p38 mitogen-activated protein kinase (MAPK) is a member of the MAPK family which is activated by cytokines and growth factors, but its role in pathogenesis of multiple myeloma (MM) is unknown. In this study, we demonstrate that the specific p38 MAPK inhibitor VX-745 inhibits interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) secretion in bone marrow stromal cells (BMSCs), without affecting their viability. Tumor necrosis factor alpha (TNF-α)–induced IL-6 secretion in BMSCs is also inhibited by VX-745. Importantly, VX-745 inhibits both MM cell proliferation and IL-6 secretion in BMSCs triggered by adherence of MM cells to BMSCs, suggesting that it can inhibit paracrine MM cell growth in the BM milieu and overcome cell adhesion–related drug resistance. These studies therefore identify p38 MAPK as a novel therapeutic target to overcome drug resistance and improve patient outcome in MM.
Introduction
Multiple myeloma (MM) cell growth, survival, drug resistance against conventional chemotherapies, and migration are mediated via cytokines in the bone marrow (BM) milieu. Specifically, interleukin 6 (IL-6)1-4 promotes multiple myeloma (MM) cell growth, survival, and drug resistance; whereas vascular endothelial growth factor (VEGF)5 induces MM cell migration. Importantly, adherence of MM cells to bone marrow stromal cells (BMSCs) up-regulates cytokine (IL-6, VEGF) secretion from both MM and BMSCs.6-8 Most excitingly, novel agents including proteasome inhibitor PS-341 and the immunodulatory derivatives of thalidomide (IMiDs) can overcome drug resistance in vitro9and in animal models10 by targeting not only tumor cells, but also host tumor cell interactions and cytokines in the BM milieu. Already these agents are achieving responses, even complete responses, in the setting of relapse and refractory MM.11 12
Recent studies have demonstrated that activation of p38 MAPK is associated with IL-6 gene expression and/or protein secretion in Sertoli cells,13 myocardial cells,14 and osteoblasts15; however, the role of p38 MAPK activation in modulating IL-6 secretion in BMSCs is undefined. In the present study, we demonstrate that the p38 MAPK inhibitor VX-745 decreases IL-6 secretion in the BMSCs of patients with MM, proliferation of MM cells adherent to BMSCs, and IL-6 secretion in BMSCs triggered by adherence of MM cells to BMSCs. These studies therefore identify p38 MAPK as a novel therapeutic target in MM, and provide the framework for clinical trials of VX-745 to improve patient outcome.
Study design
MM-derived cell lines and patient MM cells
BMSC cultures
p38 MAPK inhibitor and cytokines
A potent p38 MAPK–specific inhibitor, VX-745 (Vertex Pharmaceuticals, Cambridge, MA), was dissolved in dimethyl sulfoxide (DMSO) and kept at −20°C until use. Tumor necrosis factor alpha (TNF-α) was purchased from R&D Systems (Minneapolis, MN). SB203580 (Calbiochem, San Diego, CA) was used as a positive control for p38 MAPK inhibition.
DNA synthesis and growth inhibition assay
Proliferation was measured as previously described.16 MM cells (3 × 104cells/well) or BMSCs (5 × 104cells/well) were incubated in 96-well culture plates (Costar, Cambridge, MA) in the presence or absence of VX-745 for 48 hours at 37°C. DNA synthesis was measured by [3H]-thymidine ([3H]TdR; NEN Perkin Elmer Life Sciences, Boston, MA) uptake. Cells were pulsed with [3H]TdR (0.5 μCi/well [.0185 MBq]) during the last 8 hours of 48-hour cultures. Growth inhibition of both MM cells and BMSCs by VX-745 was also assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye absorbance, as previously described.16 All experiments were performed in quadruplicate.
Effect of VX-745 on paracrine MM cell growth in the BM
To evaluate growth stimulation and signaling in MM cells adherent to BMSCs, 3 × 104 MM.1S cells were cultured in BMSC-coated 96-well plates for 48 hours, in the presence or absence of VX-745. DNA synthesis was measured as described above. The Duoset enzyme-linked immunosorbent assay (ELISA) (R&D Systems) was used to measure IL-6 and VEGF in supernatants of 48-hour cultures of BMSCs with or without MM.1S cells, and in the presence or absence of VX-745, as previously described.19
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using the Wilcoxon signed-rank test. The minimal level of significance was P < .05.
Results and discussion
VX-745 inhibits IL-6 and VEGF secretion in BMSCs
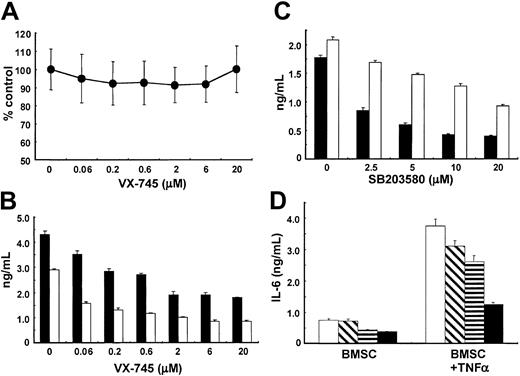
We first examined the direct effect of VX-745 on the growth of BMSCs in patients with MM, assessed by MTT assay. The growth of BMSCs in 6 patients with MM was not inhibited by VX-745 (0.05 μM-20 μM; Figure 1A). We have shown that BMSCs secrete IL-6 and VEGF,7,8 19which mediated MM cell growth, survival, drug resistance, and migration, and we therefore next examined whether VX-745 inhibits IL-6 and/or VEGF secretion from BMSCs. VX-745 significantly (P < .01) decreases IL-6 secretion in BMSCs in a dose-dependent fashion (Figure 1B), with peak inhibitory effect at 2 μM VX-745. VX-745 similarly inhibits VEGF secretion from BMSCs (Figure 1B) and MM cell lines (data not shown). To confirm that this inhibitory effect was mediated via inhibition of p38 MAPK, we used SB203580. As seen in Figure 1C, SB203580 also inhibits secretion of both IL-6 and VEGF in a dose-dependent fashion from BMSCs, suggesting that p38 MAPK regulates secretion of these cytokines from BMSCs.
Effect of VX-745 on IL-6 and VEGF secretion in BMSCs.
(A) BMSCs from patients with MM were cultured in the presence (0.06 μM-20 μM) or absence of VX-745 for 48 hours, and cell viability was assessed by MTT assay. The figure represents means (± SDs) from BMSCs of 6 patients with MM. (B) BMSCs were cultured in the presence (0.06 μM-20 μM) or absence of VX-745 for 48 hours. IL-6 (▪) and VEGF (■) in culture supernatants was measured by ELISA. (C) BMSCs were cultured in the presence or absence of SB203580 (2.5 μM-20 μM) for 48 hours. IL-6 (▪) and VEGF (■) in culture supernatants was measured by ELISA. (D) BMSCs were cultured in the presence (0.06 μM-20 μM) or absence of VX-745 for 48 hours. VEGF in culture supernatants was measured by ELISA. (D) BMSCs were cultured with TNF-α (5 ng/mL) in the absence (■) or presence of 0.05 μM (▧), 0.5 μM (▤), and 5 μM (▪) VX-745 for 48 hours. IL-6 in culture supernatants was measured by ELISA.
Effect of VX-745 on IL-6 and VEGF secretion in BMSCs.
(A) BMSCs from patients with MM were cultured in the presence (0.06 μM-20 μM) or absence of VX-745 for 48 hours, and cell viability was assessed by MTT assay. The figure represents means (± SDs) from BMSCs of 6 patients with MM. (B) BMSCs were cultured in the presence (0.06 μM-20 μM) or absence of VX-745 for 48 hours. IL-6 (▪) and VEGF (■) in culture supernatants was measured by ELISA. (C) BMSCs were cultured in the presence or absence of SB203580 (2.5 μM-20 μM) for 48 hours. IL-6 (▪) and VEGF (■) in culture supernatants was measured by ELISA. (D) BMSCs were cultured in the presence (0.06 μM-20 μM) or absence of VX-745 for 48 hours. VEGF in culture supernatants was measured by ELISA. (D) BMSCs were cultured with TNF-α (5 ng/mL) in the absence (■) or presence of 0.05 μM (▧), 0.5 μM (▤), and 5 μM (▪) VX-745 for 48 hours. IL-6 in culture supernatants was measured by ELISA.
We next examined whether VX-745 inhibits IL-6 secretion in BMSCs in the presence of TNF-α, since TNF-α is secreted by MM cells,19 activates p38 MAPK,20,21 and induces IL-6 secretion in BMSCs.19 Importantly, VX-745 inhibits TNF-α–induced up-regulation of IL-6 in BMSCs (Figure 1D). Similar results are observed in the presence of VEGF (data not shown), which is secreted by MM cells and BMSCs8 and in turn induces further IL-6 transcription22 and secretion from BMSCs.8 Since transcription and protein expression of IL-6 is regulated by NF-κB activation,6,16 these results suggest that p38 MAPK may mediate TNF-α–induced nuclear factor (NF)–κB activation and related induction of IL-6 in BMSCs, consistent with previous reports in myocytes14 and osteoblasts.15 Importantly, since both TNF-α and VEGF are secreted by MM cells,8 19 these results suggest that VX-745 can inhibit cytokine (TNF-α, IL-6, VEGF)–induced paracrine MM cell growth, survival, and drug resistance in the BM microenvironment. Ongoing studies are delineating whether VX-745 inhibits constitutive and/or TNF-α–stimulated NF-κB activation in BMSCs.
VX-745 inhibits paracrine MM cell growth in the BM
We next examined whether VX-745 inhibits MM cell growth in the BMSC microenvironment. As seen in Figure2A, VX-745 induces modest growth inhibition of MM.1S, RPMI8226, and U266 cell lines in a dose-dependent fashion, with inhibitory concentration of 50% (IC50) of 10 μM as assessed by MTT assay. This result suggests that p38 MAPK only partially mediates MM cell proliferation. Since adherence of MM cells to BMSCs promotes tumor cell growth,7,8 16 we next examined whether VX-745 overcomes growth of MM cells adherent to BMSCs. As expected, VX-745 in a dose-dependent fashion significantly (P < .01) inhibited proliferation of MM.1S cells induced by adherence to BMSCs (Figure 2B). Since binding of MM cells to BMSCs also triggers IL-6 transcription and secretion in the BM milieu, we next determined whether VX-745 similarly inhibited IL-6 secretion in MM.1S/BMSCs cultures. As seen in Figure 2C, VX-745 completely (P < .01) abrogates IL-6 secretion triggered by adherence of MM cells to BMSCs. These results demonstrate that VX-745 inhibits MM cell growth, at least in part, due to inhibition of IL-6 secretion in BMSCs. Importantly, these studies suggest that VX-745 may overcome cell adhesion–related drug resistance. They provide the framework for clinical evaluation of this agent, alone or combined with conventional or other novel therapies, to improve patient outcome in MM.
Effect of VX-745 on paracrine MM cell growth in the BM.
(A) MM.1S (●), RPMI8226 (▪), and U266 (▴) (3 × 104) cells were cultured in the presence of VX-745 (0.06 μM-20 μM) for 48 hours, and cell viability was assessed by MTT assay. (B) MM.1S cells were cultured in BMSC-coated 96-well plates for 48 hours, in the absence (■) or presence of 0.2 μM (▤), 1 μM (▧), and 5 μM (▪) VX-745. DNA synthesis was assessed by [3H]TdR uptake. (C) BMSCs were cultured with 3 × 104 MM.1S cells in the absence (■) or presence of 0.2 μM (▤), 1 μM (▧), and 5 μM (▪) VX-745 for 24 hours. IL-6 in culture supernatants was measured by ELISA.
Effect of VX-745 on paracrine MM cell growth in the BM.
(A) MM.1S (●), RPMI8226 (▪), and U266 (▴) (3 × 104) cells were cultured in the presence of VX-745 (0.06 μM-20 μM) for 48 hours, and cell viability was assessed by MTT assay. (B) MM.1S cells were cultured in BMSC-coated 96-well plates for 48 hours, in the absence (■) or presence of 0.2 μM (▤), 1 μM (▧), and 5 μM (▪) VX-745. DNA synthesis was assessed by [3H]TdR uptake. (C) BMSCs were cultured with 3 × 104 MM.1S cells in the absence (■) or presence of 0.2 μM (▤), 1 μM (▧), and 5 μM (▪) VX-745 for 24 hours. IL-6 in culture supernatants was measured by ELISA.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-06-1874.
Supported by National Institutes of Health grants PO-1 78378 and RO-1 50947 and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.), and the Multiple Myeloma Research Foundation Senior Award (T.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Dana-Farber Cancer Institute, M557, 44 Binney St, Boston, MA 02115; e-mail:kenneth_anderson@dfci.harvard.edu.

![Fig. 2. Effect of VX-745 on paracrine MM cell growth in the BM. / (A) MM.1S (●), RPMI8226 (▪), and U266 (▴) (3 × 104) cells were cultured in the presence of VX-745 (0.06 μM-20 μM) for 48 hours, and cell viability was assessed by MTT assay. (B) MM.1S cells were cultured in BMSC-coated 96-well plates for 48 hours, in the absence (■) or presence of 0.2 μM (▤), 1 μM (▧), and 5 μM (▪) VX-745. DNA synthesis was assessed by [3H]TdR uptake. (C) BMSCs were cultured with 3 × 104 MM.1S cells in the absence (■) or presence of 0.2 μM (▤), 1 μM (▧), and 5 μM (▪) VX-745 for 24 hours. IL-6 in culture supernatants was measured by ELISA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-06-1874/4/m_h80233671002.jpeg?Expires=1767860252&Signature=Yy2J-AyWE8gTP6F4FNWCAyxpr2ZjBUjmXXunWP6q8EI-3aIibCl9V1upOD6EpV49u8bTSEqn83dm3e3aXkIf~8umidUn0imxwcT~uNB1~PTAEO82Vzp9w3KHKrvbox5Iqq3L0TV16G5tVsBR-Ptk7a8nB7VxGG~SEN5Y3L0JyU6-iFLlQfLaKnE-5~upxV1aAd-LdmXqS8idNLmCmLrc1getn4OmKC2VYVDlIlFR7f3nV9LOV1IaqUbFa33lJYknc~j0KtBnhO4QjFgCwu2FsZnEJQRKR5jm5qQ4Kj3FznKLwO2lytfZStdddAXunANy~M3NBu3sutQqmZlzm-i2XA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal