Transfer of therapeutic genes to human hematopoietic stem cells (HSCs) using complex vectors at clinically relevant efficiencies remains a major challenge. Recently we described a stable retroviral vector that sustains long-term expression of green fluorescent protein (GFP) and a human β-globin gene in the erythroid progeny of transduced murine HSCs. We now report the efficient transduction of primitive human CD34+ fetal liver or cord blood cells with this vector and expression of the β-globin transgene in the erythroid progeny of these human cells for at least 2 months. After growth factor prestimulation and then a 2- to 3-day exposure to the virus, 35% to 55% GFP+ progeny were seen in assays of transduced colony-forming cells, primitive erythroid precursors that generate large numbers of glycophorin A+ cells in 3-week suspension cultures, and 6-week long-term culture-initiating cells. In immunodeficient mice injected with unselected infected cells, 5% to 15% of the human cells regenerated in the marrow (including the erythroid cells) were GFP+ 3 and 6 weeks after transplantation. Importantly, the numbers of GFP+ human lymphoid and either granulopoietic or erythroid cells in individual mice 6 weeks after transplantation were significantly correlated, indicative of the initial transduction of human multipotent cells with in vivo repopulating activity. Expression of the transduced β-globin gene in human cells obtained directly from the mice or after their differentiation into erythroid cells in vitro was demonstrated by reverse transcriptase–polymerase chain reaction using specific primers. These experiments represent a significant step toward the realization of a gene therapy approach for human β-globin gene disorders.
Introduction
Inherited disorders of β-globin gene expression pose a major health burden worldwide. Hematopoietic stem cell (HSC)–based gene therapy has long been considered an attractive treatment modality for these diseases because of the known ability of small numbers of HSCs to permanently repopulate the entire hematopoietic system.1-4 However, progress in realizing this approach has been slow, in part because of problems in designing constructs able to direct high-level, stable expression of human β-globin genes in human erythroid cells, and in part because conditions for transducing transplantable human HSCs at clinically useful efficiencies have been difficult to identify. Recent studies with murine transplant models have demonstrated the promise of oncoretroviral vectors carrying human β-globin genes under the control of the β-globin promoter and critical elements of the β-globin locus control region (LCR) in transduced murine HSCs.5,6 Advances have also occurred in HSC gene transfer procedures using oncoretroviral vectors that require rapid transit of the infected target cell into mitosis (to allow entry of the proviral integration complex into the host nucleus) without loss of HSC functional potential. These take advantage of culture conditions demonstrated to support human HSC self-renewal divisions in vitro7-11 and the delineation of the kinetics of these events12,13 as well as a number of physical and biologic strategies for enhancing virus-target cell interactions.14-18 As a result gene transfer efficiencies of more than 5% to human HSCs using relatively simple vectors have now been obtained in several clinical studies.19-22
More complex vectors have generally been less stable in viral producer cells with the generation of subgenomic and rearranged forms and typically low titers of useful virus. This has been particularly true of β-globin vectors where inclusion of genomic sequences appears necessary to achieve the sustained high-level erythroid-specific expression required for a therapeutic effect.5 Selection of transduced cells prior to transplantation greatly minimizes the problem of transgene silencing and using this strategy we were able to demonstrate the stable expression for many months of a human β-globin gene in the red cells of mice receiving transplants with HSCs that had been transduced with a murine stem cell virus (MSCV)–based β-globin vector containing the core sequence of the hypersensitive site 2 (HS2) of the β-globin LCR.6 These results led us to evaluate the ability of this virus to direct expression of the same β-globin construct in human cells. For this purpose, we chose to work initially with human fetal liver based on the observation that fetal liver cells produce larger numbers of mature erythroid cells both in vitro and in vivo23 24 and the expectation that this might facilitate detection of the transduced human β-globin gene. We found that it was possible to generate stable clones of cells producing a virus pseudotyped with the gibbon ape leukemia virus (GALV) envelope at titers sufficient to transduce primary human cells at useful efficiencies. Evidence of transduction of transplantable human cells from both fetal liver and cord blood was obtained and expression of the transduced β-globin gene in derivative erythroid cells demonstrated.
Materials and methods
Retroviral vector and packaging cell lines
The MSCV-HS2-β-globin–green fluorescent protein (GFP) vector used in these experiments has been described in detail previously6 and is shown schematically in Figure1A. To obtain virus for the human cell infections, the following procedure was followed. First, Bosc cells were transfected with the vector plasmid as previously described26 and these were then cultured in α Dulbecco modified Eagle medium (αDMEM) plus 10% fetal calf serum (FCS) for 24 hours after which the supernatant was harvested, filtered, and used for repeated infections of GPE+86 ecotropic packaging cells in the presence of 8 μg/mL of polybrene (Sigma Chemical, St Louis, MO). Single GFP+ GPE+86 cells were then isolated using the fluorescence-activated cell sorter (FACS) and expanded. The highest titer clone was identified by titration of supernatants on NIH 3T3 cells (American Type Culture Collection [ATCC], Rockville, MD) and supernatants from these cells then used to repeatedly infect confluent PG13 cells25 in the presence of 8 μg/mL polybrene. Single GFP+ PG13 cells were also cloned using the FACS and the highest titer clone (∼105/mL) identified by titration of the supernatants on K562 cells (ATCC). The PG13 producer clone was shown to be free of helper virus by a helper assay using K562 cells.27
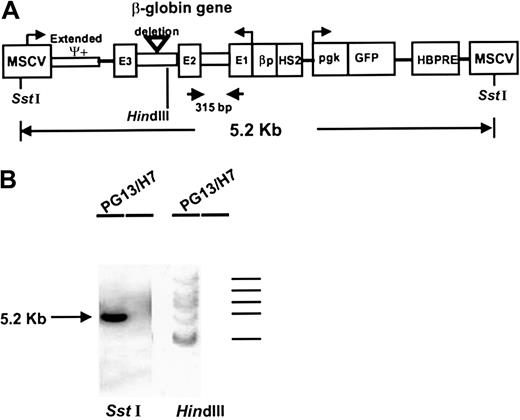
Schematic representation of the MSCV-HS2-β-globin-GFP retroviral vector and its integration into PG13 producer cells (clone H7).
Panel A shows the different parts of the vector,6 which consists of a 5.2-kb fragment flanked with 2 LTRs derived from the MSCV, a human β-globin cassette containing the HS2 domain, the β-globin promoter (βp), and the genomic β-globin gene (3 exons, 2 introns) in reverse orientation. The vector also contains a humanized EGFP gene under the control of a phosphoglycerate kinase (pgk) promoter. A hepatitis B virus posttranscriptional regulatory element (HBPRE) has been inserted to enhance RNA transport from the nucleus. The arrows show the location of the β-globin sequence to which specific primers were made and the 315-bp fragment amplified by RT-PCR for detection of transgene-derived transcripts. ψ indicates the site of the extended packaging signal. Panel B shows a Southern blot ofSstI-digested DNA (left) and HindIII-digested DNA from the PG13 producer cell clone (H7) probed with a GFP probe. TheSstI blot shows a single band corresponding to the expected full-length vector. The HindIII blot indicates that 5 copies of the vector had been integrated into these producer cells.
Schematic representation of the MSCV-HS2-β-globin-GFP retroviral vector and its integration into PG13 producer cells (clone H7).
Panel A shows the different parts of the vector,6 which consists of a 5.2-kb fragment flanked with 2 LTRs derived from the MSCV, a human β-globin cassette containing the HS2 domain, the β-globin promoter (βp), and the genomic β-globin gene (3 exons, 2 introns) in reverse orientation. The vector also contains a humanized EGFP gene under the control of a phosphoglycerate kinase (pgk) promoter. A hepatitis B virus posttranscriptional regulatory element (HBPRE) has been inserted to enhance RNA transport from the nucleus. The arrows show the location of the β-globin sequence to which specific primers were made and the 315-bp fragment amplified by RT-PCR for detection of transgene-derived transcripts. ψ indicates the site of the extended packaging signal. Panel B shows a Southern blot ofSstI-digested DNA (left) and HindIII-digested DNA from the PG13 producer cell clone (H7) probed with a GFP probe. TheSstI blot shows a single band corresponding to the expected full-length vector. The HindIII blot indicates that 5 copies of the vector had been integrated into these producer cells.
Human cells
Human fetal livers were obtained from 14- to 21-week-old aborted fetuses. The age of the human embryo was determined by a foot length measurement. Cord blood cells were obtained from normal deliveries by cesarean section. Approved institutional procedures for obtaining informed consent were followed in both cases. Single-cell suspensions were prepared from minced livers by incubation in a solution of dispase II (2.4 U/mL, Boehringer Mannheim, Laval, PQ) in phosphate-buffered saline (PBS; StemCell Technologies, Vancouver, BC) and then trituration of the pieces in an EGTA/EDTA dissociation buffer (Gibco BRL, Burlington, ON).28 Low-density (< 1.077 g/cm3) cells were isolated from both sources of human cells by density centrifugation on Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). Populations further enriched in their content of CD34+ and CD34+38− cells were then obtained by removal of cells bearing lineage markers expressed by mature cells (lin+ cells) using a StemSep column according to the supplier's directions (StemCell Technologies).
Transduction protocol and assessment of transduced cells
Cells were first washed and then stimulated for 2 days (in the absence of virus) in tissue culture dishes in serum-free medium (Iscoves modified Dulbecco medium [IMDM] plus bovine serum albumin–insulin-transferrin [BIT; StemCell Technologies]) containing 10−4 β-mercaptoethanol (Sigma) and 100 ng/mL human Flt3 ligand (FL; Immunex, Seattle, WA), 100 ng/mL Steel factor (transfected COS cell supernatant, Terry Fox Laboratory), 20 ng/mL human interleukin 3 (IL-3; Novartis, Basel, Switzerland), 20 ng/mL IL-6 (Cangene, Mississauga, ON), and 20 ng/mL human granulocyte-colony stimulating factor (G-CSF; StemCell Technologies) at 2 × 105 cells/mL. In 2 experiments, 50 ng/mL human thrombopoietin (TPO; Genentech, San Francisco, CA) was also added. Two thirds of the prestimulated cells were then washed and resuspended in freshly thawed virus-conditioned medium (VCM), or IMDM/10% FCS as a control, plus the same growth factors as before plus 5 μg/mL protamine sulfate (Sigma). For the supernatant infections, this suspension was placed into Petri dishes that had been precoated with 1 μg/cm2 CH-296 (Retronectin; Takara Biomedicals, Shiga, Japan) and then “preloaded” with virus by incubating the Retronectin-coated dishes for 60 minutes at room temperature with freshly thawed VCM (or IMDM/10% FCS [StemCell Technologies] as a control). The next day the cells were harvested, centrifuged, and resuspended in freshly thawed VCM plus growth factors and protamine sulfate and returned to the original dishes. In some cases this procedure was repeated on the third day. For the coculture infections, producer cells (or NIH 3T3 cells as a control) were plated in tissue culture dishes in αMEM/10% FCS and then irradiated (30 Gy 280 KvP x-rays) when 90% or more confluency was achieved. The growth factor–stimulated target cells were then added as in the supernatant infections. After 2 or 3 days of exposure to virus (both protocols) the infected nonadherent cells were combined with the adherent cells released by trypsin after prior depletion of most of the producers by 2 consecutive 1-hour secondary incubations at 37°C in fresh tissue culture dishes.
Aliquots of the recovered cells were then assessed by FACS directly (fetal liver) or after being cultured for another 2 days (without virus) in fresh serum-free medium containing the same growth factors as were used in the prestimulation cultures to determine the proportion of total and CD34+ GFP+ cells present. Other aliquots were plated directly into colony-forming cell (CFC) and long-term culture-initiating cell (LTC-IC) assays (see below) and the proportion of GFP+ colonies obtained directly (or in the 6-week CFC assays of the LTCs) determined by direct visualization under a fluorescent microscope. Additional aliquots were plated directly in erythroid expansion cultures (see below) and the proportion of propidium iodide (PI)− GFP+glycophorin A+ cells present 3 weeks later determined by FACS (see below). Cells injected into mice were also obtained immediately at the end of the transduction protocol without further incubation in vitro.
Flow cytometry
To determine the CD34+ cell content of starting cell populations and gene transfer efficiencies to the CD34+cells present at the end of the infection protocols, cells were first incubated for 10 minutes at 4°C in cold Hanks balanced salt solution supplemented with 2% fetal calf serum (HF; StemCell Technologies) plus 5% pooled normal human serum (HF/5% HS), to block Fc receptors and minimize nonspecific binding of labeled antibodies. The cells were then labeled for 30 minutes at 4°C with antihuman CD34–fluorescein isothiocyanate (FITC)-8G1229 or CD34-Cy5 (for analysis of retrovirally transduced cells) and CD38-phycoerythrin (PE; Becton Dickinson, San Jose, CA) or antiglycophorin A-PE 10F7 antibody. Cell suspensions were then washed twice with HF, the last wash containing 1 μg/mL PI (Sigma Chemical) and analyzed on a FACSort (Becton Dickinson) using Cell Quest software (Becton Dickinson).
Cells harvested from the marrow of immunodeficient mice that received transplants of transduced cells (see below) were stained with antihuman CD34-Cy5 plus CD19-PE and CD20-PE (Becton Dickinson) or antihuman CD15-PE (Pharmingen, Mississauga, ON, Canada) or antihuman CD45-PE (Becton Dickinson) and CD71-PE (OKT-9), or antihuman glycophorin A–PE 10F7. Cells harvested from the marrow of immunodeficient mice that received transplants with manipulated but nontransduced human cells were stained with antihuman CD34-FITC 8G12 plus CD19-PE and CD20-PE (Becton Dickinson) or antihuman CD71-PE (OKT-9) and CD45-PE plus CD15-FITC and CD66b-FITC (Pharmingen), or antihuman glycophorin-A–FITC 10F7 and Ter-119-PE (Pharmingen) to determine the proportion of mice positive for these human populations. Positivity in all of these analyses was defined as the presence in the PI− gate of more than 5 events per 2 × 104 analyzed with a fluorescence that exceeded 99.98% of that obtained with irrelevant isotype-matched control antibodies labeled with the same fluorochrome. Control suspensions of marrow cells from immunodeficient mice that did not receive transplants stained with the same antibodies were routinely negative.
In vitro progenitor assays
The CFCs were assayed in methylcellulose medium (H4330; StemCell Technologies) supplemented with 50 ng/mL human Steel factor and 20 ng/mL IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF; Novartis), IL-6, G-CSF, and 3 U/mL human erythropoietin (Epo; StemCell Technologies) as described.30 Cells were assayed for LTC-ICs in 6-week cocultures containing mixed feeders of M2-10B4 and Sl/Sl murine fibroblasts genetically engineered to produce human IL-3 (10 ng/mL), G-CSF (130 ng/mL), and Steel factor (10 ng/mL).30 Fetal liver LTC-IC values were determined by dividing the total CFC output obtained from bulk culture assays by 72 based on previous experiments showing this to be the average CFC output of LTC-ICs from human fetal liver assayed in this way.24Erythroid differentiation cultures were performed essentially as described31 by incubating the cells for the first 9 days in αDMEM/plus 15% FCS plus 1 U/mL Epo, 100 ng/mL human SF, 40 ng/mL insulinlike growth factor-1 (IGF-1; R & D systems, Minneapolis, MN), 10−6 M freshly dissolved hydrocortisone (Sigma), 10−6 M 17β-estradiol (Sigma), 1.28 μg/mL iron-saturated transferrin (StemCell Technologies) and 10−4 M β-mercaptoethanol, with half-media changes every 2 days. The cells were then harvested and cultured for another 11 days in αDMEM/plus 15% FCS, with 1 U/mL Epo, 1 μg/mL insulin (Sigma), 1.28 μg/mL iron-saturated transferrin (StemCell Technologies), and 10−4 M β-mercaptoethanol, again with half-media changes every 2 days.
Animals and in vivo studies
NOD/LtSz-scid/scid (nonobese diabetic/severe combined immunodeficient [NOD/SCID]) mice32 and NOD/LtSz-scid/scid β2-microglobulin null (NOD/SCID-β2m−/−) mice33were bred and maintained in microisolators in the animal facility of the British Columbia Cancer Research Centre (Vancouver, BC). Six- to 8-week-old mice were sublethally irradiated with 350 cGy from a137Cs source the day prior to being intravenously injected with human cells. Mice were killed 6 weeks after transplantation and cells flushed from the shafts of the 4 hind leg long bones using a syringe and 21-gauge needle prefilled with HF/5% HS and a single-cell suspension obtained by gentle aspiration. Cells were stained and assessed for their content of human cells. The number of human cells of a given phenotype per mouse was calculated by multiplying the percent positive cells by 4 times the total number of cells harvested from 2 femurs and 2 tibias (based on the report that these 4 bones contain approximately 25% of the total marrow mass of the adult mouse34). The frequency of human fetal liver cells able to repopulate sublethally irradiated NOD/SCID and NOD/SCID-β2m−/− mice with both lymphoid and myeloid cells was determined as previously described by limiting dilution analysis of the proportions of mice injected with defined numbers of cells that were not positive for both of these human populations (ie, both CD34−CD19/20+ cells and CD45/71+CD 15/66b+ cells).35,36Secondary transplantations were performed by harvesting cells from primary NOD/SCID mice and transplanting 3.5 to 35 × 106of their pooled bone marrow cells per secondary mouse (ie, 3.5%-22% of the total marrow of a primary mouse per secondary mouse based on the assumption that 2 femurs and 2 tibias represent 25% of the total mouse marrow).34 Secondary mice were 6- to 8-week-old sublethally irradiated NOD/SCID mice that were then killed for assessment of the presence of human cells in their marrow another 6 weeks later.
Reverse transcriptase–polymerase chain reaction analysis
Total RNA was extracted using a commercial kit (Trizol, Gibco BRL) and reverse transcribed (RT) by random priming using 1 μg total RNA and superscript II reverse transcriptase (Gibco BRL) at 42°C for 30 minutes, followed by denaturation at 72°C for 10 minutes and snap cooling to 4°C for 5 minutes. A polymerase chain reaction (PCR) was then performed using primers specific for the retroviral β-globin gene (5′-GAG AAG TCC GCC GTT ACT GTT-3′ and 3′-GAA GTT CTC AGG ATC CAC GT-5′) to amplify the expected 315–base pair (bp) fragment. After 40 cycles of denaturation (30 seconds at 94°C), annealing (30 seconds at 58°C), and extension (60 seconds 72°C), the PCR products were separated on a 1.5% tris acetate EDTA (TAE) agarose gel and a Southern blot performed using a β-globin probe labeled with32P by random priming.
Southern blot analysis
Southern blot analysis was performed on the selected high producer clone of PG13 cells (clone H7 (PG13H7) using standard methods for DNA isolation and SStI or HindIII digestion. SstI cuts once in each LTR to release a 5.2-kb fragment encompassing the intact proviral sequence, andHindIII cuts once within the β-globin gene insert to detect unique fragments after incorporation of the vector into host cell DNA (Figure 1A). The enhanced green fluorescent protein (EGFP) gene was labeled with 32P by random priming and used as a probe.
Results
Efficiency of gene transfer of the MSCV-HS2-β-globin-GFP virus to human progenitors detected in vitro using a supernatant infection protocol
In a first series of experiments, lin− human fetal liver cell preparations enriched for CD34+ or CD34+CD38− cells were infected with the MSCV-HS2-β-globin-GFP virus using the supernatant infection protocol described in “Materials and methods.” The frequency of gene transfer to various types of progenitors detectable in vitro was then assessed by measurements of GFP expression in their progeny. The results are summarized in Table 1. Although the proportions of all cells (and all CD34+ cells) immediately after transduction that were GFP+ were 2-fold higher in the cells that were initially selectively enriched for CD34+CD38− cells (P < .05), both target populations yielded similar frequencies (∼55%) of GFP+ colonies in the CFC assays of these cells. Similar proportions (36%) of glycophorin A+ GFP+ cells were also obtained in the 3-week erythroid differentiation cultures. LTC-IC assays were performed only in the experiments using the fetal liver cells that had been enriched for lin−CD34+CD38− cells. The transduction efficiency measured on the colonies obtained from the 6-week colony assays of these cells showed that a similar proportion (41%) of these primitive progenitors had been transduced. Results of subsequent experiments using lin− cord blood cells (enriched for CD34+ cells) were similar (Figure2).
Gene transfer efficiencies to various cell types in cultures of CD34+lin− and CD34+38−lin− human fetal liver cells after their supernatant transduction with a MSCV-HS2-β-globin-GFP virus
| Target cells . | % GFP+ total cells . | % GFP+ CD34+cells . | % GFP+ CD34+38−cells . | % GFP+ CFCs . | % GFP+ LTC-IC-derived CFCs . | % GFP+ glycophorin A+cells from 3-wk erythroid cultures . |
|---|---|---|---|---|---|---|
| CD34+lin− | 37 ± 5 | 19 ± 5 | ND | 53 ± 2 | ND | 36 ± 7 |
| (n = 3) | (n = 3) | (n = 3) | (n = 2) | |||
| CD34+38−lin− | 73 ± 6 | 47 ± 5 | 38 ± 9 | 58 ± 15 | 41 ± 27 | 36 ± 7 |
| (n = 5) | (n = 5) | (n = 5) | (n = 5) | (n = 3) | (n = 4) |
| Target cells . | % GFP+ total cells . | % GFP+ CD34+cells . | % GFP+ CD34+38−cells . | % GFP+ CFCs . | % GFP+ LTC-IC-derived CFCs . | % GFP+ glycophorin A+cells from 3-wk erythroid cultures . |
|---|---|---|---|---|---|---|
| CD34+lin− | 37 ± 5 | 19 ± 5 | ND | 53 ± 2 | ND | 36 ± 7 |
| (n = 3) | (n = 3) | (n = 3) | (n = 2) | |||
| CD34+38−lin− | 73 ± 6 | 47 ± 5 | 38 ± 9 | 58 ± 15 | 41 ± 27 | 36 ± 7 |
| (n = 5) | (n = 5) | (n = 5) | (n = 5) | (n = 3) | (n = 4) |
Values shown are the mean ± SEM percentages of the total number of cells or colonies expressing GFP after infection of lin-depleted cells (with or without removal of CD38+ cells as shown).
ND indicates not done.
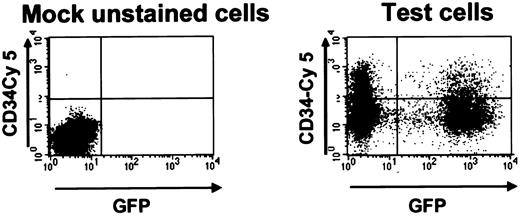
GFP+ cord blood cells after transduction with the MSCV-HS2-β-globin-GFP virus.
FACS dot plot of cells harvested 2 days after transduction and stained with antihuman CD34-Cy5. The left panel shows results for mock-transduced unstained cord blood cells and the right panel for the test cells. In this example 39% of all cells and 35% of the CD34+ cells were GFP+.
GFP+ cord blood cells after transduction with the MSCV-HS2-β-globin-GFP virus.
FACS dot plot of cells harvested 2 days after transduction and stained with antihuman CD34-Cy5. The left panel shows results for mock-transduced unstained cord blood cells and the right panel for the test cells. In this example 39% of all cells and 35% of the CD34+ cells were GFP+.
Efficiency of transfer of the MSCV-HS2-β-globin-GFP virus to transplantable human cells
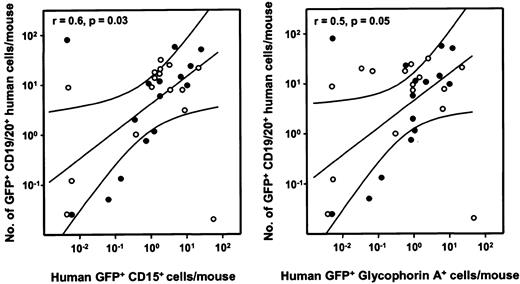
Transduced fetal liver and cord blood cells were also injected intravenously into sublethally irradiated NOD/SCID and NOD/SCID-β2m−/− mice and the presence of GFP+ human cells assessed in the marrow of these mice 6 weeks later. The frequencies of different types of human cells detected in mice injected with unselected fetal liver cells immediately after transduction are shown in Table 2. Although absolute levels of engraftment were highly variable in both types of immunodeficient hosts, significantly higher numbers of human progeny were evident in the NOD/SCID-β2m−/−mice when the human cell outputs were normalized to the number of CD34+ cells from which the transplant was derived (Table2). On the other hand, the proportions of total human cells regenerated in the 2 types of mice that were B lymphoid (CD19/20+) versus granulopoietic (CD15+) or erythroid (glycophorin A+) cells were not significantly different (P > .15 and P > .35, respectively). In mice repopulated with detectable numbers of GFP+ human cells, these were consistently represented in all of these 3 lineages of differentiating human cells. An example of the multilineage reconstitution observed in such mice is shown in Figure3 (bottom panels). For comparison, results obtained with mock-transduced cells are also shown (Figure 3, top panels). As shown in Figure 4, when the numbers of GFP+ human progeny in the B-lymphoid and granulopoietic or erythroid compartments in individual recipients were analyzed, these values could be seen to be significantly correlated.
Multilineage reconstitution of immunodeficient mice with human fetal liver cells transduced with the HS2-β-globin-GFP vector
| No. of experiments . | Target cells* . | Recipient (no. of cells injected/mouse)† . | % human cells‡ (% of human cells that are GFP+)2-153 . | |||
|---|---|---|---|---|---|---|
| CD45/71+ . | CD19/20+ . | CD15+ . | Glycophorin-A+ . | |||
| 3 | CD34+lin− | NOD/SCID | 19 ± 9 | 11 ± 5 | 4 ± 2 | 4 ± 2 |
| (15 ± 1.5 × 106) | (19 ± 4) | (12 ± 5) | (5 ± 2) | (3 ± 1) | ||
| n = 11 | ||||||
| 5 | CD34+38−lin− | NOD/SCID | 0.2 ± 0.1 | 0.05 ± 0.05 | 0.05 ± 0.05 | 0.1 ± 0.02 |
| (2 ± 0.4 × 106) | (4 ± 3) | (36 ± 19) | (4 ± 3) | (4 ± 3) | ||
| n = 4 | ||||||
| NOD/SCID-β2m−/− | 22 ± 9 | 14 ± 5 | 2 ± 1 | 3 ± 1 | ||
| (3.5 × 105) | (13 ± 4) | (12 ± 3) | (7 ± 3) | (6 ± 3) | ||
| n = 19 | ||||||
| No. of experiments . | Target cells* . | Recipient (no. of cells injected/mouse)† . | % human cells‡ (% of human cells that are GFP+)2-153 . | |||
|---|---|---|---|---|---|---|
| CD45/71+ . | CD19/20+ . | CD15+ . | Glycophorin-A+ . | |||
| 3 | CD34+lin− | NOD/SCID | 19 ± 9 | 11 ± 5 | 4 ± 2 | 4 ± 2 |
| (15 ± 1.5 × 106) | (19 ± 4) | (12 ± 5) | (5 ± 2) | (3 ± 1) | ||
| n = 11 | ||||||
| 5 | CD34+38−lin− | NOD/SCID | 0.2 ± 0.1 | 0.05 ± 0.05 | 0.05 ± 0.05 | 0.1 ± 0.02 |
| (2 ± 0.4 × 106) | (4 ± 3) | (36 ± 19) | (4 ± 3) | (4 ± 3) | ||
| n = 4 | ||||||
| NOD/SCID-β2m−/− | 22 ± 9 | 14 ± 5 | 2 ± 1 | 3 ± 1 | ||
| (3.5 × 105) | (13 ± 4) | (12 ± 3) | (7 ± 3) | (6 ± 3) | ||
| n = 19 | ||||||
Lin-depleted cells (with or without removal of CD38+ cells as indicated).
The number shown is the mean number of initial CD34+ or initial CD34+CD38− target cells from which the transduced cells injected (per mouse) were generated (± SEM).
Expressed as a percent of the total cells harvested from both femurs and both tibias of each mouse. Values shown are the mean ± SEM from the pooled data from all (n) mice.
Expressed as a percent of all human cells of the type analyzed. Values shown are the mean ± SEM from the pooled data from all (n) mice.
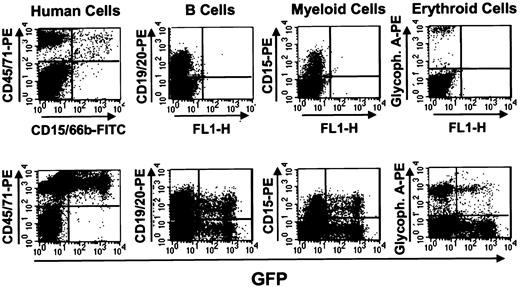
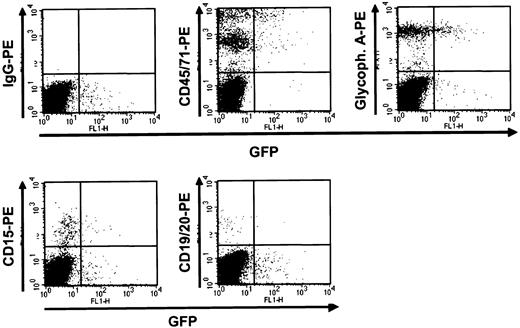
Detection of human GFP+ cells in mice that received transplants of transduced human fetal liver cells 6 weeks previously.
NOD/SCID mice that received transplants of mock-transduced (top panels) and MSCV-HS2-β-globin-GFP virus-transduced (bottom panels) human fetal liver cells were killed after 6 weeks and the marrow cells analyzed by FACS after staining with antibodies to the different human hematopoietic lineages shown. In this example a high degree of engraftment with human cells was attained in both mice (left panels) and in the recipient of transduced cells, a high proportion of all lineages of human cells produced were GFP+.
Detection of human GFP+ cells in mice that received transplants of transduced human fetal liver cells 6 weeks previously.
NOD/SCID mice that received transplants of mock-transduced (top panels) and MSCV-HS2-β-globin-GFP virus-transduced (bottom panels) human fetal liver cells were killed after 6 weeks and the marrow cells analyzed by FACS after staining with antibodies to the different human hematopoietic lineages shown. In this example a high degree of engraftment with human cells was attained in both mice (left panels) and in the recipient of transduced cells, a high proportion of all lineages of human cells produced were GFP+.
Correlation analysis of GFP+ human lymphoid and GFP+ human myeloid cells in individual recipients of transduced human fetal liver cells.
Left panel: The number of GFP+ human CD19/20+B-lymphoid cells per mouse is plotted as a function of the number of GFP+ human CD15+granulopoietic cell numbers in the same mice for 15 NOD/SCID (●) and 16 NOD/SCID-β2m−/− mice (○). A similar plot is shown on the right for GFP+ human CD19/20+B-lymphoid cells numbers as a function of GFP+ human glycophorin A+ erythroid cells in the same mice. Values shown were calculated assuming that the content of 2 tibias and 2 femurs represents 25% of the total marrow mass of a mouse.34 The correlation coefficients for each set of data are shown in the top left corner of the corresponding panel.
Correlation analysis of GFP+ human lymphoid and GFP+ human myeloid cells in individual recipients of transduced human fetal liver cells.
Left panel: The number of GFP+ human CD19/20+B-lymphoid cells per mouse is plotted as a function of the number of GFP+ human CD15+granulopoietic cell numbers in the same mice for 15 NOD/SCID (●) and 16 NOD/SCID-β2m−/− mice (○). A similar plot is shown on the right for GFP+ human CD19/20+B-lymphoid cells numbers as a function of GFP+ human glycophorin A+ erythroid cells in the same mice. Values shown were calculated assuming that the content of 2 tibias and 2 femurs represents 25% of the total marrow mass of a mouse.34 The correlation coefficients for each set of data are shown in the top left corner of the corresponding panel.
For these experiments each recipient mouse received transplants from the transduced progeny of relatively large numbers of originally lin− CD34+ or CD34+CD38− fetal liver cells (estimated to contain ∼10 multipotent cells capable of engrafting NOD/SCID mice with both lymphoid and myeloid cells for at least 6 weeks35). Nevertheless, some recipients failed to be engrafted with any human cells. We therefore undertook a series of experiments with mock-infected cells to determine the magnitude of loss of repopulating activity by human fetal liver cells under these culture conditions. The results of these assays, shown in Table3, indicated a 90-fold decrease in the lymphomyeloid cells able to repopulate NOD/SCID mice for 6 weeks at the end of the 5-day transduction protocol. This was accompanied by a reduced but still marked decrease (20-fold) in lymphomyeloid cells able to engraft NOD/SCID-β2m−/− mice for a similar period. However, cells from primary NOD/SCID recipients showing high engraftment with transduced human cells were still able to regenerate multilineage human hematopoiesis for 6 weeks when they were transferred to secondary NOD/SCID mice (data not shown). These findings indicate that the 5-day prestimulation transduction culture procedure did not eliminate the self-renewal potential of human hematopoietic cells able to engraft primary mice.
Frequencies of human fetal liver cells able to regenerate lymphomyelopoiesis in NOD/SCID and NOD/SCID-β2m−/− mice and their decrease under conditions used for retroviral transduction
| . | NOD/SCID hosts . | NOD/SCID-β2m−/−hosts . |
|---|---|---|
| Starting cells | 1/7 000 | 1/16 000 |
| (1/2 500-1/18 000) | (1/9 000-1/31 000) | |
| Posttransduction cells | 1/640 000 | 1/300 000 |
| (1/330 000-1/1 300 000) | (1/150 000-1/600 000) | |
| Loss | ∼ 90-fold | ∼ 20-fold |
| . | NOD/SCID hosts . | NOD/SCID-β2m−/−hosts . |
|---|---|---|
| Starting cells | 1/7 000 | 1/16 000 |
| (1/2 500-1/18 000) | (1/9 000-1/31 000) | |
| Posttransduction cells | 1/640 000 | 1/300 000 |
| (1/330 000-1/1 300 000) | (1/150 000-1/600 000) | |
| Loss | ∼ 90-fold | ∼ 20-fold |
All values are expressed per the number of CD34+cells injected. Values in brackets show 95% CIs for data from 34 NOD/SCID mice and 16 NOD/SCID-β2m−/− mice injected with transduced cells, from a total of 3 and 4 experiments, respectively.
Because of the likelihood that most clinical gene therapy protocols for treating human β-globin disorders would not make use of fetal liver cells as targets, we also undertook a small number of experiments with human cord blood cells. In these, lin− cord blood cells (∼60% CD34+) were infected using the supernatant transduction protocol and then immediately injected intravenously into sublethally irradiated NOD/SCID-β2m−/−mice. The mice were then killed 3 weeks later when the number of mature erythroid cells reaches a peak in these mice.36 As illustrated in Figure 5, readily detectable GFP+ cells were seen in all of the lineages of maturing human cells detected in these recipients and at similar levels. The percent GFP+ cells was 5% ± 1% for all (CD45/71+) human cells, 5% ± 2% for human (glycophorin A+) erythroid cells, 5% ± 2% for human (CD15+) granulopoietic cells, and 8% ± 3% for (CD19/20+) B-lymphoid cells. These results confirm the ability of human hematopoietic cells able to generate erythroid progeny in vivo to be transduced with the same human β-globin–encoding vector used to transduce primitive human fetal liver cells and at a similar efficiency.
GFP+ human cells present in NOD/SCID-β2m−/− mice that received transplants of MSCV-HS2-β-globin-GFP–-transduced human cord blood cells 3 weeks previously.
Mice received transplants of cells transduced as described in the text and marrow cells removed 3 weeks later were then analyzed by FACS for GFP and expression of human lineage–specific markers. Results shown here are for cells from a representative mouse. The top left panel shows results for cells not stained with the antihuman antibodies.
GFP+ human cells present in NOD/SCID-β2m−/− mice that received transplants of MSCV-HS2-β-globin-GFP–-transduced human cord blood cells 3 weeks previously.
Mice received transplants of cells transduced as described in the text and marrow cells removed 3 weeks later were then analyzed by FACS for GFP and expression of human lineage–specific markers. Results shown here are for cells from a representative mouse. The top left panel shows results for cells not stained with the antihuman antibodies.
Expression of the transduced human β-globin transgene in human erythroid cells
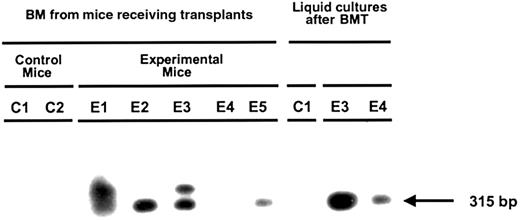
Aliquots of bone marrow cells from mice that received transplants of transduced human fetal liver cells 6 weeks previously were analyzed by RT-PCR for evidence of human β-globin gene expression directly after harvest or after being placed in liquid erythroid expansion cultures for 3 weeks as described in “Materials and methods.” As illustrated in Figure 6, about 95% of all the cells obtained after 3 weeks under these conditions were terminally differentiating human erythroid cells (glycophorin A+ Ter 119−) of which 35% were typically still expressing GFP. Figure 7 shows a representative Southern blot of the products of an RT-PCR reaction applied to RNA extracted from different sources of human cells generated from transduced fetal liver cells. Evidence of transcripts, albeit at low levels (estimated at < 1% of endogenous β-globin messenger RNA) that could be amplified only from the transgene (Figure1), was obtained both from cells harvested directly from mice reconstituted with human cells (4 of 5 mice tested) and from cells generated in 3-week erythroid expansion cultures (2 of 2 cases tested). Interestingly, in one of the latter cases, the cells obtained from the expansion culture were positive, whereas no transcripts were apparent in the directly harvested cells.
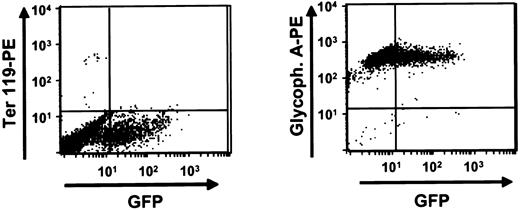
Production of human erythroid cells in liquid expansion cultures.
Marrow cells obtained from mice engrafted for 6 weeks with transduced human fetal liver cells were placed in liquid expansion cultures and maintained for 3 weeks as described in “Materials and methods” at the end of which they were stained with antihuman glycophorin A (right panel) and Ter 119 (left panel) to detect any amplified murine erythroid cells. Shown here is the FACS dot plot obtained from a representative experiment in which the cells used to initiate the culture were from an engrafted NOD/SCID-β2m−/− mouse.
Production of human erythroid cells in liquid expansion cultures.
Marrow cells obtained from mice engrafted for 6 weeks with transduced human fetal liver cells were placed in liquid expansion cultures and maintained for 3 weeks as described in “Materials and methods” at the end of which they were stained with antihuman glycophorin A (right panel) and Ter 119 (left panel) to detect any amplified murine erythroid cells. Shown here is the FACS dot plot obtained from a representative experiment in which the cells used to initiate the culture were from an engrafted NOD/SCID-β2m−/− mouse.
Expression of the MSCV-HS2-β-globin-GFP vector human β-globin transgene in the erythroid progeny of transduced human fetal liver cells.
RNA was extracted from cells harvested from the marrow of mice engrafted with transduced human fetal liver cells (E1-5) and control mice engrafted with mock-transduced human fetal liver cells (C1,2) and from 3-week cultures of human erythroid cells derived from these mice. The presence of transgene-specific β-globin messenger RNA was assessed by RT-PCR using primers that amplify the mutant sequence of the β-globin transgene (Figure 1) not present in the normal human β-globin gene.
Expression of the MSCV-HS2-β-globin-GFP vector human β-globin transgene in the erythroid progeny of transduced human fetal liver cells.
RNA was extracted from cells harvested from the marrow of mice engrafted with transduced human fetal liver cells (E1-5) and control mice engrafted with mock-transduced human fetal liver cells (C1,2) and from 3-week cultures of human erythroid cells derived from these mice. The presence of transgene-specific β-globin messenger RNA was assessed by RT-PCR using primers that amplify the mutant sequence of the β-globin transgene (Figure 1) not present in the normal human β-globin gene.
Discussion
This study represents part of a multistep effort to develop useful vectors for the gene therapy of patients with hemoglobinopathies arising from mutations in the β-globin locus. Recently we showed that a MSCV-GFP oncoretroviral construct containing a previously described human β-globin cassette5 could permanently endow transduced murine HSCs with the ability to express readily detectable levels of human β-globin protein in their erythroid progeny.6 Here we show that the same construct can be used to generate virus in the PG13 packaging line at a titer that can transduce transplantable human multilineage HSCs at a readily detectable efficiency (5%) with documentation of expression of the transduced β-globin gene in erythroblasts generated from these cells both in vitro and in vivo. To our knowledge, this represents the first report of successful transfer and expression of a human β-globin gene in primary human cells and the levels of gene transfer achieved are similar to those shown to be clinically efficacious in a recent elegant study of patients with SCID-X1 syndrome.20 22
It is important to note, however, that the ease of obtaining benefit from a gene therapy approach varies significantly from one disease setting to another. In the case of SCID patients, even low levels of transgene expression are sufficient to improve the disease and also to confer a significant selective advantage on the growth of the lymphoid progeny of the transduced HSCs. In contrast, for any β-globin gene therapy application, a number of additional challenges will need to be addressed before clinical trials can be contemplated. High levels of β-globin gene expression will be required and strategies for conferring a selective growth advantage on the transduced HSCs or their erythroid progeny will also be necessary to avoid the need for myeloablative preparative regimens. Unfortunately, in the present experiments, all attempts using high-performance liquid chromatography to detect human β-globin protein in the transduced erythroid cells generated proved unsuccessful. This indicates a need for additional improvements in vector design to achieve higher viral titers and levels of expression. Possibilities to address these latter issues include the use of other regulatory elements,37,38 other packaging systems,39,40 and other vector backbones (eg, lentiviral constructs41).
We chose to use human fetal liver cells as the first source of primary human target cells because this organ contains high numbers of erythroid precursors as well as transplantable HSCs.42Also we had recently found that human fetal liver HSCs generate much higher numbers of erythroid progenitors in NOD/SCID mice than their counterparts in cord blood or adult bone marrow,43suggesting that their use might allow easier detection of transduced β-globin gene expression in progeny erythroblasts.24,44-46 On the basis of preliminary experiments that showed conditions optimized for transducing transplantable human HSCs from cord blood,12,47-50 and adult bone marrow13 or mobilized peripheral blood21 could be applied to human fetal liver, we adopted a similar protocol for the transduction of human fetal liver. The results obtained, particularly given the low viral titer, were surprisingly good (30%-50% of progenitors detectable in vitro became GFP+) by comparison to previous studies (15% by PCR on colonies45 and 5%-10% of all types of clonogenic cells with β-gal staining44). This discrepancy with our data may be due to our use of more highly purified starting populations (that would increase the multiplicity of infection), longer exposure to cytokines, and the use of the fibronectin fragment (CH-296).44 45
Nevertheless, we also found that the price of this efficient gene transfer protocol was a huge (90-fold) loss of the most primitive type of transplantable fetal liver HSCs currently detectable51by the end of the 4- to 5-day in vitro transduction protocol. Some of this loss may be unavoidable resulting from repeated centrifugation of the cells or difficulties in harvesting all of the HSCs at the end of the infection procedure. On the other hand, it was reassuring to see that exposure to virus was not associated with any toxicity, because HSC losses were the same in mock-treated control samples. In fact, very few studies have quantitated the magnitude of the loss in HSCs that is widely known to occur during the prolonged retroviral infection protocols needed to attain the high levels of gene transfer to human HSCs reported by a number of groups.21,48,49,52 Moreover, these gene transfer efficiencies are always given for the HSCs present at the end of the transduction protocol and absolute yields of transduced HSCs are rarely reported. However, those that have indicate losses of cord blood HSCs in the order of 10- to 20-fold.53,54 The even greater losses measured here for transplantable fetal liver HSCs suggest that these HSCs are even more prone to death or differentiation in the presence of a growth factor combination that had been optimized for cord blood HSC transduction. It is interesting to note that, even in the mouse, defined culture conditions suitable for stimulating a net expansion of fetal liver HSCs in vitro have not yet been identified,55 in contrast to HSCs from adult marrow where a net expansion of these cells can be reproducibly achieved after 10 days in vitro.56 This suggests that during ontogeny, HSCs may undergo significant changes in the mechanism(s) they use to allow growth factor modulation of their continuing HSC status on being stimulated to divide.
It was therefore important to establish that the same viral vector used to transduce human fetal liver HSCs was also able to transduce human cord blood progenitors that give rise to erythroid progeny in vivo. For this we confined our studies to human myeloid-restricted short-term repopulating cell targets that give a peak but transient output of myeloid cells (including erythroid cells) 3 weeks after injection into NOD/SCID-β2m−/− mice.36 The results confirmed transduction of these cells at similar efficiencies as human fetal liver HSCs based on the proportion of human erythroid and granulopoietic progeny found to express the GFP marker transgene in the 3-week posttransplantation mice.
Taken together these experiments provide an important next step toward the successful development gene therapy approaches to the treatment of human β-globin gene disorders including β-thalassemia and sickle cell disease. Additional major advances should be possible using lentiviral vectors encoding human β-globin gene constructs based on the one used here. Indeed, recent studies in murine disease models indicate these may have curative potential due to the higher levels of product expression achievable in murine cells.41,57 The efficient transduction of human HSCs by lentiviral vectors obtainable using much shorter culture protocols38 58-61 should result in further practical benefits due to significant improvements in absolute yields of transduced HSCs.
We thank the staff of the Stem Cell Assay Service for their assistance in the initial preparation of primary human cell samples, Gayle Thornbury and Giovanna Cameron for operating the FACS, and Amy Ahamed for secretarial assistance. We are also grateful to Amgen, Cangene, Isolab, Novartis, Takara Biomedicals, and StemCell Technologies for valuable gifts of cells or reagents and Dr L. Shultz for NOD/SCID β2m−/−-mice.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI: 10.1182/blood-2002-02-0599.
Supported by a grant from the National Institutes of Health (PO1 HL55435). I-H.O. held a Postdoctoral Fellowship from the National Cancer Institute of Canada (NCIC) and C.J.E. was a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Connie J. Eaves, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail: ceaves@bccancer.bc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal