cell depletion (TCD) from allografts has been shown to be effective in reducing the incidence of GVHD. Unfortunately, TCD has been found to increase graft failure, leukemia relapse, infections, and secondary malignancies.2,3 Because of these complications, selective TCD has been proposed, in which specific T-cell subsets responsible for inducing GVHD are depleted prior to transplantation without increasing the risks related to global TCD.2,3 Indeed, several studies indicate that as many as 90% of alloreactive donor T cells can be purged ex vivo based on multiple T-cell activation markers, such as CD25 and CD69.4-7 However, in vivo animal studies show that donor T cells depleted of alloreactive CD25+ or CD69+ T cells still induce lethal GVHD in 30% to 70% of the recipient mice.8,9
In mouse models of human allo-BMT, donor CD8+ T cells are rapidly activated and induced to proliferate after infusion into lethally irradiated MHC-identical, minor histocompatibility antigen (miHA)–mismatched recipient mice, resulting in the development of acute GVHD.1-3,10-14 We have previously demonstrated that host antigen-presenting cell (APC) activation of donor CD8+ T cells is essential for initiating acute GVHD. Priming donor CD8+ T cells with allogeneic dendritic cells (DCs) for 24 hours is sufficient to induce the subsequent proliferation and differentiation of naive CD8+ T cells into GVHD effectors.12,14 However, whether all host DC-activated donor CD8+ T cells are equivalent in mediating acute GVHD or whether activation of donor CD8+ T cells by host DCs ex vivo might be useful for identifying and depleting alloreactive CD8+ T cells remains unknown. Using the C3H.SW anti-B6 (MHC-identical, miHA-mismatched) mouse model of human BMT, we now report that activation by host DCs induces the generation of 2 distinct donor T-cell subpopulations: CD44hiCD8+ and CD44loCD8+ T cells. CD44hiCD8+ T cells express T-cell activation markers but are defective in GVHD induction in MHC-matched miHA-mismatched B6 recipient mice, whereas CD44loCD8+ T cells express the phenotypes of naive T cells and are extremely potent inducers of GVHD. Interestingly, CD44hiCD8+ T cells themselves can be generated from CD44loCD8+ naive T cells by allogeneic DC stimulation ex vivo. Despite their inability to induce GVHD, CD44hiCD8+ T cells retain their ability to kill EL-4 leukemic cells in vivo.
Materials and methods
Mice
B6/SJL (H-2Db, CD45.1+), gld.B6 (H-2Db, CD45.2+), and C3H.SW (H-2Db, CD45.2+, and Ly9.1+) mice were purchased from Jackson ImmunoResearch Laboratories (Bar Harbor, ME). Drinking water of bone marrow (BM) transplant recipients was supplemented with neomycin sulfate and polymyxin B (Sigma Chemical, St Louis, MO) as we previously described.14
Antibodies, cell lines, and cytokines
Antiperforin antibody (Ab) and antigranzyme B Ab were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Biotinylated antimouse CD44 Ab and all other Abs used for immunofluorescence staining were obtained from PharMingen (San Diego, CA). Microbead-conjugated Abs and streptavidin conjugated with microbeads were purchased from Miltenyi Biotech (Auburn, CA). B6 mouse-derived EL-4 leukemic cells (T-cell acute lymphoblastic leukemia [T-ALL], H-2Db), C1498 myeloid leukemic cells (H-2Db), MC57 sarcoma cells (H-2Db), and Balb/C mouse-derived NS-1 myeloma cells (H-2Dd) were obtained from the American Type Culture Collection (ATCC; Rockville, MD). EL-4 and C1498 were grown in RPMI 1640 (Gibco, Rockville, MD) containing 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA) or in serum-free medium X-vivo 15 (BioWhittaker, Walkersville, MD). MC57 and NS-1 cells were grown in serum-free medium UltraCulture (BioWhittaker). All recombinant cytokines, interleukin 2 (IL-2), IL-4, IL-7, IL-15, granulocyte-monocyte colony-stimulating factor (GM-CSF), stem cell factor (SCF), and tumor necrosis factor α (TNF-α) were purchased from R&D Systems (Minneapolis, MN).
Cell preparations
Donor BM cells were prepared from C3H.SW mice as previously described.12,14 T cell–depleted BM (T–BM) cells were isolated with anti-CD4 and anti-CD8 Abs conjugated with magnetic microbeads. Donor CD8+ T cells were purified from spleens and lymph nodes (LNs) of C3H.SW mice by positive selection with anti-CD8 Ab conjugated with magnetic microbeads followed by magnetic cell sorting (MiniMACS; Miltenyi Biotech). CD11c+ cells were magnetically removed by anti-CD11c Ab conjugated with microbeads before purifying donor CD8+ T cells. To further separate CD44hiCD8+ and CD44loCD8+ T cells, purified CD8+ T cells were stained with phycoerythrin (PE)–conjugated anti-CD44Ab and sorted into CD44hiCD8+ and CD44loCD8+ T cells by fluorescence-activated cell sorting (FACS). The purity of sorted CD8+ T cells was consistently more than 95%. Carboxyl fluorescein succinimidyl ester (CFSE) labeling of donor CD8+ T cells was performed as previously described.14
Mature DCs were prepared from B6 BM as previously described.15 Briefly, B6 BM specimens were stained with biotinylated anti–c-kit Ab (PharMingen) followed by streptavidin-conjugated magnetic microbeads (Miltenyi Biotech). The c-kit+ hematopoietic progenitor cells were magnetically sorted and incubated for 6 days in Iscove modified Dulbecco medium (IMDM; Gibco) supplemented with 10% FBS in the presence of GM-CSF, SCF, and IL-4. CD11c+ immature DCs were magnetically sorted from this 6-day culture using anti-CD11c antibody-conjugated microbeads and stimulated with GM-CSF plus TNF-α for an additional 2 days to induce their maturation.
Ex vivo stimulation of CD8+ T cells
C3H.SW CD8+ T cells (3 × 106 cells/well) were cultured with B6 BM-derived mature DCs (1 × 106 cells/well) in 24-well plates in IMDM containing 10%FBS, with or without addition of IL-2 (5 ng/mL) plus IL-15 (3 ng/mL).
BM and CD8+ T-cell transplantation
Mice underwent allo-BMT as previously described.14 Briefly, B6 recipients were irradiated with 10.0 Gy administered in 2 fractions from a 137Cs source. C3H.SW CD8+ T cells, mixed with or without C3H.SW T–BM cells (7 × 106), were transplanted into B6 recipients via tail vein injection (5-8 mice/group/experiment) immediately after irradiation. Recipient mice were weighed twice weekly and monitored for the clinical signs of acute GVHD and survival. The clinical grading criteria for acute GVHD were followed as established.16,17
Pathologic examination of tissues
Mice were killed and specimens of liver, skin, and intestine were taken for histopathologic assessment of acute GVHD and leukemia as previously described.18
Induction and assessment of graft-versus-leukemia effect
Leukemia in lethally irradiated B6 mice was induced by the injection of 5000 EL-4 cells into their peritoneal cavity as previously described.18 After death, carcasses were fixed in formaldehyde and were subsequently evaluated for the presence of tumor masses in the retroperitoneum, mesentery, liver, spleen and kidneys. Representative tissues were also sectioned for histology confirmation.
Cytolytic assay
Cytolytic assay on target EL-4, C1498, MC57, and NS-1 cells was performed in a 12-hour 51Cr release assay as previously described.19 Donor CD8+ T cells were either freshly isolated from naive C3H.SW mice (unstimulated CD8+ T cells), sorted from the DC-stimulated cultures (DC-induced CD44hiCD8+, CD44loCD8+ T cells), or recovered from the spleens, LNs, and livers of irradiated B6 mice (CD45.1) receiving B6 DC-induced CD44hiCD8+ and CD44loCD8+ T cells by magnetic cell sorting or FACS. The cytolytic activity was directly assessed without any further ex vivo stimulation of these CD8+ T cells.
Flow cytometry analysis
Statistical analysis
Survival data were analyzed by life table methods using the Mantel-Peto-Cox summary of χ2. Differences in the number of donor CD8+ T cells that were infiltrating in various tissues or were undergoing apoptosis were evaluated using 2-way ANOVA. P values less than .05 were considered significant.
Results
DC activation induces the generation of CD44hiCD8+ and CD44loCD8+ T cells
Acute GVHD in the C3H.SW anti-B6 mouse BMT model is dependent on donor CD8+ T cells that are rapidly activated after infusion into lethally irradiated host B6 mice.12,14 To more precisely define the phenotype and function of host reactive donor CD8+ T cells, we cultured CFSE-labeled C3H.SW CD8+ T cells with B6 APCs, with or without cytokines. Over 82% of seeded CD8+ T cells survived 6 days in B6 DC-stimulated cultures, whereas more than 90% of CD8+ T cells were lost in cultures stimulated with B6 macrophages or with IL-2, IL-7, and IL-15 (Figure 1A). Flow cytometry analysis showed that B6 DCs induced C3H.SW CD8+ T cells to divide as many as 7 times in culture (Figure 1B). Of note, highly proliferative CD8+ T cells expressed high levels of CD44 (CD44hi) but low to moderate levels of CD25, CD69, and CD122 (CD25lo/modCD69lo/modCD122lo/mod; Figure 1C upper row). Most of these CD44hiCD8+ T cells were CD44hiCD62Llo (78%) and some were CD44hiCD62Lhi (22%) cells (Figure 1C upper row). In contrast, all CD8+ T cells that had undergone fewer divisions were CD44loCD62LhiCD122loCD69loCD25lo (Figure 1B-C upper row). Furthermore, these DC-induced CD44hiCD8+ T cells expressed higher levels of perforin, granzyme, and Fas ligand (FasL) than either CD44loCD8+ or unstimulated CD8+ T cells (Figure 1D). Addition of IL-2 plus IL-15 together with B6 DCs increased the number of proliferating CD44loCD8+ T cells without inhibiting the production of CD44hiCD8+ T cells (Figure 1B) or altering the expression of CD25, CD69, and CD122 on either CD44loCD8+ or CD44hiCD8+ T cells (Figure 1C). In the absence of B6 DCs, IL-2 plus IL-15 alone induced neither CD8+ T-cell proliferation nor their expression of CD44 (Figure 1B). These results show that allogeneic DC activation induces the generation of 2 distinct CD8+ T-cell subpopulations: CD44hiCD8+ effector/memory-like T cells and CD44loCD8+ naive phenotype T cells.
Mature DCs are required for the generation of CD44hiCD8+ and CD44loCD8+ T cells ex vivo. (A) CFSE-labeled C3H.SW CD8+ T cells were cultured in the presence of B6 BM-derived DCs (T cell/DC ratio = 3:1), B6 peritoneum macrophages, and cytokines including IL-2 (5 ng/mL), IL-7 (3 ng/mL), IL-15 (3 ng/mL), IL-2 plus IL-7 and IL-2 plus IL-15. Surviving T cells were numerated at day 6 after culture. All values represent means ± SD of triplicate wells. (B) CFSE-labeled whole C3H.SW CD8+ T cells and separated C3H.SW CD44loCD8+ T cells were cultured in the presence of B6 DCs, B6 DCs plus IL-2 plus IL-15, or IL-2 plus IL-15 for 5 days. Cells were collected and stained with rat anti-CD8 and anti-CD44 antibodies or control rat IgG for flow cytometry analysis. (C) C3H.SW CD8+ naive T cells were cultured in the presence of B6 DCs, with or without IL-2 plus IL-15 addition, for 5 days. Cells were harvested, stained with rat anti-CD8 Ab and other indicated Abs, and analyzed by flow cytometry. Dot plots shown are gated CD8+ T cells. Results are representative of at least 5 independent experiments. Numbers in quadrants represent percentages of total cells in each cell fraction. (D) Unstimulated CD8+ T cells and B6 DC-induced CD44hiCD8+ and CD44loCD8+ T cells were stained with anti-CD8 Ab, then permeabilized, stained with the indicated Abs, and analyzed by flow cytometry. Histograms shown are gated on CD8+ T cells.
Mature DCs are required for the generation of CD44hiCD8+ and CD44loCD8+ T cells ex vivo. (A) CFSE-labeled C3H.SW CD8+ T cells were cultured in the presence of B6 BM-derived DCs (T cell/DC ratio = 3:1), B6 peritoneum macrophages, and cytokines including IL-2 (5 ng/mL), IL-7 (3 ng/mL), IL-15 (3 ng/mL), IL-2 plus IL-7 and IL-2 plus IL-15. Surviving T cells were numerated at day 6 after culture. All values represent means ± SD of triplicate wells. (B) CFSE-labeled whole C3H.SW CD8+ T cells and separated C3H.SW CD44loCD8+ T cells were cultured in the presence of B6 DCs, B6 DCs plus IL-2 plus IL-15, or IL-2 plus IL-15 for 5 days. Cells were collected and stained with rat anti-CD8 and anti-CD44 antibodies or control rat IgG for flow cytometry analysis. (C) C3H.SW CD8+ naive T cells were cultured in the presence of B6 DCs, with or without IL-2 plus IL-15 addition, for 5 days. Cells were harvested, stained with rat anti-CD8 Ab and other indicated Abs, and analyzed by flow cytometry. Dot plots shown are gated CD8+ T cells. Results are representative of at least 5 independent experiments. Numbers in quadrants represent percentages of total cells in each cell fraction. (D) Unstimulated CD8+ T cells and B6 DC-induced CD44hiCD8+ and CD44loCD8+ T cells were stained with anti-CD8 Ab, then permeabilized, stained with the indicated Abs, and analyzed by flow cytometry. Histograms shown are gated on CD8+ T cells.
DC-induced CD44hiCD8+ T cells are deficient in mediating acute GVHD
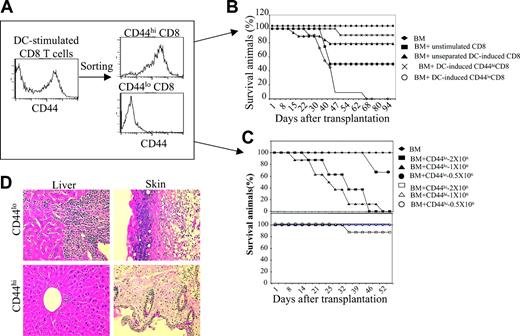
To test whether B6 DC-induced C3H.SW CD44hiCD8+ and CD44loCD8+ T cells (2 × 106) are equally able to cause GVHD, these T-cell populations were separated by FACS (Figure 2A) and transplanted along with C3H.SW T–BM into lethally irradiated B6 mice, using C3H.SW T–BM with or without unstimulated CD8+ T cells (comprised of 18.0% ± 5.0% CD44hi and 78.0% ± 3.4% CD44lo T cells) or unseparated B6 DC-induced CD8+ T cells (comprised of 45.0% ± 4.2% CD44hi and 51.0% ± 3.8% CD44lo T cells) as controls. All B6 mice receiving C3H.SW T–BM plus 2 × 106 unstimulated CD8+ T cells developed clinical GVHD, with weight loss, diarrhea, and skin lesions, and about 60% of these mice died, whereas B6 mice of C3H.SW T–BM alone did not show any sign of GVHD, as expected (Figure 2B). However, B6 mice receiving unseparated B6 DC-induced CD8+ T cells only developed moderate clinical GVHD with 23% mortality (Figure 2B). Interestingly, infusion of 1 × 106 to 2 × 106 sorted CD44loCD8+ T cells caused extremely virulent GVHD in B6 recipients, with extensive infiltration of mononuclear cells in the liver and skin (Figure 2D) and all mice dying of GVHD (Figure 2B-C). Lower doses (0.5 × 106) of added CD44loCD8+ T cells still caused GVHD, with 33.3% lethality (Figure 2C). In contrast, administration of up to 2 × 106 sorted CD44hiCD8+ T cells caused little or no clinical GVHD, and mortality was only 10% (Figure 2B-D). Thus, ex vivo allogeneic DC stimulation induced the generation of GVHD-impotent CD44hiCD8+ T cells and GVHD-potent CD44loCD8+ T cells.
DC-induced CD44hiCD8+ T cells are defective in induction of GVHD. (A) Naive C3H.SW CD8+ T cells were cultured with B6 DCs plus IL-2 plus IL-15. Five days after the culture, T cells were collected and sorted into CD44hiCD8+ and CD44loCD8+ T cells using FACS. (B) Survival of lethally irradiated B6 mice injected with C3H.SW T–BM (7 × 106) mixed with or without C3H.SW CD8+ T cells (2 × 106). ♦, T–BM (n = 8); ▪, T–BM plus unstimulated naive CD8+ (n = 8); ▴, T–BM plus unseparated B6 DC-stimulated CD8+ (n = 13); ×, T–BM plus B6 DC-induced CD44hiCD8+ (n = 19); ○, T–BM plus B6 DC-induced CD44loCD8+ (n = 15). P < .01 (▴ or × versus ○). Data shown here are pooled from 3 independent experiments. (C) Survival of lethally irradiated B6 mice injected with C3H.SW T–BM (7 × 106) mixed with or without sorted CD44hiCD8+ or sorted CD44loCD8+ T cells at the dosages ranging from 0.5 × 106 to 2 × 106. ♦, T–BM (n = 8); ▪, T–BM plus CD44loCD8+ T cells (2 × 106, n = 8); ▴, T–BM plus CD44loCD8+ T cells (1 × 106, n = 8); •, T–BM plus CD44loCD8+ T cells (0.5 × 106, n = 6); □, T–BM plus CD44hiCD8+ T cells (2 × 106, n = 8); ▵, T–BM plus CD44hiCD8+ T cells (1 × 106, n = 8); ○, T–BM plus CD44loCD8+ T cells (0.5 × 106, n = 6). Data shown here are representative of 2 independent experiments. (D) Livers and skin were harvested from mice at day 35 after allo-BMT and sectioned for histologic staining with hematoxylin and eosin; original magnification × 100.
DC-induced CD44hiCD8+ T cells are defective in induction of GVHD. (A) Naive C3H.SW CD8+ T cells were cultured with B6 DCs plus IL-2 plus IL-15. Five days after the culture, T cells were collected and sorted into CD44hiCD8+ and CD44loCD8+ T cells using FACS. (B) Survival of lethally irradiated B6 mice injected with C3H.SW T–BM (7 × 106) mixed with or without C3H.SW CD8+ T cells (2 × 106). ♦, T–BM (n = 8); ▪, T–BM plus unstimulated naive CD8+ (n = 8); ▴, T–BM plus unseparated B6 DC-stimulated CD8+ (n = 13); ×, T–BM plus B6 DC-induced CD44hiCD8+ (n = 19); ○, T–BM plus B6 DC-induced CD44loCD8+ (n = 15). P < .01 (▴ or × versus ○). Data shown here are pooled from 3 independent experiments. (C) Survival of lethally irradiated B6 mice injected with C3H.SW T–BM (7 × 106) mixed with or without sorted CD44hiCD8+ or sorted CD44loCD8+ T cells at the dosages ranging from 0.5 × 106 to 2 × 106. ♦, T–BM (n = 8); ▪, T–BM plus CD44loCD8+ T cells (2 × 106, n = 8); ▴, T–BM plus CD44loCD8+ T cells (1 × 106, n = 8); •, T–BM plus CD44loCD8+ T cells (0.5 × 106, n = 6); □, T–BM plus CD44hiCD8+ T cells (2 × 106, n = 8); ▵, T–BM plus CD44hiCD8+ T cells (1 × 106, n = 8); ○, T–BM plus CD44loCD8+ T cells (0.5 × 106, n = 6). Data shown here are representative of 2 independent experiments. (D) Livers and skin were harvested from mice at day 35 after allo-BMT and sectioned for histologic staining with hematoxylin and eosin; original magnification × 100.
These results were surprising, inasmuch as alloreactive cytotoxic T-lymphocyte (CTL) precursors are known to be highly enriched in CD44hiCD8+ T-cell populations after immunization with alloantigen.20-26 In normal mice, the CD8+ T-cell population includes CD44loCD8+ and CD44hiCD8+ T cells. When normal C3H.SW CD8+ T cells were separated into CD44loCD8+ and CD44hiCD8+ T cells and transplanted together with C3H.SW T–BM into irradiated B6 mice, respectively, severe GVHD developed in all B6 mice receiving 2 × 106 CD44loCD8+ T cells and 94% of them died (Figure 3A). In contrast, only 3 of 17 B6 mice receiving 2 × 106 CD44hiCD8+ T cells developed lethal GVHD, whereas 14 of 17 mice survived without any clinical sign of GVHD (Figure 3A), suggesting that even CD44hiCD8+ and CD44loCD8+ T cells from normal C3H.SW mice show distinct alloreactive abilities.
CD44hiCD8+ and CD44loCD8+ T cells are generated from a common precursor pool. (A) CD44hiCD8+ and CD44loCD8+ T cells were isolated from naive C3H.SW mice by the combination of magnetic cell sorting and FACS. B6 mice were irradiated and injected with C3H.SW T–BM, with or without CD8+ T cells. Survival of B6 mice after BMT: ♦, T–BM (n = 20); ▪, T–BM plus CD44hiCD8+ (n = 17); ▴, T–BM plus CD44loCD8+ (n = 17); ×, T–BM plus unstimulated CD8+ (n = 35); *P < .01 (▪ vs. ▴ or ×). Data shown here are pooled from at least 4 independent experiments. (B) Separated C3H.SW CD44loCD8+ naive T cells were cultured in the presence of B6 DCs. Five days later, B6 DC-induced CD44hiCD8+ and CD44loCD8+ T cells were sorted from the DC-induced cultures and transplanted together with C3H.SW T–BM into lethally irradiated B6 mice. Survival after BMT: ♦, T–BM (n = 8); ▪, T–BM plus CD44hiCD8+ (n = 12); ▴, T–BM plus CD44loCD8+ (n = 16). P < 0.01 (▪ versus ▴). Data shown here are pooled from 2 separate experiments.
CD44hiCD8+ and CD44loCD8+ T cells are generated from a common precursor pool. (A) CD44hiCD8+ and CD44loCD8+ T cells were isolated from naive C3H.SW mice by the combination of magnetic cell sorting and FACS. B6 mice were irradiated and injected with C3H.SW T–BM, with or without CD8+ T cells. Survival of B6 mice after BMT: ♦, T–BM (n = 20); ▪, T–BM plus CD44hiCD8+ (n = 17); ▴, T–BM plus CD44loCD8+ (n = 17); ×, T–BM plus unstimulated CD8+ (n = 35); *P < .01 (▪ vs. ▴ or ×). Data shown here are pooled from at least 4 independent experiments. (B) Separated C3H.SW CD44loCD8+ naive T cells were cultured in the presence of B6 DCs. Five days later, B6 DC-induced CD44hiCD8+ and CD44loCD8+ T cells were sorted from the DC-induced cultures and transplanted together with C3H.SW T–BM into lethally irradiated B6 mice. Survival after BMT: ♦, T–BM (n = 8); ▪, T–BM plus CD44hiCD8+ (n = 12); ▴, T–BM plus CD44loCD8+ (n = 16). P < 0.01 (▪ versus ▴). Data shown here are pooled from 2 separate experiments.
These disparate CD44hiCD8+ versus CD44loCD8+ T cells could derive from a common CD8+ precursor pool or could instead represent activation of preexisting distinct CD8+ T-cell subsets. To test these alternatives, CD44loCD8+ T cells from naive C3H.SW mice were purified and then cultured in the presence of B6 DCs. At 5 days, B6 DC-stimulated CD44loCD8+ naive T cells generated a population of highly proliferative CD44hiCD8+ T cells with the same phenotype as did unseparated CD8+ naive T cells. Addition of IL-2 plus IL-15 together with B6 DCs further enhanced the proliferation of the CD44loCD8+ T-cell pool, while maintaining the production of CD44hiCD8+ T cells (Figure 1B-C). Once again, when CD44hiCD8+ and CD44loCD8+ T cells were sorted from B6 DC-stimulated C3H.SW CD44loCD8+ T-cell cultures and transplanted into lethally irradiated B6 mice, only 2 of 12 B6 mice receiving donor CD44hiCD8+ T cells (1 × 106) developed GVHD with delayed onset. In comparison, 15 of 16 B6 recipient mice of donor CD44loCD8+ T cells (1 × 106) died from acute GVHD (Figure 3B). These results indicate that GVHD-impotent CD44hiCD8+ T cells can be generated from GVHD-potent CD44loCD8+ T cells by stimulation with allogeneic DCs.
DC-induced CD44hiCD8+ T cells mediate graft-versus-leukemia effect
To address whether GVHD-impotent CD44hiCD8+ T cells sustain detectable graft-versus-leukemia (GVL) activity, we examined the ability of these cells to kill highly virulent EL-4 leukemic cells in vivo and in vitro. Irradiated B6 mice were given transplants with C3H.SW T–BM, together with or without 2 × 106 B6 DC-induced CD44hiCD8+, B6 DC-induced CD44loCD8+, or unstimulated CD8+ T cells, at day 0, and 5000 EL-4 leukemic cells were injected into the peritoneal cavity of these B6 recipients on day 7. All irradiated B6 mice receiving C3H.SW T–BM alone died from leukemia, with a median survival time (MST) of 31.6 days. Recipients of CD44loCD8+ T cells rapidly developed lethal GVHD, and the 2 mice that survived until day 30 succumbed to leukemia. In contrast, B6 mice receiving C3H.SW T–BM plus DC-induced CD44hiCD8+ T cells did not develop GVHD, and these mice showed evidence of antileukemia protection, with MST prolonged to 43.3 days (P < .05 compared to mice receiving C3H.SW T–BM plus EL-4 cells) and 11.1% of mice survived free of leukemia and GVHD over 100 days (Figure 4A). B6 mice receiving unstimulated CD8+ T cells and EL-4 cells had an equivalent survival rate to that of DC-induced CD44hiCD8+ T cells. However, clinical and pathologic evaluation showed overwhelming leukemia infiltration in 3 of 9 mice and lethal GVHD in 5 of 9 mice (Figure 4A). Thus, although DC-induced CD44hiCD8+ T cells did not ultimately prevent virulent leukemic progression in all animals, they retained antileukemia activity in vivo without life-threatening GVHD.
DC-induced CD44hiCD8+ T cells retain the ability to kill tumor cells. (A) Naive C3H.SW CD44loCD8+ T cells were separated and stimulated with B6 DCs plus IL-2 plus IL-15. After 5 days of culture, cells were collected, sorted into CD44hiCD8+ and CD44loCD8+ T cells (CD45.2) and transplanted together with C3H.SW T–BM into lethally irradiated B6 mice (CD45.1) at day 0. At day 7 following BMT, 5000 EL-4 leukemic cells were injected into the peritoneal cavity of these B6 mice. Survival after EL-4 inoculation: ▪, T–BM (n = 12); •, T–BM plus unstimulated naive CD8+ (n = 9); ×, T–BM plus B6 DC-induced CD44loCD8+ (n = 8); ▴, T–BM plus B6 DC-induced CD44hiCD8+ (n = 18). B6 mice receiving C3H.SW T–BM plus B6 DC-induced CD44hiCD8+ but no EL-4 injection (○, n = 4) were used as controls. (B) The ability of B6 DC-induced CD44hiCD8+ (▪) and CD44loCD8+ T cells (▴) to kill EL-4 cells ex vivo was examined by 51Cr-release method either prior to or 7 days after their infusion into irradiated B6 mice. Unstimulated C3H.SW CD8+ naive T cells (♦) were used as controls, before (♦ and ×) or 7 days (♦) after infusion into B6 mice. (C) The ability of B6 DC-induced CD44hiCD8+ (♦) and CD44loCD8+ T cells (▪) to kill EL-4, C1498, MC57 and NS-1 cells that had been grown in serum-free medium was examined as mentioned in panel B. (D) Neutralizing anti–MHC-I (50 μg/mL), anti-FasL Ab (50 μg/mL), anti–MHC-II, or anti–TNF-α was added into the cytolytic assays containing 8 × 104 CD44hiCD8+ T effector cells and 1 × 104 target cells as indicated. *P < .05 and **P < .01 (anti–MHC-I Ab or anti-FasL Ab versus control IgG), respectively. Results are representative of 2 separate experiments. All values shown in B-D represent means ± SD of triplicate wells.
DC-induced CD44hiCD8+ T cells retain the ability to kill tumor cells. (A) Naive C3H.SW CD44loCD8+ T cells were separated and stimulated with B6 DCs plus IL-2 plus IL-15. After 5 days of culture, cells were collected, sorted into CD44hiCD8+ and CD44loCD8+ T cells (CD45.2) and transplanted together with C3H.SW T–BM into lethally irradiated B6 mice (CD45.1) at day 0. At day 7 following BMT, 5000 EL-4 leukemic cells were injected into the peritoneal cavity of these B6 mice. Survival after EL-4 inoculation: ▪, T–BM (n = 12); •, T–BM plus unstimulated naive CD8+ (n = 9); ×, T–BM plus B6 DC-induced CD44loCD8+ (n = 8); ▴, T–BM plus B6 DC-induced CD44hiCD8+ (n = 18). B6 mice receiving C3H.SW T–BM plus B6 DC-induced CD44hiCD8+ but no EL-4 injection (○, n = 4) were used as controls. (B) The ability of B6 DC-induced CD44hiCD8+ (▪) and CD44loCD8+ T cells (▴) to kill EL-4 cells ex vivo was examined by 51Cr-release method either prior to or 7 days after their infusion into irradiated B6 mice. Unstimulated C3H.SW CD8+ naive T cells (♦) were used as controls, before (♦ and ×) or 7 days (♦) after infusion into B6 mice. (C) The ability of B6 DC-induced CD44hiCD8+ (♦) and CD44loCD8+ T cells (▪) to kill EL-4, C1498, MC57 and NS-1 cells that had been grown in serum-free medium was examined as mentioned in panel B. (D) Neutralizing anti–MHC-I (50 μg/mL), anti-FasL Ab (50 μg/mL), anti–MHC-II, or anti–TNF-α was added into the cytolytic assays containing 8 × 104 CD44hiCD8+ T effector cells and 1 × 104 target cells as indicated. *P < .05 and **P < .01 (anti–MHC-I Ab or anti-FasL Ab versus control IgG), respectively. Results are representative of 2 separate experiments. All values shown in B-D represent means ± SD of triplicate wells.
Ex vivo cytolytic assay showed that B6 DC-induced CD44hiCD8+ T cells, either before or 7 days after their infusion into irradiated B6 mice, were able to kill EL-4 leukemic cells (Figure 4B). However, DC-induced CD44loCD8+ or unstimulated CD8+ T cells only obtained the ability to kill EL-4 cells after infusion into irradiated B6 mice (Figure 4B). To rule out the possibility that FBS in which both DCs and target EL-4 cells were grown might be responsible for an artificial GVL effect, the cytolytic activity of CD44hiCD8+ T cells was measured using target cells that had been grown in serum-free medium. We found that in the absence of FBS, DC-induced CD44hiCD8+ T cells efficiently killed B6 mouse-derived EL-4, as well as C1498 and MC57 leukemic cells, but not control Balb/c mouse-derived NS-1 cells (Figure 4C). Neutralizing anti–MHC-I or anti-FasL Ab, but not anti–MHC-II or anti–TNF-α, abrogated the cytolytic effect of CD44hiCD8+ T cells on C1498 cells and significantly inhibited CD44hiCD8+ T cell–mediated killing activity against EL-4 cells (Figure 4D), suggesting that the functional presence of MHC-I and FasL both play important roles in this CD44hiCD8+ T cell–mediated GVL effect.
CD44hiCD8+ T cells proliferate in vivo, but their accumulation is impaired
To understand possible mechanisms contributing to the inability of CD44hiCD8+ T cells to induce GVHD, CFSE-labeled B6 DC-induced C3H.SW CD8+ T-cell subsets (CD45.2) were recovered from B6 mice (CD45.1) during the 3 weeks following transplantation and analyzed for cytokine production, cell surface phenotype, and proliferative activity. Both CD44hiCD8+ and CD44loCD8+ T cells produced similarly low levels of interferon γ (IFN-γ) and TNF-α, but neither population produced IL-2 or IL-4 prior to their infusion (Figure 5A). Interestingly, low levels of IL-10 were detected in a slightly higher percentage of CD44hiCD8+ T cells (6%) than CD44loCD8+ T cells (3%) prior to their infusion (Figure 5A). However, 14 days after infusion into irradiated B6 mice, donor CD8+ T cells that were recovered from spleens of B6 mice receiving CD44hiCD8+ and B6 mice receiving CD44loCD8+ T cells produced similarly high levels of IFN-γ and low levels of IL-10 and TNF-α (Figure 5B), as did donor CD8+ T cells from the LNs and livers of these B6 mice (data not shown). Thus, production of these cytokines cannot account for the significant differences in GVHD potencies in CD44hi and CD44lo CD8+ T-cell subsets.
CD44hiCD8+ and CD44loCD8+ T cells proliferate and produce similar levels of cytokines in vivo. (A) C3H.SW CD44loCD8+ T cells were stimulated with B6 DC plus IL-2 plus IL-15. Five days later, these unseparated B6 DC-induced T cells were restimulated with anti-CD3 Ab for 16 hours and the production of intracellular cytokines IFN-γ, IL-2, IL-4, IL-10, and TNF-α was analyzed by flow cytometry prior to infusion. Numbers in quadrants represent percentages of total cells in each cell fraction. (B,D) CD44hiCD8+ and CD44loCD8+ T cells were sorted from the B6 DC-stimulated T-cell cultures at day 5 and labeled with CFSE. Then, 1 × 106 of these B6 DC-stimulated CD44hiCD8+ or CD44loCD8+ T cells (CD45.2) were mixed with 5 × 106 B6 T-BM (CD45.1) and transplanted into lethally irradiated B6 mice. Lymphocytes were recovered from the spleens these B6 recipient mice at the indicated time points after transplantation for measuring the production of intracellular cytokines (B) and phenotype (D). Numbers in quadrants represent percentages of each cell fraction. (C) The ratio of CD44hiCD62Llo/CD44hiCD62Lhi T cells among DC-induced CD44loCD8+ T cell subsets prior to infusion or donor CD8+ T cells recovered from the spleens of a group of 3 mice receiving CD44hiCD8+ and 3 mice receiving CD44loCD8+ T cells was calculated based on the number of donor CD8+ T cell–derived CD44hiCD62Llo and CD44hiCD62Lhi T cells. All values represent means ± SD. Data shown are the representative of 3 independent experiments.
CD44hiCD8+ and CD44loCD8+ T cells proliferate and produce similar levels of cytokines in vivo. (A) C3H.SW CD44loCD8+ T cells were stimulated with B6 DC plus IL-2 plus IL-15. Five days later, these unseparated B6 DC-induced T cells were restimulated with anti-CD3 Ab for 16 hours and the production of intracellular cytokines IFN-γ, IL-2, IL-4, IL-10, and TNF-α was analyzed by flow cytometry prior to infusion. Numbers in quadrants represent percentages of total cells in each cell fraction. (B,D) CD44hiCD8+ and CD44loCD8+ T cells were sorted from the B6 DC-stimulated T-cell cultures at day 5 and labeled with CFSE. Then, 1 × 106 of these B6 DC-stimulated CD44hiCD8+ or CD44loCD8+ T cells (CD45.2) were mixed with 5 × 106 B6 T-BM (CD45.1) and transplanted into lethally irradiated B6 mice. Lymphocytes were recovered from the spleens these B6 recipient mice at the indicated time points after transplantation for measuring the production of intracellular cytokines (B) and phenotype (D). Numbers in quadrants represent percentages of each cell fraction. (C) The ratio of CD44hiCD62Llo/CD44hiCD62Lhi T cells among DC-induced CD44loCD8+ T cell subsets prior to infusion or donor CD8+ T cells recovered from the spleens of a group of 3 mice receiving CD44hiCD8+ and 3 mice receiving CD44loCD8+ T cells was calculated based on the number of donor CD8+ T cell–derived CD44hiCD62Llo and CD44hiCD62Lhi T cells. All values represent means ± SD. Data shown are the representative of 3 independent experiments.
Previous studies have demonstrated that CD44hiCD62LhiCD122hi and CD44hiCD62LloCD122hi CD8+ T cells may represent central memory and effector/effector memory T cells, respectively.24,27 Flow cytometry analysis showed that B6 DC-induced CD44hiCD8+ T cells infused into irradiated B6 mice gave rise to far fewer effector/effector memory T cells than did B6 DC-induced CD44lo CD8+ T cells. After infusion of CD44hiCD8+ T cells into irradiated B6 mice, the ratio of CD44hiCD62Llo/CD44hiCD62Lhi CD8+ T cells in recipient spleens was 0.24:1 on day 7 and 0.63:1 on day 21 after transplantation (Figure 5C-D). In contrast, infusion of CD44loCD8+ T cells into irradiated B6 mice resulted in the recovery of 8.7-fold more CD44hiCD62LloCD8+ T cells than CD44hiCD62LhiCD8+ T cells in their spleens by day 21 after transplantation (Figure 5C-D). Interestingly, by day 21 after transplantation, most donor CD8+ T cells derived from both CD44hiCD8+ and CD44loCD8+ T cells expressed high levels of CD122, but not CD25 or CD69 on their surface (Figure 5D). These results imply that the ability of CD44hiCD8+ T cells to generate CD44hiCD62LloCD8+ effector/effector memory T cells is impaired in vivo.
Indeed, the overall recovery of donor CD8+ T cells in the spleens and LNs of B6 mice receiving CD44hiCD8+ T cells between 7 and 14 days following BMT was much lower than in mice receiving CD44loCD8+ T cells and mice receiving unstimulated CD8+ T cells that were unlabeled with anti-CD44 Ab (Figure 6A; P < .01). Interestingly, donor CD8+ T cells were initially detected in recipient livers 24 hours following infusion of CD44hiCD8+ T cells in higher number than following transplantation of CD44loCD8+ T cells or of unstimulated CD8+ T cells (Figure 6A). There was no significant difference in the numbers of donor CD8+ T cells recovered from the spleens and LNs of recipient mice of each CD8+ T-cell fraction (Figure 6A). In contrast, donor CD8+ T cells were not detected at 24 hours in the Peyer patch, skin, and intestine of mice receiving CD44hiCD8+, CD44loCD8+, or unstimulated CD8+ T cells (data not shown). These data confirm that in vitro treatment with anti-CD44 Ab labeling did not prevent the early homing of CD44hiCD8+ T cells to either lymphoid or target tissues. However, over the next 2 weeks CD44hiCD8+ T cells did not accumulate in the livers to the same extent as did CD44loCD8+ T cells (Figure 6A). As a result, there were 4-fold more donor CD8+ T cells in the livers of B6 mice receiving CD44loCD8+ T cells and 2.5-fold more donor CD8+ T cells in the livers of mice receiving unstimulated CD8+ T cells than in mice receiving CD44hiCD8+ T cells (Figure 6A). Despite this disparity in the number of recovered donor CD8+ T cells in GVHD target tissues, CFSE dilution analysis showed that donor CD44hiCD8+ T cells vigorously proliferated in host spleen, LNs, and liver by day 14 following their transplantation. In fact, CD44hiCD8+ T cells underwent, on average, more divisions than did CD44loCD8+ T cells (Figure 6B). These results indicate that the accumulation of CD44hiCD8+ T cells in both lymphoid and nonlymphoid tissues is impaired in vivo despite their vigorous proliferation in vivo, and this does not appear to be associated with any detectable impairment of CD44hiCD8+ T-cell ability to home to either lymphoid organs or target tissues.
The expansion of DC-induced CD44hiCD8+ T cells is impaired in vivo. CD44hiCD8+ and CD44loCD8+ T cells were sorted from the B6 DC-stimulated T-cell cultures at day 5 and labeled with CFSE. Then, 1 × 106 of these B6 DC-stimulated CD44hiCD8+, CD44loCD8+, or unstimulated CCD8+ T cells (CD45.2) were mixed with 5 × 106 B6 T–BM (CD45.1) and transplanted into lethally irradiated B6 mice (3 for each group). Donor CD8+ T cells recovered from the spleens, LNs, and livers of these B6 recipients were analyzed by flow cytometry. (A) The number of donor CD8+ T cells from the spleens, LNs, and livers of B6 mice receiving CD44hiCD8+, CD44loCD8+, or unstimulated CD8+ T cells at days 1, 7, and 14 following transplantation were calculated. *P < .01 (mice receiving CD44hiCD8+ T cells versus mice receiving CD44loCD8+ or unstimulated CD8+ T cells). All values represent means ± SD. (B) The dilution of CFSE fluorescence of donor CD8+ T cells recovered from the spleens, LNs, and livers of these B6 recipients at day 14 following their infusion were analyzed by flow cytometry. Data shown here are gated donor CD8+ T cells, which derive from 3 independent experiments. Numbers in quadrants represent percentages of each cell fraction.
The expansion of DC-induced CD44hiCD8+ T cells is impaired in vivo. CD44hiCD8+ and CD44loCD8+ T cells were sorted from the B6 DC-stimulated T-cell cultures at day 5 and labeled with CFSE. Then, 1 × 106 of these B6 DC-stimulated CD44hiCD8+, CD44loCD8+, or unstimulated CCD8+ T cells (CD45.2) were mixed with 5 × 106 B6 T–BM (CD45.1) and transplanted into lethally irradiated B6 mice (3 for each group). Donor CD8+ T cells recovered from the spleens, LNs, and livers of these B6 recipients were analyzed by flow cytometry. (A) The number of donor CD8+ T cells from the spleens, LNs, and livers of B6 mice receiving CD44hiCD8+, CD44loCD8+, or unstimulated CD8+ T cells at days 1, 7, and 14 following transplantation were calculated. *P < .01 (mice receiving CD44hiCD8+ T cells versus mice receiving CD44loCD8+ or unstimulated CD8+ T cells). All values represent means ± SD. (B) The dilution of CFSE fluorescence of donor CD8+ T cells recovered from the spleens, LNs, and livers of these B6 recipients at day 14 following their infusion were analyzed by flow cytometry. Data shown here are gated donor CD8+ T cells, which derive from 3 independent experiments. Numbers in quadrants represent percentages of each cell fraction.
FasL-mediated deletion of alloreactive CD44hiCD8+ T cells
One alternative mechanism that could explain impaired expansion of proliferative T cells in vivo is cell deletion. Indeed, we found that there were many more donor CD8+ T cells undergoing apoptosis within 14 days after BMT in the spleens of B6 mice receiving CD44hiCD8+ T cells versus CD44loCD8+ T cells (Figure 7A). Whereas less than 3% of infused CD44loCD8+ T cells stained with annexin V, 5% to 20% of donor CD8+ T cells recovered from the spleens, LNs, and livers of mice receiving CD44hiCD8+ T cells were annexin V+ during the period of 14 days after transplantation (Figure 7B; P < .05). However, when CD44hiCD8+ and CD44loCD8+ T cells were cultured ex vivo in the presence of IL-2 plus IL-15 for 24 hours without in vivo transplantation, apoptotic cell death was very low (Figure 7A). Thus, increased deletion of CD44hiCD8+ T cells occurs in vivo during their proliferation and differentiation.
DC-induced C3H.SW CD44hiCD8+ T cells induce lethal GVHD in gld.B6 mice. Highly purified C3H.SW CD44loCD8+ T cells (CD45.2) were cultured in the presence of B6 DCs and IL-2 plus IL-15 for 5 days. Cells were collected and sorted into CD44hiCD8+ and CD44loCD8+ T-cell subsets. One million of these B6 DC-stimulated CD44hiCD8+ or CD44loCD8+ T cells were mixed with 5 × 106 B6 T–BM (CD45.1) and transplanted into lethally irradiated B6 mice. (A-B) Donor CD8+ T cells prior to their infusion or recovered from the spleens, LNs, and livers of B6 recipients at days 1, 7, and 14 following transplantation were stained with annexin V for flow cytometry analysis (A) and annexin V+ donor CD8+ T cells were calculated (B). The results shown are from one of 3 experiments. *P < .05 (mice receiving CD44hiCD8+ T cells versus mice receiving CD44loCD8+ T cells). (C) Unstimulated and DC-stimulated CD44loCD8+ T cells at day 5 were stained with anti-Fas, anti-CD44, and anti-CD8 Abs followed by flow cytometry analysis. In panels A and C, the numbers represent percentages of each cell fraction. In panel B, the values represent means ± SD. (D-E) C3H.SW T–BM (CD45.1, 7 × 106), mixed with or without B6 DC-stimulated CD44hiCD8+ or CD44loCD8+ T cells (2 × 106), was transplanted into lethally irradiated gld.B6 or wild-type B6 mice. The clinical GVHD signs and survival of these recipient mice were monitored. In panel D, ♦, T–BM → gld.B6 mice (n = 6); ▪, T–BM plus CD44hiCD8+ → gld.B6 mice (n = 9); ▴, T–BM plus CD44hiCD8+ → B6 mice(n = 4). *P < .01 (▪ versus ▴). In panel E, ♦, T–BM → B6 mice (n = 6); ▪, T–BM plus CD44loCD8+ → gld.B6 mice (n = 10); ▴, T–BM plus CD44loCD8+ → B6 mice (n = 12).
DC-induced C3H.SW CD44hiCD8+ T cells induce lethal GVHD in gld.B6 mice. Highly purified C3H.SW CD44loCD8+ T cells (CD45.2) were cultured in the presence of B6 DCs and IL-2 plus IL-15 for 5 days. Cells were collected and sorted into CD44hiCD8+ and CD44loCD8+ T-cell subsets. One million of these B6 DC-stimulated CD44hiCD8+ or CD44loCD8+ T cells were mixed with 5 × 106 B6 T–BM (CD45.1) and transplanted into lethally irradiated B6 mice. (A-B) Donor CD8+ T cells prior to their infusion or recovered from the spleens, LNs, and livers of B6 recipients at days 1, 7, and 14 following transplantation were stained with annexin V for flow cytometry analysis (A) and annexin V+ donor CD8+ T cells were calculated (B). The results shown are from one of 3 experiments. *P < .05 (mice receiving CD44hiCD8+ T cells versus mice receiving CD44loCD8+ T cells). (C) Unstimulated and DC-stimulated CD44loCD8+ T cells at day 5 were stained with anti-Fas, anti-CD44, and anti-CD8 Abs followed by flow cytometry analysis. In panels A and C, the numbers represent percentages of each cell fraction. In panel B, the values represent means ± SD. (D-E) C3H.SW T–BM (CD45.1, 7 × 106), mixed with or without B6 DC-stimulated CD44hiCD8+ or CD44loCD8+ T cells (2 × 106), was transplanted into lethally irradiated gld.B6 or wild-type B6 mice. The clinical GVHD signs and survival of these recipient mice were monitored. In panel D, ♦, T–BM → gld.B6 mice (n = 6); ▪, T–BM plus CD44hiCD8+ → gld.B6 mice (n = 9); ▴, T–BM plus CD44hiCD8+ → B6 mice(n = 4). *P < .01 (▪ versus ▴). In panel E, ♦, T–BM → B6 mice (n = 6); ▪, T–BM plus CD44loCD8+ → gld.B6 mice (n = 10); ▴, T–BM plus CD44loCD8+ → B6 mice (n = 12).
Because previous studies have shown that Fas-FasL–mediated cell death is an important mechanism for T-cell deletion in vivo and may play a role in the production of GVHD,28 we asked whether Fas-FasL might be important in deleting CD44hiCD8+ T cells. First, we found that CD44hiCD8+ T cells expressed higher levels of Fas than did CD44loCD8+ T cells (Figure 7C). Next, we asked whether CD44hiCD8+ T cells could induce GVHD in FasL-deficient gld.B6 recipient mice. Six of 9 irradiated gld.B6 mice receiving C3HSW T–BM plus CD44hiCD8+ T cells developed significant clinical signs of GVHD and 4 died from acute GVHD, whereas C3HSW T–BM alone did not induce GVHD in gld.B6 mice (Figure 7D) and all 10 gld.B6 mice receiving C3HSW T–BM plus CD44loCD8+ T cells died from acute GVHD (Figure 7E). Taken together, all these results indicate that FasL-mediated deletion of DC-induced CD44hiCD8+ T cells accounts, at least in part, for their inability to induce GVHD.
Discussion
These studies demonstrate that C3H.SW CD44hiCD8+ T cells are functionally defective for inducing acute GVHD in B6 mice, whereas CD44loCD8+ T cells are potent inducers of acute GVHD. Although this marked difference in GVHD potency occurs whether the CD8+ T-cell subsets were obtained from unstimulated or allogeneic DC-stimulated C3H.SW CD8+ T cells, we found that DC activation directly induces the generation of CD44hiCD8+ T cells from CD44loCD8+ T cells. Despite their inability to produce GVHD, these DC-induced CD44hiCD8+ T cells are potent cytolytic killers with up-regulated CD25, CD69, CD122, FasL, perforin, and granzyme B in vitro, and they differentiate in vivo into CD44hiCD62LhiCD122hi CD8+ central memory-like T cells. In comparison, despite stimulation with allogeneic DCs, CD44loCD8+ T cells are phenotypically naive cells expressing low levels of CD25, CD69, and CD122 prior to infusion, but they differentiate into potent GVHD effector cells in vivo. Thus, CD44loCD8+ T cells are responsible for inducing lethal GVHD in B6 mice, whereas allogeneic DC-activated CD44hiCD8+ T cells may be excellent candidates for mediating antitumor activity in clinical BMT, without causing life-threatening GVHD.
It is somewhat surprising that DC-induced CD44hiCD8+ T cells with host-specific cytolytic activity cannot induce GVHD, whereas phenotypically naive CD44loCD8+ T cells are potent GVHD inducers. Although CD44hiCD8+ T cells in normal mice may develop following antigenic stimulation by environmental pathogens, some may be truly antigenically naive and have broad antigenic specificity.20-26 Although immunization of mice can also significantly increase the frequencies of antigen-specific CTLs in CD44hiCD8+ T cells 8- to 30-fold more than in CD44loCD8+ T cells,20,25 naive CD8+ T cells undergoing homeostatic proliferation may acquire the memory-like CD44hiCD62LhiCD25loCD69lo phenotype.21-24,26 Despite these observations, and despite the observation that allogeneic DCs did induce the generation of CD44hiCD8+ T cells from naive CD44loCD8+ T-cell precursors, we found that allogeneic DC-induced CD44hiCD8+ T cells are ineffective in mediating acute GVHD in vivo. This finding does not support strategies that selectively deplete alloreactive T cells expressing CD25 and CD69 may prevent GVHD,4-7 and may explain why donor T cells depleted of CD25+ or CD69+ (or both) alloreactive T cells can still elicit lethal GVHD in vivo in several previous animal studies.8,9 Thus, although depletion of alloreactive T cells expressing CD25 or CD69 (or both) can be achieved in vitro,4-7 this might not result in GVHD prevention in vivo, due to the existence of potentially alloreactive T cells not yet expressing T-cell activation markers and undergoing cell divisions.
The impaired expansion of donor CD44hiCD8+ T cells in vivo was paralleled and likely explained by their apoptotic deletion during their proliferation and differentiation. We found that there were significantly more donor-derived CD8+ T cells undergoing apoptosis in irradiated B6 mice receiving B6 DC-induced C3H.SW CD44hiCD8+ T cells compared with CD44loCD8+ T cells. These findings may be related to the observations of Auphan-Anezin et al that in vivo stimulation of CD8+ T cells with a weak partial agonist alloantigen leads to sustained accumulation of these T cells, whereas activation of CD8+ T cells with a strong agonist alloantigen results in their apoptotic death in vivo.29,30 Other related results have also been observed by Yu et al in the GVHD models, where anti-CD28 antibody in vivo initially induces T-cell proliferation, but prevents the expansion of T cells at later stages, leading to the inhibition of acute GVHD.31 In our experiments, ex vivo culture of C3H.SW CD8+ T cells with a high density of B6 DCs may provide both weak and strong alloantigenic signals; the weaker signals might prime donor CD8+ T cells without cell activation and division in vitro but render them resistant to apoptotic death in vivo; the stronger signals might fully activate donor CD8+ T cells to proliferate in vitro followed by apoptotic deletion after reencountering the same antigens in vivo. Such active antigen-driven T-cell death may be mediated by the expression of death cytokines such as FasL and TNF-α,28 because preparative conditioning for allo-BMT may markedly increase the production of FasL and TNF-α.1,32 We found that ex vivo stimulation of CD8+ T cells with allogeneic DCs induced higher levels of Fas expression on CD44hiCD8+ T cells than on CD44loCD8+ T cells. In contrast to their defective induction in GVHD in wild-type B6 mice, CD44hiCD8+ T cells induced lethal GVHD in 44% of gld.B6 mice that are deficient in FasL expression. These results, together with previous data,33 suggest that FasL on host tissues may play an important role in modulating the expansion and the ability to induce GVHD of CD44hiCD8+ T cells.
The inability of donor CD44hiCD8+ T cells to induce acute GVHD may have been accentuated by continued antigenic activation in the hosts. Although a substantial DC-induced C3H.SW CD44hiCD8+ T-cell population with central memory phenotype (CD62LhiCD122hiCD25lo) survived in B6 recipients, they did not induce acute GVHD. Previous studies have demonstrated that chronic antigenic stimulation can diminish antigen-specific CD8+ T-cell responses in vivo.23,24,34 For example, when lymphocytic choriomeningitis virus (LCMV) carrier mice were injected with LCMV antigen-specific CD8+ effector/memory-like T cells isolated from LCMV-immunized mice between day 8 and 15 following immunization, viral titers initially were decreased but eventually returned to pretransfer levels, and the infection was not controlled.23,24 A recent study also demonstrated that even memory CD8+ T cells can undergo peripheral tolerance. Mice preimmunized with influenza virus to induce the development of flu antigen-specific memory T cells subsequently generated weaker CD8+ T-cell responses when restimulated with a large amount of flu antigen KdHA than did control mice restimulated with phosphate-buffered saline or irrelevant antigen.34 In the present GVHD model, we have previously found that lethal irradiation of mice results in rapid activation of host APCs, which express high levels of antigen-presenting and costimulatory molecules.14 It is probable that adoptively transferred allogeneic DC-induced CD44hiCD8+ T cells may be further stimulated in vivo by these activated APCs, thereby reducing their ability to induce GVHD.
Despite their inability to cause GVHD, CD44hiCD8+ T cells retain their ability to kill EL-4 leukemic cells in vivo and in vitro. Although CD44hiCD8+ T cells did not prevent the ultimate progression of EL-4 leukemia in recipient mice, our results confirm previous observations of Sykes et al and Blazar et al that EL-4 leukemia is not eradicated by donor T cells in this setting.18,35 Interestingly, EL-4 cells expressing B7-1 appear to be more sensitive to GVL induction or targeting in vivo, with significantly prolonged survival,18 suggesting that enhanced stimulation of donor T cells by B7-1 molecules on leukemic cells can markedly augment T cell–mediated GVL activity. Mature DCs used in our culture system are specialized APCs with great ability to process various antigens and to provide both antigenic and costimulatory signals to activate T cells.36 We found that DC-activated CD44hiCD8+ T cells were able to specifically kill not only EL-4 cells but also other tumor cells, such as C1498 myeloid leukemic cells and MC57 sarcoma cells. By contrast, although unstimulated whole CD8+ T cells might potentially protect mice from EL-4 leukemia, they caused lethal GVHD in most B6 recipient mice, which precludes us from precisely quantifying their antileukemia efficiency in vivo. Thus, in vitro DC stimulation may provide a promising platform to selectively amplify tumor antigen-specific T cells ex vivo and reconstitute antitumor activity in vivo without inducing life-threatening GVHD.
Why C3H.SW CD44hiCD8+ T cells retain their ability to kill EL-4 leukemic cells despite their inability to mediate GVHD is uncertain. Given our finding that these cells proliferate but do not substantially accumulate in vivo, one possibility is that elimination of EL-4 cells does not require either as many or as long-lived tumor-specific CTLs. In contrast, the development of acute GVHD is likely a more complex process, requiring the activity of large numbers of persistent alloreactive CD8+ T cells in many tissues followed by the presence of other inflammatory cytokines and cells.1-3 Thus, the threshold for anti–EL-4 cell elimination may be much lower than for inducing GVHD. Another possibility is that EL-4 cell killing can be mediated by several low-affinity interactions between CD8+ T cells and subdominant tumor cell epitopes.37 Because T cells with higher antigen/T-cell receptor (TCR) avidity may be eliminated, whereas those T cells with lower TCR avidity can be preserved,38-40 it is possible that host mature DC-activated donor CD8+ T cells with higher TCR avidity for alloantigen could therefore be rapidly eliminated when they encounter host antigens presented by APCs in vivo after adoptive transfer. In contrast, donor CD8+ T cells with lower avidity TCR for EL-4 antigens may be spared, resulting in effective EL-4 cell killing without GVHD.
The present results support and extend recent findings of Anderson et al, who found that memory CD4+ T cells (CD44hiCD62Llo) derived from either nonrecipient antigen-immunized or normal donor B10.D2 mice do not induce GVHD in Balb/c recipient mice. Although the Anderson et al study did not address GVL per se, and indeed CD8+ T cells play a major role in antileukemic reactivity, they did find that memory CD4+ T cells can engraft in vivo and specifically respond to a third-party immunogen.41 Thus our data support their observations and indicate their applicability to CD8+ T cells and their target antigens, including epitopes responsible for GVL. In our experiments, transplanted CD44hiCD8+ effector/memory-like T cells engrafted in vivo with an ability to produce high levels of IFN-γ and to kill leukemic cells in vivo equivalent to unstimulated CD8+ T cells, although they did not prevent leukemic progression in all animals.
In addition to its potential for the prevention of GVHD in BMT, the present findings may also be significant for ex vivo DC immunization strategies that are designed to produce activated T cells for treatment of tumor and infectious diseases.24,36 We found that CD44loCD8+ T cells actually caused more severe GVHD than unmanipulated CD8+ T cells that contained 0.5 × 106 CD44hiCD8+ and 1.5 × 106 CD44loCD8+ cells, and more severe disease than the mixture of DC-induced CD8+ T cells that was composed of about 0.8 to 1.0 × 106 CD44hiCD8+ and 1.0-1.2 × 106 CD44loCD8+ cells (Figure 2B). Because 1.0 × 106 DC-induced CD44loCD8+ T cells were sufficient to cause lethal GVHD in all recipient mice (Figure 2C), it is possible that CD44hiCD8+ T cells might be able to modulate acute GVHD induced by CD44loCD8+ T cells via unknown mechanisms. Nevertheless, our findings, together with those in other antigen-specific systems,23,34,42,43 suggest that antigen-specific T cells expressing activation/memory phenotypes may be relatively ineffective in vivo in eradicating specific pathogens, so that generation or enrichment of this T-cell subset for therapy may blunt the desired antigen-specific response. Thus, we propose that purification of DC-induced CD44loCD8+ T cells rather than CD44hiCD8+ memory-like T cells following ex vivo vaccination may provide a more potent reagent for antigen-specific antiviral and antitumor therapy.
In summary, we have demonstrated that ex vivo allogeneic DC activation can separate donor CD8+ T cell–mediated GVL activity from that of GVHD. DC-induced CD44hiCD8+ T cells are defective in their ability to cause GVHD but can kill EL-4 leukemic cells in vivo. These findings suggest that this allogeneic mature DC-based culture system can be used for generating CD44hiCD8+ T cells to preserve antileukemic effects, and for identifying potent GVHD-inducing CD44loCD8+naive T cells, which can be removed prior to transplantation.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-09-3135.
Supported by a Specialized Center for Research (SCOR) grant from the Leukemia and Lymphoma Society of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal