Abstract
Nonmyeloablative allogeneic stem cell transplantation has recently been explored as a safer alternative to conventional high-dose transplant regimens. Although a high incidence of mixed chimerism after nonmyeloablative procedures has been reported, the exact kinetics of engrafting donor cells in specific cellular lineages has yet to be defined. We investigated lineage-specific chimerism in 15 patients receiving an allogeneic peripheral blood stem cell (PBSC) transplant from an HLA-identical (n = 14) or a 5/6 antigen-matched sibling donor after a preparative regimen of cyclophosphamide and fludarabine. Donor chimerism was assessed weekly in T lymphocytes and myeloid cells by polymerase chain reaction (PCR) of minisatellite regions. Eight patients survived between 121 to 409 days after transplant. Ten of 14 patients surviving more than 30 days (71.4%) had delayed disease regression consistent with a graft-versus-malignancy (GVM) effect. One patient rejected the transplant with subsequent recovery of autologous hematopoiesis. Hematological recovery was rapid (median, 11 days to ≥500 neutrophils/μL) and was initially predominantly recipient in origin. Donor myeloid chimerism gradually supplanted recipient hematopoiesis and became fully donor in all survivors by 200 days after transplantation. In contrast, T-cell engraftment was more rapid, with full chimerism in 7 patients by day 30 and in 6 further patients by day 200 after cyclosporine withdrawal and donor lymphocyte infusion. Full donor T-cell engraftment preceded donor myeloid engraftment, acute graft-versus-host disease, and disease regression, consistent with a requirement for 100% donor T-cell chimerism for full expression of the alloresponse. These results emphasize the importance of lineage-specific chimerism analysis to successfully manipulate engraftment after nonmyeloablative allogeneic PBSC transplantation.
BECAUSE OF THEIR LOW toxicity, nonmyeloablative preparative regimens are being evaluated in patients with hematological malignancies not normally considered for allogeneic stem cell transplant because of poor performance status or advanced age.1-6 While these low-intensity transplant regimens have a decreased immediate procedural mortality, the risk of graft rejection is higher because residual recipient immunity is not fully ablated, producing a mixed chimeric state. Although mixed chimerism reduces the risk of graft-versus-host disease (GVHD), it may also lead to tumor tolerance, potentially abolishing a beneficial graft-versus-malignancy (GVM) effect.7-12 Furthermore, because the preparative regimen does not contribute a significant antimalignancy effect, the risk of disease progression after transplantation is higher. Therefore, in this setting, there is a greater need to rapidly establish complete donor T-cell engraftment to confer a GVM effect. To understand the potential and the limitations of nonmyeloablative stem cell allotransplantation, it is necessary to characterize the kinetics of lymphoid and myeloid cell recovery. Furthermore, measurement of donor chimerism after transplantation is a prerequisite for manipulating engraftment favorably by altering patient immunosuppression and by donor lymphocyte infusion (DLI).
We describe here a low-intensity, nonmyeloablative transplant protocol used to establish a GVM effect in patients with a variety of hematological and nonhematological ma- lignancies. Our goal was to rapidly establish full donor chi- merism after transplant by withdrawal of immunosuppres- sion and administration of DLI. We monitored donor and recipient T-lymphocyte and myeloid cell chimerism, using a minisatellite polymerase chain reaction (PCR) technique to correlate chimerism with major transplant outcomes and to measure the efficacy of posttransplant immune manip- ulation.
MATERIALS AND METHODS
Patients gave written informed consent to the following local institutional review board approved transplantation protocols: NIH 97-H-0202 (hematologic malignancies in older individuals), NIH-97-0196 (graft-versus-tumor in metastatic renal cell carcinoma), and NIH-98-0006 (graft-versus-tumor in metastatic melanoma). Eligibility criteria for hematologic malignancies included disease with a probability of slow progression, or in remission, potentially curable by allogeneic marrow transplantation, in patients age ≥55 years. Patients with renal cell carcinoma or malignant melanoma had progressive metastatic disease refractory to standard treatment, including interleukin-2 (IL-2), α-interferon, with or without chemotherapy. Between October 1997 and November 1998, 15 patients underwent nonmyeloablative allogeneic peripheral blood stem cell (PBSC) transplantation from molecularly typed HLA identical (N = 14) or single HLA locus mismatched (n = 1) sibling donors. Patient characteristics and transplant outcome are listed in Table 1. Ages ranged from 23 to 68 years (median, 50). Eight patients had hematologic malignancies (3 had myelodysplastic syndrome; 3 had chronic myelogenous leukemia [CML], 2 in chronic phase and 1 in second chronic phase after treatment for blast crisis; 1 had treatment refractory extramedullary plasmacytomas, and 1 had relapsed diffuse large cell non-Hodgkin’s lymphoma in partial remission). Seven patients had solid tumors (4 had metastatic melanoma; 3 had metastatic renal cell carcinoma). In 9, a sex difference occurred between the patient and the donor, with 5 males receiving a transplant from a female donor.
Transplant Procedure
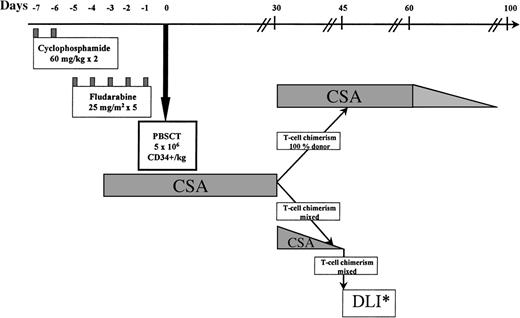
The protocol is illustrated in Fig 1. The preparative regimen consisted of cyclophosphamide 60 mg/kg on days −7 and −6, followed by fludarabine 25 mg/m2intravenously (IV) on days −5 to −1.13 14 One patient (no. 8), transplanted from a sibling donor mismatched at a single HLA locus, received additional immunosuppression with antithymocyte globulin 40 mg/kg IV daily on days −5 to −2. Cyclosporine (CSA), used to prevent graft rejection and as GVHD prophylaxis, was given from day −4, initially as an IV dose of 3 mg/kg daily. Oral CSA, 5 mg/kg twice daily, was substituted when tolerated. Levels were maintained in the therapeutic range by weekly monitoring.
Nonmyeloablative trans- plant protocol. PBSCT, peripheral blood stem cells transplant; DLI*, donor lymphocyte infusion. Patients received incremental doses of lymphocytes every 30 days until 100% donor T-cell chimerism, AGVHD, graft rejection, or a disease response occurred.
Nonmyeloablative trans- plant protocol. PBSCT, peripheral blood stem cells transplant; DLI*, donor lymphocyte infusion. Patients received incremental doses of lymphocytes every 30 days until 100% donor T-cell chimerism, AGVHD, graft rejection, or a disease response occurred.
Donors received 10 μg/kg granulocyte colony-stimulating factor (G-CSF) (filgrastim; Amgen, Thousand Oaks, CA) daily for 5 to 6 days. Mobilized PBSCs were collected by leukapheresis on day 5 and again on days 6 and 7, if necessary, to achieve a target dose of ≥5 × 106 CD34+cells/kg of recipient weight. Donor PBSC collections were prepared for fresh infusion (11 patients) or cryopreserved in 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen for subsequent thawing and infusion (4 patients). PBSC were not T-cell depleted, but in cases of major or minor ABO incompatibility between donor and recipient, were subjected to standard procedures for erythrocyte or plasma removal, respectively. CD34+ and CD3+ cells in PBSC products were quantitated by automated leukocyte counting and flow cytometry using the FACScan with CellQuest software (Becton Dickinson, Mountain View, CA), and stained with fluorochrome-labeled antibodies to CD34, CD3, and CD45 (Becton Dickinson) and concurrent staining with 7-AAD for evaluation of cell viability. The sum of the cell doses of all infused products was expressed as a dose per recipient body weight in kg. Using the same quantitation methods, donor lymphocytes collected by apheresis before the G-CSF mobilization were aliquotted into CD3+ cell doses of 2 × 106/kg and 10 × 106/kg, cryopreserved in 10% DMSO, and stored in liquid nitrogen for subsequent thawing and infusion. If required, additional DLI were collected by apheresis after the transplant.
On day 0 (and on days +1 and +2 if required to reach target cell doses), unmanipulated PBSCs were transfused to the recipient. Patients showing 100% donor T-lymphocyte chimerism by minisatellite analysis on day +30 continued CSA until day 60; thereafter CSA was tapered by 25% every 10 days and discontinued by day 100 if no GVHD developed. In patients with mixed donor/recipient T-lymphocyte chimerism on day 30, CSA was tapered off over a 2-week period and chimerism analysis repeated weekly. Patients not converting to 100% donor chimerism after CSA withdrawal received monthly escalating doses of DLI (Fig 1), with weekly reassessment of chimerism until 100% donor T-cell chimerism, GVHD, disease regression, or graft rejection occurred.
Infection prophylaxis has been described previously.15Briefly, this consisted of norfloxacin during the neutropenic period, fluconazole day −7 to day +30, high-dose acyclovir from day −5 until day +100, and Bactrim weekly beginning on day +30 and continuing to 6 months posttransplant. Cytomegalovirus (CMV) antigenemia was monitored weekly until day +100 and reactivation was treated with ganciclovir to preempt CMV disease.
Patients with symptomatic grade I acute GVHD of the skin were treated with topical steroids. Grade II or greater acute GVHD was treated with high-dose methylprednisolone 0.5 to 1.0 g/m2 IV, daily to twice daily, depending on severity, for 3 days, followed by rapid tapering in responders to a maintenance dose of 20 mg daily, continued for at least 2 weeks from the disappearance of all symptoms of GVHD.
Chimerism Assay
After transplant, serial samples of blood and marrow were analyzed for degrees of donor-recipient chimerism using PCR of informative minisatellite regions, which identified differences between recipient and donor (based on polymorphisms found in pretransplant recipient/donor samples). Chimerism was evaluated weekly in blood until day 100 and in bone marrow on days 30, 60, and 100 posttransplant. Ficoll-hypaque fractionated mononuclear cells from heparinized blood were sorted into specific lineages with immunomagnetic beads (Dynal A.S., Oslo, Norway), using a combination of anti-CD14 and anti-CD15 to select myeloid cells, and anti-CD3 to select residual (CD14/CD15-negative) T cells. In UPN 12-15, anti-CD2 beads were then used to select CD2+ natural killer (NK) cells from the remaining cells. Approximately 30 mL of bone marrow was used to select B cells, erythroid, and hematopoietic progenitor cells with immunomagnetic beads coated with monoclonal antibodies for CD19, CD71, and CD34, respectively. DNA was extracted from selected cells, suspended in Tris-acetate and EDTA at a concentration of 100 ng/μL and stored at 4°C. PCR primers flanking the repeat units of human minisatellites MS51, 33.6, 33.1, YNZ-22, 3′HVR, and λg3 were used to amplify this informative sequence in donor and patient pre and posttransplant according to a previously published method.12 16 Briefly, 25 PCR cycles were performed on all minisatellites except the MS51 and MS33.6 samples, which required 15 cycles. Varying dilutions of pretransplant patient and donor DNA were used as reference standards to quantify the proportion of donor/host chimerism after transplant. PCR products were electrophoresed in a 1.5% agarose gel at 60 V for 4 hours, then transferred to a nylon membrane and hybridized with a digoxigenin-labeled probe (Boehringer Mannheim GmbH, Mannheim, Germany). Probes were labeled with a random priming kit (MS51 and 33.6) or an oligonucleotide tailing kit (33.1, YNZ-22, 3′HVR, and λg3 (Boehringer). Membranes were subsequently treated with digoxigenin detection reagents and exposed to film (Kodak Bio-Max MR; Eastman Kodak, Rochester, NY) for different times. When a sex difference between patient and donor existed (n = 9), unsorted bone marrow samples were also karyotyped posttransplant.
Disease Evaluation
In patients with CML, monthly cytogenetic analysis of bone marrow aspirates for the Ph1 chromosome was performed until they became Ph1 negative. CML patients were also followed monthly by reverse transcriptase (RT)-PCR analysis of peripheral blood myeloid cells for the BCR-ABL fusion gene (sensitivity 1 in 106) using nested primers as previously described.17 Disease status in other leukemias was assessed on marrow aspirates by morphology and karyotypic abnormalities and by detection of recipient myeloid cells by PCR of minisatellites. Patients with solid tumors or lymphomas were followed by sequential computerized tomography scans on posttransplant days 30, 60, 100, and every 2 months thereafter. One patient with multiple extramedullary plasmacytomas (no. 15) was followed by computerized tomography scans as above and monthly posttransplant serum and urine protein electrophoresis with immunofixation.
RESULTS
The outcomes of 15 patients undergoing nonmyeloablative allogeneic SCT are listed in Table 1. Apart from transient nausea, the preparative regimen was well-tolerated with no mucositis or hemorrhagic cystitis. One patient (no. 6) developed bilateral interstitial pulmonary infiltrates at the time of neutrophil recovery, attributed to fludarabine, which resolved rapidly with steroids. One patient with RAEBT (refractory anemia with excess blasts in transformation) myelodysplastic syndrome (MDS) (no. 11) who had previously received an autologous bone marrow transplant (BMT) developed transient hyperbilirubinemia (maximum total bilirubin 9.0 mg/dL) attributed to mild veno-occlusive disease.
Patient Characteristics and Outcome After Nonmyeloablative Allogeneic Transplant
| No. . | UPN . | Dx . | Age/ Sex . | Transplant Dose/kg . | RBC/Plt Tx . | Days to ANC > 500 . | Discharge Day . | Survival Day . | |
|---|---|---|---|---|---|---|---|---|---|
| CD34 × 106 . | CD3 × 108 . | ||||||||
| 1 | 77 | Mel | 23/M | 13.5 | 2.1 | 1/1 | 11 | 13 | 85: DP |
| 2 | 79 | Mel | 27/M | 7.8 | 4.0 | 2/1 | 9 | NA | 15: DP |
| 3 | 80 | CML (2CP) | 68/M | 12.2 | 3.8 | 0/1 | 15 | 21 | 106: DP |
| 4 | 85 | Mel | 42/F | 9.5 | 5.2 | 0/0 | 9 | 12 | 199: DP |
| 5 | 86 | RCC | 50/M | 9.1 | 2.0 | 1/0 | 11 | 23 | >409: CR |
| 6 | 89 | Mel | 50/F | 5.1 | 1.1 | 2/3 | 10 | NA | 59: TRM |
| 7 | 91 | CML-CP | 56/F | 6.6 | 1.1 | 2/0 | 12 | 21 | >379: CR |
| 8 | 93 | RCC | 46/M | 5.8 | 1.4 | 0/3 | 11 | 13 | >351: CRU |
| 9 | 95 | MDS (RAEBT) | 60/M | 6.8 | 1.8 | 0/0 | 10 | 10 | 205: Bacterial sepsis |
| 10 | 102 | Relapsed NHL | 55/F | 7.5 | 2.7 | 0/0 | 14 | 13 | >220: CR |
| 11 | 107 | MDS (RAEBT) | 58/M | 3.4 | 3.5 | 2/3 | 14 | 20 | 248: DP |
| 12 | 109 | CMML | 56/M | 6.2 | 5.2 | 2/0 | 11 | 21 | >180: CR |
| 13 | 113 | RCC | 48/M | 6.0 | 1.6 | 1/0 | 11 | 1 | >160: PR |
| 14 | 115 | CML-CP | 28/M | 3.7 | 4.6 | 1/0 | 13 | 4 | >159: CR |
| 15 | 118 | EMP | 34/F | 6.5 | 3.3 | 2/0 | 11 | 17 | >121: Relapse |
| No. . | UPN . | Dx . | Age/ Sex . | Transplant Dose/kg . | RBC/Plt Tx . | Days to ANC > 500 . | Discharge Day . | Survival Day . | |
|---|---|---|---|---|---|---|---|---|---|
| CD34 × 106 . | CD3 × 108 . | ||||||||
| 1 | 77 | Mel | 23/M | 13.5 | 2.1 | 1/1 | 11 | 13 | 85: DP |
| 2 | 79 | Mel | 27/M | 7.8 | 4.0 | 2/1 | 9 | NA | 15: DP |
| 3 | 80 | CML (2CP) | 68/M | 12.2 | 3.8 | 0/1 | 15 | 21 | 106: DP |
| 4 | 85 | Mel | 42/F | 9.5 | 5.2 | 0/0 | 9 | 12 | 199: DP |
| 5 | 86 | RCC | 50/M | 9.1 | 2.0 | 1/0 | 11 | 23 | >409: CR |
| 6 | 89 | Mel | 50/F | 5.1 | 1.1 | 2/3 | 10 | NA | 59: TRM |
| 7 | 91 | CML-CP | 56/F | 6.6 | 1.1 | 2/0 | 12 | 21 | >379: CR |
| 8 | 93 | RCC | 46/M | 5.8 | 1.4 | 0/3 | 11 | 13 | >351: CRU |
| 9 | 95 | MDS (RAEBT) | 60/M | 6.8 | 1.8 | 0/0 | 10 | 10 | 205: Bacterial sepsis |
| 10 | 102 | Relapsed NHL | 55/F | 7.5 | 2.7 | 0/0 | 14 | 13 | >220: CR |
| 11 | 107 | MDS (RAEBT) | 58/M | 3.4 | 3.5 | 2/3 | 14 | 20 | 248: DP |
| 12 | 109 | CMML | 56/M | 6.2 | 5.2 | 2/0 | 11 | 21 | >180: CR |
| 13 | 113 | RCC | 48/M | 6.0 | 1.6 | 1/0 | 11 | 1 | >160: PR |
| 14 | 115 | CML-CP | 28/M | 3.7 | 4.6 | 1/0 | 13 | 4 | >159: CR |
| 15 | 118 | EMP | 34/F | 6.5 | 3.3 | 2/0 | 11 | 17 | >121: Relapse |
Abbreviations: RCC, renal cell carcinoma (metastatic); Mel, melanoma (metastatic); CML-CP, chronic myelogenous leukemia in chronic phase; MDS, myelodysplastic syndrome; RAEBT, refractory anemia with excess blasts; NHL, diffuse large cell non-Hodgkin’s lymphoma (stage IV); CMML, chronic myelomonocytic leukemia; EMP, extramedullary plasmacytoma (multiple, unresectable, and treatment refractory); DP, disease progression; CR, complete response; CRU, complete response unsure; TRM, treatment-related mortality; PR, partial response.
The infused allograft contained a median of 6.6 × 106CD34+ cells/kg (range, 3.4 to 13.5) and 2.7 × 108 CD3+ cells/kg (range, 1.1 to 5.2). Hematological recovery was prompt. Neutrophils decreased to <100/μL in all patients and recovered to >500/μL at a median of 11 (range, 9 to 15) days posttransplant. All patients received IV antibiotics to treat febrile neutropenia. The median time to a platelet count >50,000/μL was 8 days (range, 0 to 10) posttransplant with 10 of 15 patients never dropping their platelet count below 20,000/μL. Six patients required 1 to 4 (median, 1) single-donor plateletpheresis transfusions. Median red blood cell transfusion requirement was 2 U (range, 0 to 4 U). Six patients reactivated CMV, 5 were asymptomatic, and 1 developed esophagitis. All responded to ganciclovir.
Eight patients survived between 121 and 409 (median, 200) days posttransplant. Five died of progressive disease (3 melanoma, 1 CML, 1 MDS) and 2 of transplant-related causes (idiopathic encephalitis, day 59 and bacterial sepsis, day 205).
Disease Response
At day +30 posttransplant, all 14 surviving patients had evidence of residual disease. Subsequently, 10 patients (71.4%) had disease regression, 5 of whom remain in complete remission (Table 2). Of note, 1 patient with extensive renal cell cancer,18 and 2 patients with CML remain disease-free 13, 12, and 5 months, respectively, posttransplant.
Characteristics of Patients Showing a Disease Response
| Patient No. . | Disease . | Day T-Cell Chimerism 100% Donor . | Disease Response . |
|---|---|---|---|
| 5 | RCC | 60 | Regression of metastatic disease noted on CT scan day 86. CR day 110. |
| 6 | Melanoma | 14 | Regression of metastatic disease noted by physical exam and confirmed on CT scan day 36. |
| 7 | CML-CP | 85 | Philadelphia chromosome and RT-PCR for BCR/ABLnegative day 100. |
| 8 | RCC | 14 | Regression of metastatic disease noted on CT scan day 220. CRU day 280. |
| 9 | MDS (RAEBT) | 164 | Negative for recipient myeloid cells by minisatellite analysis day 185. |
| 10 | Relapsed NHL | 30 | Regression of metastatic disease on CT scan day 60. CR day 100. |
| 12 | CMML | 65 | Resolution of cytogenetic abnormality (20q−) and negative for recipient myeloid cells by minisatellite analysis day 83. |
| 13 | RCC | 14 | Regression of metastatic disease on CT scan day 128. Disease regression ongoing on CT scan day 160. |
| 14 | CML-CP | 30 | Philadelphia chromosome negative day 100. RT-PCR negative day 128. |
| 15 | EMP | 21 | Resolution of paraproteinemia and regression of multiple plasmacytomas on CT scan day 60. Relapsed disease on CT scan day 100. DLI day 120. |
| Patient No. . | Disease . | Day T-Cell Chimerism 100% Donor . | Disease Response . |
|---|---|---|---|
| 5 | RCC | 60 | Regression of metastatic disease noted on CT scan day 86. CR day 110. |
| 6 | Melanoma | 14 | Regression of metastatic disease noted by physical exam and confirmed on CT scan day 36. |
| 7 | CML-CP | 85 | Philadelphia chromosome and RT-PCR for BCR/ABLnegative day 100. |
| 8 | RCC | 14 | Regression of metastatic disease noted on CT scan day 220. CRU day 280. |
| 9 | MDS (RAEBT) | 164 | Negative for recipient myeloid cells by minisatellite analysis day 185. |
| 10 | Relapsed NHL | 30 | Regression of metastatic disease on CT scan day 60. CR day 100. |
| 12 | CMML | 65 | Resolution of cytogenetic abnormality (20q−) and negative for recipient myeloid cells by minisatellite analysis day 83. |
| 13 | RCC | 14 | Regression of metastatic disease on CT scan day 128. Disease regression ongoing on CT scan day 160. |
| 14 | CML-CP | 30 | Philadelphia chromosome negative day 100. RT-PCR negative day 128. |
| 15 | EMP | 21 | Resolution of paraproteinemia and regression of multiple plasmacytomas on CT scan day 60. Relapsed disease on CT scan day 100. DLI day 120. |
Abbreviations: RCC, renal cell carcinoma (metastatic); CML-CP, chronic myelogenous leukemia in chronic phase; MDS, myelodys- plastic syndrome; RAEBT, refractory anemia with excess blasts; NHL, diffuse large cell non-Hodgkin’s lymphoma (stage IV); CMML, chronic myelomonocytic leukemia; EMP, extramedullary plasmacytoma (multiple, unresectable, and treatment refractory); CR, complete response; CRU, complete response unsure; TRM, treatment-related mortality; DLI, donor lymphocyte infusion.
GVHD
Acute GVHD grade ≥II occurred in 9 patients (5 when not receiving CSA). Six had grade II and 3 grade III GVHD with a median onset of 45 days (range, 28 to 195) posttransplant. GVHD responded to treatment with methylprednisolone and reinstitution of CSA. One patient developed grade II gastrointestinal acute GVHD on day +195, 50 days after a third DLI for persistent mixed T-cell chimerism. He responded promptly to steroid treatment, but died (day +205) of sepsis associated with disseminated intravascular coagulation (DIC). Four patients developed mild chronic GVHD; 3 limited to the skin and 1 in the liver. All 4 responded to low doses of alternate day CSA and prednisone.
Chimerism
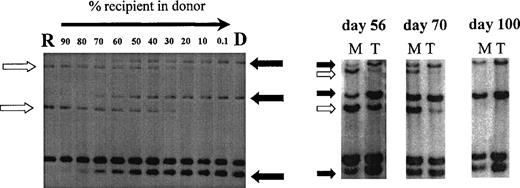
Using PCR analysis of minisatellite sequences with up to 6 primer pairs, informative recipient and donor-specific bands were obtained in all patients. Sensitivity of detection of specific bands was 0.1% for minisatellites MS51 and 33.6 and 1% for mini- satellites 33.1, YNZ-22, 3′HVR, and λg3. Figure 2 illustrates chimerism data in 1 patient using this minisatellite technique.
Chimerism analysis of patient no. 7 using primers MS51 and 33.6. Left, recipient DNA (R) was diluted in donor DNA (D) to estimate the chimerism level in posttransplant samples (right). Three donor-specific bands (solid arrow) and 2 recipient-specific bands (open arrow) were identified. Posttransplant samples were sorted into myeloid cells (M) and T cells (T) on days 56, 70, and 100 and showed donor T-cell engraftment preceding donor myeloid engraftment.
Chimerism analysis of patient no. 7 using primers MS51 and 33.6. Left, recipient DNA (R) was diluted in donor DNA (D) to estimate the chimerism level in posttransplant samples (right). Three donor-specific bands (solid arrow) and 2 recipient-specific bands (open arrow) were identified. Posttransplant samples were sorted into myeloid cells (M) and T cells (T) on days 56, 70, and 100 and showed donor T-cell engraftment preceding donor myeloid engraftment.
Sequential measurements of donor chimerism.
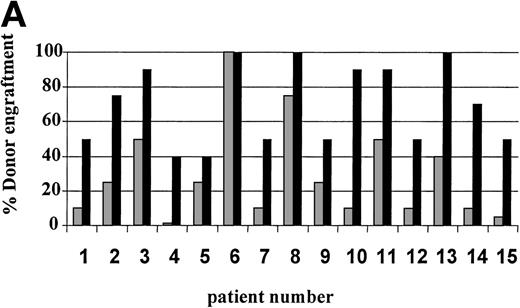
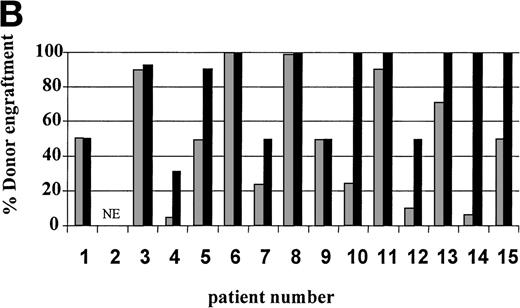
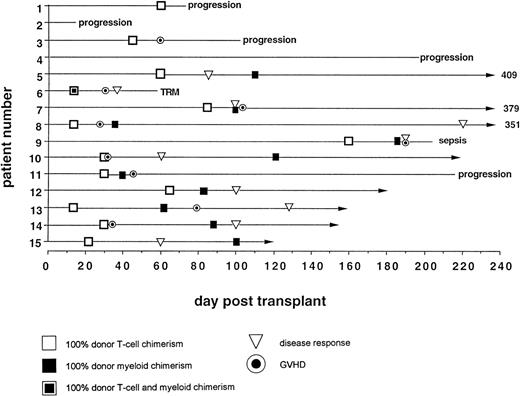
On day 14 posttransplant, the percentage of donor T-cell (CD3+) chimerism ranged from 40% to 100%. In contrast, myeloid (CD14+/CD15+) recovery at the same time was found to be ≥50% recipient (autologous) in 13 of 15 patients (Fig 3A). Subsequent time points showed that donor myeloid engraftment usually lagged behind T-cell engraftment, although the exact patterns of recovery varied considerably among patients (Fig 4). The median time to complete donor T-cell chimerism was 30 days (range, 14 to 164). By day 100, donor T cells reached 100% in 10 of 12 evaluable patients and by day 200, all 6 evaluable patients had achieved 100% donor T-cell chimerism. CD34+ and CD71+ cell chimerism closely approximated myeloid (CD14+ and CD15+) chimerism. NK (CD2+, CD3−) cell chimerism paralleled T- (CD3+) cell chimerism, but B- (CD19+) cell recovery was distinct from myeloid and T-cell lineages. Correlation between recovery of different lineages is shown in Fig 5.
Percentage of donor T (CD3+ cells, ▪) and myeloid (CD14+ and CD15+, ░) cells in 15 individual patients on day 14 (A) and day 30 (B) posttransplant. NE, nonevaluable.
Percentage of donor T (CD3+ cells, ▪) and myeloid (CD14+ and CD15+, ░) cells in 15 individual patients on day 14 (A) and day 30 (B) posttransplant. NE, nonevaluable.
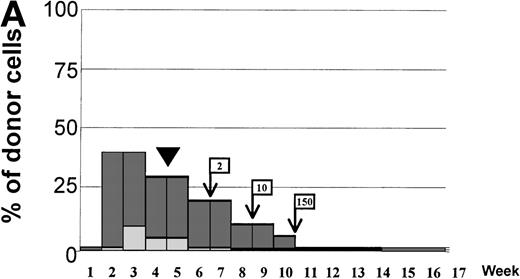
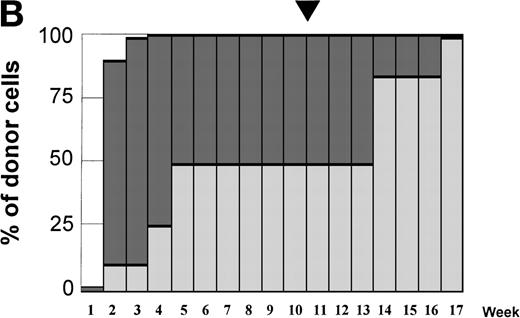
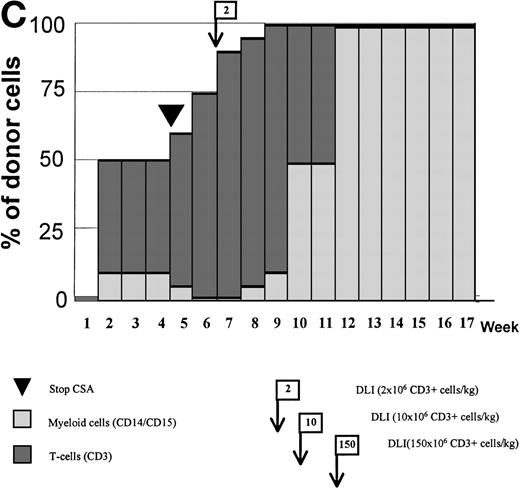
Variations in donor myeloid and T-cell engraftment posttransplant and relationship with CSA reduction and DLI. (A) Patient 4: poor T-cell engraftment (maximum, 40%) followed by graft rejection despite CSA withdrawal and DLI. (B) Patient 10: rapid and complete donor T-cell engraftment by week 3; prolonged mixed myeloid chimerism (initially predominantly recipient) reaching 100% donor by week 17 after CSA withdrawal. (C) Patient 12: partial T-cell engraftment increasing to 100% donor after CSA withdrawal and DLI followed by complete donor myeloid engraftment by week 12.
Variations in donor myeloid and T-cell engraftment posttransplant and relationship with CSA reduction and DLI. (A) Patient 4: poor T-cell engraftment (maximum, 40%) followed by graft rejection despite CSA withdrawal and DLI. (B) Patient 10: rapid and complete donor T-cell engraftment by week 3; prolonged mixed myeloid chimerism (initially predominantly recipient) reaching 100% donor by week 17 after CSA withdrawal. (C) Patient 12: partial T-cell engraftment increasing to 100% donor after CSA withdrawal and DLI followed by complete donor myeloid engraftment by week 12.
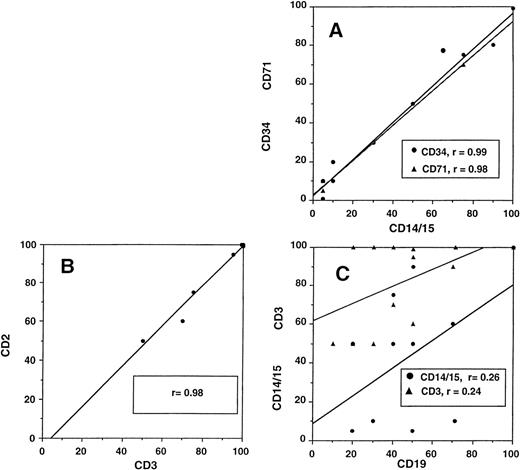
Relationship between different cellular lineages during engraftment. (A) Close correlation of myeloid cells (DC14+and CD15+) with CD34+ and CD71+cells and (B) T lymphocytes (CD3+) with NK cells (CD2+, CD3−). (C) No correlation of B-cell (CD19+) recovery with either myeloid or T cells.
Relationship between different cellular lineages during engraftment. (A) Close correlation of myeloid cells (DC14+and CD15+) with CD34+ and CD71+cells and (B) T lymphocytes (CD3+) with NK cells (CD2+, CD3−). (C) No correlation of B-cell (CD19+) recovery with either myeloid or T cells.
Relationship of Posttransplant Events to Donor T-Cell Chimerism
Engraftment.
Day 14 posttransplant chimerism analysis showed donor T-cell and myeloid engraftment in all 15 patients (Figs 3A and 6). However, 1 of 12 patients evaluable ≥100 days posttransplant (no. 4), who never achieved more than 40% donor T-cell and 10% donor myeloid chimerism, rejected the transplant by day 72, recovering 100% recipient T and myeloid cells with no detectable cytopenias during autologous hematological recovery (Fig 4A). Patient no. 8, who received a single HLA locus-mismatched transplant, engrafted rapidly and achieved complete donor chimerism in all cellular lineages by day 36.
GVHD.
Acute GVHD occurred only in patients who achieved 100% donor T-cell chimerism (Fig 6). The median time from the detection of 100% donor T-cell chimerism to the onset of acute GVHD was 15 days (range, 7 to 65 days). Five of 9 patients had mixed myeloid chimerism at the time of grade ≥II GVHD. Four of these 5 rapidly converted to complete donor myeloid chimerism, consistent with a delayed graft-versus-host hematopoiesis effect.
GVM effect.
In responders, disease regression occurred only after 100% donor T-cell chimerism was achieved (Fig 6). Median time between the establishment of full donor T-cell chimerism and first signs of disease regression was 27.5 days (range, 15 to 206).
Effects of Immune Modulation on Donor Chimerism
Seven of 14 patients had mixed (donor range, 40% to 90%) T-cell chimerism on day +30 (Fig 3B). After CSA withdrawal, 3 additional patients became 100% donor T-cell chimeric. The 4 remaining patients with mixed T-cell chimerism 2 to 4 weeks after CSA withdrawal received 1 to 3 DLI given at 30-day intervals. Three achieved 100% donor T-cell chimerism. One patient, who only reached a maximum of 40% donor T cells, showed a progressive decrease in percentage of donor T cells despite 3 DLIs (2, 10, and 150 × 106 CD3+cells/kg) and completely rejected the transplant by day 72. Overall, 13 of 14 patients established 100% donor T-cell chimerism (Table 3).
Impact of Posttransplant Immune Manipulation on T-Cell Chimerism in 14 Patients Evaluable 30 Days After Transplant
| . | T-Cell Chimerism . | |
|---|---|---|
| Mixed . | 100% Donor3-150 . | |
| Day 30 | 7 | 7 |
| Stop CSA | 4 | 10 |
| DLI 1 | 2 | 12 |
| DLI 2 | 2 | 12 |
| DLI 3 | 1 (rejection) | 13 |
| . | T-Cell Chimerism . | |
|---|---|---|
| Mixed . | 100% Donor3-150 . | |
| Day 30 | 7 | 7 |
| Stop CSA | 4 | 10 |
| DLI 1 | 2 | 12 |
| DLI 2 | 2 | 12 |
| DLI 3 | 1 (rejection) | 13 |
Cumulative number of patients achieving 100% chimerism.
At day 100, 6 patients, who achieved 100% donor T-cell chimerism by day 30, were evaluable. Four were successfully weaned off CSA, while 2 required ongoing treatment because of GVHD.
DISCUSSION
The optimum low-intensity allogeneic stem cell transplant technique, which maximizes engraftment and GVM effects while minimizing transplant-related complications, has yet to be defined. In selecting a low-intensity preparative regimen, we chose to use 2 chemotherapeutic agents with proven immunosuppressive, but nonmyeloablative effects. To improve the chance of donor engraftment, daily apheresis of donor T-lymphocyte–rich PBSC collections continued until a minimum of 5 × 106 CD34+ progenitors were harvested.19 The aim of the experimental protocol was to determine whether this regimen had low toxicity, while establishing a competent donor immune system able to exert a GVM effect.20,21 The transplant procedure was targeted to individuals who would not be expected to tolerate a standard high-intensity transplant for reasons of advanced age or poor performance status; or to individuals with solid tumors, where the lack of any established efficacy from allogeneic PBSC transplants would deter the investigative use of such transplants due to an unacceptably high risk of treatment-related mortality.22,23 A further goal was to attempt to rapidly establish 100% donor T-cell chimerism on order to favor GVM effects.7-10
Our data indicate that this cyclophosphamide-fludarabine preparative regimen is well-tolerated and highly immunosuppressive, with a 93% chance of resulting in complete donor T-cell engraftment. Only 2 patients died of transplant-related causes. This low toxicity confirms other reports of nonmyeloablative transplant outcome1-5 and should be viewed in the context of a patient population with a high median age and high risk of transplant-related mortality. Results compare favorably with an anticipated 200-day transplant-related mortality of 30% to 35% using conventional intensive allotransplants in this age group.24 Furthermore, the disease responses seen in both hematological and nonhematological malignancies occurred 2 to 7 months posttransplant. This characteristic delay implies that the low-intensity conditioning regimen used was inactive against these malignancies and emphasizes the central role of the GVM effect in mediating these dramatic tumor regressions.
Karyotypic analysis of bone marrow aspirates in sex-mismatched patients correlated with myeloid chimerism by minisatellite analysis (data not shown). However, our data show that with lineage-specific analysis of chimerism, a more detailed and complex picture of engraftment emerges. We found 3 distinct recovery patterns in T cells and NK cells, B cells, and myeloid cells (including granulocytes, erythroid, and progenitor cells). Three separate cellular sorting assays, using immuno- magnetic beads for CD14/15, CD3, and CD19, are sufficient to delineate the recovery of these lineages. Our lineage-spe- cific chimerism analysis confirmed that the regimen was more immunosuppressive than myelosuppressive, as T-cell engraftment occurred more promptly than myeloid engraft- ment.
The rapid recovery of largely recipient myelopoiesis was striking and accounted for the very brief period of posttransplant pancytopenia. Although there was mixed chimerism early after transplant, half of the evaluable patients achieved full donor T-cell chimerism by day +30 and, with withdrawal of CSA (followed by DLI if necessary), all but 1 patient ultimately achieved 100% donor T-cell engraftment. Such preferential expansion of allogeneic lymphocytes after CSA withdrawal suggests that residual recipient T cells may have become dysfunctional after immunosuppressive conditioning. However, it is important to note that transplant-related events, including the evolution of lineage-specific chimerism, may be time-dependent. Therefore, despite the strong temporal relationship between these immune manipulations and subsequent events, there is no direct proof these interventions were responsible for the transplant outcomes or changes in donor chimerism observed. Nevertheless, in contrast to prior reports of similar regimens,4 5 this protocol, which included posttransplant immune manipulation, was capable of establishing a complete donor immune system in the majority of transplanted patients. Our results suggest that the establishment of full donor T-lymphoid chimerism was an important factor in determining GVHD, GVM, and full donor myeloid engraftment.
This transplant approach has advantages over conventional transplant regimens: first, acute GVHD is delayed and usually occurs after patients have fully recovered from conditioning-related toxicity. This delay may mitigate the severity of GVHD-related tissue damage. Although 60% of patients developed acute GVHD, it was easily controlled with steroids, and chronic GVHD was mild and limited. Second, the ability to modify chimerism by posttransplant CSA withdrawal or DLI allows flexibility in selecting the optimum pace of donor immune recovery, with its attendant benefit from GVM effects and its risk of GVHD induction. While these data must be considered preliminary, they support favorable results reported in other studies using less intense transplant regimens.1-5 Our results emphasize the importance of monitoring posttransplant chimerism following nonmyeloablative procedures and lay a foundation for future studies investigating the therapeutic potential of the GVM effect in specific diseases.
N.C. was supported by a grant from The Foundation de France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R. Childs, MD, Hematology Branch, NHLBI, Bldg 10, Room 7C103, 9000 Rockville Pike, Bethesda MD 20892.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal