Abstract
Postgrafting cyclosporine (CSP) given for 35 days resulted in establishment of stable marrow grafts from DLA-identical canine littermates after otherwise suboptimal, but nevertheless, lethal conditioning with 450 cGy of total body irradiation (TBI). We now asked whether sustained allografts could be achieved after sublethal TBI or without TBI. Five groups of recipients were studied. Dogs in group 1 were given 200 cGy TBI and postgrafting CSP, 15 mg/kg twice daily by mouth on days −1 to 35 posttransplant. Dogs in group 2 were given 200 cGy TBI and methotrexate (MTX), 0.4 mg/kg intravenously (IV) on days 1, 3, 6, and 11 along with CSP. Dogs in group 3 were given 200 cGy TBI and CSP along with mycophenolate mofetil (MMF ), 10mg/kg twice daily subcutaneously (SC) on days 0 to 27 after transplant, a novel immunosuppressive combination. Dogs in group 4 were given 100 cGy TBI and MMF/CSP. Dogs in group 5 were not given TBI and they received MMF/CSP posttransplant. Allografts were assessed by (Ca)n dinucleotide repeat polymorphism studies in cells from peripheral blood, lymph nodes, and marrow. Dogs in group 1 had transient mixed donor-host hematopoietic chimerism for no more than 4 weeks. Three of six dogs in group 2 had transient mixed chimerism for 3 to 11 weeks, and three have remained stable mixed chimeras for up to 60 weeks now. Four of five dogs in group 3 have remained stable mixed chimeras for 54 to 57 weeks now, while one lost the allograft after 12 weeks. All dogs in groups 4 and 5 rejected their allografts after 2 to 12 weeks. In summary, the establishment of stable mixed hematopoietic chimerism following nonmyelosuppressive and nontoxic conditioning programs has remained a difficult goal. Here we present evidence in a large random-bred animal species that this goal may be achievable with pharmacological immunosuppression postgrafting, capable of inhibiting both host-versus-graft (HVG) and graft-versus-host (GVH) reactions in the setting of DLA-identical grafts.
HEMATOPOIETIC STEM CELL transplantation requires the crossing of a double barrier that is composed of host-versus-graft (HVG) and graft-versus-host (GVH) reactions. Traditionally, myeloablative and immunosuppressive high-dose conditioning programs have been used before transplant to overcome HVG reactions, while postgrafting immunosuppression has dealt with the second barrier, GVH reactions. In the setting of genotypically major histocompatibility complex (MHC)-identical related grafts, both HVG and GVH reactions are thought to be mediated by T lymphocytes. Given that assumption, we hypothesized that postgrafting immunosuppression, which is effective in preventing GVH disease (GVHD) should also be capable of enhancing hematopoietic grafts across non-MHC barriers by suppressing HVG reactions.
We tested the hypothesis in a canine marrow graft model and showed that administration of postgrafting cyclosporine (CSP) for no more than 35 days resulted in stable and consistent engraftment of marrow from DLA-identical littermates after otherwise suboptimal conditioning with 450 cGy of total body irradiation (TBI),1 a dose that was still in the supralethal range.
The current study extended the previous observation and asked whether sustained hematopoietic grafts from DLA-identical littermates could be achieved after sublethal TBI (200 cGy and 100 cGy, respectively) or without TBI. Engraftment would be manifested either as all donor-type hematopoiesis or mixed donor/host chimerism. Postgrafting immunosuppression consisted either of CSP alone or CSP combined with one of two “antimetabolites,” methotrexate (MTX) or mycophenolate mofetil (MMF ). These drug combinations were chosen since both MTX2,3 and MMF4 were synergistic with CSP in preventing GVHD after hematopoietic stem cell transplantation.
MATERIALS AND METHODS
Litters of beagles, harriers, Walker hounds, pit bull/beagle crossbreeds, and other mixed breeds were either raised at the Fred Hutchinson Cancer Research Center (Seattle, WA) or purchased from commercial kennels in the state of Washington. The dogs weighed from 7.8 to 18.2 kg (median, 11.7 kg) and were 7 to 12 months old (median, 8 months). They were observed for disease for at least 60 days before entering the study. All were immunized for leptospirosis, distemper, hepatitis, and parvovirus. Research was performed according to the principles outlined in the Guide for Laboratory Animal Facilities and Care prepared by the National Academy of Sciences, National Research Council. The research protocols were approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. The kennels are certified by the American Association for Accreditation of Laboratory Animal Care.
Twenty-three matched littermate donor/recipient pairs were chosen on the basis of identity for highly polymorphic MHC class I and class II microsatellite markers.5,6 Specific DLA DRB1 allelic identity was determined by direct sequencing.7
Marrow for transplantation was aspirated under general anesthesia through needles inserted into humeri and femora. Recipients were given TBI at doses of 200 cGy and 100 cGy, respectively, delivered at 7 cGy/minute from two opposing 60Co sources.8 Two recipients were not given TBI. Marrow was infused intravenously (IV) at doses of 1.9 to 4.4 × 108 cells/kg (median, 4.0 × 108/kg) within 4 hours of TBI. The day of marrow grafting was designated as day 0. Recipients were given standard postgrafting care that included twice daily oral nonabsorbable antibiotics, neomycin sulfate and polymyxin sulfate, which were administered from day −5 until the day of recovery of white blood cell counts to >1,000/μL. Prophylactic systemic ceftazidime was administered twice daily from day 0 until the day of white cell count recovery to >1,000/μL. None of the dogs required transfusion support. The dogs' clinical status was assessed twice daily. When studies were completed, dogs were killed and underwent complete autopsies including histopathological examinations.
Five groups of recipients were studied. Dogs in groups 1 to 3 were given 200 cGy TBI before transplant, dogs in group 4 100 cGy TBI, and dogs in group 5 no TBI. Dogs in all five groups were given CSP, 15 mg/kg twice daily orally on days −1 to 35 after transplant. The four dogs in group 1 received CSP alone. The five dogs in group 2 received, in addition to CSP, MTX, 0.4 mg/kg IV on days 1, 3, 6, and 11. Dogs in groups 3 to 5 received, in addition to CSP, MMF, 10 mg/kg twice daily subcutaneously (SC), on days 0 to 27.
Marrow engraftment was assessed by sustained recoveries of granulocyte and platelet counts after the postirradiation nadir, by histologic features of the marrow from biopsy or autopsy specimens, and by documentation of donor (CA)n repeat polymorphisms in cells from peripheral blood, popliteal lymph nodes, and marrow. Conversely, graft rejection was defined as reappearance of cells with host (CA)n repeat polymorphisms. Marrow aspirates from the humoral head and popliteal lymph node biopsies were done under general anesthesia. The (CA)n dinucleotide repeats were assessed in a polymerase chain reaction-based assay.9 The technique detects between 2.5% to 97.5% mixtures of donor and host cells. Mixed hematopoietic chimerism was quantified by estimating the proportion of donor specific DNA among host DNA using the storage phosphor imaging technique.10 In brief, an imaging screen was placed over the gel of the (CA)n dinucleotide repeats in a manner similar to autoradiography with film. Radioactive decay electrons from the sample interacted with the europium-doped screen matrix forming a stable latent image of the distribution and intensity of radioactivity on the chromatogram. After an exposure was completed, the latent image was read by scanning the screen with a red laser to release the stored energy, which was proportional to the absorbed energy. The information generated was stored in a digitized format that greatly enhanced sample evaluation through the use of image analysis programs. The events (reading from PhosphorImage analysis) from a donor specific band (D) and a host specific band (H) were added together as total events (T = D + H). The percentage of donor origin DNA was calculated as D% = (D)/(T) × 100%.
The immune function of mixed chimeras was assessed by established techniques. These included testing of the dogs' lymphocyte responsiveness to alloantigens in a standard mixed lymphocyte culture and to concanavalin A in vitro11 and determining the numbers of lymphocytes with CD4 and CD8 phenotypes.12 Also, their antibody responses to sheep red blood cells in vivo were assessed. To this purpose, dogs were given an IV injection of 1 ml of a 10% suspension of sheep red blood cells in 0.15 mol/L NaCl. Antibody titers were determined as described.11
RESULTS
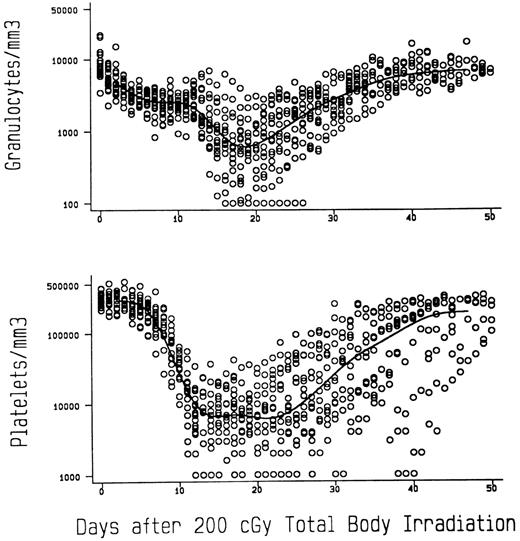
TBI controls.To illustrate that 200 cGy TBI is sublethal in dogs and to show the degree of myelosuppression achieved with that dose, Fig 1 presents the granulocyte and platelet changes in 18 previously reported8 and concurrent dogs given 200 cGy TBI at 7 (n = 5) and 10 (n = 13) cGy/min, respectively, and no marrow infusion. The granulocyte nadirs were reached by day 20 after TBI, and the mean count leveled off at approximately 750/μL before recovery, which was complete by day 40. The platelet nadirs were reached between days 15 and 24, and the mean count leveled off at approximately 7,500/μL before recovery, which was complete by day 50. Seventeen of the 18 dogs survived, while 1 given TBI at 10 cGy/min died on day 26 of pneumonia.
Median granulocyte and platelet changes in dogs given 200 cGy TBI at either 7 (n = 5) or 10 (n = 13) cGy/min and no subsequent marrow infusions.
Median granulocyte and platelet changes in dogs given 200 cGy TBI at either 7 (n = 5) or 10 (n = 13) cGy/min and no subsequent marrow infusions.
DLA-identical marrow grafts.Table 1 summarizes results of allogeneic marrow transplants. Recipients in groups 1 to 3 were conditioned by 200 cGy TBI, those in group 4 by 100 cGy TBI, and those in group 5 were given no TBI.
Marrow Grafts From DLA-Identical Littermates After Conditioning With Sublethal TBI Delivered at 7 cGy/min or No Conditioning
| Group . | TBI Dose (cGy) . | Postgrafting Immunosuppression . | Recipient No. . | Sustained Allograft . | GVHD . | Rejection . | Complete Autologous Recovery . | Duration of Mixed Hematopoietic Chimerism by (CA)n Dinucleotide Repeat Marker Studies . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Acute . | Chronic . | . | . | (weeks after marrow graft) . |
| 1 | 200 | CSP* | D902 | No | — | — | Yes | Yes | 4 |
| D944 | No | — | — | Yes | Yes | 4 | |||
| E026 | No | — | — | Yes | Yes | 4 | |||
| E027 | No | — | — | Yes | Yes | 4 | |||
| 2 | 200 | MTX†/CSP* | E126 | No | — | — | Yes | Yes | 7 |
| E127 | No | — | — | Yes | Yes | 2 | |||
| E156 | No | — | — | Yes | Yes | 11 | |||
| E157 | Yes | No | No | No | No | >8ρ | |||
| E200 | Yes | No | No | No | No | >60 | |||
| E203 | Yes | No | No | No | No | >60 | |||
| 3 | 200 | MMF‡/CSP* | E131 | Yes | No | No | No | No | >571-155 |
| E219 | Yes | No | No | No | No | >54 | |||
| E220 | Yes | No | No | No | No | >54 | |||
| E066 | Yes | No | No | No | No | >56 | |||
| E069 | No | No | No | Yes | Yes | 12 | |||
| 4 | 100 | MMF‡/CSP* | E165 | No | — | — | Yes | Yes | 12 |
| E166 | No | — | — | Yes | Yes | 10 | |||
| E202 | No | — | — | Yes | Yes | 3 | |||
| E204 | No | — | — | Yes | Yes | 3 | |||
| E227 | No | — | — | Yes | Yes | 10 | |||
| E228 | No | — | — | Yes | Yes | 10 | |||
| 5 | None | MMF‡/CSP* | E242 | No | — | — | Yes | Yes | 2 |
| E244 | No | — | — | Yes | Yes | 2 | |||
| Group . | TBI Dose (cGy) . | Postgrafting Immunosuppression . | Recipient No. . | Sustained Allograft . | GVHD . | Rejection . | Complete Autologous Recovery . | Duration of Mixed Hematopoietic Chimerism by (CA)n Dinucleotide Repeat Marker Studies . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Acute . | Chronic . | . | . | (weeks after marrow graft) . |
| 1 | 200 | CSP* | D902 | No | — | — | Yes | Yes | 4 |
| D944 | No | — | — | Yes | Yes | 4 | |||
| E026 | No | — | — | Yes | Yes | 4 | |||
| E027 | No | — | — | Yes | Yes | 4 | |||
| 2 | 200 | MTX†/CSP* | E126 | No | — | — | Yes | Yes | 7 |
| E127 | No | — | — | Yes | Yes | 2 | |||
| E156 | No | — | — | Yes | Yes | 11 | |||
| E157 | Yes | No | No | No | No | >8ρ | |||
| E200 | Yes | No | No | No | No | >60 | |||
| E203 | Yes | No | No | No | No | >60 | |||
| 3 | 200 | MMF‡/CSP* | E131 | Yes | No | No | No | No | >571-155 |
| E219 | Yes | No | No | No | No | >54 | |||
| E220 | Yes | No | No | No | No | >54 | |||
| E066 | Yes | No | No | No | No | >56 | |||
| E069 | No | No | No | Yes | Yes | 12 | |||
| 4 | 100 | MMF‡/CSP* | E165 | No | — | — | Yes | Yes | 12 |
| E166 | No | — | — | Yes | Yes | 10 | |||
| E202 | No | — | — | Yes | Yes | 3 | |||
| E204 | No | — | — | Yes | Yes | 3 | |||
| E227 | No | — | — | Yes | Yes | 10 | |||
| E228 | No | — | — | Yes | Yes | 10 | |||
| 5 | None | MMF‡/CSP* | E242 | No | — | — | Yes | Yes | 2 |
| E244 | No | — | — | Yes | Yes | 2 | |||
CSP, 15 mg/kg bid orally on days −1 to 35.
MTX, 0.4 mg/kg IV on days 1, 3, 6, and 11.
MMF, 10 mg/kg bid SC on days 0-27.
ρ Dog was euthanized because of massive papillomata on feet related to CSP-induced immunosuppression.
Dog was euthanized at the completion of the study.
The four dogs in group 1 given postgrafting CSP showed mixed hematopoietic chimerism among nucleated peripheral blood cells at weeks 2, 3, and 4 after transplant. In all four, only host-type cells were seen at weeks 6 and 7 after transplant.
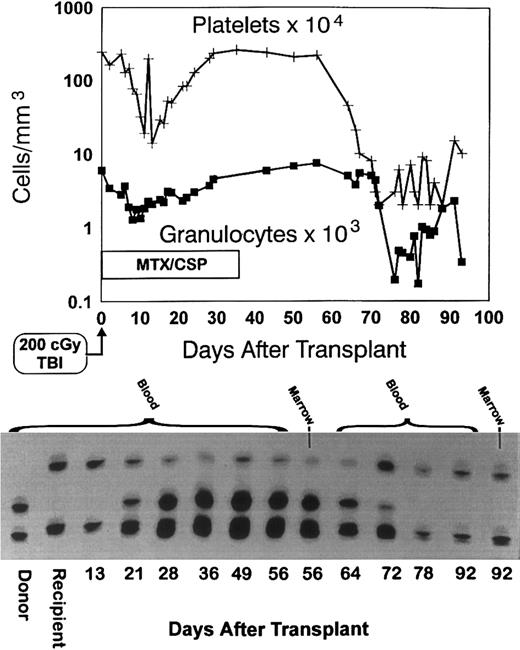
All six dogs in group 2 given MTX/CSP as postgrafting immunosuppression had mixed hematopoietic chimerism in the peripheral blood at week 2. One dog (E127) showed all host cells at weeks 3 and 4. One of the remaining four dogs (E126) remained a mixed chimera through week 7 and subsequently rejected the graft. Dog E157 was killed at 8 weeks because of persisting extensive CSP-associated papillomata on all foot pads. This dog was a mixed hematopoietic chimera at the time of euthanasia. Dog E156 lost its graft after week 11. Figure 2 shows the hematopoietic changes in that dog (note the depression of peripheral blood counts at the time of graft rejection). Also shown are the results of the (CA)n dinucleotide repeat marker studies in donor and recipient cells before transplant and in recipient cells after transplant through day 92. The graft loss is evident by the disappearance of the donor band. The remaining two dogs given MTX/CSP have remained mixed donor/host hematopoietic chimeras for more than 49 weeks now after transplant.
Graft rejection. Granulocyte and platelet changes in dog E156 conditioned with 200 cGy TBI, given a marrow graft from a DLA-identical littermate on day 0 and postgrafting immunosuppression with methotrexate/cyclosporine (MTX/CSP) for no more than 35 days (top panel). The bottom panel shows the results of testing for (CA)n dinucleotide repeats of donor and recipient cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3 to 14).
Graft rejection. Granulocyte and platelet changes in dog E156 conditioned with 200 cGy TBI, given a marrow graft from a DLA-identical littermate on day 0 and postgrafting immunosuppression with methotrexate/cyclosporine (MTX/CSP) for no more than 35 days (top panel). The bottom panel shows the results of testing for (CA)n dinucleotide repeats of donor and recipient cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3 to 14).
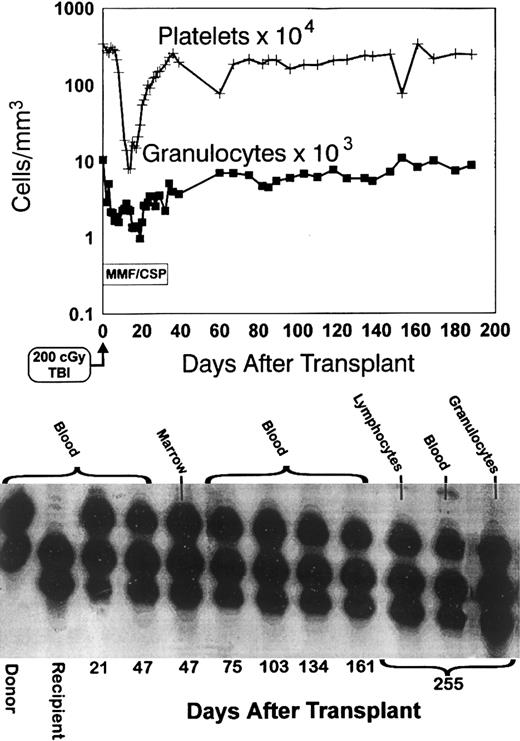
Four of the five dogs in group 3 given MMF/CSP have remained stable mixed chimeras through weeks 44 to 56 after transplant. One dog, E069, lost the allogeneic graft beyond week 12. Figure 3 shows the platelet and granulocyte changes in one of the stable mixed chimeras (E131) along with the results of the (CA)n dinucleotide repeat marker studies through day 255, demonstrating the presence of the donor band.
Sustained engraftment. Granulocyte and platelet changes in dog E131 conditioned with 200 cGy TBI, given a marrow graft from a DLA-identical littermate on day 0 and postgrafting immunosuppression with mycophenolate mofetil/cyclosporine (MMF/CSP) for no more than 35 days. The bottom panel shows the results of testing for (CA)n dinucleotide repeats of donor and recipient cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3 to 12).
Sustained engraftment. Granulocyte and platelet changes in dog E131 conditioned with 200 cGy TBI, given a marrow graft from a DLA-identical littermate on day 0 and postgrafting immunosuppression with mycophenolate mofetil/cyclosporine (MMF/CSP) for no more than 35 days. The bottom panel shows the results of testing for (CA)n dinucleotide repeats of donor and recipient cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3 to 12).
All six dogs in group 4, which were conditioned with only 100 cGy TBI and given MMF/CSP after transplant, rejected their allografts after 3 to 12 weeks.
The two dogs in group 5 given no TBI and MMF/CSP after transplant were found to have mixed chimerism among their nucleated peripheral blood cells by week 2, but no donor cells were seen on repeated testing thereafter.
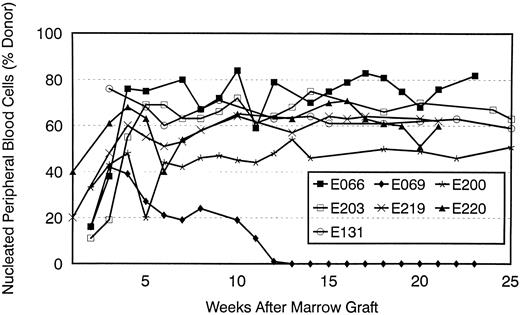
Figure 4 estimates of the percent of donor-derived nucleated peripheral blood cells for seven recipients in groups 2 and 3, as based on phosphor image analyses of results of (CA)n dinucleotide repeat studies over time. In all dogs, the proportions of donor cells increased over the first 5 weeks after transplant. They have remained stable in six of the seven dogs, while declining to zero in one (E069). Popliteal lymph node cells from biopsies done between 302 and 384 (median, 308) days after transplant showed results that were similar to those seen in the peripheral blood for the seven dogs studied (data not shown).
Percent donor peripheral blood cells after transplant in seven dogs given 200 cGy TBI and DLA-identical marrow grafts. Two dogs received MTX/CSP (E200, E203) and five MMF/CSP (E066, E069, E131, E219, and E220) after transplant. Results were estimated by phosphor image analysis of (CA)n dinucleotide repeat marker results.
Percent donor peripheral blood cells after transplant in seven dogs given 200 cGy TBI and DLA-identical marrow grafts. Two dogs received MTX/CSP (E200, E203) and five MMF/CSP (E066, E069, E131, E219, and E220) after transplant. Results were estimated by phosphor image analysis of (CA)n dinucleotide repeat marker results.
Table 2 shows phosphor image analyses results of amplified DNA from marrow and peripheral blood cell subpopulations in five of the recipients from groups 2 and 3. The highest proportions of donor cells were seen among granulocytes, the lowest among lymphocytes.
Dogs Given a Sublethal Dose of TBI (200 cGy) and Marrow Grafts From DLA-Identical Littermates
| Dog No. . | Postgrafting Immunosuppression . | Day of Test . | % Donor Cells Estimated by Image Analysis of (CA)n Dinucleotide Repeat Marker Results . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | Marrow . | Peripheral Blood . | . | . | ||
| . | . | . | . | Buffy Coat Cells . | Lymphocytes . | Granulocytes . | . | . |
| E200 | MTX/CSP | 135 | 85 | 86 | 54 | 91 | ||
| E203 | 135 | 85 | 86 | 55 | 92 | |||
| E131 | MMF/CSP | 180 | 44 | 55 | 49 | 54 | ||
| E219 | 98 | 54 | 61 | 36 | 69 | |||
| E220 | 98 | 53 | 60 | 35 | 68 | |||
| Dog No. . | Postgrafting Immunosuppression . | Day of Test . | % Donor Cells Estimated by Image Analysis of (CA)n Dinucleotide Repeat Marker Results . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | . | Marrow . | Peripheral Blood . | . | . | ||
| . | . | . | . | Buffy Coat Cells . | Lymphocytes . | Granulocytes . | . | . |
| E200 | MTX/CSP | 135 | 85 | 86 | 54 | 91 | ||
| E203 | 135 | 85 | 86 | 55 | 92 | |||
| E131 | MMF/CSP | 180 | 44 | 55 | 49 | 54 | ||
| E219 | 98 | 54 | 61 | 36 | 69 | |||
| E220 | 98 | 53 | 60 | 35 | 68 | |||
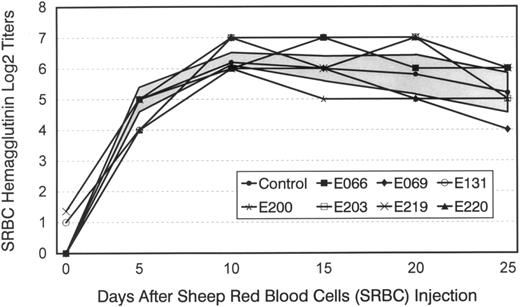
None of the mixed hematopoietic chimeras developed evidence of GVHD at any time after transplant. The lymphocyte responsiveness to alloantigens and to concanavalin A of seven of the chimeras was tested between 219 and 301 (median, 224) days after transplant (data not shown), and no significant differences were seen from controls. Flow cytometric analyses of the dogs' peripheral blood lymphocytes showed normal T4/T8 ratios in all cases ranging from 1.25 to 3.9 (median, 1.6). Figure 5 shows the chimeras' primary humoral antibody titers in response to sheep red blood cells, which were not significantly different from those of five concurrently injected controls.
Hemagglutinin titers after a single injection of sheep red blood cells (SRBC) in five normal dogs, six mixed hematopoietic chimeras (E066, E131, E200, E203, E219, and E220), and one recipient that had rejected the marrow allograft (E069). Shown for controls are mean titers ± 1 standard deviation (SD) (shaded area).
Hemagglutinin titers after a single injection of sheep red blood cells (SRBC) in five normal dogs, six mixed hematopoietic chimeras (E066, E131, E200, E203, E219, and E220), and one recipient that had rejected the marrow allograft (E069). Shown for controls are mean titers ± 1 standard deviation (SD) (shaded area).
DISCUSSION
Current clinical and experimental approaches for suppressing HVG reactions have relied on pretransplant conditioning programs, and their intensity has been increased to a point at which dose-limiting toxicities are frequently seen. Also, because of regimen-related, virtually complete marrow ablation, survival of patients so treated depends on the prompt function of the hematopoietic graft. In contrast, the current study accomplished engraftment by combining a nonmyleoablative conditioning regimen of sublethal TBI that had little toxicity with postgrafting immunosuppression, which inhibited both HVG and GVH reactions.
The current observations extended previous ones that showed that CSP postgrafting established either mixed or entirely donor-type hematopoietic chimerism in dogs conditioned with a low, but still lethal, dose of 450cGy TBI.1 CSP alone did not suffice to accomplish sustained chimerism after a sublethal TBI dose of 200 cGy. By comparison, MTX/CSP, shown to be synergistic with regard to GVHD prevention,2 3 led to prolonged mixed hematopoietic chimerism in all cases, although at least half of the dogs studied ultimately lost their grafts. By comparison, four of five dogs given MMF/CSP have become long-term mixed chimeras, while one rejected the graft beyond 4 months. Thus, results provide proof of principle that postgrafting immunosuppression given for no more than 35 days is capable of establishing mixed donor/host hematopoietic chimerism following a sublethal dose of TBI. None of the surviving mixed chimeras showed evidence of acute or chronic GVHD, and they were immunologically competent as assessed by lymphocyte responses to alloantigens and to concanavalin A, antibody formation to sheep red blood cells, and normal T4/T8 ratios in peripheral blood.
When the TBI dose was reduced to 100 cGy, four of six MMF/CSP-treated dogs showed mixed chimerism for extended periods of time before graft rejection occurred in all cases. Mixed chimerism was also transient in two MMF/CSP-treated dogs given no TBI. The lack of success in establishing sustained mixed chimerism after 100 cGy TBI could be explained in at least two different ways, suboptimal recipient immunosuppression or suboptimal creation of marrow “space” effected by the lessened radiation dose. Further studies are required to distinguish between the two explanations.
The transplanted dogs' peripheral blood granulocyte and platelet counts generally did not decline to levels after TBI, which required transfusion support or which raised concerns about potentially lethal infections. Thus, for human patients such transplants would be safe, relatively inexpensive, and could be conducted in the ambulatory care setting. Also, as shown for patients with aplastic anemia, mixed chimeras are less prone to develop GVHD.13 The approach of establishing mixed chimerism could be applied to patients undergoing transplantation for aplastic anemia, and genetic diseases, eg, thalassemia and sickle cell disease. Mixed chimerism would be curative for patients in the latter two categories.14-16
Observations of mixed donor/recipient hematopoietic microchimerism have been made by solid organ transplant surgeons, whereby it is as yet not clear whether mixed chimerism is an epiphenomenon associated with the life-long use of immunosuppression in patients with kidney or liver transplants, or whether it is a major contributor to the long-term survival of transplanted organs.17-21 At any event, these findings, as well as the original observations by Main and Prehn22 in mice that “radiation chimeras” tolerate organ transplants from their hematopoietic stem cell donors, have raised speculation that establishment of mixed hematopoietic chimerism could serve as a prelude to transplantation of solid organs without the need for prolonged recipient immunosuppression.
Most preclinical studies involving induction of mixed allogeneic chimerism have been conducted in inbred strains of mice. Most have used in vivo T-cell depletion with antibodies combined with TBI at high, but nevertheless sublethal, doses. In other cases, TBI was combined with thymic irradiation and postgrafting CSP or high-dose cyclophosphamide. The first murine study was that of Cobbold et al23 in 1986 in which pretransplant treatment with anti-CD4 and anti-CD8 monoclonal antibodies was combined with 600 to 850 cGy TBI delivered at 35 cGy/min. In a recent study, non-obese diabetic (NOD) mice received ≥750 cGy of TBI followed by very large doses of allogeneic marrow cells, ≥30 × 106.24 In another study, mice were conditioned for allografts by 500 cGy TBI and given 100 or 200 mg cyclophosphamide/kg after transplant.25 Sharabi et al26 combined anti-CD4 and CD8 monoclonal antibodies with 3 Gy TBI and 7 Gy thymic irradiation to assure engraftment. Most recently, that group of investigators reported that the need for thymic radiation could be avoided by additional anti-CD4 and CD8 monoclonal antibody injections after transplant.27,28 A recent study, using A/J → B10 and B10.A → B10 murine marrow grafts, showed persistent chimerism when recipients were given anti-CD4 and CD8 monoclonal antibodies and 3Gy TBI (administered at 100 cGy/min) before transplant, and an antibody against natural killer cells after transplant for at most 16 weeks.29
One study in cynomolgus monkeys included conditioning with antithymocyte globulin, 300 cGy TBI (dose rate unknown), 700 cGy of thymic irradiation, and postgrafting CSP at an initial dose of 15 mg/kg/day for a total of 4 weeks.30 Marrow donors were MHC-mismatched, and mixed chimerism was observed for 18 to 51 days after transplantation. Maximum levels of donor-type chimerism ranged from 1.5% to 57.8%. The more favorable current results in dogs are most likely related to the use of MHC-matched littermate donor/recipient pairs compared with the unrelated monkey transplants. Of interest, marrow donor kidney grafts persisted in monkeys, even after marrow grafts had been rejected. Another study in DLA-incompatible littermate dogs used conditioning with 15 Gy of total lymphoid irradiation over 10 days delivered at 2 Gy/min and FK506 for 3 months after marrow transplant.31 While chimerism data were not shown, prolonged kidney and skin graft survivals were reported.
In summary, the establishment of sustained mixed hematopoietic chimerism following nonmyelosuppressive and nontoxic conditioning programs has remained a difficult goal. Here we present evidence in random-bred dogs that this goal can be achieved with the help of effective postgrafting immunosuppression, capable of suppressing both HVG and GVH reactions.
ACKNOWLEDGMENT
The authors are grateful to Michelle Fisher, Cindy Davis, Jill Johnson, Doug Jones, James Works, and Susan DeRose for technical expertise, to Bonnie Larson, Harriet Childs, and Lori Ausburn for secretarial help. We also would like to thank Barbara Johnston, DVM, and the technicians of the hematology, pathology, and bacteriology laboratories of the Fred Hutchinson Cancer Research Center for their assistance.
Supported by Grants No. CA15704, CA31787, HL36444, and DK42716 from the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD. The laboratory was also supported through a prize from the Josef Steiner Krebsstiftung, Bern, Switzerland, awarded to R.S.
Address reprint requests to Rainer Storb, MD, Fred Hutchinson Cancer Research Center, 1124 Columbia St, M318, Seattle, WA 98104-2092.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal