Abstract
The purpose of this study was to investigate the efficacy and safety of the monoclonal antibody, rituximab, in relapsed or refractory hairy cell leukemia (HCL). Fifteen patients with relapsed or primary refractory HCL after nucleoside analogs received rituximab 375 mg/m2 weekly for a total of 8 planned doses. An additional 4 doses could be administered to responders who had not achieved complete response (CR). The overall response rate was 80%. Eight patients (53%) achieved CR, 2 (13%) attained CR by hematologic parameters with residual marrow disease (1% to 5% marrow hairy cells), and 2 (13%) had a partial response. Of the 12 responders followed for a median of 32 months (range, 8 to 45+ months), 5 patients (42%) had progression of disease 8, 12, 18, 23, and 39 months from the start of therapy. Three patients failed to respond (after 4, 6, or 8 doses). Reductions in serum interleukin-2 receptor (sIL-2R) levels correlated with response. Toxicity was minimal, and no infectious episodes were observed. Rituximab has significant activity and minimal toxicity in HCL and warrants further study. Rituximab should be explored further in HCL with regard to eradication of minimal residual disease and in combination with nucleoside analogs.
Introduction
Hairy cell leukemia (HCL), or leukemic reticuloendotheliosis, is a chronic B-cell lymphoproliferative disorder characterized by circulating mononuclear cells that exhibit cytoplasmic projections and have a typical pattern of marrow and spleen infiltration.1,2 Splenectomy was the first standard treatment, because it improved peripheral cytopenias; however, most patients required systemic therapy within a year.3,4 Interferon-α (IFN-α) produced response rates of 80%, but most (70%) responses were partial remissions (PRs). Relapses were frequent after cessation of therapy.5-8 The activity of nucleoside analogs in HCL was demonstrated, and treatment with a single course of 2-chlorodeoxyadenosine (2-CdA) produced complete remission (CR) rates of 80% to 90%.9-15 Responses after 2-CdA were durable, with reported relapse-free survival rates ranging from 70% to 85% at 4 to 5 years.
With longer follow-up, relapses after 2-CdA or deoxycoformycin16-18 (DCF) were common. We had reported a 7-year relapse-free survival rate of 60% after a single 7-day course of 2-CdA (median follow-up, 7 years).19 Response rates after retreatment with 2-CdA ranged from 70% to 90%, but remissions were less durable.12-14,20 Increased risks of repetitive administration of nucleoside analogs include: persistent CD4 lymphocytopenia, marrow aplasia and prolonged cytopenias requiring transfusion support, neutropenic febrile episodes and infections, and neurotoxicity that may be life-threatening.15,21-24 Alternative salvage strategies were sought at our institution after 2 patients with long initial CRs (5 and 7 years, respectively) developed prolonged myelosuppression after retreatment with 2-CdA for relapsed HCL and succumbed to fatal systemic fungal infections.
Ginaldi et al25 have shown that HCL exhibited the highest CD20 antigen density of the malignancies studied to date (eg, 312 ± 110 × 103 CD20 molecules per cell for HCL compared with 65 ± 11 × 103 for chronic lymphocytic leukemia [CLL]). In addition to the expression of CD25 on hairy cells, soluble forms of serum interleukin-2 receptor (sIL-2R) can be detected both in vitro and in vivo. The sIL-2R level is extremely high at initial diagnosis of HCL, with reported mean levels of 19 310 U/mL (range, 1726 to 81 042 U/mL).26-29 Decreases in sIL-2R levels correlate with improvement in hematologic parameters, reduction in splenomegaly, and decrease in the percentage of marrow hairy cells.26-29
Rituximab is a chimeric immunoglobulin G1 (IgG1) κ monoclonal antibody (MoAb) that targets the CD20 antigen expressed on most malignant B-cell leukemias and lymphomas.30-32 Rituximab induces apoptosis in addition to complement- and antibody-mediated cellular cytotoxicity.32-34 It has activity (with minimal toxicity) in both low-grade lymphoma (LGL)35-38 and CLL.39,40 In the pivotal trial of previously treated LGL, 4 weekly doses of 375 mg/m2 resulted in an overall response rate of 48%.32 Piro et al38 reported a 57% overall response rate in the phase 2 trial of 8 weekly standard doses of rituximab in relapsed or refractory LGL, suggesting that extended dosing of rituximab may improve outcome.
Owing to the efficacy reported in other lymphoproliferative disorders, rituximab was considered an attractive agent to study in HCL. A pilot study of rituximab was thus designed for previously treated HCL, with serial assessments of sIL-2R levels during and after completion of therapy. Herein we update the results of our initial findings41 demonstrating the significance of rituximab given weekly for 8 doses in HCL.
Patients and methods
Eligibility
Patients had previously treated HCL with active disease. Morphologic diagnosis of HCL was obtained from peripheral blood and/or bone marrow aspirate/biopsy and supported by positive tartrate-resistant acid phosphatase (TRAP) stain with characteristic immunophenotyping (eg, coexpression of CD22 and CD11c and CD103 positivity). Active disease was defined as 1 or more of the following: (1) hemoglobin (Hgb) less than 100 g/L (10 g/dL) or transfusions of at least 2 units of packed red blood cells per month, absolute neutrophil count (ANC) less than 1.5 × 109/L, platelet (PLT) count less than 100 × 109/L, or more than 25% decline from baseline over 3 months in 1 or more cell lines; (2) circulating hairy cells at least 1 × 109/L or extramedullary HCL; and/or (3) bone marrow hairy cells at least 10%. Recurrent infections, progressive decline in performance status, or symptomatic splenomegaly with any of the above features were additional indications for therapy. Patients were required to have a performance status of 3 or less (Zubrod scale) and adequate liver (bilirubin 51.3 μM [3.0 mg/dL] or less and aspartate aminotransferase/alanine aminotransferase [AST/ALT] less than 3 times the upper limit of normal [ULN] [or less than 5 times the ULN if considered due to tumor]) and renal function (creatinine 265.2 μM [3.0 mg/dL] or less).
Patients had to have received nucleoside analog therapy. They were eligible if they were (1) refractory, (2) responsive but with short remission(s), or (3) responsive but with comorbid conditions or absolute CD4 counts 0.2 × 109/L or less precluding retreatment with nucleoside analogs in the judgment of the treating physician. Level of absolute CD4 lymphopenia was arbitrarily chosen based on suspected potential for further immunosuppression and opportunistic infections if retreated with 2-CdA. Patients refusing chemotherapy were also eligible.
Study group
Between March 1999 and May 2001, 15 patients with relapsed (n = 13) or primary refractory (n = 2) HCL were enrolled after obtaining informed consent according to institutional guidelines. All patients had received prior therapy with 2-CdA; 6 had received retreatment with failure to respond (3), shorter remissions (2), or toxicity (1). Four patients had been treated with IFN-α, 3 with DCF, and 2 had splenectomy. Median time from diagnosis to rituximab therapy was 6 years (range, 6 months to 10 years). Pretreatment characteristics of the study group are detailed in Table 1. Seventy-three percent of the patients were older than 50 years of age, and 53% had received more than 1 prior therapy. All but 1 patient had Zubrod performance status 1 or better. Extramedullary disease was present in 4 patients.
Pretreatment characteristics of 15 patients with relapsed or refractory HCL
Patient no. . | Age, y/sex . | Prior therapy . | Time from diagnosis, mo . | Granulocytes, × 109/L . | Hgb, g/dL . | Platelets, × 109/L . | Absolute CD4 count . | β2-microglobulin, mg/dL . |
|---|---|---|---|---|---|---|---|---|
| 1 | 60/M | IFN-α, 2-CdA × 2 | 103 | 3.6 | 13.4 | 196 | 484 | 2.6 |
| 2 | 47/M | Splenectomy, DCF | 72 | 1.1 | 7.2 | 311 | 343 | 1.4 |
| 3 | 66/F | 2-CdA | 111 | 1.5 | 11.7 | 92 | 474 | 2.3 |
| 4 | 49/M | 2-CdA | 80 | 2.9 | 15.9 | 167 | 481 | 1.3 |
| 5 | 44/M | 2-CdA | 39 | 2.2 | 13.4 | 231 | 595 | 1.7 |
| 6 | 41/F | 2-CdA | 72 | 1.2 | 13.0 | 75 | 569 | 1.5 |
| 7 | 35/M | 2-CdA | 4 | 0.1 | 9.2 | 59 | — | 1.4 |
| 8 | 60/M | 2-CdA × 2, splenectomy, IFN-α | 72 | 0.6 | 10.0 | 104 | — | 3.3 |
| 9 | 52/M | DCF, IFN-α, fludarabine, 2-CdA × 3 | 84 | 1.1* | 12.2 | 103 | 119 | 1.5 |
| 10 | 58/M | IFN-α, 2-CdA, IFN-α, 2-CdA | 126 | 1.2 | 11.2 | 88 | — | — |
| 11 | 59/M | 2-CdA | 107 | 1.3 | 11.4 | 99 | 306 | 1.5 |
| 12 | 71/F | 2-CdA × 2 | 46 | 2.3* | 10.6 | 90 | 260 | 3.7 |
| 13 | 28/M | 2-CdA | 6 | 1.5* | 14.8 | 79 | 1430 | 1.7 |
| 14 | 63/M | 2-CdA | 106 | 0.1 | 7.2 | 29 | 225 | 3.9 |
| 15 | 65/M | Splenectomy, IFN-α, DCF, 2-CdA | 225 | 0.9 | 13.4 | 121 | — | 1.8 |
Patient no. . | Age, y/sex . | Prior therapy . | Time from diagnosis, mo . | Granulocytes, × 109/L . | Hgb, g/dL . | Platelets, × 109/L . | Absolute CD4 count . | β2-microglobulin, mg/dL . |
|---|---|---|---|---|---|---|---|---|
| 1 | 60/M | IFN-α, 2-CdA × 2 | 103 | 3.6 | 13.4 | 196 | 484 | 2.6 |
| 2 | 47/M | Splenectomy, DCF | 72 | 1.1 | 7.2 | 311 | 343 | 1.4 |
| 3 | 66/F | 2-CdA | 111 | 1.5 | 11.7 | 92 | 474 | 2.3 |
| 4 | 49/M | 2-CdA | 80 | 2.9 | 15.9 | 167 | 481 | 1.3 |
| 5 | 44/M | 2-CdA | 39 | 2.2 | 13.4 | 231 | 595 | 1.7 |
| 6 | 41/F | 2-CdA | 72 | 1.2 | 13.0 | 75 | 569 | 1.5 |
| 7 | 35/M | 2-CdA | 4 | 0.1 | 9.2 | 59 | — | 1.4 |
| 8 | 60/M | 2-CdA × 2, splenectomy, IFN-α | 72 | 0.6 | 10.0 | 104 | — | 3.3 |
| 9 | 52/M | DCF, IFN-α, fludarabine, 2-CdA × 3 | 84 | 1.1* | 12.2 | 103 | 119 | 1.5 |
| 10 | 58/M | IFN-α, 2-CdA, IFN-α, 2-CdA | 126 | 1.2 | 11.2 | 88 | — | — |
| 11 | 59/M | 2-CdA | 107 | 1.3 | 11.4 | 99 | 306 | 1.5 |
| 12 | 71/F | 2-CdA × 2 | 46 | 2.3* | 10.6 | 90 | 260 | 3.7 |
| 13 | 28/M | 2-CdA | 6 | 1.5* | 14.8 | 79 | 1430 | 1.7 |
| 14 | 63/M | 2-CdA | 106 | 0.1 | 7.2 | 29 | 225 | 3.9 |
| 15 | 65/M | Splenectomy, IFN-α, DCF, 2-CdA | 225 | 0.9 | 13.4 | 121 | — | 1.8 |
Patient nos. 2 and 14 both transfusion-dependent of packed red blood cells, with the latter also platelet transfusion dependent. Patient nos. 6, 7, 8, and 12 had splenomegaly more than 5 cm below costal margin. 2-CdA × denotes number of salvage attempts with 2-CdA; and —, not done.
Circulating hairy cells
Therapy
Rituximab 375 mg/m2 was given over 6 to 12 hours for the first dose. Patients were premedicated with diphenhydramine and acetaminophen. The dose rate was escalated from 50 mg/h to a maximum of 300 mg/h. If fever, chills, hypotension, or other toxicity was observed, the infusion was held until resolution of symptoms. Rituximab was then resumed and re-escalated as tolerated. Hydrocortisone was given to ameliorate symptoms at the physician's discretion. Doses were repeated weekly at 375 mg/m2 for a total of 8 doses. An additional 4 doses (maximum of 12 doses of rituximab) could be administered if patients had not yet achieved a CR but showed signs of continued hematologic improvement.
Assessments
Hematologic profiles and toxicity assessments were usually performed weekly prior to each dose of rituximab. Absolute CD4 counts were obtained at baseline in 12 patients and after completion of therapy in 10 patients. Imaging was performed as clinically indicated.
Bone marrow assessments with immunophenotyping were obtained within 1 to 3 months of the last dose of rituximab. Immunostaining with the anti-CD20 antibodies L26 and DBA.44 was performed on bone marrows of patients achieving CR when feasible. Immunostaining with the novel anti-pan-B-cell marker Pax-5, a B-cell-specific transcription factor, was retrospectively performed (BD Biosciences San Diego, CA) on bone marrow biopsies.42
Serial sIL-2R levels were measured by an immunoenzymatic assay. Performance characteristics were determined by the reference sites. Prior to December 1999, these studies were performed at MRL Reference Laboratory (Cypress, CA) (sIL-2R reference range: normal, less than 970 U/mL; linearity, more than 5868 U/mL without titration). Thereafter, assays were performed at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA) (reference range, 200-1100 U/mL; linearity, more than 6684 U/mL without titration; samples were titrated when above linearity after May 2001).
Supportive care
Prophylactic antibiotics were not routinely administered except if judged appropriate by the treating physician. Neither granulocyte-macrophage colony-stimulating factor (GM-CSF) nor G-CSF was administered concomitantly with the rituximab. Transfusion support with irradiated and filtered packed red blood cells and/or platelets was provided when indicated.
Criteria for response
Complete response (CR) was defined as the absence of hairy cells in the bone marrow (BM) or the presence of less than 1% atypical cells (not definitively called hairy cells) and the disappearance of all evidence of HCL on physical examination. Hematologic parameters for CR required an ANC of 1.5 × 109/L or greater; Hgb at least 120 g/L [12.0 g/dL] (at least 110 g/L [11.0 g/dL] for females); and PLT at least 100 × 109/L without growth factor or transfusion support. CR with residual disease (CR-RD) was defined as for CR but with persistence of 1% to 5% hairy cells in the marrow (but no circulating hairy cells). Partial response (PR) was defined as (1) meeting the peripheral blood count criteria for CR/CR-RD with more than 5% residual hairy cells in the marrow or (2) at least 50% improvement or correction of at least 1 cytopenia without decrease in any of the cell counts; reduction in palpable abnormalities on physical examination by at least 50%; and reduction in circulating or bone marrow hairy cells by at least 50%. All other responses were considered failures.
Statistical considerations
This pilot study was designed to assess the efficacy and safety of rituximab in up to 18 patients with previously treated HCL meeting the eligibility criteria stated previously. Associations between patient characteristics and response outcome were evaluated by Fisher exact χ2 tests.43 Cut points for quantitative variables were those commonly used for defining abnormal values. Distributions of overall survival and relapse-free survival were estimated by the methods of Kaplan and Meier.44 Survival was measured from the start of therapy until death from any cause. Relapse-free survival was measured from the start of therapy until detection of relapse or progressive disease. Relapse was defined as detection of hairy cells on routine morphologic marrow evaluation in patients who had achieved CR. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0.
Results
Response
The overall response rate was 80% (Table 2). Eight patients achieved CR after 8 doses of rituximab (except for 2 patients after treatment with 4 and 12 doses, respectively). Three of 5 patients with pretreatment cytopenias met hematologic criteria for CR after 4 doses but did not have bone marrows performed to assess for CR (not required by protocol). Of the 8 responders, 1 was primary refractory to 2-CdA, and 1 had failed 2-CdA retreatment prior to rituximab. Two patients achieved CR-RD (one after 4 and one after 8 doses), and 2 patients achieved PR (one after 4 and one after 12 doses). Two of the responders (CR-RD and PR) received only 4 of the 8 planned doses based on the judgment of the treating physician. Three patients failed to respond after 4, 6, and 8 doses, respectively.
Response to rituximab by number of prior therapies and prior therapy response
. | . | . | . | . | Bone marrow HCs, % . | . | . | Bone marrow flow cytometry CD20, % . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | No. of prior treatments . | Prior response* . | Rituximab response (no. of doses)† . | RR, % (response) . | Before therapy . | After therapy . | Immuno/cytochemical marrow stains after therapy . | Before therapy . | After therapy . | ||
| 1 | 3 | CR | CR | 53 (CR) | 11 | 0 | Rare TRAP+ CD20— | 95.7 | 0.8 | ||
| 2 | 3 | NR‡ | CR | 3 | 0 | TRAP— CD20— | 78.4 | 0.0 | |||
| 3 | 1 | CR | CR | 18 | 0 | Rare TRAP+ | 49.2 | 0.0 | |||
| 4 | 1 | CR | CR | 5 | 0 | Rare TRAP+ | 59.9 | ND | |||
| 5 | 1 | CR | CR | 7 | 0 | TRAP— Pax-5+ 9% | 76.7 | 1.8 | |||
| 6 | 1 | CR | CR | 6 | 0 | Rare TRAP+ Pax-5+ 10% | 87.3 | 0.2 | |||
| 7 | 1 | NR‡ | CR | 5 | 0 | Rare TRAP+ Pax-5+ 11% | 85.1 | 3.1 | |||
| 8 | 2 | NR‡ | CR (12) | 10 | 0 | Rare TRAP+ Pax-5+ 14% | 95.4 | 1.4 | |||
| 9 | 8 | PR | CR-RD | 13 (CR-RD) | 22 | 4 | ND | 96.2 | 0.8 | ||
| 10 | 4 | NR | CR-RD (4) | 24 | 2 | ND | 91.8 | ND | |||
| 11 | 1 | CR | PR (4) | 13 (PR) | 33 | 7 | ND | 89.6 | 4.6 | ||
| 12 | 2 | NR‡ | PR (12) | 15 | 4 | ND | 87.2 | 67.5 | |||
| 13 | 1 | NR‡ | NR | 20 (NR) | 30 | 28 | ND | 88.5 | 91.3 | ||
| 14 | 3 | CR | NR (6) | 71 | 90 | ND | 93.5 | 0.4 | |||
| 15 | 4 | PR | NR (4) | 7 | 17 | ND | 88.4 | ND | |||
. | . | . | . | . | Bone marrow HCs, % . | . | . | Bone marrow flow cytometry CD20, % . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | No. of prior treatments . | Prior response* . | Rituximab response (no. of doses)† . | RR, % (response) . | Before therapy . | After therapy . | Immuno/cytochemical marrow stains after therapy . | Before therapy . | After therapy . | ||
| 1 | 3 | CR | CR | 53 (CR) | 11 | 0 | Rare TRAP+ CD20— | 95.7 | 0.8 | ||
| 2 | 3 | NR‡ | CR | 3 | 0 | TRAP— CD20— | 78.4 | 0.0 | |||
| 3 | 1 | CR | CR | 18 | 0 | Rare TRAP+ | 49.2 | 0.0 | |||
| 4 | 1 | CR | CR | 5 | 0 | Rare TRAP+ | 59.9 | ND | |||
| 5 | 1 | CR | CR | 7 | 0 | TRAP— Pax-5+ 9% | 76.7 | 1.8 | |||
| 6 | 1 | CR | CR | 6 | 0 | Rare TRAP+ Pax-5+ 10% | 87.3 | 0.2 | |||
| 7 | 1 | NR‡ | CR | 5 | 0 | Rare TRAP+ Pax-5+ 11% | 85.1 | 3.1 | |||
| 8 | 2 | NR‡ | CR (12) | 10 | 0 | Rare TRAP+ Pax-5+ 14% | 95.4 | 1.4 | |||
| 9 | 8 | PR | CR-RD | 13 (CR-RD) | 22 | 4 | ND | 96.2 | 0.8 | ||
| 10 | 4 | NR | CR-RD (4) | 24 | 2 | ND | 91.8 | ND | |||
| 11 | 1 | CR | PR (4) | 13 (PR) | 33 | 7 | ND | 89.6 | 4.6 | ||
| 12 | 2 | NR‡ | PR (12) | 15 | 4 | ND | 87.2 | 67.5 | |||
| 13 | 1 | NR‡ | NR | 20 (NR) | 30 | 28 | ND | 88.5 | 91.3 | ||
| 14 | 3 | CR | NR (6) | 71 | 90 | ND | 93.5 | 0.4 | |||
| 15 | 4 | PR | NR (4) | 7 | 17 | ND | 88.4 | ND | |||
RR indicates response rate; HCs, hairy cells; CR, complete response; TRAP, tartrate-resistant acid phosphatase stain; —, _____; NR, no response; ND, not done; PR, partial response; and CR-RD, CR with residual marrow hairy cells 1% to 5%.
Response to last therapy received prior to rituximab
Fewer than 8 doses due to patient request, lack of response, or judgment of physician
Refractory to last 2-CdA
Serum IL-2 receptor levels
Reductions in sIL-2R levels appeared to correlate with response, and rising levels often preceded development of overt progression of disease (Table 3). The sIL-2R levels generally normalized during the first 2 to 3 months of therapy in patients who achieved CR, although the early response (within 4 weeks) sIL-2R kinetics cannot be determined owing to limited sampling. Patients who had residual disease or failed to respond to rituximab maintained elevated sIL-2R levels. One nonresponder who demonstrated a minor reduction in sIL-2R level (patient no. 15) failed to meet the criteria for PR, although there was a hematologic improvement in the platelet count after 4 doses of rituximab.
Serum IL-2 receptor levels by response
. | . | Serum IL-2 receptor levels, U/mL* . | . | . | |
|---|---|---|---|---|---|
| Patient no. . | Rituximab response (no. of doses) . | Before therapy . | After therapy (months after start of therapy) . | Disease status (mo) . | |
| 1 | CR | > 5868 | 4184 (2) | CR (44+) | |
| < 24 (5) | |||||
| 553 (8) | |||||
| 587 (12) | |||||
| 663 (16) | |||||
| 498 (20) | |||||
| 723 (24) | |||||
| 904 (28) | |||||
| 713 (44) | |||||
| 2 | CR | > 6432 | 419 (4) | CR (32+) | |
| 300 (8) | |||||
| 3 | CR | > 5868 | < 24 (5) | CR (45+) | |
| 4 | CR | > 5868 | 429 (4) | CR (30+) | |
| 454 (12) | |||||
| 5 | CR | 2895 | 939 (1) | CR (33+) | |
| 585 (2) | |||||
| 470 (6) | |||||
| 6 | CR | 16 509 | 664 (2) | CR (23+) | |
| 456 (9) | |||||
| 7 | CR | 5364 | 1586 (2) | PD (18) | |
| 1195 (9) | |||||
| 8 | CR (12) | > 5868 | 1775 (3) | CR (40+) | |
| 9 | CR-RD | 5645 | 575 (3) | PD (12) | |
| 1889 (12) | |||||
| 10 | CR-RD (4) | 2957 | 982 (5) | PD (23) | |
| 2078 (13) | |||||
| 2316 (15) | |||||
| 11 | PR (4) | > 5868 | ND (—) | PD (39) | |
| 12 | PR (12) | 20 468 | 23 106 (1) | ||
| 23 640 (2) | PD (8) | ||||
| 77 827 (6) | |||||
| 13 | NR | > 6684 | > 6684 (1) | NA | |
| 14 | NR (6) | > 6684 | > 6684 (12) | NA | |
| 15 | NR (4) | > 5868 | 4845† (1) | SD (24+) | |
. | . | Serum IL-2 receptor levels, U/mL* . | . | . | |
|---|---|---|---|---|---|
| Patient no. . | Rituximab response (no. of doses) . | Before therapy . | After therapy (months after start of therapy) . | Disease status (mo) . | |
| 1 | CR | > 5868 | 4184 (2) | CR (44+) | |
| < 24 (5) | |||||
| 553 (8) | |||||
| 587 (12) | |||||
| 663 (16) | |||||
| 498 (20) | |||||
| 723 (24) | |||||
| 904 (28) | |||||
| 713 (44) | |||||
| 2 | CR | > 6432 | 419 (4) | CR (32+) | |
| 300 (8) | |||||
| 3 | CR | > 5868 | < 24 (5) | CR (45+) | |
| 4 | CR | > 5868 | 429 (4) | CR (30+) | |
| 454 (12) | |||||
| 5 | CR | 2895 | 939 (1) | CR (33+) | |
| 585 (2) | |||||
| 470 (6) | |||||
| 6 | CR | 16 509 | 664 (2) | CR (23+) | |
| 456 (9) | |||||
| 7 | CR | 5364 | 1586 (2) | PD (18) | |
| 1195 (9) | |||||
| 8 | CR (12) | > 5868 | 1775 (3) | CR (40+) | |
| 9 | CR-RD | 5645 | 575 (3) | PD (12) | |
| 1889 (12) | |||||
| 10 | CR-RD (4) | 2957 | 982 (5) | PD (23) | |
| 2078 (13) | |||||
| 2316 (15) | |||||
| 11 | PR (4) | > 5868 | ND (—) | PD (39) | |
| 12 | PR (12) | 20 468 | 23 106 (1) | ||
| 23 640 (2) | PD (8) | ||||
| 77 827 (6) | |||||
| 13 | NR | > 6684 | > 6684 (1) | NA | |
| 14 | NR (6) | > 6684 | > 6684 (12) | NA | |
| 15 | NR (4) | > 5868 | 4845† (1) | SD (24+) | |
CR indicates complete remission; PD, progressive disease requiring further therapy; CR-RD, CR with residual marrow hairy cells 1% to 5%; ND, not done, NR, no response; NA, not applicable, and SD, stable disease.
Reference range, less than 970 U/mL or 200 to 1100 U/mL (depending on assay); for conversion to pg/mL, multiply by factor of 8.85
Marrow hairy cells unchanged with improvement of platelet count
Toxicity
Most adverse events were grade 1 and 2 expected infusional events that occurred during the first dose of rituximab (Table 4). Sixty percent of the patients experienced fever and chills, and one fourth had nausea and vomiting. Grade 2 infusional events with the first dose included shortness of breath, hypotension, and palpitations. Rapid resolution occurred with temporary cessation of the infusion; 1 patient required hydrocortisone for shortness of breath. Subsequent doses were generally administered without incidents except in 2 patients: 1 had recurrence of palpitations with the second dose only, and 1 had grade 3 myalgias for 24 to 48 hours after each dose, requiring narcotic use. Other mild complaints included back pain, fatigue, and rash. No infections were observed, and no patient required hospitalization. No significant reductions in absolute CD4 lymphocyte counts (median pretreatment level, 0.481 × 109/L [481/μL]; posttreatment, 0.357 × 109/L [357/μL]) or immunoglobulin levels (median pretreatment IgG level, 10.4 g/L [1040 mg/dL]; posttreatment, 11.9 g/L [1190 mg/dL]) were observed in patients who had serial measurements performed (n = 10).
Toxicity with rituximab, n = 15
Toxicity . | No. . | Grade . | Dose no. . |
|---|---|---|---|
| Fever and chills | 9 | 1 | 1 |
| Nausea and vomiting | 4 | 1 | 1,2 |
| Cardiovascular | 2 | — | — |
| Hypotension | 1 | 1 | 1 |
| Palpitations | 1 | 2 | 1,2 |
| Pulmonary: shortness of breath | 1 | 2 | 1 |
| Myalgias | 1 | 3 | All 8 |
| Fatigue | 2 | 1,2 | 1,2 |
| Back pain | 1 | 1 | 1 |
| Rash | 1 | 1 | 1 |
| Infection | 0 | — | — |
Toxicity . | No. . | Grade . | Dose no. . |
|---|---|---|---|
| Fever and chills | 9 | 1 | 1 |
| Nausea and vomiting | 4 | 1 | 1,2 |
| Cardiovascular | 2 | — | — |
| Hypotension | 1 | 1 | 1 |
| Palpitations | 1 | 2 | 1,2 |
| Pulmonary: shortness of breath | 1 | 2 | 1 |
| Myalgias | 1 | 3 | All 8 |
| Fatigue | 2 | 1,2 | 1,2 |
| Back pain | 1 | 1 | 1 |
| Rash | 1 | 1 | 1 |
| Infection | 0 | — | — |
— indicates not applicable.
Duration of response and salvage therapy
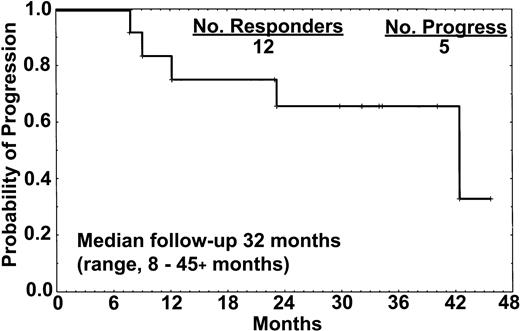
The median follow-up was 32 months (range, 8 to 45+ months). Five patients (42%) had progression of disease 8, 12, 18, 23, and 39 months from the start of therapy. One of 8 patients who achieved CR developed disease progression after 18 months and commenced retreatment with rituximab (Table 3). One patient (no. 1) had minimal residual disease (MRD) detectable in the marrow by immunostaining with L26 and DBA.44 at 12 months, and a slight increment in the sIL-2R level that had previously been undetectable, but no other signs of disease. All 4 patients who attained CR-RD or PR (2 treated with only 4 doses) developed disease progression at 8, 12, 23, and 39 months (Figure 1). These patients had active disease that required therapy at the time of progression.
Relapse-free survival of the 12 responders. One of the 8 patients who achieved CR relapsed after 18 months, whereas all 4 patients who achieved either CR-RD or PR have progressed. All 15 patients treated with rituximab remained alive at the time of this report.
Relapse-free survival of the 12 responders. One of the 8 patients who achieved CR relapsed after 18 months, whereas all 4 patients who achieved either CR-RD or PR have progressed. All 15 patients treated with rituximab remained alive at the time of this report.
Patient no. 9 had failed to respond to etanercept (recombinant soluble human tumor necrosis factor receptor p75-Fc fusion protein) and then achieved a brief CR with a combination of rituximab and 2-CdA as the tenth salvage regimen (prior splenectomy, IFN-α, DCF, fludarabine, and three 2-CdA retreatments). Patient no. 10 failed etanercept and then achieved a PR with 2-CdA retreatment (previously refractory to 2-CdA). Patient no. 11 failed retreatment with 2-CdA. Patient no. 12 was undergoing evaluation for the immunotoxin BL-22 at the time of this report.45 Of the 3 nonresponders to rituximab, 2 received salvage therapy. Patient no. 13 was first treated with single-agent alemtuzumab, and then etanercept without success, but achieved PR with BL-22. Patient no. 14 achieved PR with 2-CdA retreatment.
Discussion
Hairy cell leukemia (HCL) is a chronic B-cell lymphoproliferative disorder with a high response rate to a single course of 2-CdA.9-15 Although the efficacy of 2-CdA has been confirmed by multiple investigators, an increasing proportion of recurrences has been observed with longer follow-up, raising the issue of whether 2-CdA can truly eradicate HCL.19 Treatment of actively relapsed or refractory HCL can be challenging. Current salvage strategies are limited. Options include (1) retreatment with nucleoside analogs,12-15,20 (2) IFN-α,46 (3) splenectomy,3,4 or (4) investigational agents such as novel chemotherapy drugs, monoclonal antibodies, immunotoxins (eg, LMB22 or BL-22),45,47 TNF-α inhibitors,48 and angiogenesis inhibitors.49 The anti-CD20 MoAb rituximab has demonstrated activity with minimal toxicity in both low-grade lymphomas35-38 and CLL39,40 and was thus investigated in HCL.
This report illustrates significant activity of extended-dosing single-agent rituximab in HCL. Eighty percent of the patients responded, with a 53% CR rate. These responses appeared durable with extended follow-up (median, 32 months; longest, 45+ months). Progression was observed in 1 of 8 patients with CR. All patients with CR-RD or PRs progressed. Rituximab was well tolerated, with most toxicities being grade 1 infusional events during the first dose, as expected (Table 4). No patient required hospitalization, and no infectious episodes were observed.
Levels of the soluble forms of the IL-2 receptor (CD25) are extremely high at initial diagnosis of HCL, and decreasing levels of sIL-2R have correlated with improvement in hematologic parameters, reduction in splenomegaly, and decrease in the percentage of marrow hairy cells.26-29 In patients treated with 2-CdA, the decrease in sIL-2R could be observed as early as 48 hours into therapy, with the maximal decrease 2 to 3 weeks after 2-CdA. With other agents such as DCF and IFN-α, the time for nadir of the sIL-2R levels was 4 to 6 months and 12 months, respectively. Increasing sIL-2R levels may predict relapse prior to the development of clinically detectable cytopenias, with elevations in sIL-2R levels detectable from 4 months to 3 years prior to overt disease.50
In our study, sIL-2R levels appeared to correlate with response. In a few patients who achieved CR, the sIL-2R levels became undetectable. Those who achieved PR had reductions in levels from baseline but with persistent elevation (Table 3). Nonresponders had no significant declines in sIL-2R levels, indicating that this parameter could be a surrogate marker to determine timing of bone marrow aspiration and response.
Alternative antigen targets to CD20 have been recently explored in HCL. Hairy cells (except variant HCL) express the Tac antigen, which corresponds to the p55 α chain of the interleukin-2 receptor (IL-2R) or CD25 antigen.51,52 It is normally expressed on the surface of activated T and B cells and on various malignant cells. The recombinant immunotoxin composed of the Fv portion of the anti-Tac antibody fused to a truncated form of Pseudomonas exotoxin A (LMB-2) has demonstrated preliminary activity in 4 patients with refractory hairy cell leukemia treated in a phase 1 trial.47 Although all 4 patients responded (1 CR, 3 PRs), toxicities included grade 1-2 fever, grade 1 elevation in transaminases, gastrointestinal disturbances such as grade 1 nausea or grade 3 diarrhea, and a transient grade 4 cardiomyopathy. In addition, neutralizing antibodies were observed in 6 of the 36 patients treated with various malignancies, a phenomenon currently not observed with rituximab.
Kreitman et al45 reported an 80% response rate (69% CR rate) in 16 patients with previously treated HCL given BL-22 (a recombinant immunotoxin containing an anti-CD22 variable domain [Fv] fused to truncated Pseudomonas exotoxin), with half of the patients also receiving rofecoxib and infliximab to ameliorate a cytokine release syndrome. Toxicities included observation of reversible hemolytic-uremic syndrome in 2 patients. Three of the 11 CRs relapsed after a median follow-up of 16 months (range, 10 to 23 months) and were retreated with BL-22. All 3 patients achieved a second complete remission. Neutralizing antibodies developed in 4 of the 16 patients and may be a limitation in the design of future clinical trials.
Rituximab appears to be a good therapeutic option in HCL, although its optimal dose and schedule remains unknown. The use of single-agent rituximab 375 mg/m2 given weekly for 4 doses has been studied in previously treated HCL. Hagberg and Lundholm53 reported a response rate of 75% (CR rate, 62%) in 8 previously treated HCL patients. Of 3 untreated patients, only 1 achieved CR with rituximab. None of the responders had relapsed after a median follow-up of 14 months (range, 1 to 34 months). In contrast, Lauria et al54 reported a response rate of 50% (CR rate, 10%) in 10 previously treated patients, with criteria for CR including negative immunostaining of the bone marrow. In our study, 4 patients in morphologic CR were positive for minimal residual disease by Pax-5/CD20 immunostaining (2 others were negative by standard CD20 immunostaining). Three of the 4 patients remained in CR (23+, 33+, 40+ months); 1 patient recurred after 18 months. Clinical significance of positivity for MRD by Pax-5/CD20 immunostaining after therapy requires prospective testing.
Although the numbers of patients evaluated in these 2 studies were small, the results of our study and that of Lauria et al54 suggest that 4 weekly standard doses may be inadequate to achieve the optimal response in some patients. Review of pretreatment characteristics in these studies suggests a lower response rate in patients with higher pretreatment hairy cell leukemia marrow infiltration (these data were not provided in the Hagberg and Lundholm study53 ). Cytokine modulation to up-regulate the CD20 antigen may improve the efficacy of rituximab with little additional toxicity.55 Synergy of rituximab with other nucleoside analogs such as fludarabine has been observed and applied to combined modality therapy of CLL with success.56,57 Rituximab could be administered in combination with 2-CdA and/or other agents such as IFN-α to exploit this synergy and to reduce the currently observed relapse rates in HCL.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-02-0630.
Presented in part at the 41st annual meeting of the American Society of Hematology Meeting, New Orleans, LA, December 3-7, 1999.41
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Sherry Pierce, RN, for her instrumental role in the preparation of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal