Abstract
Cytomegalovirus (CMV) infection and Epstein-Barr virus (EBV)-induced lymphoproliferative disease are serious complications associated with allogeneic stem cell transplantation. Immunotherapy using ex vivo expanded, virus-specific cytotoxic T lymphocytes (CTL) has been explored and proven to be effective in therapeutic or prophylactic regimens for CMV and EBV infections. To generate CTL specific for both CMV and EBV, we engineered EBV-transformed B-lymphoblastoid cell lines (BLCL) to express CMV pp65 for use as antigen-presenting cells (APC). BLCL were transduced with a recombinant retrovirus encoding pp65, the immunodominant CMV polypeptide. Western blot analysis and immunocytochemistry confirmed the expression of pp65 in the transduced cells. Peripheral blood mononuclear cells (PBMC) from healthy CMV seropositive donors were stimulated with autologous pp65-expressing BLCL weekly for 3 weeks. Chromium release assays showed that the resulting CTL cultures possessed specific cytotoxicity against EBV and CMV. Recombinant vaccinia viruses encoding individual CMV peptides were used to demonstrate that this CMV-specific cytotoxicity was specific for pp65. Assays on CD4- and CD8-depleted CTL fractions indicated that CD8+ CTL mediated the pp65-specific cytotoxicity. These CMV/EBV-specific CTL recognized CMV- and EBV-infected targets sharing HLA class I antigens, but not HLA mismatched targets. Our results demonstrate that BLCL can be used as APC to stimulate expansion of EBV- and CMV-specific CTL simultaneously. These findings have potential implications for posttransplant CMV and EBV immunotherapy in recipients of allogeneic stem cell transplants.
HUMAN CYTOMEGALOVIRUS (CMV) and Epstein-Barr virus (EBV) are members of the herpesvirus family, and in the normal host these viruses cause self-limited disease.1,2 In immunocompromised individuals, especially recipients of allogeneic, T-cell–depleted (TCD) stem cell transplants (SCT), CMV and EBV are responsible for significant morbidity and mortality.3-8 The incidence of CMV reactivation has been reported to be between 70% to 80% after SCT.3,6,7,9 The likelihood of developing EBV-induced lymphoproliferative disease (EBV-LPD) can be as high as 20% after a TCD graft, depending on the patient’s extent of immunosuppression.10,11 In these patients, cellular immunity, which plays a major role in preventing and controlling viral infections, is compromised as a result of immunosuppression aimed at preventing graft-versus-host disease (GVHD) and graft rejection.10,12,13 For example, more than 65% of patients at day 40 posttransplant are deficient in CD8+T-cell responses to CMV.13,14 Similarly, there is a correlation between the peak incidence of EBV-LPD and levels of EBV-specific cytotoxic T lymphocytes (CTL) in SCT patients, who are most susceptible to developing this complication between days 60 to 90 posttransplant.10 Thus, the restoration of cellular immunity is necessary to prevent viral reactivation.12,13,15,16 Although pharmacological agents exist to treat CMV infections, these medications have several side effects, the most serious of which is myelosuppression.7 17-19 Therefore, alternative therapies that restore host cellular immunity to CMV and EBV are of interest.
The infusion of ex vivo expanded, EBV-specific CTL has been proven to be safe and efficacious for the treatment and prophylaxis of EBV-LPD.15,16 To expand donor-derived, EBV-specific CTL, peripheral blood mononuclear cells (PBMC) are cocultivated ex vivo with autologous B-lymphoblastoid cell lines (BLCL), which are latently infected with EBV. Similar protocols have been developed to generate CMV-specific CTL cultures by cocultivating donor PBMC with CMV-infected autologous skin fibroblasts (SF), the only cell type supporting CMV replication in vitro.20-22 CD8+, CMV-specific CTL used for patient infusion are cloned from bulk CTL cultures due to the high allogeneic cytotoxicity in the latter.14Walter et al23 and Riddell et al24demonstrated that the administration of CMV-specific CTL clones resulted in the restoration of CMV-specific cellular immunity in SCT patients at risk for CMV disease. Current strategies for posttransplant, antiviral adoptive immunotherapy may be limited by the need for cultivating separate CTL for each important target antigen.
BLCL can be readily established in the laboratory by EBV immortalization. These lines phenotypically resemble the transformed B cells in posttransplant EBV-LPD and express immunogenic EBV peptides,25 making them ideal stimulators for expanding EBV-specific CTL. Among the more than 150 polypeptides encoded by CMV, several are known to be immunogenic.26 Those targeted by CTL include the immediate-early protein, virion envelope glycoprotein B, and the internal matrix proteins pp65 and pp150.27-31CMV pp65 has been identified as the immunodominant antigen, targeted by 70% to 90% of CMV-specific CTL.29,32 33 Because SCT patients are at risk for both EBV and CMV disease, a CTL preparation specific for both EBV and CMV would be of potential benefit, especially if CMV/EBV-specific CTL could be expanded simultaneously in the same culture. To this end, we developed a novel system, using BLCL as antigen-presenting cells (APC) to present both EBV and CMV epitopes. BLCL were transduced with a recombinant retrovirus encoding CMV pp65 and were then used to prime autologous PBMC. We demonstrated that (1) CTL cultures stimulated with pp65-expressing BLCL had CMV- and EBV-specific cytotoxicity and (2) this cytotoxicity was mediated by CD8+ CTL.
MATERIALS AND METHODS
Donors.
Four normal CMV-seropositive donors (AE, RB, SQ, and ST) and 2 seronegative donors (AD and SB) provided PBMC, SF, and serum for this study under protocols approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB). CMV serostatus was determined by the UAB Core Immunology Laboratory with an IgG enzyme-linked immunosorbent assay (Abbott Laboratories, Chicago, IL). Major histocompatibility complex (MHC) class I and II typing was performed by the UAB Histocompatibility Laboratory.
Expression vectors and viruses.
To construct the pp65-encoding recombinant retrovirus MSCVpp65, an 1,800-bp EcoRI-BamHI fragment containing the entire CMV pp65 coding sequence (a gift from J.A. Zaia,34 City of Hope Medical Center, Duarte, CA) was subcloned into theEcoRI-BglII linearized retroviral vector MSCV2.1 (a gift from R.G. Hawley,35 University of Toronto, Toronto, Canada). CMV pp65 was placed under the control of the long terminal repeat (LTR), while the internal human phosphosglycerole kinase promoter drove the expression of the selection marker bacterial neomycin phosphotransferase.
To generate replication-defective recombinant retroviruses, CsCl-purified construct DNA was electroporated into psi-cre cells, an ecotropic packaging cell line.36 Culture medium from the transfected psi-cre cells was used to infect the amphotropic producer cell line GP+envAM12.37 The producer cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Herndon, VA) with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), and were selected with G418 (Geneticin, BRL-Life Technologies, Gaithersburg, MD) at 600 μg/mL for 7 days. The virus-containing media was collected after the producer cell line was grown to confluence, and this supernatant was used to infect either human SF or BLCL. Retrovirus titer was determined by G418-selectable colonies in human SF and ranged from 5 × 105 to 106/mL.
Recombinant vaccinia viruses encoding CMV pp65 or pp150 were used as previously described.33 Briefly, virus stocks were prepared in BSC-40 cells (American Type Culture Collection [ATCC], Rockville, MD). After infection at a multiplicity of infection (MOI) of 0.1, the cells were cultured for 48 hours and then harvested and underwent 3 cycles of freeze and thaw. Virus suspension was cleared of debris by centrifugation and stored in aliquots at −20°C. Vaccinia virus titers ranged from 1 × 107 to 108/mL as determined by plaque assays in BSC-40 cells.
AD 169 human CMV was obtained from ATCC and virus was propagated in human SF. The initial infection was at an MOI of 0.1. Five days after cytopathological effects appeared in more than 90% cells, the virus-containing media were collected, passed through a 0.45-mm filter, and frozen in liquid nitrogen as aliquots. CMV titer was between 1 × 106 and 107/mL by plaque assays in human SF.
Cell lines, retroviral transduction, and virus infection.
PBMC were separated from heparinized whole blood by Histopaque (Sigma, St Louis, MO) gradient centrifugation and washed with RPMI 1640 (BRL-Life Technologies). BLCL were established from PBMC by EBV transformation and maintained in RPMI 1640 with 10% FBS, as described previously.10 BLCL were transduced with recombinant retroviruses by a 2-hour incubation with retrovirus-containing media. G418 selection was applied 48 hours after infection at 400 μg/mL for 14 days.
Primary human dermal fibroblast cell lines were established from skin biopsies from forearms. Human SF were grown in F12/DMEM (Mediatech) with 20% FBS and transduced with retroviruses by a 1-hour incubation with retrovirus-containing media. G418 selection was applied 24 hours after infection at 200 μg/mL for 5 days. When growth was sufficient, the cell lines were tested for mycoplasma contamination by a mycoplasma detection kit (BRL-Life Technologies). When used as targets in chromium-51 (51Cr) release assays, SF were treated with 100 IU/mL interferon-γ for 48 hours and were infected overnight with either recombinant vaccinia virus at an MOI of 5 or with CMV stock at an MOI of 10.
Protein analysis.
For immunocytochemical detection, the cells were fixed in 96-well plates with a 50% mixture of acetone and methanol for 20 minutes. A monoclonal antibody against human CMV-pp65,38 205, was used to stain the cells for 60 minutes at a dilution of 1:25. Immunoperoxidase staining was performed with a kit as instructed by the manufacturer (Immunopure Ultra-sensitive ABC Peroxidase Staining Kit; Pierce, Rockford, IL).
For immunoblotting, 0.2 × 106 cells were lysed in a buffer containing 4% sodium dodecyl sulfate (SDS), 0.1 mol/L Tris pH 6.8, 0.2% Brilliant Blue G250, 280 mmol/L 2-mercaptomethanol, and 20% glycerol. After boiling for 5 minutes, the samples were separated on an 8% SDS-polyacrylamide gel and transferred to a piece of Hybond-P membrane (Amersham Lifescience, Arlington Heights, IL). The blots were blocked with 5% nonfat milk in PBS-T (0.1 mol/L phosphate buffer, pH7.4, 200 mmol/L NaCl, 0.05% Tween 20), and probed with the monoclonal antibodies against pp65 (65-8)39 at 1:100 dilution and/or EBNA2 (PE2)40 at 1:500 dilution (Dako, Carpenteria, CA) for 60 minutes at room temperature. A horseradish peroxidase-labeled sheep antimouse IgG antibody (Amersham) was used as the secondary antibody at a dilution of 1:2,000. Enhanced chemiluminescence (ECL) detection was performed with ECL detection agents (Amersham) and recorded on Hyperfilm-ECL film (Amersham).
Ex vivo expansion of cytotoxic T lymphocytes.
Both pp65-expressing BLCL and SF were used as stimulators for ex vivo CTL expansion. PBMC were cocultivated with autologous pp65-expressing BLCL in 24-well plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) in RPMI 1640 supplemented with 10% FBS and 50 μmol/L 2-mercaptoethanol (Sigma). BLCL were exposed to 100 Gy of gamma irradiation before use as stimulator cells. The CTL cultures were primed weekly following a regimen of decreasing responder:stimulator ratios from 40:1 at day 0, 20:1 at day 7, and 5:1 on day 14 over a period of 3 weeks. pp65-expressing SF were used as stimulators following conditions described previously using CMV-infected SF.13 20 Briefly, PBMC were dispensed at 10 × 106 cells/well in 6-well plates containing 0.5 × 106 pp65-expressing SF in RPMI 1640 with 10% human CMV seronegative AB serum and 50 μmol/L 2-mercaptoethanol (Sigma). Weekly the CTL cultures were collected, washed with RPMI 1640, and replated with the same number of fresh SF stimulators at a ratio of 20:1 using autologous gamma-irradiated PBMC as feeder cells. For both BLCL and SF stimulators, interleukin-2 (IL-2) (Collaborative Biomedical Products, Bedford, MA) was added 10 days after the initial stimulation to a final concentration of 2.5 IU/mL, and medium was then changed every 3 days by replacing one half of the supernatant with fresh medium.
Chromium release assays.
Chromium release assays were performed as previously described.10 Target cells included the following: BLCL and BLCL transduced with MSCVpp65 or the backbone MSCV; SF, SF transduced with MSCVpp65 or the backbone MSCV, SF infected with CMV, and SF infected with recombinant vaccinia viruses encoding either pp65 or pp150. Target cells were labeled with 51Cr (New England Nuclear, Boston, MA) overnight (100 μCi/106 cells) and labeled cells were harvested, either by trypsinizing for SF or by centrifugation for BLCL. Cells were then washed in phosphate-buffered saline (PBS) and dispensed in triplicates into 96-well V-bottom plates (ICN, Costa Mesa, CA) at 4 × 103 cells/well. The effector:target (E:T) ratios were 12.5:1, 5:1, and 2.5:1. Spontaneous and total release for each target were used to calculate percent specific release using the following formula:
CD4+ and CD8+ cell isolation.
To isolate CD4+ and CD8+ populations of polyclonal CMV/EBV-specific CTL, bulk culture CTL were depleted of either CD4+ or CD8+ cells and natural killer (NK) cells using monoclonal antibodies specific for CD4/CD56 or CD8/CD56 (Pharmingen, San Diego, CA). Briefly, cells were placed in RPMI 1640 at 4°C at a concentration of 5 × 106/mL, and the monoclonal antibody was added for a final concentration of 20 μg/mL. After a 30-minute incubation at 4°C, the cells were washed twice in RPMI 1640, and Dynabeads (Dynal, Lake Success, NY) were added following the manufacturer’s instructions. After 30 minutes incubation at 4°C, the cell-bead suspension was placed on a magnet and the supernatant was removed, according to the manufacturer’s guidelines.
Cytofluorometry.
The cell-surface phenotype of EBV/CMV CTL was determined by staining with directly conjugated monoclonal antibodies and analyzing on a cytometer (FACScan; Becton Dickinson, San Jose, CA). These antibodies (Becton Dickinson) were specific for the following: CD3, CD4, CD8, and CD16/56 (simultest). Cells were washed with PBS supplemented with 3% FBS and 0.01% sodium azide. Dilutions and incubations for the antibodies followed the manufacturer’s instructions.
RESULTS
APC presenting both EBV and CMV antigens.
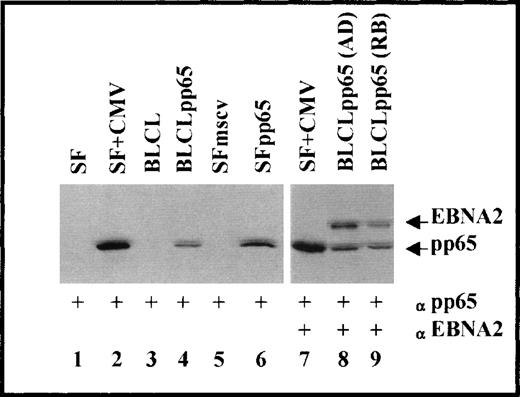
After selection with G418, BLCL and SF transduced with either the retrovirus MSCVpp65 (BLCLpp65 and SFpp65, respectively) or MSCV (BLCLmscv or SFmscv, respectively) continued to proliferate, whereas uninfected cells did not survive. Western blot analysis (Fig 1) of the transduced cells showed that the pp65-specific monoclonal antibody 65-8 detected a single band from BLCLpp65 and SFpp65 (lanes 4 and 6), but not in the MSCV backbone vector-transduced or the untransduced parental cells (lanes 1, 3, and 5). A band of similar size was also observed in the SF infected with CMV (lane 2), confirming the expression and the physical integrity of pp65 in the cells transduced with MSCVpp65. In addition, using a monoclonal antibody specific for EBV nuclear antigen 2 (EBNA2), a specific band was detected in the two BLCLpp65 tested (lane 8, from AD; lane 9, from RB), both of which also expressed pp65. Because EBNA2 is 1 of the immunogenic EBV polypeptides expressed in BLCL, the detection of both pp65 and EBNA2 from BLCLpp65 was consistent with our expectation that BLCLpp65 would present epitopes derived from both CMV and EBV.
Immunoblot analysis for the expression of CMV pp65 and EBV EBNA2. Lanes 1 through 6, probed against CMV pp65; lanes 7 through 9, probed against both EBV EBNA2 and CMV pp65.
Immunoblot analysis for the expression of CMV pp65 and EBV EBNA2. Lanes 1 through 6, probed against CMV pp65; lanes 7 through 9, probed against both EBV EBNA2 and CMV pp65.
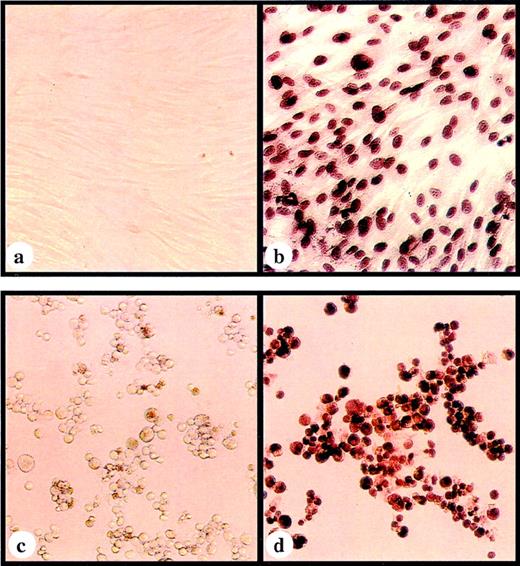
To examine the expression of pp65 at the single cell level, a CMV pp65–specific monoclonal antibody (205)38 was used to stain transduced cells immunocytochemically. The specific immunoperoxidase staining was localized to the nuclei in both SFpp65 and BLCLpp65 (Fig 2b and d). In addition to a diffuse staining pattern, some cells (most clearly in the SFpp65) displayed the characteristic inclusion body-like structure seen in CMV-infected cells.39 While almost all of the SFpp65 showed positive staining, the percentage of G418-resistent BLCLpp65 cells positive for immunoperoxidase staining varied from approximately 50% to 90%. This may be related to intrinsic differences in these two types of cells and their level of differentiation. Contrary to the relatively homogeneous nature of SF cultures, BLCL are regarded as heterogeneous, proliferating B cells at different stages of differentiation. The expression of the pp65, which is controlled by MSCV LTR, might be subject to the differentiation status of BLCL, as it has been reported that the promoter activity of MSCV LTR is associated with the differentiation status of hematopoietic cells.41
Immunoperoxidase staining using a pp65-specific monoclonal antibody with (a) SF transduced with MSCV; (b) SF transduced with MSCVpp65; (c) BLCL transduced with MSCV; and (d) BLCL transduced with MSCVpp65.
Immunoperoxidase staining using a pp65-specific monoclonal antibody with (a) SF transduced with MSCV; (b) SF transduced with MSCVpp65; (c) BLCL transduced with MSCV; and (d) BLCL transduced with MSCVpp65.
Generation of CTL cultures.
The transduced cells were cocultivated with autologous PBMC ex vivo to stimulate preferential expansion of EBV- and pp65-specific CTL. Before coculturing, the stimulators were confirmed for pp65 expression by immunoperoxidase staining. Pilot experiments included BLCLpp65 and SFpp65 as stimulators. Approximately 3 days after cocultivation, colonies composed of irregularly shaped cells were observed, most abundantly in the BLCLpp65-primed cultures. Although pp65-specific cytotoxicity was detected from CTL cultures using either cell line as an APC, SFpp65 were discontinued to be used as stimulators due to our limited access to human CMV seronegative AB serum, which the SF-based protocols called for.13,20 42
Cytotoxic specificity of the BLCLpp65-stimulated CTL cultures.
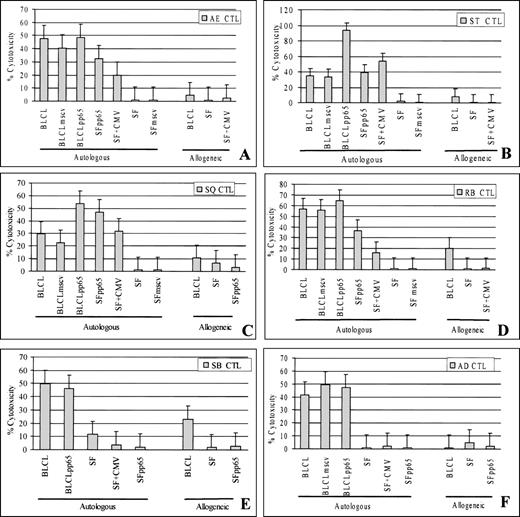
51Cr release assays were used to test CTL cultures against a panel of autologous and allogeneic target cells. Figure 3 shows that CTL cultures from all 6 donors lysed specific lysis of BLCL, as expected from their positive EBV-serology. In addition, CTL cultures from the 4 CMV-seropositive donors (AE, RB, SQ, and ST), but not the 2 CMV seronegative donors (AD and SB), also displayed specific cytotoxicity against MSCVpp65-transduced cells. Similar results were obtained for CTL cultures from day 14 and day 35 (data not shown). Although the stimulator BLCLpp65 should express the neomycin phosphotransferase, as indicated by the G418 selectibility, there was no specific killing against SFmscv in 51Cr release assays. The failure of ex vivo expansion of CTL specific for this bacterial protein, in contrast to the viral pp65, may be due to its weak immunogenecity, and/or a very low precursor frequency.
CTL cultures at 21 days after ex vivo stimulation with autologous BLCLpp65 were tested for specific cytotoxicity against EBV-transformed BLCL, CMV-infected SF, and MSCVpp65-transduced cells in chromium release assay. (A through D) CTL activity from 4 CMV seropositive/EBV seropositive individuals. (E through F) CTL activity from 2 CMV seronegative/EBV seropositive individuals. (E:T ratio, 12.5:1).
CTL cultures at 21 days after ex vivo stimulation with autologous BLCLpp65 were tested for specific cytotoxicity against EBV-transformed BLCL, CMV-infected SF, and MSCVpp65-transduced cells in chromium release assay. (A through D) CTL activity from 4 CMV seropositive/EBV seropositive individuals. (E through F) CTL activity from 2 CMV seronegative/EBV seropositive individuals. (E:T ratio, 12.5:1).
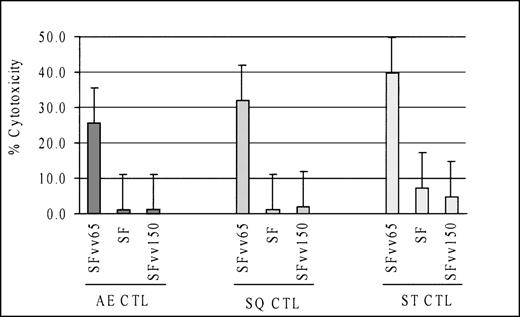
CTL cultures showing pp65-specific cytotoxicity also lysed autologous SF targets infected with CMV, suggesting that the pp65-specific cytotoxicity was effective in a setting close to natural CMV infection. However, a legitimate concern against this interpretation would be that residual CTL specific to other CMV antigens may be the cause of, or contribute to, the killing of the CMV-infected cells. To exclude this possibility, SF used as targets were infected with recombinant vaccinia viruses encoding either pp65 or pp150. CMV pp150 is another CMV matrix protein that is often targeted by specific CTL.31 Although all 3 CTL cultures lysed the pp65-vaccinia infected SF, none lysed pp150-vaccinia infected targets (Fig 4), indicating that the CMV-specific cytotoxicity was against pp65.
Cytotoxicity against CMV-infected cells by BLCLpp65-primed CTL cultures was mediated via pp65. CTL cultures lysed autologous fibroblasts infected with a recombinant vaccinia virus encoding CMV pp65, but not the one encoding CMV pp150. (E:T ratio, 12.5:1)
Cytotoxicity against CMV-infected cells by BLCLpp65-primed CTL cultures was mediated via pp65. CTL cultures lysed autologous fibroblasts infected with a recombinant vaccinia virus encoding CMV pp65, but not the one encoding CMV pp150. (E:T ratio, 12.5:1)
Effector cells of the CMV/EBV-specific cytotoxicity.
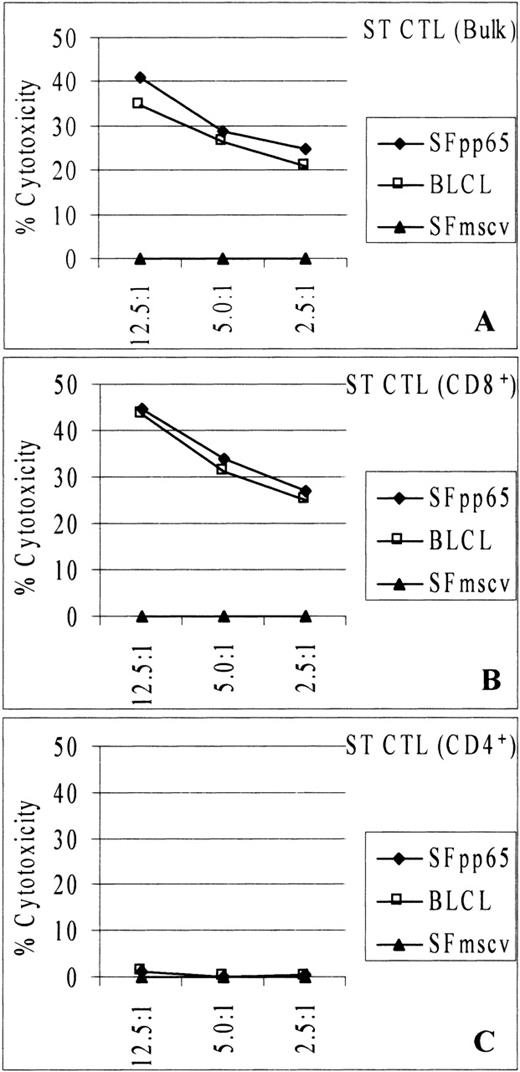
To discern the nature of the EBV/CMV-specific cytotoxicity, bulk CTL cultures from donor ST were selectively depleted of either CD4+/CD56+ cells or CD8+/CD56+ cells. Resulting cells were greater than 96% positive for CD8+ and greater than 80% positive for CD4+ cells, respectively. Cell fractions were tested in51Cr release assays against autologous SFpp65, BLCL, or SFmscv (Fig 5). CD8-enriched cells lysed both SFpp65 and BLCL, but not SFmscv (Fig 5B). A similar cytotoxicity profile was observed from bulk culture CTL (Fig 5A). In contrast, the CD4-enriched fraction failed to lyse any of these targets (Fig 5C). Furthermore, bulk culture and CD8-enriched CTL sustained specific cytotoxicity against BLCL and SFpp65 at 3 E:T ratios (12.5:1, 5:1, and 2.5:1). The CD4-enriched fraction failed to show any lysis of the BLCL or SFpp65 at any E:T ratios. These data indicated that the CMV/EBV-specific cytotoxicity was mediated mostly, if not entirely, by CD8+ CTL.
Cytotoxicity against EBV-transformed BLCL and MSCVpp65-transduced SF was mediated by CD8+ CTL. Bulk, CD8-enriched, or CD4-enriched CTL culture from donor ST were tested at different E:T ratios in chromium release assay. CD8-enriched CTL (B) retained the cytotoxicity displayed by the bulk CTL (A), while the CD4-enriched CTL (C) did not.
Cytotoxicity against EBV-transformed BLCL and MSCVpp65-transduced SF was mediated by CD8+ CTL. Bulk, CD8-enriched, or CD4-enriched CTL culture from donor ST were tested at different E:T ratios in chromium release assay. CD8-enriched CTL (B) retained the cytotoxicity displayed by the bulk CTL (A), while the CD4-enriched CTL (C) did not.
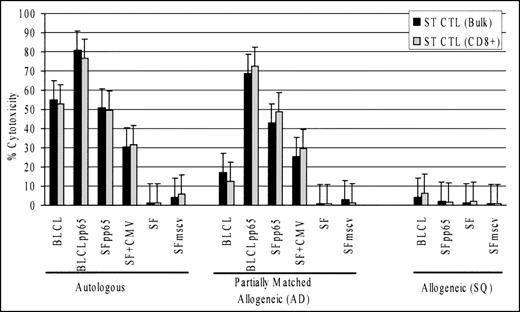
The differential cytotoxicity towards the autologous and allogeneic pp65-expressing targets by BLCLpp65-stimulated CMV/EBV CTL was consistent with a pattern of HLA-restricted cytotoxicity (Fig 3). We confirmed this by comparing specific cytotoxicity against a panel of targets consisting of autologous, partially matched allogeneic, or completely mismatched allogeneic cells (Fig6). CTL from donor ST were used, which shared A2,B44 with AD, a CMV seronegative donor of which the CTL culture showed no cytotoxicity against autologous pp65-expressing target cells. Bulk and CD8-enriched CTL from donor ST lysed all the 3 pp65-expressing targets from AD almost as well as the autologous counterparts (ST). The same CTL cultures failed to lyse the completely mismatched targets from donor SQ (Fig 6) or targets from 2 other mismatched individuals (data not shown). Note the EBV and CMV targets from SQ were lysed by the autologous CMV/EBV CTL (Fig 3D). This suggested that the pp65-specific cytotoxicity was HLA A2 and/or B44 restricted. The CMV/EBV-specific CTL from donor ST also showed cytotoxicity against the partially matched allogeneic BLCL, although the activity was lower than that for the autologous BLCL. This may be explained by the fact that BLCL express at least 8 EBV antigens, and it is possible that not all of the epitopes were presented by the 2 shared HLA alleles.
CMV pp65-specific cytotoxicity was HLA class I-restricted. Bulk and CD8-enriched CTL from donor ST showed cytotoxicity against HLA class I partially matched allogeneic targets (AD), which shared HLA A2 and B44 with donor ST. There was no specific lysis of the complete allogeneic targets (SQ). (E:T ratio, 12.5:1)
CMV pp65-specific cytotoxicity was HLA class I-restricted. Bulk and CD8-enriched CTL from donor ST showed cytotoxicity against HLA class I partially matched allogeneic targets (AD), which shared HLA A2 and B44 with donor ST. There was no specific lysis of the complete allogeneic targets (SQ). (E:T ratio, 12.5:1)
DISCUSSION
CMV disease and EBV-LPD are 2 of the most serious and potentially life-threatening viral complications after allogeneic, TCD-SCT.5-8 Previous studies have shown that adoptive immunotherapy with virus-specific CTL can be used to prevent complications related to these viruses.15 24 Current methodologies are limited by a requirement for developing CTL cultures using separate APC. This report demonstrated the feasibility of using retroviral technology to transduce EBV BLCL and achieve expression of CMV pp65. These BLCL present EBV peptides, as well as CMV pp65, and resulted in polyclonal, bispecific CTL cultures when cocultured with autologous PBMC. These findings are of importance to centers applying adoptive immunotherapy for viral infections in SCT patients.
Over the past decade, there has been a decrease in morbidity and mortality caused by CMV disease in posttransplant SCT, and this is largely attributable to the availability of pharmacological agents effective against CMV infections.43,44 Ganciclovir has been used to prevent CMV reactivation, either at the onset of laboratory evidence of CMV infection (by culture, polymerase chain reaction [PCR], or antigenemia assay) or as prophylaxis.7,17,18However, ganciclovir-related granulocytopenia has been reported in 30% to 60% of bone marrow transplant patients, complicating the routine use of this drug for prophylaxis in all CMV seropositive patients.7,17-19 This side effect is of particular concern in patients for whom slow engraftment is a potential problem and the myelosuppressive effects of this medication could lead to graft failure. As a result of drug-induced cytopenia, patients receiving ganciclovir can have increased transfusion requirements. The use of this drug has also been associated with delayed reconstitution of CMV-specific CTL posttransplant and an increased incidence of late CMV disease.45 The limitations of anti-CMV pharmacotherapy have promoted interest in adoptive immunotherapy as an alternative means for the prevention and treatment of CMV disease in SCT patients.
The application of adoptive immunotherapy has evolved as a result of an increased understanding of the cellular immune response to viral pathogens. Infected cells process endogenously synthesized viral polypeptides and present viral epitopes on the cell surface.46,47 CD8+ CTL recognize these epitopes in an HLA class I–restricted context and are activated to proliferate and lyse the infected cells. Thus, the prerequisite for adoptive immunotherapy is to have suitable APC that can present relevant viral epitopes. Regarded as professional APC, BLCL express high levels of class I and II HLA molecules and costimulatory molecules such as intercellular adhesion molecule (ICAM)-1, B7/BB1, and lymphocyte function-associated antigen (LFA)-3,48-50 in addition to key EBV antigens. Probably due to this reason, BLCL have been used to generate EBV-specific CTL cultures reliably by different laboratories, and as a result, adoptive immunotherapy is now performed routinely at some centers for EBV-LPD in TCD-SCT patients. The relative ease with which EBV CTL are cultivated using donor BLCL led us to investigate the possibility of using the same APC to generate CMV-specific CTL.
CMV pp65 was chosen as CMV-specific antigen based on its immunological and virological properties. The majority of CMV-specific CTL isolated from CMV seropositive patients recognize pp65.29,32,33 As a component of the virion tegument, pp65 is introduced into the host cells during virus entry. The exogenous pp65 has been shown to be presented by HLA class I molecules and targeted by CD8+CTL, possibly before the viruses multiply.51 Therefore, targeting this antigen may limit the spread of CMV infection at an early time point of CMV reactivation. Recently, attempts have been made to elucidate CMV HLA allele-specific epitopes for developing peptide vaccines. One nonamer pp65 epitope was shown to be immunogenic when presented in the context of HLA 0201.42 Although peptide-pulsed APC would be a promising tool to generate CTL culture for adoptive immunotherapy, it is limited, at least at this stage of development, to certain HLA alleles. In contrast, BLCLpp65 have the potential to present various pp65 epitopes, and it would be universally applicable to donors with diverse HLA alleles.
We chose a well-characterized recombinant retrovirus (MSCV) to achieve pp65 expression in BLCL. Retroviral vectors transduce rapidly dividing cells, such as BLCL, with high efficiency,35 making it possible to establish pp65-expressing BLCL in a timely fashion. All of the BLCL that we tested were readily transduced by this virus, and all of the pp65-transduced BLCL stimulated specific cytotoxicity in the EBV/CMV seropositive CTL cultures regardless of the heterogeneity of pp65 expression. Furthermore, MSCV is replication-defective, and careful selection of producer cells should eliminate the problem of contamination with replication-competent viruses resulting from fortuitous recombination. MSCVpp65 encoded only 2 products, the target antigen pp65 and a selection marker that appeared not to elicit a strong CTL reaction. A minimal number of transgenes encoded by a vector translates into fewer antigens presented and hence fewer nonrelevant CTL expanded.
Our method for generating CMV/EBV-specific CTL cultures has unique advantages and is feasible in terms of both resources and time. This approach would technically simplify adoptive T-cell transfer, as EBV- and CMV-specific CTL are generated simultaneously in the same culture. The use of BLCL as APC circumvents the problem of procuring skin biopsies from the donors and the time to grow fibroblasts. In addition, CMV/EBV CTL primed by BLCLpp65 can be cultivated in FBS, which is readily available, does not carry the risk of exposure to human infectious agents, and is presently in use by other investigators in adoptive immunotherapy. The CMV/EBV CTL generated in this study were not reactive against noninfected, allogeneic SF, suggesting that the lengthy procedure of CTL cloning may not be necessary. Time required to generate bispecific CTL cultures could be further shortened by transducing BLCL with high-titer retrovirus produced by the new generation of vector production systems.52,53 High titer retrovirus should greatly improve the transduction efficiency and as a result dispense with the need for G418 selection of the transduced cells.52 53 Alternatively, retroviral transduction could be performed at the same time as EBV transformation, eliminating the time required to generate pp65-expressing BLCL.
It should be noted that with BLCLpp65 as stimulators, CMVpp65-specific CTL cultures were generated only from CMV-seropositive, but not seronegative, individuals. Similar results have also been observed by others when CMV-infected fibroblasts were used as APC.20These results appear to suggest that B cells, similar to fibroblasts, are able to reactivate the memory CTL, but not to prime naı̈ve T cells. Thus, BLCL may not be suitable as APC to present CMV antigens for CMV seronegative individuals. Work is ongoing in our laboratory to determine which APC can prime in vitro expansion of CMV-specific CTL from seronegative donors.
Supported by Grant No. CRTG 97-043-EDT from the American Cancer Society and Grant No. R01 CA75566-01 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenneth Lucas, MD, Bone Marrow Transplantation Program, University of Alabama at Birmingham, 1900 University Blvd, THT 541, Birmingham, AL 35294; e-mail: klucas@uabmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal