Abstract
Although nonmyeloablative conditioning regimen transplantations (NMTs) induce engraftment of allogeneic stem cells with a low spectrum of toxicity, graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality. In vivo T-cell depletion, using alemtuzumab, has been shown to reduce the incidence of GVHD. However, this type of maneuver, although reducing GVHD, may have an adverse impact on disease response, because NMTs exhibit their antitumor activity by relying on a graft-versus-malignancy effect. To explore the efficacy of alemtuzumab compared with methotrexate (MTX) for GVHD prophylaxis, we have compared the results in 129 recipients of a sibling NMT enrolled in 2 prospective studies for chronic lymphoproliferative disorders. Both NMTs were based on the same combination of fludarabine and melphalan, but the United Kingdom regimen (group A) used cyclosporin A plus alemtuzumab, whereas the Spanish regimen (group B) used cyclosporin A plus MTX for GVHD prophylaxis. Patients receiving alemtuzumab had a higher incidence of cytomegalovirus (CMV) reactivation (85% versus 24%,P < .001) and a significantly lower incidence of acute GVHD (21.7% versus 45.1%, P = .006) and chronic GVHD (5% versus 66.7%, P < .001). Twenty-one percent of patients in group A and 67.5% in group B had complete or partial responses 3 months after transplantation (P < .001). Eighteen patients in group A received donor lymphocyte infusions (DLIs) to achieve disease control. At last follow-up there was no difference in disease status between the groups with 71% versus 67.5% (P = .43) of patients showing complete or partial responses in groups A and B, respectively. No significant differences were observed in event-free or overall survival between the 2 groups. In conclusion, alemtuzumab significantly reduced GVHD but its use was associated with a higher incidence of CMV reactivation. Patients receiving alemtuzumab often required DLIs to achieve similar tumor control but the incidence of GVHD was not significantly increased after DLI.
Introduction
Nonmyeloablative transplants (NMTs) are currently being evaluated in patients with hematologic malignancies who are considered poor candidates for conventional allogeneic transplantation because of their advanced age or other concurrent medical conditions. The rationale for NMTs relies on previous observations that adoptive transfer of alloreactive donor lymphocytes may eradicate refractory or recurrent disease.1-5Therefore, in an effort to reduce the toxicity associated with high-dose chemotherapy,6-11 several groups have designed less toxic regimens that might exhibit their antitumor effect relying on a graft-versus-malignancy effect rather than on myeloablative chemotherapy.12-16 Although these regimens have been shown to permit engraftment of allogeneic hemopoietic stem cells with a low spectrum of toxicity, graft-versus-host disease (GVHD) remains a significant cause of increased morbidity and mortality.4-20 The ideal approach for GVHD prophylaxis remains uncertain and several agents have been introduced to reduce incidence and severity. The United Kingdom collaborative group has incorporated alemtuzumab (Campath-1H) as part of a fludarabine-based protocol and a low incidence of GVHD has been reported in both related and unrelated donor transplantation.21 However, this type of intervention to reduce the incidence and severity of GVHD may have an adverse impact on disease response, because several studies have suggested a positive correlation between GVHD and graft-versus-leukemia (GVL) effect,6,15,16,19,22-25 both in myeloid and in lymphoid malignancies18,20,25,26 and higher relapse rates have been reported after syngeneic or T-cell–depleted transplantation.27 28 Thus, the ideal approach is to establish the optimum balance between the risk and benefit of decreasing GVHD versus an increase in the risk of relapse.
To explore the efficacy of alemtuzumab compared with methotrexate (MTX; both in combination with cyclosporine) for GVHD prophylaxis and its impact on the outcome of patients receiving transplants for lymphoproliferative disorders, we have retrospectively compared the results of 2 prospective studies carried out in the United Kingdom21 and Spain.29 Both NMT schedules were based on the same combination of fludarabine and melphalan, but the United Kingdom regimen (group A) used cyclosporin A (CsA) plus alemtuzumab, whereas the Spanish regimen (group B) used CsA plus MTX. Patients included in this study underwent transplantation from February 1998 to January 2001 (group A) and from February 1999 to May 2001 (group B).
Patients and methods
Patient characteristics
We have analyzed data on 129 patients with chronic lymphoproliferative disorders who were included in both registries and received a peripheral blood stem cell transplant from HLA-matched siblings. Both studies included patients considered candidates to receive a transplant from a related donor but who had a high risk of transplantation-related mortality (TRM) due to advanced age (≥ 45 years old, n = 77) or other concurrent medical condition (relapse after a previous autologous transplantation, n = 30; severe organ disfunction, active fungal infection, or heavily pretreated patients, n = 22). Patients gave written informed consent for inclusion in the protocols, which were approved by all local ethical review boards as well as the Spanish (protocol 99-0151) and the United Kingdom (study no. 974/0127) drug agencies.
No significant differences were observed between the 2 groups in median age, diagnosis, or previous transplantation. Disease status at transplantation was categorized as low risk (patients in CR1 and CR2), high risk (patients with refractory or progressive disease and more than first partial response [PR]) and intermediate risk (the rest of cases, ie, more than second complete response [CR]); no differences were observed in terms of disease status. In contrast, there was a sex mismatch in 56% of the alemtuzumab group (A) compared with 35% in MTX group (B; P = .031). In addition, in 37% of cases in group A both donor and recipient were cytomegalovirus (CMV) negative compared with only 9% in group B (P = .009). Other patient characteristics are specified in Table1.
Patient and disease characteristics
| . | Fludarabine/melphalan/alemtuzumab (n = 78) . | Fludarabine/melphalan/MTX (n = 51) . | P . |
|---|---|---|---|
| Age, y (median, SD) | 44 (10,7) | 47 (11,3) | .94 |
| Previous transplantation | 35 (45%) | 26 (51%) | .49 |
| Sex mismatched | 34 (55.7%) | 18 (35.3%) | .031 |
| Patient CMV+ | 37 (53%) | 27 (81.8%) | |
| Patient CMV− donor CMV+ | 7 (10%) | 3 (9.1%) | .009 |
| Both CMV− | 26 (37%) | 3 (9.1%) | |
| Disease status at transplantation categorized* | |||
| Low risk | 7 (9.1%) | 7 (13.7%) | .25 |
| CR1 | 6 | 2 | |
| CR2 | 1 | 5 | |
| Intermediate risk | 53 (67.5%) | 27 (52.9%) | |
| More than CR2 | 7 | 2 | |
| 1st PR | 44 | 18 | |
| CR1/2 after 3rd line | 2 | ||
| Untreated relapse/SD treatment | 7 | ||
| High risk | 18 (23.4%) | 17 (33.3%) | |
| Refractory/progressive | 15 | 13 | |
| More than 1st PR | 3 | 4 | |
| Disease | |||
| HD | 15 | 10 | .52 |
| CLL | 8 | 7 | |
| MM | 19 | 16 | |
| MCL | 6 | ||
| Low-grade NHL | 11 | 7 | |
| High-grade NHL | 12 | 4 | |
| T-NHL | 2 | 4 | |
| OCLPDs | 7 | 4 | |
| Follow-up (median, SD) | 400 (273) | 253 (200) | .05 |
| . | Fludarabine/melphalan/alemtuzumab (n = 78) . | Fludarabine/melphalan/MTX (n = 51) . | P . |
|---|---|---|---|
| Age, y (median, SD) | 44 (10,7) | 47 (11,3) | .94 |
| Previous transplantation | 35 (45%) | 26 (51%) | .49 |
| Sex mismatched | 34 (55.7%) | 18 (35.3%) | .031 |
| Patient CMV+ | 37 (53%) | 27 (81.8%) | |
| Patient CMV− donor CMV+ | 7 (10%) | 3 (9.1%) | .009 |
| Both CMV− | 26 (37%) | 3 (9.1%) | |
| Disease status at transplantation categorized* | |||
| Low risk | 7 (9.1%) | 7 (13.7%) | .25 |
| CR1 | 6 | 2 | |
| CR2 | 1 | 5 | |
| Intermediate risk | 53 (67.5%) | 27 (52.9%) | |
| More than CR2 | 7 | 2 | |
| 1st PR | 44 | 18 | |
| CR1/2 after 3rd line | 2 | ||
| Untreated relapse/SD treatment | 7 | ||
| High risk | 18 (23.4%) | 17 (33.3%) | |
| Refractory/progressive | 15 | 13 | |
| More than 1st PR | 3 | 4 | |
| Disease | |||
| HD | 15 | 10 | .52 |
| CLL | 8 | 7 | |
| MM | 19 | 16 | |
| MCL | 6 | ||
| Low-grade NHL | 11 | 7 | |
| High-grade NHL | 12 | 4 | |
| T-NHL | 2 | 4 | |
| OCLPDs | 7 | 4 | |
| Follow-up (median, SD) | 400 (273) | 253 (200) | .05 |
Low risk: CR1, CR2; high risk: refractory, progressive, more than 1st PR.
CR1 indicates 1st complete remission; CR2, 2nd complete remission; 1st PR, first partial response; SD, stable disease; HD, Hodgkin disease; CLL, chronic lymphoid leukemia; MM, multiple myeloma; MCL, mantle cell lymphoma; and OCLPDs, other chronic lymphoproliferative disorders, including Waldenstrom (n = 1/1), T-prolymphocytic leukemia (n = 1), low-grade transformed to high-grade HNL (n = 5/1), Richter (n = 0/2).
Conditioning regimen and GVHD prophylaxis
In both trials the conditioning regimen consisted of fludarabine 30 mg/m2 intravenously for 5 days followed by melphalan 140 mg/m2 intravenously in 1 dose or divided in 2 doses: fludarabine 30 mg/m2 days −7 to −3 plus melphalan 140 mg/m2 on day −2 in group A and fludarabine 30 mg/m2 days −8 to −4 plus melphalan 140 mg/m2divided in 2 doses on days −3 and −2 in group B.21 29
Acute and chronic GVHD were similarly graded by established criteria in both trials.30 31 In group A, GVHD prophylaxis consisted of CsA 3 mg/kg intravenously starting on day −1 and switched to an oral dose as soon as the patient could tolerate oral medications. The median duration of administration was 4 months for the first 40 patients; however, the observed low incidence of GVHD led physicians to taper the dose by the end of the second month for the rest of the patients. In patients without evidence of GVHD, CsA was discontinued by the end of the third month. alemtuzumab was administered at a dose of 20 mg/d by intravenous infusion over 8 hours on days −8 to −4.
In group B, CsA was started on day −7. In the absence of grade II or greater acute GVHD, CsA was tapered 10% weekly starting on day +90 and discontinued by day +150. In both groups CsA levels were monitored starting on day +1 and were maintained in the therapeutic range (150-300 ng/mL) until tapering. MTX in group B was given at a dose of 10 mg/m2 intravenously on days +1, +3, and +6, followed by folinic acid rescue (10 mg intravenously every 6 hours for 4 doses starting 24 hours after each dose of MTX).
Patients with relapsed/progressive disease after transplantation or those not evolving to 100% donor chimerism after discontinuing immunosuppression were considered eligible for donor lymphocyte infusion (DLI) in both studies.
An escalated dose regimen, starting at 1 × 106CD3+ T cells/kg with dose escalation at intervals of 3 months, was used in group A. The median interval from transplantation to the first DLI was 230 days (range, 182-781 days). Overall, 18 patients received DLI in group A. Fifteen patients received DLI for persistent disease after transplantation and for mixed chimerism. In group B, also an escalated dose regimen at intervals of 3 months was used; however, the starting dose was 1 × 107 CD3+ T cells/kg. In this group, 4 patients received DLI due to persistent disease (n = 3) or progression (n = 1). The median interval to start DLI after transplantation was +210 days (range, 120-243 days). Data on chimerism analysis and DLI (S.M., manuscript in preparation) have already been reported.21,29 Protocols for the prevention and treatment of transplantation complications and supportive care were similar in both groups.21 29
Statistical methods
Comparison of relapse rate and GVHD between groups was performed using the χ2 and Fisher exact test. Events analyzed were calculated from the time of transplantation using Kaplan-Meier product-limit estimates. For TRM, patients were censored at last follow-up. Disease-free survival (DFS) was calculated from transplantation until disease progression for those patients reaching CR after transplantation and also for those reaching CR after DLI. Patients who suffered TRM and those who were still alive without progression at the time of reporting were censored at death and last follow-up, respectively. Event-free survival (EFS) was calculated from transplantation until disease progression or death and those patients who did not reach disease response (CR or PR) any time after transplantation were considered events. Overall survival (OS) was calculated from transplantation until death from any cause, and surviving patients were censored at last follow-up. The incidence and time to onset of chronic GVHD were calculated in patients followed for at least 90 days.
Results
Engraftment
Both regimens induced an early and sustained engraftment.21 28 As shown in Table2, granulocyte recovery was faster in group A patients; the median time for more than 500 granulocytes/mm3 was 12.8 versus 14.7 days (P < .001) and for more than 1000 granulocytes/mm3 13.7 versus 17.6 days (P < .001) for groups A and B, respectively. In contrast, the use of alemtuzumab was associated with a significant delay in platelet recovery: median of 14.4 versus 11.2 days to reach more than 20 × 109/L platelets (P = .009) and 21.7 versus 14.1 days to reach more than 50 × 109/L platelets (P = .001) in groups A and B, respectively.
Transplantation-related morbidity and mortality
| . | Fludarabine/melphalan/alemtuzumab (n = 78) . | Fludarabine/melphalan/MTX (n = 51) . | P . |
|---|---|---|---|
| Days to reach | |||
| More than 500 granulocytes | 12.88 (2.84) | 14.79 (2.14) | < .001 |
| More than 1 000 granulocytes | 13.7 (3.89) | 17.68 (3.09) | < .001 |
| More than 20 000 platelets | 14.49 (6.49) | 11.25 (5.34) | .009 |
| More than 50 000 platelets | 21.73 (14.1) | 14.14 (5.38) | .001 |
| Acute GVHD | Yes vs no .001 | ||
| No | 64 (82.1%) | 28 (54.9%) | 0-I vs II-IV |
| Grade I | 9 (11.5%) | 1 (2%) | < .001 |
| Grade II | 5 (6.4%) | 15 (29.4%) | 0-II vs III-IV |
| Grade III-IV | 2 (2.6%) | 7 (13.7%) | .015 |
| Overall acute GVHD (before + after DLI) | Yes vs no .006 | ||
| No | 60 (76.9%) | 28 (54.9%) | 0-I vs II-IV |
| Grade I | 10 (12.8%) | 1 (2%) | < .001 |
| Grade II | 3 (3.8%) | 15 (29.4%) | 0-II vs III-IV |
| Grade III-IV | 4 (5.1%) | 7 (13.7%) | .092 |
| Chronic GVHD | |||
| Limited | 1 | 9 | < .001 |
| Extensive | 1 | 13 | |
| CMV reactivation | 27 (46.6%) | 10 (22.7%) | .018 |
| CMV+ patient or donor | 26 (86.7%) | 7 (25%) | < .001 |
| CMV− patient and donor | 0 | 0 | |
| TRM | 8 (10.3%) | 10 (20%) | .12 |
| . | Fludarabine/melphalan/alemtuzumab (n = 78) . | Fludarabine/melphalan/MTX (n = 51) . | P . |
|---|---|---|---|
| Days to reach | |||
| More than 500 granulocytes | 12.88 (2.84) | 14.79 (2.14) | < .001 |
| More than 1 000 granulocytes | 13.7 (3.89) | 17.68 (3.09) | < .001 |
| More than 20 000 platelets | 14.49 (6.49) | 11.25 (5.34) | .009 |
| More than 50 000 platelets | 21.73 (14.1) | 14.14 (5.38) | .001 |
| Acute GVHD | Yes vs no .001 | ||
| No | 64 (82.1%) | 28 (54.9%) | 0-I vs II-IV |
| Grade I | 9 (11.5%) | 1 (2%) | < .001 |
| Grade II | 5 (6.4%) | 15 (29.4%) | 0-II vs III-IV |
| Grade III-IV | 2 (2.6%) | 7 (13.7%) | .015 |
| Overall acute GVHD (before + after DLI) | Yes vs no .006 | ||
| No | 60 (76.9%) | 28 (54.9%) | 0-I vs II-IV |
| Grade I | 10 (12.8%) | 1 (2%) | < .001 |
| Grade II | 3 (3.8%) | 15 (29.4%) | 0-II vs III-IV |
| Grade III-IV | 4 (5.1%) | 7 (13.7%) | .092 |
| Chronic GVHD | |||
| Limited | 1 | 9 | < .001 |
| Extensive | 1 | 13 | |
| CMV reactivation | 27 (46.6%) | 10 (22.7%) | .018 |
| CMV+ patient or donor | 26 (86.7%) | 7 (25%) | < .001 |
| CMV− patient and donor | 0 | 0 | |
| TRM | 8 (10.3%) | 10 (20%) | .12 |
GVHD and infectious complications
Patients receiving alemtuzumab had a significantly lower incidence of acute GVHD (20.5%) when compared with patients receiving MTX (45.1%; P = .001). In addition, in patients receiving alemtuzumab the incidence of grades II to IV and III to IV acute GVHD was significantly lower compared with patients receiving MTX: 9% versus 43.1% grades II to IV acute GVHD in groups A and B, respectively (P < .001) and 2.6% versus 13.7% grades III to IV acute GVHD in groups A and B, respectively (P = .015). Interestingly, 18 patients received DLI in group A due to relapse/persistence of disease or mixed chimerism and only 3 of these patients developed GVHD (1 each grade I, grade IV, and limited chronic GVHD), so that a significantly lower incidence of acute GVHD persisted in patients receiving alemtuzumab even after DLI. However, there was no significant difference in terms of severe acute GVHD (grades III and IV) after DLI (P = .09; Table2).
There were also differences in the incidence of chronic GVHD between the groups. Only 2 patients receiving alemtuzumab developed chronic GVHD (1 limited, 1 extensive) in contrast to 22 (66.7% of patients at risk) patients receiving MTX (13 cases of extensive chronic GVHD;P < .001).
Patients were monitored for CMV reactivation by weekly CMV antigenemia (group B) or PCR assay (group A). Statistically significant differences were observed in the incidence between both groups. Although a lower percentage of patients in group B were CMV−, only 22.7% developed CMV reactivation as compared with 47% in group A (P = .018). Even more strikingly, among CMV+patients, 87% had CMV reactivation in group A versus 25% in group B (P < .001). Despite the high incidence of reactivation only one patient died of CMV disease in group A and none in group B.
TRM
No significant differences were observed in terms of TRM between the groups, although a trend toward a higher mortality rate was observed in group B (10.2% versus 20% for groups A and B, respectively; P = .12; log-rank, P = .06; Figure 1).
Transplantation-related mortality.
Graph measures days from transplantation to death.
Transplantation-related mortality.
Graph measures days from transplantation to death.
Overall 25.6% of patients in group A died compared with 29.4% in group B (P = .6); 33.4% of deaths in group B were related to GVHD compared with 5% in group A. By contrast, the most frequent cause of death in patients receiving alemtuzumab was disease relapse/progression (60% of deaths versus 20%). Data concerning morbidity and mortality are specified in Table 2.
Disease response
Disease status was evaluated 3 months after transplantation (Table3). After 3 months, 21.1% of patients in group A were in CR/PR in contrast to 67.5% in group B (P < .001). Interestingly, disease status at transplantation significantly influenced response to transplantation in patients receiving alemtuzumab but not in those receiving MTX. The most important differences in response were observed in patients categorized as high risk according to disease status before transplantation with no patient reaching CR or PR in group A versus 52.9% in group B (P < .001). In the intermediate-risk group of patients, 18.8% reached CR or PR in group A compared with 55.5% in group B (P < .001). In contrast, no significant differences were observed in the low-risk group, although the number of patients in this subgroup was too small to reach any conclusion. As previously stated, 18 patients received DLI in group A because of relapse/persistence of disease or mixed chimerism versus 4 patients in group B. Disease status at last follow-up (after CsA taper and DLI) is summarized in Table 3. Disease status at last follow-up was not significantly different between both groups for the overall series of patients. In addition, no significant differences were observed according to pretransplantation disease status, because in the high-risk group 53.8% of patients receiving alemtuzumab reached CR/PR versus 50% in the group of patients receiving MTX (P = .45). Also in the intermediate-risk group 55.8% of patients receiving alemtuzumab reached CR/PR compared with 56.5% of patients receiving MTX (P = .76).
Disease response and transplantation outcome
| . | Fludarabine/ melphalan/ alemtuzumab (n = 78) . | Fludarabine/ melphalan/MTX (n = 51) . | P . |
|---|---|---|---|
| Disease response at 3 mo after transplantation | |||
| CR | 15 (21.1%) | 19 (47.5%) | < .001 |
| PR | 0 | 8 (20%) | |
| Stable/progression free | 48 (67.6%) | 9 (22.5%) | |
| Progression | 8 (11.3%) | 4 (10%) | |
| Disease status at last follow-up3-150 | |||
| CR | 28 (47.4%) | 19 (47.5%) | .43 |
| PR | 14 (23.7%) | 8 (20%) | |
| Stable/progression free | 16 (27.1%) | 9 (22.5%) | |
| Progression | 1 (1.8%) | 4 (10%) |
| . | Fludarabine/ melphalan/ alemtuzumab (n = 78) . | Fludarabine/ melphalan/MTX (n = 51) . | P . |
|---|---|---|---|
| Disease response at 3 mo after transplantation | |||
| CR | 15 (21.1%) | 19 (47.5%) | < .001 |
| PR | 0 | 8 (20%) | |
| Stable/progression free | 48 (67.6%) | 9 (22.5%) | |
| Progression | 8 (11.3%) | 4 (10%) | |
| Disease status at last follow-up3-150 | |||
| CR | 28 (47.4%) | 19 (47.5%) | .43 |
| PR | 14 (23.7%) | 8 (20%) | |
| Stable/progression free | 16 (27.1%) | 9 (22.5%) | |
| Progression | 1 (1.8%) | 4 (10%) |
Disease status after CsA taper and DLI.
On analyzing all patients (Table 4), we found an association between development of acute GVHD and disease response (55.2% of patients developing acute GVHD reached CR/PR compared with 29.5% for those who did not develop acute GVHD,P = .025) but this association disappeared after DLI. The same occurred when we analyzed patients developing grades II to IV acute GVHD. To evaluate whether these results could be attributed to any of the GVHD prophylaxis regimens both groups were separately analyzed. No clear relationship between acute GVHD and response to transplant or disease status after DLI was observed in either group A or B, although this might be related to the lower number of cases included, when both series were separately analyzed. In addition, 74% of patients developing chronic GVHD achieved CR/PR compared with 29% of patients who did not develop it (P < .001). In this case, the impact of DLI could not be evaluated because only 2 patients in group A developed chronic GHVD.
Response to transplantation according to GVHD development
| . | Yes . | No . | P . |
|---|---|---|---|
| Acute GVHD | |||
| Response to transplantation | |||
| CR/PR | 16 (55.2%) | 26 (29.5%) | .025 |
| Stable/progression | 13 (44.8%) | 56 (70.5%) | |
| Disease status at last follow-up4-150 | |||
| CR/PR | 21 (75%) | 47 (67.1%) | .61 |
| Stable/progression | 7 (25%) | 23 (32.9%) | |
| Chronic GVHD | |||
| Response to transplantation | |||
| CR/PR | 17 (74%) | 23 (29%) | .016 |
| Stable/progression | 6 (23%) | 57 (71%) |
| . | Yes . | No . | P . |
|---|---|---|---|
| Acute GVHD | |||
| Response to transplantation | |||
| CR/PR | 16 (55.2%) | 26 (29.5%) | .025 |
| Stable/progression | 13 (44.8%) | 56 (70.5%) | |
| Disease status at last follow-up4-150 | |||
| CR/PR | 21 (75%) | 47 (67.1%) | .61 |
| Stable/progression | 7 (25%) | 23 (32.9%) | |
| Chronic GVHD | |||
| Response to transplantation | |||
| CR/PR | 17 (74%) | 23 (29%) | .016 |
| Stable/progression | 6 (23%) | 57 (71%) |
Disease status after CsA taper and DLI.
Finally, results from both registries were analyzed according to the type of underlying disease: in multiple myeloma both response to transplant and disease status after DLI were improved in patients receiving MTX (52.6% of patients in group A reached CR/PR at last follow-up compared with 68.8% in group B, P = .029). In patients with multiple myeloma there was no clear relation between acute GVHD and disease control; however, 50% of those patients who developed acute GVHD reached CR/PR compared with 25% for those who did not develop GVHD (P = .25).
In patients with Hodgkin disease, the initial response was better in group B; however, disease status after DLI was similar in both groups (53.3% of patients reached CR/PR in group A compared with 50% in group B, P = .21). Finally, within patients diagnosed with high-grade non-Hodgkin lymphoma (NHL; n = 21), only 2 were categorized as low-risk disease at transplantation, whereas the rest were categorized as intermediate (n = 9) and high risk (n = 10). Response to transplantation was significantly better for patients receiving MTX but, after examining disease status after DLI, no significant differences were observed between the 2 groups, although we observed a trend toward better disease control for patients in group B (26.7% of patients receiving alemtuzumab in CR/PR versus 50% for those receiving MTX). In view of the low number of patients no other disease categories were analyzed.
OS and DFS
Disease-free survival was first calculated identifying only those patients reaching a CR after transplantation. Median DFS was not reached for patients receiving alemtuzumab and not computed for those receiving MTX because no relapses were observed among patients reaching CR. No significant differences were found. When we also considered patients achieving CR after DLI, DFS at 2 years was 76% for patients in group A versus 100% in group B (P = .19). When we compared both groups according to pretransplantation disease status, no significant differences were found for any of the risk categories when we considered disease status after DLI.
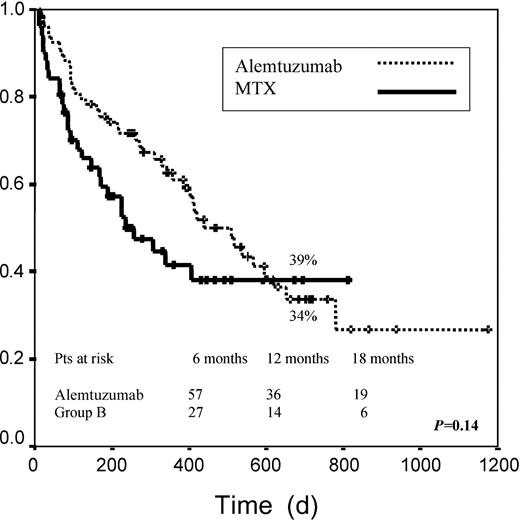
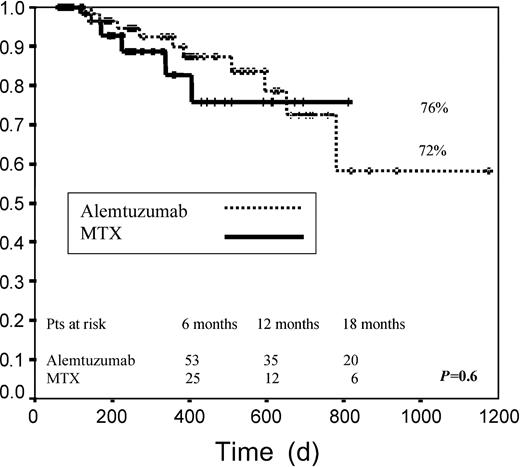
Median EFS among patients receiving alemtuzumab was 438 days versus 267 days for patients receiving MTX. EFS at 2 years was 34% in group A versus 39% in group B (P = .14; Figure2). When both groups were examined according to pretransplantation disease status, no significant differences were found for any of the risk categories when we considered disease status after DLI. When we analyzed only those patients achieving any response (CR, PR, or stable disease), median EFS was not reached for any of the 2 groups. For these patients EFS at 2 years was 72% and 76% for groups A and B, respectively (P = .6; Figure 3).
Event-free survival.
Graph measures days from transplantation to death or relapse. Patients who did not reach CR or PR were considered events.
Event-free survival.
Graph measures days from transplantation to death or relapse. Patients who did not reach CR or PR were considered events.
Event-free survival.
Graph measures days from transplantation to death or to relapse for patients who reached CR, PR, or SD after transplantation.
Event-free survival.
Graph measures days from transplantation to death or to relapse for patients who reached CR, PR, or SD after transplantation.
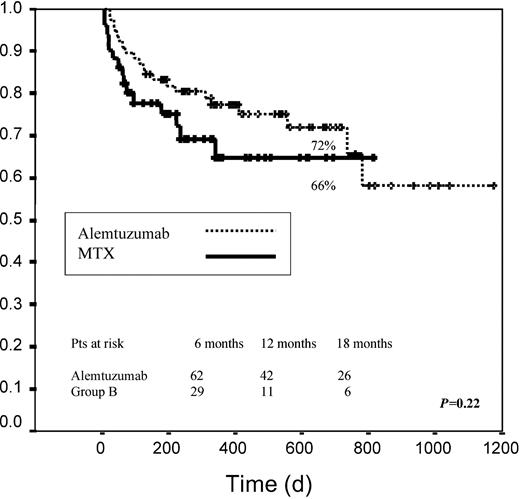
With regard to OS, at the present time the median survival has not been reached for either of the 2 groups. No significant differences were observed between both groups (72% OS at 2 years in group A versus 66% in group B, P = .22) even when we considered the different risk categories (Figure 4).
Discussion
It has been well established that NMTs may offer a reduced risk of procedure-associated mortality,6,14-16,21,29 compared with conventional transplantation.8-10 However, despite the considerably lower toxicity, GVHD still remains a major limitation for this strategy.6,14-16 29 To explore the impact of alemtuzumab on the prevention of GVHD and the efficacy of NMT, we have performed the first comparative study between 2 prospective national studies in patients diagnosed with lymphoproliferative disorders.
Both conditioning regimens21 29 were based on the same doses of fludarabine and melphalan, with the only difference being the use of alemtuzumab (group A) or MTX (group B) in addition to CsA to prevent GVHD. Characteristics of patients at transplantation were similar, except for a higher incidence of sex-mismatched pairs in the group of patients receiving alemtuzumab and a lower percentage of CMV seronegativity in the group of patients receiving MTX.
This comparative study has confirmed that alemtuzumab significantly reduces the overall incidence of acute GVHD when compared with MTX. In addition, this reduction can be maintained even after DLI. Because the efficacy of this type of transplantation relies primarily on the graft-versus-tumor effect,6-10,24 any attempt to decrease GVHD may be offset by a higher risk of relapse and the results should be cautiously interpreted. Disease response at 3 months was significantly better in the group of patients receiving MTX. In addition, patients who developed GVHD obtained a better disease response after transplantation. Nevertheless, 18 patients in group A received DLI to generate a tumor response and our analysis has shown that disease status at last follow-up did not differ between both groups. Interestingly, disease response after DLI was not associated with a significant increase in GVHD because differences in GVHD incidence between both groups remained even after DLI. In addition, correlation between GVHD and response was not significant when we considered disease status after DLI. These results are in accordance with previous reports suggesting that the approach of using delayed DLI can induce disease response and may be associated with less GVHD compared with early infusion34 and also support previous reports showing that the use of DLI can contribute to overcome the negative effect on GVL effect of in vitro T-cell depletion using alemtuzumab in myeloid as well as in lymphoid malignancies.35
This study also confirmed that lymphomas, chronic lymphoid leukemia, and multiple myeloma are susceptible to graft-versus-tumor effect.18,20,25,26 36-40 This effect is not equally potent across tumor subtypes and disorders with low proliferative capacity may respond better when compared to more aggressive forms of disease. We were also able to demonstrate a correlation between disease response and severity of GVHD and, not surprisingly, in our series of myeloma patients, response to transplantation was significantly better in group B. Interestingly, the improved outcome in group B remained even after DLI in group A, suggesting that an early onset of GVHD might be more beneficial in multiple myeloma.
Although the use of T-cell depletion combined with cyclosporine may have a deleterious effect on transplantation outcome,41recent reports have unexpectedly shown that Campath and cyclosporin can reduce the risk of death due to infections 6 months after the transplantation.42 Therefore, further studies will be needed to explore the mechanism of the cyclosporin effect and the potential benefit in combination with T-cell depletion.
As previously reported, the use of less intensive regimens may have a beneficial effect on infections.43 In the immediate posttransplantation period, the short duration of neutropenia may result in reduced incidence of bacterial infections. In the present study, alemtuzumab recipients experienced increased incidence of CMV reactivation compared with MTX, although only one patient in the former group died because of CMV disease. This difference in terms of CMV reactivation cannot be attributed to the different techniques used in both trials because several studies have demonstrated that antigenemia and PCR assay have similar predictive values and show a high linear correlation.44,45 According to these findings, the Centers for Disease Control and Prevention (CDC) have recently recommended that any of both techniques should be selected to determine the need for preemptive treatment.46 On the other hand, we were able to show that the use of MTX, as expected, was associated with delayed neutrophil engraftment, although unexpectedly, platelet engraftment was delayed in patients receiving alemtuzumab. These differences were not clinically important. The delay in platelet engraftment in group A might be related to the in vivo T-cell depletion induced by alemtuzumab, because T lymphocytes may be an important element for platelet engraftment,47 or in addition, alemtuzumab might have direct toxicity on megakaryocytic progenitors.
Although a major concern about the use of anti-T monoclonal antibodies is the increased incidence of posttransplantation lymphoproliferative disorders (PTLDs), in this study, none of the patients developed PTLDs. This is in contrast to previous reports on other anti-T monoclonal antibodies48 49 and is probably related to the anti–B-cell effect induced by alemtuzumab.
In conclusion, both nonmyeloablative regimens offer good results in patients considered poor candidates to undergo a conventional allogeneic transplantation, but a different pattern of complications was observed. Alemtuzumab significantly reduced GVHD but it was related to a higher incidence of CMV reactivation. These findings have prompted us to initiate a study to establish an optimum dose of alemtuzumab to maintain its efficacy for GVHD prophylaxis without increasing CMV reactivation.50 However, patients receiving alemtuzumab required DLI to achieve a similar response rate compared with those receiving MTX, but the incidence of GVHD did not significantly increase after DLI, thus suggesting the possibility of separating GVHD from GVL effect. Finally, better responses were observed early after transplantation in patients with multiple myeloma or with active disease before transplantation, indicating that these patients may benefit from MTX-containing regimens. Considering these results, a randomized trial comparing both protocols of GVHD prophylaxis is warranted.
The authors wish to thank Jose Manuel Garcı́a de Cecilia, Statistician of the SEK University (Segovia), for his support in the statistical analysis.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0701.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
José A. Pérez-Simón, Servicio de Hematologı́a, Hospital Universitario de Salamanca, Paseo de San Vicente s/n, 37007, Spain; e-mail: pesimo@usal.es.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal