Abstract
Primary systemic amyloidosis (AL) is a plasma cell dyscrasia resulting in multisystem failure and death. High-dose chemotherapy with peripheral blood stem cell transplantation (PBSCT) has been associated with higher response rates and seemingly higher overall survival than standard chemotherapy. Selection bias, however, confounds interpretation of these results. We performed a case-match-control study comparing overall survival of 63 AL patients undergoing transplantation with 63 patients not undergoing transplantation. Matching criteria included age, sex, time to presentation, left ventricular ejection fraction, serum creatinine, septal thickness, nerve involvement, 24-hour urine protein, and serum alkaline phosphatase. According to design, there was no difference between the groups with respect to sex (57% males), age (median, 53 years), left ventricular ejection fraction (65%), number of patients with peripheral nerve involvement (17%), cardiac interventricular septal wall thickness (12 mm), serum creatinine (1.1 mg/dL [97.24 μmol/L]), and bone marrow plasmacytosis (8%). Sixty-six patients have died (16 cases and 50 controls). For PBSCT and control groups, respectively, the 1-, 2-, and 4-year overall survival rates are 89% and 71%; 81% and 55%; and 71% and 41%. Outside a randomized clinical trial, these results present the strongest data supporting the role of PBSCT in selected patients with AL.
Introduction
Primary systemic amyloidosis (AL) is a rare plasma cell dyscrasia that is a progressive, fatal multisystem disease. Improvement in median overall survival with standard alkylator-based chemotherapy is 6 months.1 Clinical response rates are only about 20%, but patients who do achieve response have a median survival of 89 months.2 Peripheral blood stem cell transplantation (PBSCT) was introduced as a therapeutic option in the late 1990s, but transplantation-related mortality was high.3-6 With better patient selection, transplantation-related mortality rates dropped to approximately 15%7,8 to 25%,9 but concern over whether the improved outcome was an artifact of selection grew. We previously demonstrated that, if strict transplantation eligibility criteria were applied to patients with amyloid, only 16% of patients were candidates for transplantation and that the highly selected patients had a 24-month survival comparable to that reported in the literature for patients undergoing PBSCT.10 With longer follow-up, however, there is a suggestion that the 48-month survival is better in patients undergoing transplantation (60%)8 than in selected, but conventionally treated patients (43%).10 Intuitively, the increased hematologic and organ response rates observed with PBSCT should result in superior survival, but this has not yet been demonstrated in a randomized controlled trial. We therefore sought to address the question in a different fashion— that is, a case-control study matching those patients who have received high-dose chemotherapy with peripheral blood stem cell transplantation (cases) to those who have comparable clinical features but who have not undergone transplantation (controls).
Patients, materials, and methods
The retrospective study was approved by the Mayo Clinic Institutional Review Board according to the regulations of the Declaration of Helsinki. All patients had supplied written consent for review of their medical records for research purposes. The diagnosis of amyloidosis was made as previously described.11 All patients who received PBSCT were evaluated by 1 of 3 physicians (M.A.G., M.Q.L., A.D.) to determine eligibility. For patients with borderline organ function, final eligibility decisions were made by consensus and melphalan dose reduced accordingly.
Seventy-one consecutive (July 26, 1996, through May 29, 2001) AL patients undergoing transplantation were initially selected as the case group. Seven were excluded because they received a cadaveric heart transplant, and 1 other was excluded because of the lack of echocardiogram data, leaving 63 cases. To minimize the effect of potentially confounding variables, the transplantation patients were matched one-to-one to amyloid patients who did not receive transplants. For each transplantation patient, the set of potential controls was restricted to those of the same sex, such that the control patient's time from initial diagnosis to being seen at our institution was comparable to that of the case (to control for biases in referral patterns), and who were alive and under our care for at least as long as the time to transplantation of the associated case (deaths very soon after referral would never have been eligible for transplantation). Within this group of potential controls, that subject was chosen who best matched the case in terms of clinical risk variables, including level of nerve involvement, serum creatinine, septal thickness, ejection fraction, alkaline phosphatase, and urine protein. Matching can never be exact on such a long list of factors; the relative importance of each of these was based on the coefficients from a survival model that was fit to the combined set of cases and potential controls.
The analysis focused on comparing PBSCT cases versus matched controls that did not receive PBSCT. Demographic and baseline clinical and laboratory data of the 2 groups were compared using χ2 tests12 and Fisher exact tests13 for categoric variables and rank sum tests14 for continuous variables. Survival was estimated using the method of Kaplan and Meier,15 and the groups were compared using log-rank tests.16 Hazard ratios with 95% confidence intervals were obtained from Cox regression.17 All statistical tests were 2-sided, and P values less than .05 were considered significant. All analyses were performed in SAS version 8.2 on a SunOS 5.8 platform (Sun Microsystems, Palo Alto, CA).
Results
There were 63 people each in the case (PBSCT) and the control (non-PBSCT) groups. Detailed demographic information is shown in Table 1. Median time from diagnosis to our treatment center was 1.5 months for the PBSCT group and 1.4 months for the nontransplantation group. Median time from AL diagnosis to transplantation was 4.4 months (range, 1.3 to 74.6 months); median time to other therapies in the control group was 1.4 months (range, 0.0 to 13.2 months). According to design, there was no significant difference between the groups with respect to sex (57% men), age (median, 53 years), left ventricular ejection fraction (65%), number of patients with peripheral nerve involvement (17%), cardiac septal wall thickness (12 mm), serum creatinine (1.1 mg/dL [97.24 μmol/L]), serum alkaline phosphatase (196 U/L), serum M-spike (0 g/dL [0 g/L]), and bone marrow plasmacytosis (8%).
Characteristics of patients and controls
. | Median (range or percentage) . | . | . | ||
|---|---|---|---|---|---|
. | Patients, n = 63 . | Controls, n = 63 . | All, n = 126 . | ||
| Time from diagnosis to presentation to Mayo, mo | 1.5 (0-71.6) | 1.4 (0-11.0) | 1.4 (0-71.6) | ||
| Diagnosis to transplantation/treatment, mo | 4.4 (1.3-74.6)* | 1.4 (0-13.2) | 2.7 (0-74.6) | ||
| No. of males | 36 (57%) | 36 (57%) | 72 (57%) | ||
| Age, y | 53 (30-69) | 53 (32-69) | 53 (30-69) | ||
| No. with nerve involvement | 11 (17%) | 11 (17%) | 22 (17%) | ||
| Left ventricular ejection fraction | 65% (30%-79%) | 65% (30%-80%) | 65% (7%-80%) | ||
| No. with left ventricular ejection fraction less than or equal to 50% | 4 (6%)* | 12 (19%) | 16 (13%) | ||
| Interventricular septum, mm | 12 (7-23) | 12 (7-25) | 12 (7-25) | ||
| No. with interventricular septum greater than 15 mm | 11 (17%) | 10 (16%) | 21 (17%) | ||
| Serum alkaline phosphatase level, U/L | 196 (57-1195) | 196 (76-1926) | 196 (57-1926) | ||
| No. with serum alkaline phosphatase level greater than twice normal | 8 (13%) | 4 (6%) | 12 (10%) | ||
| Serum creatinine level, mg/dL | 1.1 (0.5-2.1) | 1.0 (0.6-10.3) | 1.0 (0.5-10.3) | ||
| Serum M protein level, g/dL | 0.5 (0.0-2.9) | 0.0 (0.0-2.8) | 0.0 (0.0-2.9) | ||
| Serum albumin level, g/dL | 2.7 (1.4-3.8) | 2.7 (0.9-4.1) | 2.7 (0.9-4.1) | ||
| 24-h urine protein level, g/24 h | 4.6 (0.0-20.7) | 5.3 (0.1-24.7) | 4.8 (0.0-24.7) | ||
| No. with 24-h urine protein level greater than 3 g/24 h | 37 (59%) | 37 (59%) | 74 (59%) | ||
| 24-h urine M protein level | 0.1 (0.0-3.1) | 0.2 (0.0-4.0) | 0.2 (0.0-4.0) | ||
| Bone marrow plasma cells | 10% (1%-91%) | 8% (1%-66%) | 8% (1.0%-91%) | ||
| No. with bone marrow plasma cells greater than 30% | 8 (16%) | 3 (5%) | 11 (10%) | ||
. | Median (range or percentage) . | . | . | ||
|---|---|---|---|---|---|
. | Patients, n = 63 . | Controls, n = 63 . | All, n = 126 . | ||
| Time from diagnosis to presentation to Mayo, mo | 1.5 (0-71.6) | 1.4 (0-11.0) | 1.4 (0-71.6) | ||
| Diagnosis to transplantation/treatment, mo | 4.4 (1.3-74.6)* | 1.4 (0-13.2) | 2.7 (0-74.6) | ||
| No. of males | 36 (57%) | 36 (57%) | 72 (57%) | ||
| Age, y | 53 (30-69) | 53 (32-69) | 53 (30-69) | ||
| No. with nerve involvement | 11 (17%) | 11 (17%) | 22 (17%) | ||
| Left ventricular ejection fraction | 65% (30%-79%) | 65% (30%-80%) | 65% (7%-80%) | ||
| No. with left ventricular ejection fraction less than or equal to 50% | 4 (6%)* | 12 (19%) | 16 (13%) | ||
| Interventricular septum, mm | 12 (7-23) | 12 (7-25) | 12 (7-25) | ||
| No. with interventricular septum greater than 15 mm | 11 (17%) | 10 (16%) | 21 (17%) | ||
| Serum alkaline phosphatase level, U/L | 196 (57-1195) | 196 (76-1926) | 196 (57-1926) | ||
| No. with serum alkaline phosphatase level greater than twice normal | 8 (13%) | 4 (6%) | 12 (10%) | ||
| Serum creatinine level, mg/dL | 1.1 (0.5-2.1) | 1.0 (0.6-10.3) | 1.0 (0.5-10.3) | ||
| Serum M protein level, g/dL | 0.5 (0.0-2.9) | 0.0 (0.0-2.8) | 0.0 (0.0-2.9) | ||
| Serum albumin level, g/dL | 2.7 (1.4-3.8) | 2.7 (0.9-4.1) | 2.7 (0.9-4.1) | ||
| 24-h urine protein level, g/24 h | 4.6 (0.0-20.7) | 5.3 (0.1-24.7) | 4.8 (0.0-24.7) | ||
| No. with 24-h urine protein level greater than 3 g/24 h | 37 (59%) | 37 (59%) | 74 (59%) | ||
| 24-h urine M protein level | 0.1 (0.0-3.1) | 0.2 (0.0-4.0) | 0.2 (0.0-4.0) | ||
| Bone marrow plasma cells | 10% (1%-91%) | 8% (1%-66%) | 8% (1.0%-91%) | ||
| No. with bone marrow plasma cells greater than 30% | 8 (16%) | 3 (5%) | 11 (10%) | ||
P < .05.
There was overlap between periods of diagnosis for the cases (June 1992 to November 2000) and the controls (March 1983 to August 2000). Despite the fact that our first AL stem cell transplantation was performed March 1996, less than a third of the 63 cases underwent transplantation before 1999. Only 14% of the controls were diagnosed in 1998 or later. Reasons for not performing transplantation in the controls during the overlap period included the stricter transplantation eligibility criteria used during the middle to late 1990s, patient preference, and financial restrictions.
Twenty-seven of the patients undergoing transplantation received some therapy prior to transplantation conditioning: 8, melphalan and prednisone; 15, dexamethasone or prednisone; 3, vincristine, doxorubicin, and dexamethasone (VAD); and 1, 4′-iodo-4′-deoxydoxorubicin (IDOX). Peripheral blood stem cells were mobilized with cyclophosphamide and granulocyte-macrophage colony-stimulating factor (GM-CSF) (n = 33) or G-CSF alone (n = 30). Seventeen received melphalan (140 mg/m2) and total body radiation (12 Gy), 37 melphalan 200 mg/m2, 5 melphalan 140 mg/m2, and 4 melphalan 100 mg/m2, followed by the usual supportive care.7 Initial therapies in the control group included alkylator-based oral chemotherapy regimens (n = 52), high-dose steroid-based therapy (n = 4), colchicine (n = 5), or experimental regimens (n = 2: 1 IDOX and 1 vitamin E). Transplantation-related mortality—death within 100 days of transplantation—was 13% (8 of 63).
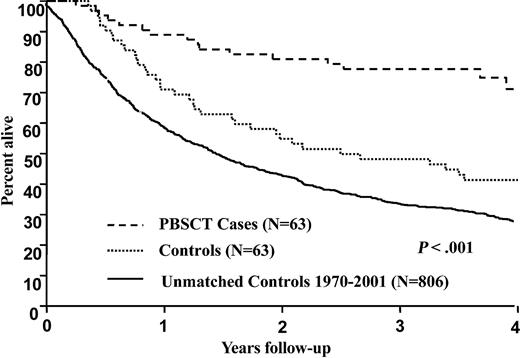
The median follow-up from diagnosis was 3.8 years for the cases and 8.8 years for the controls. Sixteen patients have died in the PBSCT group whereas 50 have died in the control group. Patients in the PBSCT group were diagnosed with amyloidosis between 1992 and 2001 (median, 1999) whereas patients in the control group were diagnosed between 1983 and 2000 (median, 1992). In the survival model used for matching patients to controls, the relative risks for individual patients ranged from 1.1 to 5.3. For 57 (90%) of the 63 matched pairs, the difference in risk was 30% or less. For PBSCT and control groups, respectively, the 1-, 2-, and 4-year overall survival rates from diagnosis are 89% and 71%; 81% and 55%; and 71% and 41%, P < .001. The Kaplan-Meier survival plots are demonstrated in Figure 1. The 1-, 2-, and 4-year survivals calculated from date of transplantation (cases) or initiation of therapy (controls) were as follows: 82% and 68%; 81% and 53%, and 70% and 40%, P < .001. Even when the 7 patients who received nonstandard therapies (colchicine, IDOX, or vitamin E) were excluded, the survival of the control group was inferior at 1, 2, and 4 years (89% versus 73%, 82% versus 58%, and 75% versus 43%), P < .001. There were 4 sets of patients (8 total) who were age 66 to 69. Interestingly, in this older subset, only 1 of the cases has died, at 6.3 months, with the other 3 cases alive at 35.5, 36.5, and 38 months; all of the controls have died at 6.6, 6.8, 11.6, and 42.6 months. Although suggestive, no firm conclusions about the outcomes of 66- to 69-year-old amyloid patients can be drawn given the small sample size.
Survival from initial diagnosis of 932 patients with immunoglobulin light chain amyloid. Case-control comparison, P < .001. Cases (n = 63) received peripheral blood stem cell transplantation. Controls (n = 63) did not undergo transplantation. Unmatched cases (n = 806) are also included for comparison.
Survival from initial diagnosis of 932 patients with immunoglobulin light chain amyloid. Case-control comparison, P < .001. Cases (n = 63) received peripheral blood stem cell transplantation. Controls (n = 63) did not undergo transplantation. Unmatched cases (n = 806) are also included for comparison.
Discussion
Our study of 126 AL patients demonstrates that patients receiving PBSCT enjoy better overall survival than their matched counterparts not undergoing this procedure. Although our data do not suggest that PBSCT is the best therapy for all amyloid patients, they do illustrate that patients deemed candidates for the procedure at an amyloid treatment facility do better with the procedure than without. One must recall that the initial treatment-related mortality figures for AL patients undergoing transplantation approached the sobering value of 50% 4,18 but, with more stringent selection criteria, are now approximately 15%7,8,19 at specialty amyloid transplantation centers and 25% at other centers.9
Controversy has reigned regarding the therapy of AL since its recognition as a plasma cell dyscrasia in 1931.20 For years, it was questioned as to whether therapy provided any benefit. In 1978, Kyle and Greipp published a randomized controlled trial demonstrating a superior overall survival in patients receiving at least a year of melphalan and prednisone as compared with placebo.21 The utility (or lack thereof) of colchicine was the topic of the next major treatment dispute. Subsequent randomized studies showed a doubling of survival in patients treated with melphalan-containing regimens as compared with colchicine, suggesting the ineffectiveness of the latter.1,22,23 Because hematologic response rates are higher in myeloma patients receiving vincristine, carmustine, melphalan, cyclophosphamide, and prednisone (VBMCP), Gertz et al performed another randomized trial, comparing VBMCP with MP.24 This study was negative—survival in the 2 arms was comparable. Phase 2 studies using VAD, dexamethasone, and thalidomide have yielded variable results, and their role in the treatment of AL is not settled.25-28
Over the last decade, the role of PBSCT for AL patients has been questioned. Because a randomized controlled trial comparing oral melphalan and prednisone to PBSCT is not likely to be done, we performed a case-control study matching patients who underwent PBSCT to those with similar clinical characteristics who did not. A superior overall survival in the transplantation group is demonstrated. These findings complement our previous report, which illustrated that eligibility for PBSCT was a favorable prognostic factor for survival,10 and are the logical extension for this crucial question. Herein, we match not only eligibility and measurements of organ involvement but also time to treatment. It is striking that only 45% of patients underwent transplantation within 6 months of diagnosis and 87% underwent transplantation within a year of diagnosis. We have addressed the potential bias of the cases possessing sufficiently indolent disease to survive to their transplantation date within our matching algorithm; both time to our treatment facility (1.5 and 1.4 months for cases and controls, respectively) and time to transplantation were included.
Another potential limitation of this study is that the follow-up for the control group was longer than that for the cases. Although the median follow-up in the control group was more than twice as long as for the cases, we report the Kaplan-Meier estimates to only 4 years (the median follow-up time of the transplantation group is 3.8 years) to minimize this bias. At 3 years, 7 of the cases are censored as compared with 2 of the controls. At 4 years, 29 of the cases are censored in contrast with 3 of the controls. Despite this difference, the 95% confidence intervals estimating survival at 4 years do not overlap (cases 71%, 95% confidence interval [CI], 59% to 85%; versus controls 41%, 95% CI, 31% to 55%). Although this study cannot substitute for a randomized controlled study, it does lend additional evidence to the belief that in selected patients PBSCT results in superior overall survival as compared with standard chemotherapy.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-12-4192.
Supported in part by CA 62242 and CA 91561 from the National Cancer Institute and by the Robert A. Kyle Hematologic Malignancies Fund.
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Carol Shipman for her years of work managing the database.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal