Abstract
Fifty-six patients, 10 to 66 years of age, with idiopathic myelofibrosis (IMF) or end-stage polycythemia vera or essential thrombocythemia received allogeneic hematopoietic cell transplants from related (n = 36) or unrelated (n = 20) donors. Forty-four patients were prepared with busulfan plus cyclophosphamide and 12 with total body irradiation plus chemotherapy. The source of stem cells was marrow in 33 and peripheral blood in 23 patients. All but 3 patients achieved engraftment. While 50 patients showed complete donor chimerism, 3 patients were found to be mixed chimeras at 26, 48, and 86 months after transplantation, respectively. Two patients died from relapse/progressive disease, and 18 died from other causes. There are 36 patients surviving at 0.5 to 11.6 (median, 2.8) years, for a 3-year Kaplan-Meier estimate of 58% (CI, 43%-73%). Dupriez score, cytogenetic abnormalities, and degree of marrow fibrosis were the most significant risk factors for posttransplantation mortality. Patients conditioned with a regimen of busulfan targeted to plasma levels of 800 to 900 ng/mL plus cyclophosphamide had a higher probability of survival (76% [CI, 62%-91%]) than other patients. Results with unrelated donors were comparable with those with HLA-identical sibling transplants. Thus, allogeneic hematopoietic cell transplantation offers long-term relapse-free survival for patients with myelofibrosis.
Introduction
Idiopathic myelofibrosis (IMF) is characterized by a leukoerythroblastic peripheral blood smear, splenomegaly, and extramedullary hematopoiesis. With conservative management, life expectancy may range from only a few months to more than a decade.1 Eventually, progressive hematopoietic failure or leukemic transformation leads to the patients' demise. A similar clinical picture may develop in 15% to 20% of patients with advanced polycythemia vera (P vera) and 10% of patients with essential thrombocythemia (ET).2,3 At that stage, treatment options for those diseases are limited, and the prognosis is poor.
Anemia, thrombocytopenia, increase in immature white blood cells in circulation, and hepatomegaly are some of the poor-risk prognostic factors identified in patients with myelofibrosis.4-6 Patients with IMF with either a hemoglobin (Hgb) level less than 100 g/L (10 g/dL) or a white blood cell (WBC) count less than 4 × 109/L or more than 30 × 109/L have a median life expectancy of about 2 years.7 Patients who “require” splenectomy (because of massive spleen enlargement, transfusion-dependent anemia, or thrombocytopenia) also have a median life expectancy of approximately 2 years. Presumably the indications that lead to splenectomy are manifestations of disease progression.8 In addition, abnormal karyotype and osteomyelosclerosis are considered poor prognostic features.
As these disorders are clonal diseases of hematopoietic stem cells, it should be possible to cure them by hematopoietic cell transplantation (HCT). The marrow fibrosis is thought to be a reactive process of nonclonal fibroblasts, possibly mediated by transforming growth factor-β released by megakaryocytes, and other signals.9 Thus, removal of the abnormal clone and replacement by normal precursors from healthy donors should eliminate the stimulus for a reaction of the marrow environment, and the fibrosis should regress. This notion is supported by preliminary observations.10-17
Here we summarize results in 56 patients with myelofibrosis treated at a single institution and who received transplants from HLA-identical siblings or alternative related or unrelated donors.
Patients and methods
Patients
Patients with IMF or with myelofibrosis developing with P vera or ET, and patients with myeloproliferative disorders that were not otherwise specified but were associated with myelofibrosis, were referred to the Fred Hutchinson Cancer Research Center (FHCRC) for HCT because of peripheral blood cytopenias, spent-phase disease, or leukemic transformation. From February 1980 through May 2002, 56 patients underwent transplantation, all but 3 since 1994. While 12 patients were prepared with radiation-containing conditioning regimens, 44 patients were enrolled prospectively in a trial that used busulfan (BU) in combination with cyclophosphamide (CY). Patient characteristics are summarized in Table 1. Karyotypes were normal in 28 patients, and in 6 patients material was insufficient for analysis. Clonal cytogenetic abnormalities in marrow cells were present in 22 patients: +8 (± other abnormalities) in 5, 20q- or -20 in 3, 13q- in 1, 7q- in 1, various translocations in 5, and other structural or numeric abnormalities in 7 patients. At various intervals before HCT 20 patients had undergone splenectomy, generally because of symptoms related to massive splenomegaly. There were 20 patients who had been transfused with red blood cells, platelets, or both; 14 had received hydroxyurea, 8 interferon, 5 anagrelide, 4 corticosteroids, 4 erythropoietin, 2 chemotherapy other than hydroxyurea; 11 had been given a variety of other therapies, and 19 patients had not received prior therapy.
Patient and disease characteristics
Data . | No. . |
|---|---|
| Number of patients studied | 56 |
| Patient age, y, range (median) | 10-66 (43) |
| Patient sex, M/F | 28/28 |
| Disease duration, mo, range (median) | 3-312 (33) |
| Primary diagnosis | |
| IMF | 33 |
| ET with myelofibrosis | 10 |
| P vera with myelofibrosis | 5 |
| Myelofibrosis with increased blasts* | 5 |
| Other† | 3 |
| Degree of marrow fibrosis‡ | |
| 1 (mild) | 21 |
| 2 (moderate) | 17 |
| 3 (severe) | 18 |
| Dupriez classification at transplantation§ | |
| Good | 25 |
| Intermediate | 17 |
| Poor | 13 |
| Platelet count at transplantation¶ | |
| 100 × 109/L or higher | 40 |
| Less than 100 × 109/L | 15 |
| Prior splenectomy | |
| Yes/no | 20/36 |
Data . | No. . |
|---|---|
| Number of patients studied | 56 |
| Patient age, y, range (median) | 10-66 (43) |
| Patient sex, M/F | 28/28 |
| Disease duration, mo, range (median) | 3-312 (33) |
| Primary diagnosis | |
| IMF | 33 |
| ET with myelofibrosis | 10 |
| P vera with myelofibrosis | 5 |
| Myelofibrosis with increased blasts* | 5 |
| Other† | 3 |
| Degree of marrow fibrosis‡ | |
| 1 (mild) | 21 |
| 2 (moderate) | 17 |
| 3 (severe) | 18 |
| Dupriez classification at transplantation§ | |
| Good | 25 |
| Intermediate | 17 |
| Poor | 13 |
| Platelet count at transplantation¶ | |
| 100 × 109/L or higher | 40 |
| Less than 100 × 109/L | 15 |
| Prior splenectomy | |
| Yes/no | 20/36 |
IMF indicates idiopathic myelofibrosis; ET, essential thrombocythemia; and P vera, polycythemia vera.
All 5 patients had IMF
One patient had IMF that evolved to CML. There were 2 patients who had evidence of NHL in lymph nodes, suggesting the synchronous occurrence of 2 malignancies
According to Guardiola et al.13
Data were incomplete for one patient
Counts were not available for one patient
The indications for transplantation included single or multilineage peripheral blood cytopenias (platelet count, < 100 × 109/L; Hgb level, < 100 g/L [10 g/dL]; neutrophil count, < 1.5 × 109/L) (n = 35), leukemic transformation (n = 5), or “spent phase” of P vera or ET (n = 15). One patient presented originally with a myeloproliferative disorder consistent with IMF (normal karyotype on 2 determinations) and received supportive therapy only. Prolonged follow-up showed a rise in leukocytes, and eventually cytogenetic analysis revealed a t(2; 9; 22), a picture consistent with the development of chronic myeloid leukemia on the background of IMF. In 2 patients, the pretransplantation work-up revealed evidence for non-Hodgkin lymphoma on cervical lymph node biopsies. The possibility of lymphoma-associated myelofibrosis could not be categorically excluded. However, the extent of lymphoma was minimal, and the patients were considered to have 2 synchronous malignancies and remained in the study. All patients had given informed consent according to the requirements of the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Data on 13 of these patients were included in a previous report.11
Disease classification
For the purpose of risk analysis, the disease was classified on the basis of pretransplantation findings, using the criteria proposed by Dupriez et al for IMF:7 Hgb values (≥ 100 g/L [10 g/dL] [favorable] vs < 100 g/L [10 g/dL]) and WBC counts (> 4 and < 30 × 109/L [favorable] vs either < 4 or > 30 × 109/L). Patients with both parameters in the favorable range were classified as good risk (score 1), those with one favorable parameter as intermediate risk (score 2), and those without favorable parameters as high risk (score 3). In addition, we considered platelet counts (≥ 100 × 109/L vs < 100 × 109/L), cytogenetic abnormalities, and degree of marrow fibrosis (graded as 1, 2, or 3, with 3 including patients who showed evidence of osteosclerosis) using a grading scheme as reported recently.13
Donor selection
HLA typing was carried out as described.18 Thirty-six patients had suitable related donors (genotypically HLA-identical siblings in 31 patients, and family members who were HLA genotypically identical for one haplotype but differed for 1 or 2 HLA antigens on the second haplotype in 5 patients). Unrelated donors were identified for 20 patients; 14 were HLA-matched with the patient, and 6 were HLA-mismatched as determined by intermediate- or high-resolution typing.19,20
Transplantation
Donor and transplant characteristics are summarized in Table 2. There were 44 patients conditioned with a combination of BU, 1 mg/kg given orally every 6 hours over 4 days (16 mg total), followed by CY, 60 mg/kg intravenously for 2 consecutive days. In 39 of these patients, BU doses were adjusted to achieve steady-state plasma levels of 800 to 900 ng/mL (targeted BU) as described.21,22 The remaining patients, enrolled in the initial phase of this trial, were conditioned with total body irradiation (TBI) in combination with either BU or CY as described.23,24 The source of stem cells was bone marrow in 33 patients and granulocyte colony-stimulating factor (G-CSF)-mobilized cells from peripheral blood in 23 patients. In all but 3 patients, graft-versus-host disease (GVHD) prophylaxis consisted of a combination of cyclosporine (CSP) and methotrexate (MTX) or CSP and mycophenolate mofetil (MMF). Acute and chronic GVHD were evaluated according to established criteria.25,26
Donor and transplant characteristics
Data . | No. . |
|---|---|
| Donor age, y, range (median) | 6-65 (39) |
| Donor sex, M/F | 31/25 |
| Relationship | |
| Related | 36 |
| HLA-identical sibling | 31 |
| HLA-nonidentical relative* | 5 |
| Unrelated | 20 |
| HLA-identical | 14 |
| HLA-nonidentical† | 6 |
| Source of stem cells | |
| Marrow | 33 |
| Peripheral blood | 23 |
| Conditioning regimen | |
| BUCY (targeted) | 44 (39) |
| BUTBI (1200) | 7 |
| CYTBI (1200) | 5 |
| GVHD prophylaxis | |
| MTX + CSP | 50 |
| MMF + CSP | 3 |
| MTX + FK506 | 2 |
| MTX | 1 |
| CMV status, patient/donor‡ | |
| +/+ | 10 |
| +/- | 10 |
| -/+ | 6 |
| -/- | 21 |
Data . | No. . |
|---|---|
| Donor age, y, range (median) | 6-65 (39) |
| Donor sex, M/F | 31/25 |
| Relationship | |
| Related | 36 |
| HLA-identical sibling | 31 |
| HLA-nonidentical relative* | 5 |
| Unrelated | 20 |
| HLA-identical | 14 |
| HLA-nonidentical† | 6 |
| Source of stem cells | |
| Marrow | 33 |
| Peripheral blood | 23 |
| Conditioning regimen | |
| BUCY (targeted) | 44 (39) |
| BUTBI (1200) | 7 |
| CYTBI (1200) | 5 |
| GVHD prophylaxis | |
| MTX + CSP | 50 |
| MMF + CSP | 3 |
| MTX + FK506 | 2 |
| MTX | 1 |
| CMV status, patient/donor‡ | |
| +/+ | 10 |
| +/- | 10 |
| -/+ | 6 |
| -/- | 21 |
BU indicates busulfan; TBI, total body irradiation (cGy); MTX, methotrexate; CSP, cyclosporine; MMF, mycophenolate mofetil; and FK506, tacrolimus.
Of the donors, 1 was HLA phenotypically matched; 2 were mismatched for HLA-B, 1 for DR, and 1 for DR and DQ
Of the donors, 2 were mismatched for DQB1, 1 each for HLA-A or HLA-B, and 2 for DR and DQ
In 9 patient/donor pairs, the information on CMV was incomplete
Criteria for engraftment and response
The day of engraftment was defined as the first of 3 consecutive days on which blood granulocytes rose to 0.5 × 109/L.27 Donor cell engraftment was documented by sex chromosome analysis (in patients with opposite sex donor) or by analysis of variable number tandem repeats for which patient and donor differed.
In patients with marrow fibrosis, transplantation-mediated complete remission was defined as 100% donor cell engraftment and evidence of regression of fibrosis as determined by sequential bone marrow biopsies (the tempo of regression of fibrosis varied considerably). In patients with myelodysplastic features or with frank leukemia, regression of marrow fibrosis, absence of leukemic blasts, and disappearance of dysplastic changes were required for complete remission.13
Statistical analysis
Proportional hazards regression models were used to assess the association of various factors with the hazard of failure for time-to-event end points such as overall mortality and relapse. Time to engraftment was compared between groups with the 2-sample t test among patients who engrafted. Proportions of patients who engrafted were compared with the chi-square test. Survival estimates were obtained using the method of Kaplan and Meier. Cumulative incidence estimates were used to summarize the probability of GVHD and relapse, where deaths without GVHD and deaths without relapse were regarded as competing events for the respective end points.28 Reported P values associated with regression models were derived from the Wald test, and all P values are 2-sided. No adjustments were made for multiple comparisons. Results were analyzed as of June 1, 2002.
Results
Engraftment and relapse
Granulocyte engraftment as defined in “Patients and methods” was achieved in 53 (95%) of 56 patients, and platelet engraftment in 40 patients by the time they left the transplantation center. Eventually all surviving patients except one recovered normal platelet counts. The median time to granulocyte engraftment was 17 days among splenectomized patients, and 24 days among patients without splenectomy (P = .01). The corresponding figures for platelet engraftment were 16 and 24 days, respectively (P = .06).
All patients who received transplants from HLA-identical related donors had sustained engraftment. One of 5 patients who received transplants from HLA-nonidentical related donors and 2 of 20 who received transplants from unrelated donors experienced primary graft failure; all 3 had been conditioned with targeted BU and CY (tBUCY) and received marrow as a source of stem cells. An additional 3 patients, 1 who received a transplant from an HLA-nonidentical family member and 2 from HLA-identical unrelated donors, showed initial complete donor cell engraftment. However, chimerism studies on marrow cells obtained 26, 48, and 86 months after transplantation, respectively, showed “mixed chimerism” with 80% to 98% host cells. In 2 of these patients the underlying diagnosis was IMF, and in 1 the diagnosis was P vera. The patient with P vera also showed a rising hematocrit level. The donor component among marrow CD33+ cells increased again from a low of 15% to 80% and among CD3+ cells from 50% to 99% over the ensuing 9 months, and the hematocrit level normalized without therapeutic interventions. The 2 patients with IMF have remained mixed chimeras without morphologic or cytogenetic evidence of disease.
Thus, failure of sustained engraftment occurred in 6 (26%) of 25 patients who received transplants from alternative donors (P = .04; hazard ratio, 0.1; CI, 0.01-0.9). All relapses and failures of sustained engraftment occurred among the 33 patients who received transplants of marrow cells, leading to a difference in the proportion of failures compared with patients who received transplants of peripheral blood progenitors (P = .04). Among 20 patients with prior splenectomy, 4 failed to achieve sustained engraftment, compared with 2 of 36 without splenectomy (P = .17). Also, none of 21 cytomegalovirus (CMV)-negative patients who received transplants from CMV-negative donors failed to engraft, compared with 5 of 26 transplantations where either the patient or the donor (or both) was CMV positive, and 1 of 9 in whom CMV information was incomplete.
GVHD
Thirty-eight patients developed acute GVHD grades II to IV for a cumulative incidence of 68% (12 patients [21%] had grades III-IV). Incidence rates were similar for HLA-identical related (21 of 31) and alternative donor (17 of 25) transplants. Chronic GVHD occurred in 31 among 54 evaluable patients for a cumulative incidence of 59% at 2 years; it was limited in 3 patients and extensive in 28 patients. At 6 months to 3 years after transplantation, 16 patients still require immunosuppressive therapy.
Toxicity and causes of death
Twenty of the patients have died (Table 3), 8 within 100 days of transplantation, and 12 between 4 months and 3 years after transplantation. The most frequent causes of death were pneumonia and disseminated infections, often caused by viral or fungal organisms (7 patients dying within 100 days, and 6 patients dying later). There were 2 patients who died of progressive disease. GVHD was the primary cause of death in 2 patients, and 1 patient developed a lymphoma (in host cells) one year after transplantation. Of 12 patients prepared with TBI-containing regimens, 9 died, compared with 2 of 5 prepared with BUCY without dose adjustment, and 9 of 39 given BUCY with BU dose adjustment. Steady-state BU levels (BUss), determined in 37 patients, ranged from 682 to 1154 (mean, 845; SD ± 97) ng/mL. Among 8 of these patients who died fromtransplant-related causes, BUSS levels were 830 ± 80 ng/mL compared with 849 ± 101 ng/mL among 29 patients who survived.
Causes of death
Causes . | No. of patients . |
|---|---|
| Progressive disease/relapse | 2 |
| Nonrelapse causes | 18 |
| Pneumonia/IPS | 5* |
| Other infections | 8 |
| Invasive aspergillosis | 3 |
| Aspergillosis + GVHD | 2 |
| Encephalitis ± TTP | 2 |
| VZV + HUS | 1 |
| Intracranial hemorrhage | 1 |
| GVHD | 3 |
| NHL | 1 |
Causes . | No. of patients . |
|---|---|
| Progressive disease/relapse | 2 |
| Nonrelapse causes | 18 |
| Pneumonia/IPS | 5* |
| Other infections | 8 |
| Invasive aspergillosis | 3 |
| Aspergillosis + GVHD | 2 |
| Encephalitis ± TTP | 2 |
| VZV + HUS | 1 |
| Intracranial hemorrhage | 1 |
| GVHD | 3 |
| NHL | 1 |
IPS indicates idiopathic pneumonia syndrome; GVHD, graft-versus-host disease; TTP, thrombotic thrombocytopenic purpura; VZV, varicella zoster virus; HUS, hemolytic uremic syndrome; and NHL, non-Hodgkin lymphoma.
Cytomegalovirus was isolated in one patient
Survival and responses
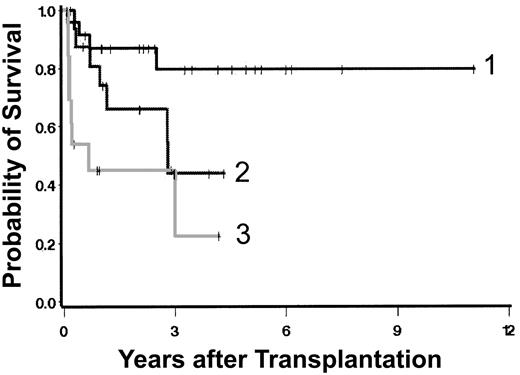
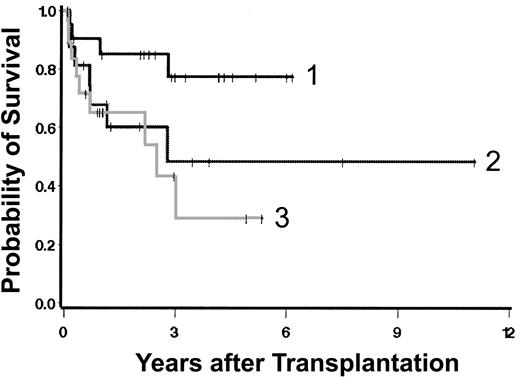
Currently, 36 patients are surviving 0.5 to 11.6 (median, 2.8) years after transplantation (Figure 1A), including 2 patients with initial graft failure. One received a successful second transplant using G-CSF-mobilized peripheral blood cells (rather than marrow) from the original donor after conditioning with tBUCY. The second patient is in remission on interferon therapy. There are 3 additional patients who are “mixed chimeras.” Survival was superior in patients conditioned with a targeted BUCY regimen (Figure 1B). Table 4 summarizes univariate regression models for the outcome “overall mortality.” Neither type of donor, source of stem cells, presence of excess blasts, duration of disease prior to HCT, nor splenectomy was statistically significantly associated with the hazard of mortality. Because of the small numbers of patients and events in some of the resulting groups, the possibility that clinically relevant differences exist but failed to reach statistical significance must be considered. Increasing severity by Dupriez classification (Figure 2) and clonal cytogenetic abnormalities were significantly associated with posttransplantation mortality. The degree of marrow fibrosis was suggestively associated with the hazard of mortality, with increasing fibrosis leading to worse outcome (Figure 3; Table 4). There was also a suggestion of increasing hazard of death with increasing age in univariate analysis (P = .07) and in several multivariable models. Higher platelet counts at transplantation were associated with improved outcome (Table 4).
Overall survival. (A) All patients; (B) survival by conditioning regimen. Shown are results in patients prepared with a regimen of targeted BUCY ([t]BUCY) compared with those prepared with other regimens. Surviving patients are indicated by tick marks.
Overall survival. (A) All patients; (B) survival by conditioning regimen. Shown are results in patients prepared with a regimen of targeted BUCY ([t]BUCY) compared with those prepared with other regimens. Surviving patients are indicated by tick marks.
Univariate regression models for overall mortality
Factor (no. of patients dying/all patients) . | Hazard ratio . | 95% Cl . | P . |
|---|---|---|---|
| Conditioning regimen | |||
| Other regimens (11/17) | 1 | — | — |
| Targeted BUCY (9/39) | 0.3 | 0.1-0.8 | .01 |
| Platelet count | |||
| × 109/L or higher (10/40) | 1 | — | — |
| Less than × 109/L (9/15) | 3.6 | 1.4-8.9 | .006 |
| Dupriez classification | |||
| 1 (4/25) | 1 | — | — |
| 2 (7/17) | 2.9 | 0.9-10.1 | .09 |
| 3 (8/13) | 6.5 | 2.0-22.0 | .002 |
| Degree of fibrosis | |||
| 1 (4/21) | 1 | — | — |
| 2 (7/17) | 2.9 | 0.8-9.9 | .09 |
| 3 (9/18) | 3.7 | 1.1-12.1 | .03 |
| Karyotype | |||
| Normal (6/29) | 1 | — | — |
| Clonal (11/21) | 5 | 1.5-18.2 | .009 |
| Age, y | |||
| Modeled as a continuous linear variable | Increasing age, increasing hazard | — | .07 |
| Age, y | |||
| Younger than 40 (5/19) | 1 | — | — |
| 40 to 50 (7/20) | 1.3 | 0.4-4.2 | .61 |
| Older than 50 (8/17) | 2.5 | 0.8-7.6 | .12 |
| Splenectomy | |||
| No (14/36) | 1 | — | — |
| Yes (6/20) | 0.8 | 0.3-2.0 | .58 |
| Excess blasts | |||
| No (17/51) | 1 | — | — |
| Yes (3/5) | 1.8 | 0.5-6.2 | .34 |
| Source of stem cells | |||
| BM (13/33) | 1 | — | — |
| PBSC (7/23) | 1.1 | 0.4-2.8 | .88 |
| Donor | |||
| Unrelated or mismatched related (10/25) | 1 | — | — |
| HLA-identical sibling (10/31) | 0.7 | 0.3-1.7 | .42 |
| Disease duration, modeled as a continuous linear variable | — | — | .85 |
Factor (no. of patients dying/all patients) . | Hazard ratio . | 95% Cl . | P . |
|---|---|---|---|
| Conditioning regimen | |||
| Other regimens (11/17) | 1 | — | — |
| Targeted BUCY (9/39) | 0.3 | 0.1-0.8 | .01 |
| Platelet count | |||
| × 109/L or higher (10/40) | 1 | — | — |
| Less than × 109/L (9/15) | 3.6 | 1.4-8.9 | .006 |
| Dupriez classification | |||
| 1 (4/25) | 1 | — | — |
| 2 (7/17) | 2.9 | 0.9-10.1 | .09 |
| 3 (8/13) | 6.5 | 2.0-22.0 | .002 |
| Degree of fibrosis | |||
| 1 (4/21) | 1 | — | — |
| 2 (7/17) | 2.9 | 0.8-9.9 | .09 |
| 3 (9/18) | 3.7 | 1.1-12.1 | .03 |
| Karyotype | |||
| Normal (6/29) | 1 | — | — |
| Clonal (11/21) | 5 | 1.5-18.2 | .009 |
| Age, y | |||
| Modeled as a continuous linear variable | Increasing age, increasing hazard | — | .07 |
| Age, y | |||
| Younger than 40 (5/19) | 1 | — | — |
| 40 to 50 (7/20) | 1.3 | 0.4-4.2 | .61 |
| Older than 50 (8/17) | 2.5 | 0.8-7.6 | .12 |
| Splenectomy | |||
| No (14/36) | 1 | — | — |
| Yes (6/20) | 0.8 | 0.3-2.0 | .58 |
| Excess blasts | |||
| No (17/51) | 1 | — | — |
| Yes (3/5) | 1.8 | 0.5-6.2 | .34 |
| Source of stem cells | |||
| BM (13/33) | 1 | — | — |
| PBSC (7/23) | 1.1 | 0.4-2.8 | .88 |
| Donor | |||
| Unrelated or mismatched related (10/25) | 1 | — | — |
| HLA-identical sibling (10/31) | 0.7 | 0.3-1.7 | .42 |
| Disease duration, modeled as a continuous linear variable | — | — | .85 |
BM indicates bone marrow; PBSC, peripheral blood stem cell.
Survival by Dupriez category. Surviving patients are indicated by tick marks.
Survival by degree of marrow fibrosis. Group 3 includes patients who had evidence of osteosclerosis.
Survival by degree of marrow fibrosis. Group 3 includes patients who had evidence of osteosclerosis.
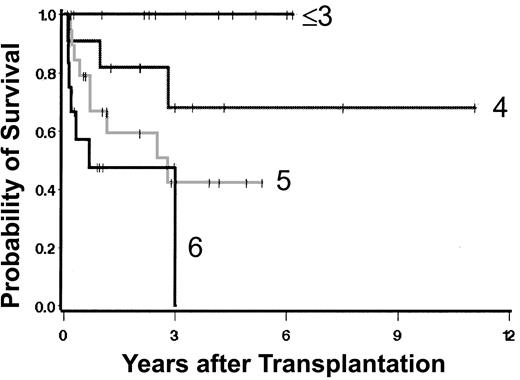
Several of the variables associated with outcome were also correlated with each other. Due to the small number of events (20 deaths), the ability to fit multivariable regression models was limited. Nonetheless, several models were fit, some of which are summarized in Table 5. In general, the qualitative conclusions resulting from the univariate models remained after examining each of the multivariable models (data not shown). This finding indicates that each of the factors that yielded a suggestive or statistically significant association with the hazard of mortality in the univariate models actually was associated with mortality, and not simply through an association with other factors. Of note were 2 models in particular. One considered Dupriez classification and degree of myelofibrosis and the second, Dupriez classification and peripheral blood platelet counts. Both models suggest that factors added to the Dupriez classification improved prognostic accuracy for posttransplantation outcome (Figure 4).
Multivariable regression models for overall mortality
. | Hazard ratio . | 95% Cl . | P . |
|---|---|---|---|
| Model 1 | |||
| Conditioning regimen | |||
| Other regimens | 1 | — | — |
| Targeted BUCY | 0.3 | 0.1-0.8 | .01 |
| Dupriez classification | |||
| 1 | 1 | — | — |
| 2 | 2.3 | 0.7-8.1 | .19 |
| 3 | 7.2 | 2.1-25.0 | .002 |
| Model 2 | |||
| Platelet count | |||
| × 109/L or higher | 1 | — | — |
| Less than × 109/L | 2.2 | 0.8-6.0 | .13 |
| Dupriez classification | |||
| 1 | 1 | — | — |
| 2 | 2.6 | 0.8-9.1 | .13 |
| 3 | 4.5 | 1.2-16.9 | .03 |
| Model 3 | |||
| Degree of fibrosis | |||
| 1 | 1 | — | — |
| 2 | 3.2 | 0.9-11.4 | .08 |
| 3 | 2.8 | 0.8-9.4 | .11 |
| Dupriez classification | |||
| 1 | 1 | — | — |
| 2 | 2.6 | 0.8-9.3 | .13 |
| 3 | 6.5 | 1.8-22.9 | .004 |
| Model 4 | |||
| Karyotype | |||
| Normal | 1 | — | — |
| Clonal | 5.3 | 1.5-18.2 | .009 |
| Dupriez | |||
| 1 | 1 | — | — |
| 2 | 0.8 | 0.2-3.6 | .82 |
| 3 | 3.4 | 0.9-12.3 | .06 |
. | Hazard ratio . | 95% Cl . | P . |
|---|---|---|---|
| Model 1 | |||
| Conditioning regimen | |||
| Other regimens | 1 | — | — |
| Targeted BUCY | 0.3 | 0.1-0.8 | .01 |
| Dupriez classification | |||
| 1 | 1 | — | — |
| 2 | 2.3 | 0.7-8.1 | .19 |
| 3 | 7.2 | 2.1-25.0 | .002 |
| Model 2 | |||
| Platelet count | |||
| × 109/L or higher | 1 | — | — |
| Less than × 109/L | 2.2 | 0.8-6.0 | .13 |
| Dupriez classification | |||
| 1 | 1 | — | — |
| 2 | 2.6 | 0.8-9.1 | .13 |
| 3 | 4.5 | 1.2-16.9 | .03 |
| Model 3 | |||
| Degree of fibrosis | |||
| 1 | 1 | — | — |
| 2 | 3.2 | 0.9-11.4 | .08 |
| 3 | 2.8 | 0.8-9.4 | .11 |
| Dupriez classification | |||
| 1 | 1 | — | — |
| 2 | 2.6 | 0.8-9.3 | .13 |
| 3 | 6.5 | 1.8-22.9 | .004 |
| Model 4 | |||
| Karyotype | |||
| Normal | 1 | — | — |
| Clonal | 5.3 | 1.5-18.2 | .009 |
| Dupriez | |||
| 1 | 1 | — | — |
| 2 | 0.8 | 0.2-3.6 | .82 |
| 3 | 3.4 | 0.9-12.3 | .06 |
Additional models considered were as follows: conditioning regimen and fibrosis; conditioning regimen and platelet count; platelet count and fibrosis; conditioning regimen and age; Dupriez classification and age; fibrosis and age; and platelet count and age. Those models did not appear to contribute substantially to the data shown above.
Impact of degree of marrow fibrosis and Dupriez score on posttransplantation survival. The numeric scores indicate the sum of Dupriez score (1, 2, or 3) and degree of fibrosis (1, 2, or 3).
Impact of degree of marrow fibrosis and Dupriez score on posttransplantation survival. The numeric scores indicate the sum of Dupriez score (1, 2, or 3) and degree of fibrosis (1, 2, or 3).
At the most recent follow-up, 6 months to 10 years after transplantation, patients had WBC counts of 2.5 to 13.6 (median, 6.8) × 109/L, absolute neutrophil counts (ANCs) of 1.68 to 5.9 (median, 2.9) × 109/L, platelet counts of 49 to 313 (median, 190) × 109/L, and hematocrit levels of 25 to 42.3 (median, 39). Follow-up marrow biopsies were available in 49 patients, 6 months to 8 years after transplantation. Among these, 30 showed no fibrosis or only traces of marrow fibrosis (some as early as 12 months after transplantation), and 13 were staged as showing grade 2, and 6 as grade 3 even 2 years after transplantation. Among 27 survivors without splenectomy, in 18 the spleen was of normal size, in 7 the spleen was “palpable” at the costal margin, in 2 the spleen reached to 14 cm (at 6 months) and 21 cm (at 10 months), respectively, below the costal margin, and in 2 no information was available.
Discussion
This study of HCT in patients with myelofibrosis of various etiologies extends previous reports that suggest that HCT offers curative therapy for these disorders.13 Most patients enrolled in this trial had received conventional management, frequently over extended periods of time, and came to transplantation only when other therapeutic measures were not or no longer effective. Other patients had not been treated, and by the Dupriez criteria, would be considered good-risk patients. However, as outlined above, other factors such as the degree of marrow fibrosis and clonal cytogenetic abnormalities also affected outcome. The results show that in these patients with advanced disease successful HCT is possible not only with related but also with unrelated donors. Patients with lower Dupriez scores, higher platelet counts, less severe marrow fibrosis, and normal karyotype fared better than patients with more advanced disease. Multivariable analyses showed that incorporation of the degree of marrow fibrosis and peripheral blood platelet counts, in addition to the Dupriez severity score (which considers hemoglobin and leukocyte count only), into a grading scheme allowed for refinement of prognostic accuracy. Peripheral blood CD34+ cell counts, identified by Barosi et al as a measure of disease progression,29 were not available consistently and, thus, were not included in the analysis. All 12 patients who had low-risk Dupriez scores along with grade I (mild) fibrosis and platelet counts 100 × 109/L or more are surviving in remission. The probability of survival decreased in parallel to an increase in the cumulative scores of all these parameters. The Dupriez score correlated with platelet counts: 2 (9%) of 23 patients with a score of 1 had platelet counts less than 100 × 109/L, compared with 3 (21%) of 14 with a score of 2, and 8 (73%) of 11 with a score of 3.
It is not clear why the degree of marrow fibrosis had an effect on outcome. In the nontransplantation context, the degree of marrow fibrosis has not been found to correlate with prognosis. Conceivably the expansion of extramedullary hematopoiesis with more severe myelofibrosis results in tissue damage, for example in the form of fibrosis, in organs such as the lungs or liver. As a consequence, these organs may be more susceptible to transplantation-related complications, while in the absence of conditioning therapy (ie, in patients who did not undergo transplantation) such an effect would not become manifest.
Second only to the Dupriez score, an abnormal karyotype had the strongest negative effect on survival. While only 6 of 31 patients with normal karyotypes died, 11 of 19 with clonal cytogenetic abnormalities succumbed to various complications. The significance of clonal cytogenetic abnormalities for prognosis in patients with myelofibrosis is not clear. Tefferi et al observed abnormal karyotypes in 57% of 165 patients, in particular 20q-, 13q-, +8, +9, 12p-, and abnormalities of chromosomes 1 and 7.30 The presence of +8, found in 5 patients in the current series, or 12p- was associated with inferior survival. While additional work is needed, the data presented here support the concept that an abnormal karyotype represents a high-risk feature, certainly with HCT.
Among factors other than disease characteristics, the transplantation conditioning regimen had a significant effect on outcome. It is of note, however, that there was some correlation between Dupriez score and conditioning regimen: while 18 (78%) of 23 patients with a score of 1 were conditioned with targeted BUCY, 10 (71%) of 14 with a score of 2, and 5 (45%) of 11 with a score of 3 received that regimen. Since all patients with myelofibrosis, regardless of severity, were enrolled into the same protocol, and the targeted BUCY regimen was used consistently in the patients who more recently underwent transplantation, the observed association of conditioning regimen and disease score apparently reflects a recent trend toward earlier referral of patients for transplantation. Nevertheless, the beneficial effect of a targeted BUCY regimen remained even after adjusting for the Dupriez score. Furthermore, patients prepared with targeted BUCY tended to be older than patients conditioned with other regimens (median, 45.7 vs 40.4 years; P = .07). These results with a targeted BUCY regimen are in agreement with observations in patients with chronic myelogenous leukemia (CML)21 and with myelodysplastic syndrome,24 which suggest that maintenance of BU plasma levels within a narrow range contributes to improved regimen tolerance. There was a suggestion that transplantation outcome was inferior in patients older than 50 years (Tables 4, 5). As myelofibrosis is frequently a disease of older patients who may experience more treatment-related toxicity, tolerability of the conditioning regimen is of central importance. BU targeting appears to contribute to that objective. Devine et al recently reported on 4 patients with myelofibrosis who underwent transplantation after “reduced-intensity” conditioning with fludarabine and melphalan.31 All 4 patients achieved engraftment and were surviving 11 to 19 months after transplantation. Whether these results can be confirmed in larger series or with other nonmyeloablative regimens that have been used for various indications32 remains to be determined.
Primary graft failure occurred in 3 patients in the present study who received transplants of marrow cells from alternative donors. Delayed mixed chimerism was observed in another 3 patients, for an overall incidence of failure of complete and sustained donor cell engraftment of 10%. One of the patients with mixed chimerism with a primary diagnosis of P vera showed a rising hematocrit level about 7 years after transplantation and underwent several phlebotomies. Subsequently, without further intervention, blood cell counts normalized, and the proportion of host cells, both lymphoid and myeloid, progressively declined. The mechanism for such a phenomenon is unclear; the kinetics suggest a transient activity of a host clone surviving for years after transplantation. No disease marker was present in the other 2 mixed hematopoietic chimeras.
Concern about graft failure was a reason for the initial reluctance to carry out transplantations in patients with myelofibrosis. The present study and other publications show, however, that graft failure is a problem only in a minority of patients, particularly those who received transplants from “alternative” donors and were given marrow as a source of stem cells. Despite this complication, overall outcome among patients who received transplants from unrelated donors was comparable with that of HLA-identical sibling donors. This result is in keeping with reports that show that selection of unrelated donors on the basis of high-resolution HLA typing can yield transplantation results comparable with those achieved with HLA genotypically identical sibling donors.24
In conclusion, allogeneic hematopoietic cell transplantation offers curative therapy for patients with myelofibrosis, generally with structural normalization of the marrow. Results with HLA-identical unrelated donors are comparable with those with related donors, and HCT can be carried out successfully in patients in their 50s and 60s. The data suggest that, in patients who are interested in pursuing transplantation, HCT should be carried out before severe marrow fibrosis, clonal cytogenetic abnormalities, and severe abnormalities of hematologic parameters develop.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-06-1856.
Supported in part by grants HL36444, CA18029, CA15704, and CA87948 from the National Institutes of Health, Bethesda, MD. E.H.W. is supported by a Lilly Clinical Investigator Award from the Damon Runyon Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We thank all physicians and staff who have cared for these patients; the patients and their physicians for their willingness to participate in these studies; Dr Eileen Bryant and her staff for providing cytogenetic data; Dr Ch. Bagley for the documentation of late mixed chimerism in one patient; Dr Joseph Prchal for carrying out in vitro hematopoietic studies in one patient with P vera; Christine Kane for her help in compiling long-term follow-up observations; and Bonnie Larson and Helen Crawford for manuscript preparation.

![Figure 1. Overall survival. (A) All patients; (B) survival by conditioning regimen. Shown are results in patients prepared with a regimen of targeted BUCY ([t]BUCY) compared with those prepared with other regimens. Surviving patients are indicated by tick marks.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-06-1856/6/m_h82335313001.jpeg?Expires=1765120022&Signature=Ax7q8Y03myy4k9mQu1SvPc0l-1aCDCbmZXRjazfq4DsVeN5OKx7x~VLNtzpBqoJELdPv8YFXMqrm~QZzG1eXA3ucDLlCX0VdUQVXAPaLPV4nZKTXW6T6yWrNAW3Kk9h~hCtmiPE9-GrF2SiONq1YZOB9XW6qXJ4Xv2skXPJ9Vt8HFCDtDp9zz3QUB3FD6Kp9PhFK8hvPRHpe8zCV5kDRGWopNuiuSEuvJzYYZ6IE~0qGj~y7es4SHGS1KzI0qFLG6PgbuMKKk9d2waSFBYer0RsSEoSWVJdNBR274AX39yid-TXfiHgPDhdtdtkw2mctV0Hfm7QpAzFt8SjrpUJ1kQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal