Abstract
Xenotransplantation from pigs could provide a potential solution to the severe shortage of allogeneic donor organs. Because xenogeneic tissues are subject to vigorous immune rejection, tolerance induction is likely to be essential to the success of clinical xenotransplantation. Here we explore the possibility of inducing human T-cell tolerance to porcine xenografts through mixed chimerism. We previously showed that NOD/SCID-Tg mice expressing porcine cytokine transgenes permit the induction of durable porcine hematopoietic chimerism. In this study we achieved human T-cell development in these mice by engrafting human fetal thymus/liver tissues. In porcine hematopoietic chimeras, human thymus grafts were populated with porcine class IIhigh cells in addition to human cells, and human T cells were tolerant of the porcine hematopoietic donor as measured by mixed lymphocyte reaction assay and skin grafting. This study proves the principle that porcine chimerism induces tolerance of xenoreactive human T cells.
Introduction
The severe shortage of allogeneic organ donors currently limits the number of transplantations performed.1,2 This supply-demand disparity could be corrected by the ability to use organs from other species (xenografts). In view of the ethical issues and impracticalities associated with the use of nonhuman primates, interest has focused on other species, in particular the pig, as the most suitable organ donor species for humans. In addition to organ size and physiologic similarities to humans, the ability to rapidly breed and inbreed pigs makes them particularly amenable to genetic modifications that could improve their ability to function as organ donors to humans.3-7 However, organ transplantations across discordant species barriers are subject to vigorous immunologic rejection.1,8 One might, therefore, expect the amount of nonspecific immunosuppression that would be required to overcome xenograft rejection to be so great that recipients would succumb to infections. Thus, it would be highly desirable to eliminate the immune response to xenografts through the induction of tolerance.
Mixed chimerism has been proven to be a powerful and reliable approach for tolerance induction across allogeneic and closely related xenogeneic barriers.9-14 Our recent studies showed that this approach also induces tolerance in a pig-to-mouse, highly disparate xenogeneic combination.15 However, the ability of mixed chimerism to induce human T-cell tolerance to porcine xenografts has yet to be explored because of the lack of a suitable model system. It has been unclear whether or not genetic incompatibilities in immune cytokines,16,17 accessory molecules,17-19 and other unknown molecules between the 2 species might impede the induction of human T-cell tolerance to porcine xenografts through mixed chimerism. To address these questions, we have developed a murine model that permits the induction of both sustained porcine hematopoietic chimerism and active human thymopoiesis. Our results demonstrate the principle that mixed hematopoietic chimerism can mediate deletion of donor antigen-reactive human thymocytes in the human thymus and induce human T-cell tolerance to porcine xenografts.
Materials and methods
Animals and human fetal tissues
Immunodeficient nonobese diabetic/severe combined immunodeficient–transgenic (NOD/SCID-Tg) mice expressing porcine cytokine transgenes (interleukin 3 [IL-3], granulocyte-macrophage colony-stimulating factor [GM-CSF], and stem cell factor [SCF])20 were housed in a specific pathogen-free microisolator environment and used at 6 to 10 weeks of age. Inbred Massachusetts General Hospital miniature swine (3-5 months old)3 were used as porcine bone marrow donors. Human fetal thymus and liver tissues (17- to 20-week-old fetuses) were obtained from Advanced Bioscience Resource (Alameda, CA). Protocols involving human tissues and animals were approved by the Massachusetts General Hospital human research committee and subcommittee on research animal care, respectively.
Porcine bone marrow transplantation and human tissue implantation
NOD/SCID-Tg mice were conditioned with 3 Gy whole body irradiation (WBI) and given intravenous transplants of 1 × 108 porcine bone marrow cells (BMCs) on the same day.20 Human fetal thymus and liver (Thy/Liv) fragments (∼1 mm3) were implanted under the recipient kidney capsule 3 to 10 days after WBI. In some experiments, mice were additionally injected intravenously with human CD34+ cells (1 × 105/mouse) purified from the fetal liver of the same fetuses on the day of human Thy/Liv implantation. Levels of porcine and human chimerism were determined by multicolor flow cytometric (FCM) analysis of blood cells and single-cell suspensions of the recipient tissues and human Thy/Liv grafts. To prepare hepatic mononuclear cells, livers were perfused with heparinized phosphatebuffered saline (PBS), removed, gently crushed using a syringe plunger, and pressed through nylon mesh. Mononuclear cells were separated from liver cells by centrifugation using Histopaque (Sigma, St Louis, MO). Porcine antigen-specific monoclonal antibodies20 (mAbs) used in this study were 1030H-1-19 (pig pan-tissue), 74-22-15 (SWC3, expressed on monocytes, macrophages, and granulocytes),21 MSA4 (CD2), 898H2-6-15 (CD3), 74-12-4 (CD4), 76-2-11 (CD8), G7 (CD16, kindly provided by Dr Y. B. Kim, Chicago, IL), and BB6-11C9.6 (CD21; Southern Biotechnology, Birmingham, AL). Anti–HLA class I mAb (W6/32) was obtained from Leinco (St Louis, MO). All other mAbs, including antihuman mAbs (CD3, CD4, CD8, CD19, CD20, CD34, CD45, and CD45RA), antimouse CD45, and isotype control mAbs were purchased from BD PharMingen (San Diego, CA). Analysis was performed on a FACSCalibur (Becton Dickinson, Mountain View, CA) and dead cells were excluded by gating out low forward scatter plus high propidium iodide-retaining cells.
Confocal scanning laser microscopy
Human Thy/Liv grafts and normal human and porcine thymic tissues were cryosectioned and fixed with cold acetone for 10 minutes. After blocking with 1% bovine serum albumin (BSA) for 20 minutes, tissue sections were incubated with pretitrated primary mouse antibodies (anti–HLA DR [G46-6, IgG2a; BD PharMingen]; antiswine leukocyte antigen [SLA] DR [1053H2-18-1, IgG2a]; or isotype control mAb HOPC [IgG2a; BD PharMingen]) for 45 minutes at room temperature, washed, and incubated with fluorescein isothiocyanate (FITC)–conjugated rat antimouse IgG2a or biotinylated rat antimouse IgG2a plus Texas red-avidin (BD PharMingen). After washing with PBS, the coverslips were mounted onto slides using Dako fluorescent mounting medium (Dako, Carpinteria, CA). Specimens were examined by confocal imaging using a Leica DM IRBE microscope and the Leica TCS 4D confocal system.
Mixed lymphocyte reaction assay
Single-cell suspensions of the human Thy/Liv grafts and the mouse recipient spleens were prepared and used as responder cells. Triplicate wells containing 4 × 105 responders with irradiated (30 Gy) stimulators consisting of porcine (4 × 105) or human (4 × 105) peripheral blood mononuclear cells (PBMCs) were incubated in AIM-V medium containing 10% human AB serum (Sigma) at 37° C in 5% CO2. Cultures were pulsed with [3H]-TdR on day 3 and harvested 18 hours later. Data are expressed as stimulation index (cpm of stimulated culture/cpm of unstimulated culture). Unstimulated cultures were the same responder cells incubated with medium or irradiated autologous human fetal liver cells (cryopreserved at the time of human Thy/Liv implantation). Proliferation of unstimulated responder cells ranged between 300 and 1000 cpm.
Skin grafting
Split thickness (2.3 mm) porcine skin samples from a donor SLA-matched to the BMC donor pig and SLA-mismatched (third-party) pigs were grafted on the lateral thoracic wall 7 weeks after human tissue transplantation.15 Skin grafts were evaluated daily from day 7 onward to 4 weeks and then at least one inspection every third day thereafter. Grafts were defined as rejected when less than 10% of the graft remained viable.
Results
Induction of porcine hematopoietic chimerism and human T-cell development in NOD/SCID-Tg mice
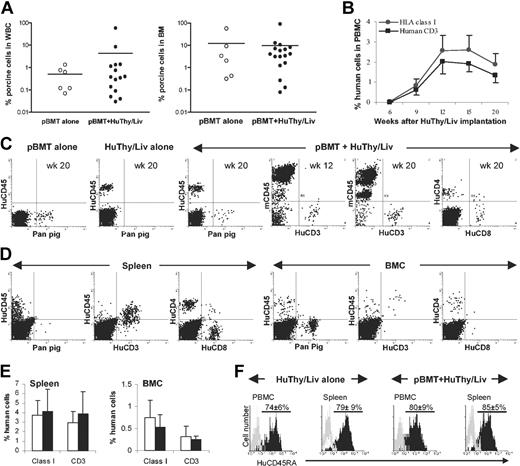
We have previously developed a strain of immunodeficient NOD/SCID-Tg mice expressing porcine cytokine transgenes (IL-3, GM-CSF, and SCF) and demonstrated that durable porcine hematopoietic stem cell (HSC) engraftment and chimerism can be achieved in these animals after 3 Gy WBI.20 To study the potential of porcine hematopoietic chimerism to induce human T-cell tolerance, we attempted to achieve human thymopoiesis and T-cell development in NOD/SCID-Tg mice with pre-established porcine hematopoietic chimerism. Porcine hematopoietic chimeras were prepared by porcine bone marrow transplantation (BMT) in 3 Gy WBI-conditioned NOD/SCID-Tg mice as previously described.20 Some of these mice and conditioned NOD/SCID-Tg mice that did not undergo porcine BMT were subsequently given implants of human fetal Thy/Liv fragments to achieve human thymopoiesis and T-cell development. FCM analysis revealed that long-term porcine hematopoietic chimerism was achieved in all porcine BM transplant recipients (Figure 1A). The development of human T cells in porcine hematopoietic chimeras (ie, mice that underwent porcine BMT prior to human Thy/Liv implantation) did not affect porcine hematopoietic repopulation (Figure 1A) and both porcine and human chimerism were durably maintained in these mice (Figure 1B-F). Animals that received porcine BM transplants alone and those given implants with human Thy/Liv tissues without prior porcine BM transplants showed repopulation with only porcine or human cells, respectively (Figure 1C). In human Thy/Liv-transplanted mice, human cells (identified as HLA class I+ or human CD45+) consisting mainly of CD3+ T cells (including both CD4+ and CD8+ T cells) became detectable by 6 weeks and reached peak levels by 12 weeks in blood after human tissue implantation (Figure 1B-C). Human T cells were also detected in the recipient spleen and BM at the time they were killed, 20 weeks after human Thy/Liv implantation (Figure 1D-E). The majority of human T cells were CD45RA+ cells in human Thy/Liv-transplanted mice, regardless of whether or not a porcine BM transplant was given, consistent with a “naive” status (Figure 1F).
Durable mixed porcine and human chimerism in NOD/SCID-Tg mice receiving porcine BM transplants and subsequent human Thy/Liv implants. (A) Porcine hematopoietic chimerism in the white blood cells (WBCs) and BM of NOD/SCID-Tg mice that received porcine BM transplants alone (○) or porcine BM transplants plus human Thy/Liv grafts (•) at week 20 after human Thy/Liv transplantation. Each symbol represents an individual animal. Horizontal lines indicate the mean chimerism level for each group of mice. (B) Kinetics of human chimerism in the PBMCs of NOD/SCID-Tg mice given transplants with porcine BMCs and human Thy/Liv tissues (mean ± SD; n = 17). (C) Peripheral blood cells were collected from NOD/SCID-Tg mice that received porcine BM transplants alone (pBMT, n = 6), human Thy/Liv alone (HuThy/Liv, n = 8), or porcine BM transplants plus human Thy/Liv (n = 17) at the indicated times after human Thy/Liv implantation, and stained with various combinations of antihuman (CD45, CD3, CD4, and CD8), antipan pig tissue (Pan pig), and antimouse CD45 (mCD45) mAbs. Shown are representative FCM profiles. (D) FCM profiles showing porcine and human chimerism in the spleen and BM of NOD/SCID-Tg mice given transplants of porcine BMCs plus human Thy/Liv tissues at week 20 after human Thy/Liv implantation. (E) Percentages (means ± SD) of human class I+ and CD3+ cells in the spleen and BMCs of NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv grafts (□; n = 17) or human Thy/Liv tissues alone (▪; n = 8) at week 20 after human Thy/Liv implantation. (F) Expression of human CD45RA on gated human CD4+ cells. Black and gray histograms are staining profiles of anti-CD45RA and isotype control mAbs, respectively. The percentages (means ± SD) of CD45RA+ cells in the gated human CD4+ cell population were comparable between HuThy/Liv alone and pBMT plus HuThy/Liv groups (P = .25 and P = .17 for PBMCs and spleen, respectively).
Durable mixed porcine and human chimerism in NOD/SCID-Tg mice receiving porcine BM transplants and subsequent human Thy/Liv implants. (A) Porcine hematopoietic chimerism in the white blood cells (WBCs) and BM of NOD/SCID-Tg mice that received porcine BM transplants alone (○) or porcine BM transplants plus human Thy/Liv grafts (•) at week 20 after human Thy/Liv transplantation. Each symbol represents an individual animal. Horizontal lines indicate the mean chimerism level for each group of mice. (B) Kinetics of human chimerism in the PBMCs of NOD/SCID-Tg mice given transplants with porcine BMCs and human Thy/Liv tissues (mean ± SD; n = 17). (C) Peripheral blood cells were collected from NOD/SCID-Tg mice that received porcine BM transplants alone (pBMT, n = 6), human Thy/Liv alone (HuThy/Liv, n = 8), or porcine BM transplants plus human Thy/Liv (n = 17) at the indicated times after human Thy/Liv implantation, and stained with various combinations of antihuman (CD45, CD3, CD4, and CD8), antipan pig tissue (Pan pig), and antimouse CD45 (mCD45) mAbs. Shown are representative FCM profiles. (D) FCM profiles showing porcine and human chimerism in the spleen and BM of NOD/SCID-Tg mice given transplants of porcine BMCs plus human Thy/Liv tissues at week 20 after human Thy/Liv implantation. (E) Percentages (means ± SD) of human class I+ and CD3+ cells in the spleen and BMCs of NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv grafts (□; n = 17) or human Thy/Liv tissues alone (▪; n = 8) at week 20 after human Thy/Liv implantation. (F) Expression of human CD45RA on gated human CD4+ cells. Black and gray histograms are staining profiles of anti-CD45RA and isotype control mAbs, respectively. The percentages (means ± SD) of CD45RA+ cells in the gated human CD4+ cell population were comparable between HuThy/Liv alone and pBMT plus HuThy/Liv groups (P = .25 and P = .17 for PBMCs and spleen, respectively).
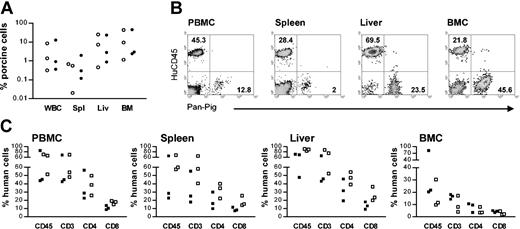
Durable coengraftment of porcine and human hematopoietic cells was also observed in mice that were given grafts of human Thy/Liv tissues under the subcapsular space of both kidneys (Figure 2). Compared to NOD/SCID-Tg mice that received human Thy/Liv grafts under the capsule of a single kidney (Figure 1), these mice showed markedly increased repopulation by human T cells (Figure 2). At week 25 after human Thy/Liv implantation, both porcine and human cells were detected in multiple tissues of the NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv tissues (Figure 2A-B). Despite the high levels of human T-cell repopulation, the levels of porcine chimerism in these mice were comparable to those in the recipients of porcine BM transplants alone (Figure 2A). In addition, the recipients of porcine BM transplants plus human Thy/Liv grafts and the mice that received human Thy/Liv alone, showed comparable levels of human CD45+, CD3+, CD4+, and CD8+ cells in all tissues examined, including blood, spleen, liver (hepatic mononuclear cells), and BM (Figure 2C). The lack of rejection of porcine hematopoietic cells in the recipients of porcine BM transplants plus human Thy/Liv tissues (Figures 1, 2) suggested that human T cells that developed in the porcine hematopoietic chimeras might be tolerant of the porcine donor.
Persistence of porcine chimerism and enhanced human T-cell development in NOD/SCID-Tg mice grafted with human Thy/Liv tissues under the subcapsular space of both kidneys. Shown are porcine and human chimerisms in various tissues at 25 weeks after human Thy/Liv implantation. (A) Porcine hematopoietic chimerism in the WBCs, spleen (Spl), liver (Liv), and BM of NOD/SCID-Tg mice that received porcine BM transplants alone (○) or porcine BM transplants plus human Thy/Liv grafts (•). Each symbol represents an individual animal. (B) FCM profiles showing coengraftment of porcine and human cells in various tissues of a NOD/SCID-Tg mouse recipient of porcine BM transplants plus human Thy/Liv grafts. Numbers indicate the percentages of the corresponding cell populations. (C) Percentages of human CD45+, CD3+, CD4+, and CD8+ cells in various tissues of NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv (▪) or human Thy/Liv alone (□). Each symbol represents an individual animal.
Persistence of porcine chimerism and enhanced human T-cell development in NOD/SCID-Tg mice grafted with human Thy/Liv tissues under the subcapsular space of both kidneys. Shown are porcine and human chimerisms in various tissues at 25 weeks after human Thy/Liv implantation. (A) Porcine hematopoietic chimerism in the WBCs, spleen (Spl), liver (Liv), and BM of NOD/SCID-Tg mice that received porcine BM transplants alone (○) or porcine BM transplants plus human Thy/Liv grafts (•). Each symbol represents an individual animal. (B) FCM profiles showing coengraftment of porcine and human cells in various tissues of a NOD/SCID-Tg mouse recipient of porcine BM transplants plus human Thy/Liv grafts. Numbers indicate the percentages of the corresponding cell populations. (C) Percentages of human CD45+, CD3+, CD4+, and CD8+ cells in various tissues of NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv (▪) or human Thy/Liv alone (□). Each symbol represents an individual animal.
In both the recipients of porcine BM transplants plus human Thy/Liv grafts and the NOD/SCID-Tg mice that received human Thy/Liv tissues alone, human Thy/Liv grafts underwent significant growth (Figure 3A) and maintained a normal thymic structure on histology, including cortex, medulla, and Hassall corpuscles (Figure 3B). In addition, FCM analysis showed a normal distribution of human CD4+CD8–, CD4–CD8+, and CD4+CD8+ thymocyte subsets (Figure 3C-D). The grafts removed from transplanted mice 20 weeks after implantation consisted mainly of human cells (CD45+), of which most cells were HLA class Ilow and over half expressed detectable surface levels of human CD3 (Figure 3C-D). Although small percentages of porcine cells were also detectable in human thymic grafts from porcine hematopoietic chimeras (0.62%-1.71%; Figure 3C), these cells did not express pig CD4, CD8, or CD3, indicating the absence of porcine T-cell development in the human thymic grafts in these mice. In contrast to the Thy/Liv grafts, human Thy tissue grafted without Liv tissue remained small and fibrous and failed to achieve sustained human T-cell repopulation in NOD/SCID-Tg mice (data not shown). These results are consistent with previous reports that a source of lymphoid progenitors is required to achieve human thymopoiesis in immunodeficient mice.22-24 In addition, human T cells were not detected in any mice given grafts with human fetal liver only (n = 5, data not shown). Together, these data indicate that functional thymopoiesis and de novo human T-cell development were achieved in human fetal Thy/Liv-grafted NOD/SCID-Tg mice.
Histologic and phenotypic analysis of human thymus grafts from NOD/SCID-Tg mice that received human Thy/Liv implants. Thy/Liv grafts were removed at week 20 after human tissue implantation from the recipients of porcine BM transplants plus human Thy/Liv grafts (n = 13) or the NOD/SCD-Tg mice that received human Thy/Liv tissues alone (n = 7). (A-C) Data shown are results of a representative graft. (A) Macroscopic appearance. (B) Histology (hematoxylin and eosin; original magnifications × 10 [left panel] and × 40 [right panel]); C indicates cortex; M, medulla; H, Hassall corpuscles. (C) Phenotypic analysis by FCM. (D) Percentages (means ± SD) of human thymocytes expressing various surface markers in the Thy/Liv grafts from NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv (□; n = 13) or human Thy/Liv tissues alone (▪, n = 7).
Histologic and phenotypic analysis of human thymus grafts from NOD/SCID-Tg mice that received human Thy/Liv implants. Thy/Liv grafts were removed at week 20 after human tissue implantation from the recipients of porcine BM transplants plus human Thy/Liv grafts (n = 13) or the NOD/SCD-Tg mice that received human Thy/Liv tissues alone (n = 7). (A-C) Data shown are results of a representative graft. (A) Macroscopic appearance. (B) Histology (hematoxylin and eosin; original magnifications × 10 [left panel] and × 40 [right panel]); C indicates cortex; M, medulla; H, Hassall corpuscles. (C) Phenotypic analysis by FCM. (D) Percentages (means ± SD) of human thymocytes expressing various surface markers in the Thy/Liv grafts from NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv (□; n = 13) or human Thy/Liv tissues alone (▪, n = 7).
Population of human thymic grafts with porcine class II+ cells
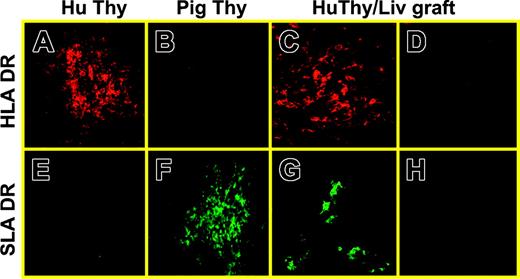
Histologic analysis was performed to detect the population of human thymic grafts with human and porcine antigen-presenting cells (APCs). In this experiment, human Thy/Liv grafts were removed from NOD/SCID-Tg mice that received both porcine BM transplants and human Thy/Liv fragments at week 20 following transplantation and analyzed for human and porcine MHC class II+ cells using confocal microscopy. The presence of SLA class IIhigh cells was clearly identified in tissue sections from 5 of 8 Thy/Liv grafts examined (Figure 4). SLA class II+ cells were detected mainly in the medulla of human thymus grafts and showed morphology suggestive of dendritic cells (DCs) or macrophages.
Reconstitution of human thymus grafts with porcine class II+ cells in NOD/SCID-Tg mice given transplants of porcine BMCs plus human Thy/Liv tissues. (A,E) Normal human fetal thymus; (B,F) normal porcine thymus; (C-D,G-H) a human Thy/Liv graft removed at week 20 after implantation from a NOD/SCID-Tg mouse that received porcine BMCs and human Thy/Liv tissues. Panels A-C, HLA DR; E-G, SLA DR; D,H, isotype control. Original magnifications: × 20 (A-B, E-F); × 40 (C-D, G-H).
Reconstitution of human thymus grafts with porcine class II+ cells in NOD/SCID-Tg mice given transplants of porcine BMCs plus human Thy/Liv tissues. (A,E) Normal human fetal thymus; (B,F) normal porcine thymus; (C-D,G-H) a human Thy/Liv graft removed at week 20 after implantation from a NOD/SCID-Tg mouse that received porcine BMCs and human Thy/Liv tissues. Panels A-C, HLA DR; E-G, SLA DR; D,H, isotype control. Original magnifications: × 20 (A-B, E-F); × 40 (C-D, G-H).
Human T cells that develop in porcine hematopoietic chimeras show donor-specific nonresponsiveness in mixed lymphocyte reaction assay
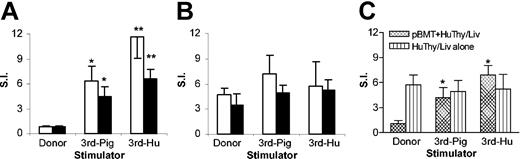
We next assessed the xenoreactivity and tolerance of human T cells that developed in human Thy/Liv-transplanted mice, using a mixed lymphocyte reaction (MLR) assay. Single-cell suspensions of the human thymus grafts and the recipient spleens were prepared at week 20 or 25 after human Thy/Liv implantation and their proliferative responses to stimulators from the porcine BM transplant donor, a SLA-mismatched third-party pig, and an allogeneic human were measured. We have previously shown that NOD/SCID-Tg mice do not support the development of porcine T and B cells and that porcine BMT does not induce “leakiness,” so that NOD/SCID-Tg mouse recipients of a porcine BM transplant remain immunodeficient and show no detectable allogeneic or xenogeneic responses.15,20 Thus, MLR responses, if detected in human Thy/Liv-grafted mice, are expected to be mediated by human T cells. As shown in Figure 5, both human thymocytes and splenocytes from porcine chimeras responded to the third-party pig and allogeneic human stimulators, but not to the donor pig cells (Figure 5A,C). In contrast, human thymocytes and splenocytes from human Thy/Liv-grafted NOD/SCID-Tg mice that did not receive porcine BM transplants showed MLR responses against both the BM transplant donor and third-party pigs, as well as allogeneic human stimulators (Figure 5B,C). Consistent with our previous studies,15 splenocytes from NOD/SCID-Tg mice that received porcine BM transplants alone (n = 8) showed no detectable proliferation in response to any of these stimulators (data not shown).
Human T cells developing in porcine hematopoietic chimeras are specifically tolerant to the porcine hematopoietic donor. (A-B) Human Thy/Liv graft cells (□) and recipient spleen cells (▪) were prepared from NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv (A; n = 9) or human Thy/Liv alone (B; n = 4) at week 20 after human Thy/Liv implantation. (C) Spleen cells were prepared from NOD/SCID-Tg mice that received double amount of human Thy/Liv tissues with (pBMT + HuThy/Liv) or without (HuThy/Liv alone) porcine BM transplants at week 25 after human tissue implantation (these are the same mice shown in Figure 2C). Shown are MLR responses (mean ± SE) against the porcine BM transplant donor (donor), third-party pig (3rd-Pig), or third-party human (3rd-Hu). SI indicates stimulation index. *P < .05, **P < .01 compared to the antidonor pig response of the same responder cells.
Human T cells developing in porcine hematopoietic chimeras are specifically tolerant to the porcine hematopoietic donor. (A-B) Human Thy/Liv graft cells (□) and recipient spleen cells (▪) were prepared from NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv (A; n = 9) or human Thy/Liv alone (B; n = 4) at week 20 after human Thy/Liv implantation. (C) Spleen cells were prepared from NOD/SCID-Tg mice that received double amount of human Thy/Liv tissues with (pBMT + HuThy/Liv) or without (HuThy/Liv alone) porcine BM transplants at week 25 after human tissue implantation (these are the same mice shown in Figure 2C). Shown are MLR responses (mean ± SE) against the porcine BM transplant donor (donor), third-party pig (3rd-Pig), or third-party human (3rd-Hu). SI indicates stimulation index. *P < .05, **P < .01 compared to the antidonor pig response of the same responder cells.
Mixed chimerism induces human T-cell tolerance to porcine skin grafts
We next explored the possibility of inducing human T-cell tolerance to porcine skin grafts through mixed hematopoietic chimerism. Our initial studies showed that NOD/SCID-Tg mice that grafted with human fetal Thy/Liv tissues were incapable of fully rejecting porcine skin grafts, despite the fact that human T cells from these mice could mount antipig responses in vitro in MLR assay (Figure 5). A recent study demonstrated that postthymic self-recognition is needed to maintain the antigen reactivity of mature T cells and interruption of T-cell contact with self-peptide major histocompatibility complex (MHC) ligands leads to a rapid decline in signaling and response sensitivity to foreign stimuli.25 In addition, BM-derived APCs play an important role in the induction of antigen-specific immune responses of T cells.26,27 Thus, the lack of active human hematopoiesis in the recipient BM may be responsible for the insufficient in vivo immune responses of human T cells in human Thy/Liv-grafted mice. To address this question, we recently performed combined transplantation of human Thy/Liv and human HSCs and demonstrated that the addition of CD34+ HSC transplantation can lead to sustained repopulation with multilineage human hematopoietic cells (including T cells, B cells, and DCs) and improved in vivo function of human T cells in NOD/SCID mice given transplants of human fetal Thy/Liv tissues (P.L. et al, manuscript in preparation). Thus, in this experiment NOD/SCID-Tg mice received additional CD34+ cells purified from the liver of the same human fetus. NOD/SCID-Tg mice were conditioned with 3 Gy WBI and given transplants of porcine BMCs on the same day. Some of these porcine BM transplant recipients were subsequently given human fetal Thy/Liv tissues (under the kidney capsule) and CD34+ fetal liver cells intravenously 3 days after porcine BMT. Skin grafting from a donor SLA-matched to the BM donor pig and SLA-mismatched (third-party) pigs was performed 7 weeks after human Thy/Liv/HSC transplantation. FCM analysis confirmed that porcine cells were detectable in the blood of all porcine BM transplant recipients prior to skin grafting, and the levels of porcine cell chimerism were comparable between the recipients of porcine BMCs alone and the mice that received porcine BMCs plus human Thy/Liv and CD34+ cells (6.2% ± 2.5% versus 6.3% ± 4.6% at week 6 after human Thy/Liv/HSC transplantation). As presented in Table 1, neither the donor SLA-matched nor the third-party pig skin grafts were rejected in mice that received porcine BM transplants alone (group A). However, except for one mouse that died on day 40 with surviving skin grafts, all mice that received porcine BM transplants and human Thy/Liv/HSC tissues rejected third-party pig skin grafts (day 14, 38, and 55) while maintaining the BM donor SLA-matched grafts (group B). In contrast, mice that received human Thy/Liv/HSCs without porcine chimerism (group C) rejected both SLA-matched and third-party skin grafts. Thus, by the stringent test of skin grafting, porcine BM transplants induced transplantation tolerance in mice with human T cells developing in human thymus grafts.
Skin graft survival in reconstituted NOD/SCID-Tg mice
. | . | Individual graft survival, d . | . | |
|---|---|---|---|---|
| Group . | Transplant . | SLA-matched . | Third party . | |
| A (n = 5) | pBMT | > 120 | > 120 | |
| B (n = 4)* | pBMT; HuThy/Liv/HSC | > 120 (n = 3), > 40† | 14, 38, 55, > 40† | |
| C (n = 3)* | HuThy/Liv/HSC | 14, 38, > 120‡ | 38, 31, > 120‡ | |
. | . | Individual graft survival, d . | . | |
|---|---|---|---|---|
| Group . | Transplant . | SLA-matched . | Third party . | |
| A (n = 5) | pBMT | > 120 | > 120 | |
| B (n = 4)* | pBMT; HuThy/Liv/HSC | > 120 (n = 3), > 40† | 14, 38, 55, > 40† | |
| C (n = 3)* | HuThy/Liv/HSC | 14, 38, > 120‡ | 38, 31, > 120‡ | |
FCM analysis at week 10 after human tissue/cell transplantation confirmed that mice in groups B and C had comparable levels of human cell chimerism (HLA class 1+ cells in blood were 2%-37% for group B and 2%-31% for group C).
Died on day 40 with surviving grafts.
Histologic examination showed severe mononuclear cell infiltration in the graft removed on day 120.
Discussion
Mixed hematopoietic chimerism is a powerful and reliable approach to the induction of T- and B-cell tolerance.28 However, difficulties in achieving long-term hematopoietic engraftment have been a major obstacle to evaluating the ability of mixed hematopoietic chimerism to induce tolerance across discordant xenogeneic barriers. Studies using a pig-to-primate combination have shown prolonged survival of porcine kidney grafts in cynomolgus monkey recipients preinfused with donor marrow cells. However, long-lasting hematopoietic chimerism was not achieved in any of these animals, and all kidney grafts were ultimately rejected.29,30 Because donor hematopoietic stem cells may fail to engraft in discordant xenogeneic recipients, even in recipients in which T and B cells are absent,31 the rejection of pig kidney grafts in these studies does not distinguish between the failure of donor hematopoietic cells to engraft and an inability of chimerism to induce tolerance across highly disparate species barriers. By using a murine model, in which human immune reconstitution was achieved by transplantation of human fetal Thy/Liv fragments (or Thy/Liv plus CD34+ HSCs) in NOD/SCID-Tg mice with pre-established porcine hematopoietic chimerism, we have now shown that human T cells that developed in mice with porcine hematopoietic chimerism showed specific nonresponsiveness to the porcine donor, as evidenced by the lack of rejection of porcine hematopoietic cells and antidonor pig MLR responses, as well as the acceptance of donor SLA-matched skin grafts. The capacity of human T cells from the same chimeric mice to reject third-party porcine skin grafts and to respond to third-party pig and allogeneic human antigens in MLR assay indicates that the human T cells were immunocompetent and that the tolerance is not generic to all pig antigens, but specific only to those of the BM donor. This was further supported by the observation that human T cells developing in mice without porcine chimerism responded to both porcine donor and third-party xenogeneic and allogeneic antigens.
The capacity of hematopoietic chimerism to induce T-cell tolerance results in large part from the ability of donor cells to induce intrathymic clonal deletion of maturing donor-reactive thymocytes,28,32 resulting in the generation of a T-cell repertoire that is tolerant of the hematopoietic cell donor. Previous studies have shown that intrathymic clonal deletion may occur across species. It has been reported that porcine thymus grafts can support the development of mouse T cells in athymic recipients33,34 or human T cells in immunodeficient mice,24 which are functional and tolerant to the porcine thymus donor antigens. Although these studies demonstrate the potential of tolerance induction by thymus transplantation, the determinants of tolerance induction in the thymus transplantation model differ from those in the setting of mixed chimerism. The latter relies on the efficiency of donor cell-mediated central deletion of donor antigen-reactive T cells in the recipient thymus. Although cross-species incompatibilities in adhesion molecules may limit the thymic homing of donor cells in discordant xenogeneic recipients,35-38 such defects have not been detected in a highly disparate pig-to-mouse combination. We have previously shown that long-term repopulation of host thymus with SLA class II+ cells can be achieved in NOD/SCID-Tg mice by porcine BMT,20 and that the induction of porcine hematopoietic chimerism is capable of inducing T-cell tolerance to porcine hematopoietic donor antigens in mice.15 The population of human thymic grafts with porcine SLA class II+, morphologically DC-like, cells in porcine hematopoietic chimeras (Figure 4) suggests that porcine SLA class II+ cells or progenitors that give rise to class II+ cells in the human thymus are capable of migrating to and seeding the human thymus. Although mouse angiogenesis contributes to the vascularization of human thymic grafts in the model used in this study, the retention of DCs in the thymus and the intrathymic DC development likely depend on signals from thymic epithelial cells,39-42 which were of human origin in the human thymus grafts. Although the present study cannot rule out the role of other mechanisms (eg, anergy and peripheral immunoregulation), the presence of SLA class II+ cells and the lack of donor antigen-reactive human T cells in the human thymic grafts (Figures 4, 5) suggest that intrathymic deletion is critical in the induction of xenograft tolerance through mixed chimerism, at least in the present model, in which the recipient has no pre-existing mature T cells.
The present study shows that human T cells developing in porcine hematopoietic chimeria mice were specifically tolerant of the porcine hematopoietic donor. Given the formidable immunologic and genetic barriers between discordant species, the induction of porcine hematopoietic chimerism in humans would be much more difficult than in the humanized NOD/SCID-Tg mouse model. In addition to immunologic rejection, donor hematopoietic cells also have a competitive disadvantage in the hematopoietic microenvironment of highly disparate xenogeneic recipients. Inhibition of porcine hematopoiesis was observed when porcine BMCs were cocultured with human or monkey hematopoietic cells in long-term BM cultures on human or monkey, but not porcine, stromal layers.43,44 Similar results were obtained in vivo using a pig-to-NOD/SCID-Tg mouse BMT model, in which injection of immunodeficient NOD/SCID-Tg mouse BMCs into porcine hematopoietic chimeras eliminated pre-established porcine chimerism.15 The present study proving the principle that mixed hematopoietic chimerism is capable of inducing human T-cell tolerance to porcine xenoantigens should stimulate the development of methods of improving the function of porcine hematopoietic progenitor and stem cells in human recipients, so that mixed chimerism can fulfill its potential as an approach to clinical xenotransplantation.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-10-3697.
Supported by grants from Juvenile Diabetes Foundation International (1-1999-573 and 1-2001-770), the National Institutes of Health (RO1 HL54038), and a sponsored research agreement between Massachusetts General Hospital and Immerge BioTherapeutics Inc (Cambridge, MA).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs Boris Nikolic and Gerald L. Waneck for critical review of the manuscript, Longjuan Zhang for technical assistance, and Robin Laber for expert assistance with the manuscript.

![Figure 3. Histologic and phenotypic analysis of human thymus grafts from NOD/SCID-Tg mice that received human Thy/Liv implants. Thy/Liv grafts were removed at week 20 after human tissue implantation from the recipients of porcine BM transplants plus human Thy/Liv grafts (n = 13) or the NOD/SCD-Tg mice that received human Thy/Liv tissues alone (n = 7). (A-C) Data shown are results of a representative graft. (A) Macroscopic appearance. (B) Histology (hematoxylin and eosin; original magnifications × 10 [left panel] and × 40 [right panel]); C indicates cortex; M, medulla; H, Hassall corpuscles. (C) Phenotypic analysis by FCM. (D) Percentages (means ± SD) of human thymocytes expressing various surface markers in the Thy/Liv grafts from NOD/SCID-Tg mice that received porcine BM transplants plus human Thy/Liv (□; n = 13) or human Thy/Liv tissues alone (▪, n = 7).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/10/10.1182_blood-2003-10-3697/6/m_zh80100461390003.jpeg?Expires=1765916885&Signature=TO0HPHYdZtXHN62BhgdZmMHNdrHgf4JvYiDQt5PYFwBKVx4fwzpaQWQ2N6iyidk9KCJl2W4nhM1F~9O-66GJmksAXq4jWXVDdS2kqS-A8YOldvZ75cucSHHovqmUMpZeV6rH-8R1xh72NX3vYIeCqv7sbhc-Xe7un~EcYkqYTBok7-wOWQvmd7dUXxQolUAg5s0UCYFm3gNQn-zV0FX7KLhxUZMd1cMsubnS4VsZZO6WRJQUBJWOniqzse5topws9HgrknpnLYRp5C71Oh5z9ZrcFkk5ZRJAsoKm2x0jVLzJp5Hu-N3U8zFq8Ffhse77jkHTRyaFsJZC2um0u9ChJQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal